Abstract
Background
Intrathecal neostigmine produces analgesia, but also severe nausea. In contrast, epidural neostigmine enhances opioid and local anesthetic analgesia without causing nausea. Previous studies examined only single epidural neostigmine bolus administration and did not assess the efficacy of continuous epidural infusion or several aspects of maternal and fetal safety. We therefore tested the hypothesis that epidural neostigmine in combination with bupivacaine by continuous infusion during labor would reduce the amount of bupivacaine required.
Methods
Twelve healthy women scheduled for elective cesarean delivery were assigned to receive epidural neostigmine, 40 μg (first 6 subjects) or 80 μg (second 6 subjects) as a single bolus, with fetal heart rate and uterine contractions monitored for 20 minutes. In a subsequent experiment, 40 healthy laboring women were randomized to receive bupivacaine 1.25 mg/mL alone or with neostigmine 4 μg/mL by patient-controlled epidural analgesia. The primary outcome measure was hourly bupivacaine use.
Results
Epidural neostigmine bolus did not alter baseline fetal heart rate, induce contractions or produce nausea. Epidural neostigmine infusion reduced bupivacaine requirement by 19% in all patients and 25% in those with > 4 hours of treatment (P<0.05 for both), but might have contributed to the incidence of mild sedation. Mode of delivery, incidence of maternal nausea and fetal heart rate abnormality were similar between groups.
Conclusions
These data show that adding epidural neostigmine 4 μg/mL reduces the hourly bupivacaine requirement by 19% to 25% with patient-controlled epidural analgesia during labor. Administered as a bolus and by continuous infusion at the studied doses, epidural neostigmine does not cause nausea and does not induce uterine contractions or fetal heart rate abnormalities, but mild sedation can occur.
Introduction
Although local anesthetics alone provide effective epidural labor analgesia, their use can be complicated by maternal hypotension and motor block, especially with prolonged infusions. Dense motor block is unwanted and uncomfortable in this setting, and may increase the risk of instrumental delivery, as suggested by controlled studies and meta-analysis.1 For this reason, adjuvant opioids are added to decrease local anesthetic use and to minimize motor block.2 Neuraxial opioids, however, produce pruritus, increase the incidence of respiratory depression, and decrease fetal heart rate (FHR) variability.3 Additionally, extra time may be required to document use of a controlled substance.
Other drugs have been introduced as adjuvants to local anesthetics for neuraxial analgesia. Intrathecal injection of the cholinesterase inhibitor neostigmine increases extracellular acetylcholine concentrations within the spinal cord, leading to increased stimulation of spinal muscarinic and possibly nicotinic receptors to produce analgesia.4 After animal toxicity studies5 and Phase I safety studies,6,7 intrathecal neostigmine entered clinical trials, including laboring parturients, in which it demonstrated efficacy but also severe nausea and vomiting.8-10 In contrast, more recent studies of single epidural bolus administration of neostigmine have shown it to produce effective analgesia without severe nausea and vomiting.11-13
Few studies have examined the safety and efficacy of epidural neostigmine in obstetrics, and these are limited to single bolus studies, which demonstrated an analgesic effect associated with mild sedation without severe nausea or vomiting.14-16 Fetal bradycardia and enhanced uterine contractions have been noted with systemic or IV-administered neostigmine.17,18 Significant effects of neostigmine on FHR or uterine contractions could be overlooked during active labor. We therefore performed a 2-phase study. The purpose of the first phase was to assess safety by examining the effects of epidural neostigmine on uterine activity and FHR in term pregnant non-laboring women. For this study, we used a classic open label, dose escalating safety study design. The goal and power of this first phase was to observe for common (10-20% or more) and significant side effects, if any, and to help determine a safe neostigmine dose for initiation and continuous infusion of labor epidural analgesia.
In phase 2 of our study, we tested the hypothesis that continuous epidural neostigmine infusion would significantly reduce bupivacaine requirement in women during maintenance patient-controlled epidural analgesia (PCEA) for labor.
Methods
Regulatory Issues
Neostigmine intrathecal toxicity studies supporting bolus intrathecal administration have been reported previously,4,5 and the authors hold an Investigational New Drug approval from the United States Food and Drug Administration for clinical investigation of intrathecal neostigmine. Since fibrosis of epidural catheters develops rapidly in rats,19 we were unable to repeat toxicity testing by this route in the rat model. We are unaware of any drugs which are safe when administered intrathecally but neurotoxic when administered epidurally, even though the converse may occur.20 An independent Data Safety Monitoring Board reviewed the results, including an obstetrician's review of the FHR uterine contraction tracings, at the conclusion of each group of 10 subjects using standard criteria.21,22 This study consisted of 2 phases: (I) pilot safety assessment of epidural neostigmine prior to elective cesarean delivery in non-laboring patients, and (II) double-blind, randomized, controlled trial of continuous epidural neostigmine with bupivacaine compared to bupivacaine alone for labor analgesia. Neostigmine methylsulfate with preservatives (1mg/mL, American Regent, Inc, Shirley, NY, USA) and preservative-free bupivacaine (2.5mg/mL, Hospira, Inc, Lake Forest, IL USA), together with normal saline, were used to make the epidural study solutions. Intrathecal safety assessment for neostigmine methylsulfate with preservatives had been previously reported by the authors.6,7 The IRB, Wake Forest University School of Medicine, Winston-Salem, NC, approved both phases of the study.
I. Pilot Safety Assessment prior to elective cesarean delivery
Written informed consent for study participation was obtained from 12 healthy adult parturients scheduled for elective cesarean delivery. Peripheral IV access was established and all patients received a 15 mL/kg bolus of lactated Ringer's solution. A multiport epidural catheter was inserted at the L3-4 or L4-5 interspace. A 3 mL epidural test dose of lidocaine 20 mg/mL plus epinephrine 5 μg/mL was injected and, 5 min later, the first 6 patients received epidural neostigmine 40 μg in a 2 mL solution, while the next 6 patients received 80 μg. These doses were chosen to encompass the bolus dose and the likely hourly exposure patients would receive in the second phase of the study.
Continuous FHR, uterine contractions (with external tocodynometry), maternal vital signs and SpO2 were recorded prior to epidural catheter insertion and every 5 min for 20 min thereafter. Women were also asked to rate pain, nausea, pruritus and sedation using a verbal rating scale every 5 min for 20 min, and immediately prior to transfer to the operating room and in the postanesthesia care unit. The verbal rating scale ranged from 0 to 10 by self-assessment with 0 being no pain, nausea, pruritus, or sedation, respectively, and 10 being the worst pain imaginable, worst nausea, worst pruritus or asleep.
At the end of this 20-min observation period, women received 2–chloroprocaine 30 mg/mL by incremental epidural injection to establish anesthesia. Maternal vital signs were recorded every 5 min or more frequently, if needed, during initiation of epidural anesthesia and surgery. Medication administered and obstetrical, surgical and neonatal outcomes were recorded. FHR, external tocodynometry and SpO2 were continuously monitored until transfer of the patients to the operating room. Patients were queried regarding onset or presence of uterine contractions every 5-10 min, and maternal vital signs were recorded every 5 min. FHR and uterine contraction tracings were evaluated at a later time by an obstetrician blinded to type of drug treatment. Supplemental intraoperative anesthesia consisted of additional epidural 2-chloroprocaine and fentanyl and intravenous morphine as needed. Once in the postanesthesia care unit dermatomal level of sensory analgesia was evaluated at 15 min intervals until sensory blockade to pinprick receded to the T10 dermatome.
II. Double-Blind, Randomized, Controlled Trial in Labor
Written informed consent for study participation was obtained prior to patient's request for labor epidural analgesia from 50 ASA physical status I or II women, weight < 114 kg, with a single fetus in active labor and cervical dilation ≤ 6 cm. Epidural analgesia was initiated at patient request and with a minimum verbal pain score of 4 or more. A lumbar epidural catheter was inserted and a combined IV and intrathecal test dose consisting of lidocaine 45 mg and epinephrine 15 μg was administered. A computer-generated random number table was used to provide randomization of patient allocation which was concealed in sealed envelopes. At the time of patient's request for epidural analgesia administration, an attending anesthesiologist (who was familiar with the study protocol but not involved with the care of the patient or data collection) then opened the sealed envelope for group assignment and prepared the study solution. The patients, the investigators, and the research personnel involved in the data collection, as well as the personnel involved in the medical care of the patients, were all blinded to the assignment and study drug. Patients were randomized to receive 15 mL (in 5 mL increments) of bupivacaine 1.25 mg/mL or bupivacaine 1.25 mg/mL with neostigmine, 0.004 mg/mL. If the patient failed to achieve analgesia (failed analgesia was defined as verbal pain score > 3) 20 minutes after this injection, they were excluded from the study and the epidural catheter replaced.
After successful analgesia (verbal pain score ≤3) was achieved, maintenance epidural analgesia was initiated with the assigned solution via PCEA (basal infusion rate: 6 mL/h, PCEA bolus: 5 mL, lockout interval: 10 min, maximum dose: 30 mL/h). Breakthrough pain in the first stage of labor was treated with an anesthesiologist-administered bolus of 5-10mL bupivacaine 2.5mg/mL to comfort (verbal pain score ≤3) and 2-chloroprocaine 20 mg/mL or lidocaine 20 mg/mL, 5-10 mL (in 5 mL increments) in the second stage of labor. Patients were excluded from the study if they required more than 1 anesthesiologist-administered dose per 2 hours or were not comfortable (verbal pain score > 3) after receiving 10 mL bupivacaine 2.5mg/mL during the first stage of labor.
Pain was assessed before epidural catheter insertion, at 5 min after test dose, every 5 min for the first 20 min after the initial study drug administration, then every 2 hours until delivery. Maternal vital signs were recorded at the same times as pain assessments or more frequently, if indicated. Dermatomal level of sensory block to pinprick testing, degree of motor block (using a 0-3 scale 23), maternal sedation and nausea (using a 0-10 verbal rating scale self-assessment) were assessed at the same times as pain assessments. The patients were instructed to rate their sedation based on their subjective feeling of sedation/sleepiness using a verbal rating score with 0 being wide awake and not feeling sleepy at all to 10 being asleep. Nausea was rated with 0 being no nausea to 10 being worst nausea imagined. Maternal hypotension was defined as a decrease of 20% from systolic blood pressure obtained at admission. The presence of shivering (by patient self-assessment), maternal hypotension and abnormalities of FHR pattern,21,22 mode of delivery, infant weight, 1 and 5 min Apgar scores, total volume of study solution, PCEA bolus demands and anesthesiologist-administered bolus doses and any medication administered were recorded. FHR and uterine contraction tracings for the duration of labor after initiation of epidural analgesia were evaluated by an obstetrician21,22 blinded to patient group assignment. After delivery before patients returned to the postpartum ward, the patients were asked to rate the overall degree of satisfaction of epidural analgesia for the duration of labor using a 1-3 verbal scale (1=unsatisfactory analgesia, 2=satisfactory analgesia, and 3=excellent analgesia).
Data Analysis
Data are presented as mean ± SD or incidence (%). Based on our obstetric unit quality assurance data and previous observations,2 we estimated the total hourly consumption of bupivacaine (including anesthesiologist-administered boluses) during labor to be the equivalent of 14 mL of bupivacaine 1.25mg/mL with a standard deviation of 3.0 mL. For the labor study, a prior sample size analysis with the above assumption and with a β of 0.20 and α of 0.05 demonstrated that a sample size of 20 per group would allow us to detect a 20% difference in total epidural drug required per hour. Prior to study initiation, we planned a separate analysis of drug use among those with labor analgesia > 4 h. The primary outcome variable analyzed in the labor study was hourly bupivacaine use, using a 2-tailed t test. Incidence and proportion data were compared between groups using Chi Square or Fisher's exact test. Sedation, shivering, pruritus, nausea and motor block were considered present if the score was more than zero. Nonparametric data were analyzed with Mann-Whitney U tests. Change in continuous measures over time within each dose group was determined using one way analysis of variance for repeated measures, and 2-way analysis of variance for comparison between groups. P < 0.05 was considered significant.
Results
I. Pilot Safety assessment prior to cesarean delivery
Study participants were 30 ± 7-years-old, 164 ± 8 cm tall, and 82 ± 19 kg in weight. The median gravidity and parity were 2 and 1, respectively, and median gestational age was 39 weeks. Neostigmine was injected 5-10 min after the epidural test dose in all but 1 case, in which there was a 58 min interval due to an obstetric delay. At the time of neostigmine injection, a sensory level to pinprick was present in 8 of 12 women from the epidural test dose, with a median dermatomal level of T11 bilaterally. Fifteen min after neostigmine injection, 9 of 12 women exhibited hypesthesia to pinprick testing, with a median dermatomal level of T10 on the left and T8 on the right. Two women had intermittent uterine contractions prior to neostigmine injection and had no interval change in frequency of contractions over the next 15 min after neostigmine injection. The obstetrician evaluating the FHR and uterine contraction tracings did not consider the uterine contractions in these 2 women to have temporal relationship or cause-effect relationship with the neostigmine injection. The mean FHR was 137 ± 18 bpm at the time of neostigmine injection, and 139 ± 18 bpm 15 min later. There were no episodes of fetal bradycardia or alterations in long-term beat-to-beat variability as assessed by external monitoring. Maternal arterial blood pressure, heart rate, and level of pain, pruritus and sedation were not altered after epidural neostigmine (data not shown). One patient in each dose group experienced nausea after neostigmine injection, but in each case it was rated 1 on the 0-10 scale. The mean interval from neostigmine injection to local anesthetic injection was 22 min (excluding 1 case in which the neostigmine injection to local anesthetic injection was 98 min due to an obstetrical delay). Uterine incision occurred 68 ± 31 min after neostigmine injection. Maternal arterial blood pressure and heart rate did not differ between neostigmine dose groups, and 4 of 6 women in each group received ephedrine for hypotension after epidural administration of local anesthetic. All neonates had a 1 min Apgar score > 7 and a 5 min Apgar score > 8.
II. Randomized Controlled trial in labor
Fifty women were recruited, with 10 excluded from study due to no block and inadequate analgesia from the initial epidural dose (3 in bupivacaine + neostigmine group), epidural catheter dislodgement (1 in bupivacaine + neostigmine group), failure of the epidural analgesia requiring ≥ 2/2h anesthesiologist administered boluses during labor (1 in each group), recognition of breech presentation after epidural catheter was inserted (1 in bupivacaine alone group), protocol violation due to IV butorphanol administration within 30 min prior to epidural catheter placement (2 excluded before randomization for group assignment), or informed consent obtained but the study never initiated (1 excluded before randomization for group assignment). The bupivacaine alone (n=20) and bupivacaine + neostigmine (n=20) groups of the remaining 40 women who completed the study did not differ in demographic or labor characteristics (Table 1).
Table 1. Patient and Labor Characteristics of Laboring Patients.
| Study Group (Neostigmine + Bupivacaine) (N= 20) | Control Group (Bupivacaine Alone) (N = 20) | |
|---|---|---|
| Age (year) | 27±6 | 29±7 |
| Height (cm) | 166±5 | 164±9 |
| Weight (kg) | 84±14 | 84±15 |
| Gestation (weeks) | 39±1 | 39±1 |
| Parous (%) | 45 % | 45 % |
| Oxytocin Use (%) | 55 % | 75 % |
| Oxytocin Infusion Rate (mU/min) at time of epidural catheter placementa | 7.0±10.3 | 8.4±7.6 |
| Fetal weight (grams) | 3350±540 | 3370±450 |
| VRS score - baselineb | 7.6 ± 1.8 | 6.9 ± 2.2 |
| VRS score -20 minc | ||
| Mean ± SD | 0.6 ± 1.3 | 0.8 ± 1.3 |
| Median | 0 | 0 |
| Mode | 0 | 0 |
| Range | 0 – 3 | 0 – 3 |
| Cervical dilation prior epidural placement (cm) | 3.1 ± 1.3 | 3.3 ± 1.3 |
| Cervical dilation 2 hr after epidural placement (cm) | 4.7 ± 1.5 | 4.3 ± 1.5 |
Average of all patients including those with or without oxytocin use at time of epidural catheter placement.
VRS before initiation of epidural analgesia.
20 min after injection of epidural study solution.
VRS = verbal rating scale. Data are presented as mean ± SD or incidence (%) unless otherwise indicated. There are no statistical differences between groups for any variable.
Safety and outcome of labor
Maternal arterial blood pressure was similar in the 2 groups prior to initiation of analgesia and was reduced to a similar degree during initiation of epidural analgesia (Figure 1A). Five patients in the bupivacaine alone group and 7 in the bupivacaine + neostigmine group received treatment for hypotension (P=NS). Maternal heart rate between contractions before and after epidural analgesia was similar between groups and was not affected by epidural analgesia (data not shown). FHR was similar between groups before and after epidural analgesia (Figure 1B), and there was 1 case in each group of variable FHR decelerations within the 20 min of initial epidural dose administration. One patient in each group underwent cesarean delivery for non-reassuring FHR. The obstetrician (blinded to group/drug assignment) evaluating the FHR and uterine contraction tracings in the context of the patient's obstetric history and clinical presentation did not find any of these changes to be temporally or directly associated with the study or control drug administration. Groups did not differ in Apgar scores and there were no 1-minute scores of <7 or 5-minute scores of <8 in either group.
Figure 1.
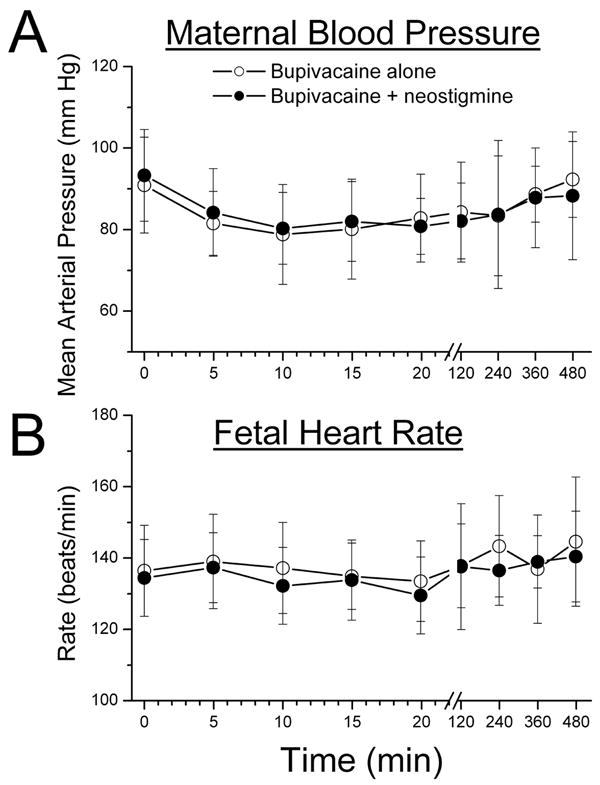
A) Mean maternal arterial blood pressure and B) Mean fetal heart rate after epidural analgesia, initiated at time 0, with bupivacaine, 1.25mg/mL, alone (open circles) or with neostigmine, 4 μg/mL (filled circles). No difference between groups by 2-way analysis of variance. The whisker represents the standard deviation of the data.
Progress of labor was unaffected by the addition of neostigmine. Cervical dilation was similar between groups at the time of initiation of analgesia and 2 hr later (Table 1). Additionally, for all those who reached complete cervical dilation, the time until complete cervical dilation did not differ between bupivacaine alone (5.1±3.8 h) and bupivacaine + neostigmine (4.6±.2.2 h). The mode of delivery did not differ between groups, with 4 cesarean deliveries in each, and 1 forceps and 1 vacuum extraction in the bupivacaine + neostigmine group.
The sedation score was increased compared to baseline from 5 to 20 min after initiation of analgesia in the bupivacaine + neostigmine group (P<0.05), but not in the bupivacaine-alone group. The incidence of sedation also increased over the first 20 min in the bupivacaine + neostigmine group, but not in the bupivacaine alone group (Figure 2A); P < 0.05. There was no significant difference in sedation scores between groups at any time. The incidence of sedation in the bupivacaine + neostigmine group was not increased after the initial dosing period (Figure 2A). The intensity of sedation among those reporting a non-zero score did not differ at any time between the groups. However, the median maximum sedation scores were 0 (range 0-10) for bupivacaine alone group and 3 (range 0-6) for bupivacaine + neostigmine group, while the mode was 0 for both groups. Groups also did not differ in incidence or severity of motor block at any time during the study period. The median and mode of the non-zero motor blockade score were 1 for both groups. The mode and median maximum motor blockade scores from each patient were both 1 (range 0-2) for both groups. Groups did not differ in the incidence of pruritus or shivering at any time, and the incidence of these effects did not increase after initiation of analgesia (data not shown). The incidence of nausea was not different between groups (Figure 2B). One patient in the bupivacaine alone group, received treatment for nausea.
Figure 2.
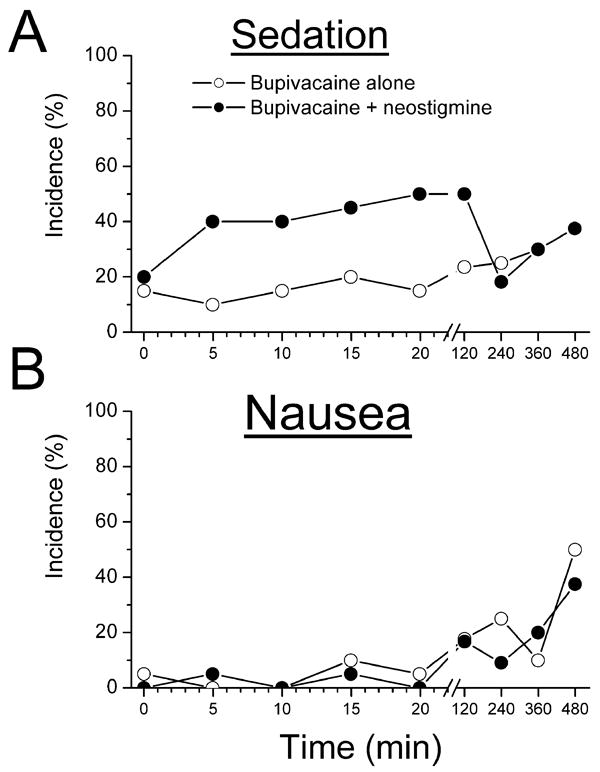
Incidence of A) sedation (non-zero verbal sedation scores) and B) nausea (non-zero verbal nausea scores) after epidural analgesia, initiated at time 0, with bupivacaine, 1.25mg/mL, alone (open circles) or with neostigmine, 4 μg/mL (filled circles). No difference between groups by 2-way analysis of variance. The sedation score was increased compared to baseline from 5 to 20 min after initiation of analgesia in the bupivacaine + neostigmine group by 1-way repeated measures analysis of variance. (P<0.05)
Efficacy
Pain scores rapidly decreased (Table 1) after initiation of analgesia, and did not differ between groups at any time during the study. The median number of patient demands with PCEA was greater in the bupivacaine alone group than in the bupivacaine + neostigmine group (13 vs. 4 respectively; P<0.05). The groups did not differ, however, in the number of patients requiring physician-administered top-up doses (9 vs. 7 respectively) or the number of top-up doses among those who received at least 1 physician-administered dose (median value 1, range 1 and 3, and interquartile range 1 and 2, for both groups). The mean bupivacaine dose was 14.7 ± 4.7 mL/h in the bupivacaine group versus 11.9 ± 3.0 mL/h in the bupivacaine + neostigmine group (P<0.05) in all patients, and 15.6 ± 3.3 mL/h versus 11.7 ± 2.7 mL/h, respectively, in patients with prolonged (> 4hr) analgesia (P < 0.05). Twenty-five patients (12 in bupivacaine group and 13 in bupivacaine + neostigmine group) received prolonged analgesia. The addition of neostigmine reduced the mean hourly epidural dose of bupivacaine by 19% in the entire population and by 25% in those receiving study solution for more than 4 hr (both P < 0.05; Figure 3). Groups did not differ in satisfaction with analgesia (data not shown).
Figure 3.
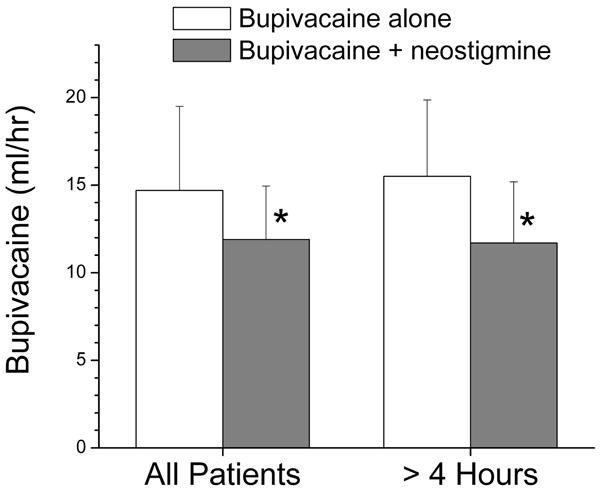
Mean hourly bupivacaine use (mL/h) in women receiving epidural analgesia with bupivacaine, 1.25mg/mL, alone (open bars) or with neostigmine, 4 μg/mL (filled bars). Data are presented for all patients in the study and for those with study drug duration > 4 hr. *P < 0.05 compared to bupivacaine alone. The whisker represents the standard deviation of the data.
Discussion
This study demonstrates an absence of a significant direct effect on FHR and uterine contractions from epidural neostigmine (up to 80 μg bolus) at term gestation in the absence of labor or analgesia. This study also demonstrates a bupivacaine-sparing effect with continuous administration of low concentration (4 μg/mL) epidural neostigmine similar to that observed with fentanyl2 without the presence of nausea, pruritus or apparent adverse FHR or neonatal effect. Interestingly, we confirmed previous observations14 of neostigmine-induced sedation after a neostigmine bolus, but this side effect was mild and not sustained with continued treatment.
Intrathecal neostigmine in large doses for prolonged periods failed to cause neurotoxicity in animals,4, 5 and clinical trials of intrathecal and epidural neostigmine have raised no concerns regarding neurotoxicity. Intrathecal neostigmine has been associated with severe nausea and vomiting.6-10 This side effect is absent when neostigmine is administered by the epidural route, although the reasons for this difference are obscure.11-13 Nonetheless, the current study supports previous observations that epidural neostigmine does not increase the incidence of nausea after a bolus during labor15,16 or after surgery11,13,14 and now extends this finding to the non-laboring women at term gestation (without the confounding effects of labor, analgesia and anesthesia) and to laboring women who receive neostigmine via patient-controlled continuous epidural infusion for labor analgesia.
Intrathecal9,10 and epidural14 bolus administration of neostigmine increases sedation scores, although no more than a moderate degree of sedation has been noted. Transient sedation with initiation of epidural or spinal analgesia in labor has been frequently observed, and may reflect maternal relaxation from the prolonged stress of sustained pain during labor or, in the case of opioids, from the drug itself. We observed an increase in incidence of sedation in the 20 min after initiation of labor analgesia when neostigmine was added to bupivacaine compared to bupivacaine alone, although the degree was mild (median maximum sedation score from each patient was 0 for bupivacaine alone group and 3 for bupivacaine + neostigmine group). Since degrees of pain relief did not differ between groups, we conclude that this sedation was at least in part due to neostigmine. Groups did not differ in Apgar scores and there were no 1-minute scores of <7 or 5-minute scores of <8 in either group. Although sedation was mild and shorter than 2 hours in duration, without any apparent detrimental maternal or neonatal effects, future studies are needed to determine more precisely whether the incidence, duration, and severity of this side effect would increase with doses and duration above what we studied. Prior to the study, we had anticipated the sedative effect of low dose epidural neostigmine to be very minimal if any. Therefore, we chose to use a self-assessed verbal rating score especially for measuring the low level of sedative effect as perceived by the patients. This form of self-assessed verbal rating sedation score has been used and validated with high correlation with Observer's Assessment of Alertness/Sedation score and Bispectral Index, especially for measuring the mild degree of sedation from epidural or spinal anesthesia.24,25 In the bupivacaine + neostigmine group, the highest maximum sedation score obtained for anyone was 6, whereas the median maximum sedation score was only 3. However, such self-assessed sedation verbal rating score has its limitation at the high end of the score (such as 9 or 10), in which the patient is asleep or too sleepy to accurately differentiate the different level of much deeper sedation. If much deeper sedation level (such as in difficult to arouse patients) is anticipated, observer's assessment, such as Observer's Assessment of Alertness/Sedation, may be more appropriate than self-assessment.
Stimulation of spinal cholinergic receptors can increase sympathetic nervous system activity,18 which may lead to decreased uteroplacental perfusion. We found no evidence for this effect with neostigmine at the doses we studied; there were no differences from the control group in maternal arterial blood pressure or heart rate or differences in the incidence of FHR abnormalities. The lack of effect of neostigmine on blood pressure may be related to dose of neostigmine or relative contributions of neostigmine and bupivacaine at the doses and concentrations studied. Additionally, systemic absorption of neostigmine could result in stimulation of myometrial activity18 or, if transferred in adequate amounts to the fetus,17 in fetal bradycardia. We found no evidence of either effect in either patients about to undergo elective cesarean delivery or in patients in labor. We were, however, limited to detecting only major changes in uterine contraction frequency and could not detect changes in intensity since nearly all our study patients had external monitors. These observations are reassuring and fail to raise concerns regarding the safety of epidural neostigmine in obstetrics. Nonetheless, this study, as are previous ones in this setting,14-16 was inadequately powered to exclude uncommon side effects, and we believe that epidural neostigmine should remain an investigational therapy at this time.
This is the first study to examine continuous infusion of epidural neostigmine for labor analgesia. Others have demonstrated a much higher bolus dose of neostigmine (4 μg/kg), when added to ropivacaine 10mg, provided equivalent efficacy to ropivacaine 20 mg alone.15 Roelants and Lavand'homme also demonstrated that epidural neostigmine administered by bolus in combination with sufentanil was effective in reducing the dose of sufentanil needed to initiate labor analgesia, but they did not investigate the use of neostigmine by continuous infusion for maintenance of labor analgesia.16 We designed the labor phase of the study to investigate whether a low neostigmine dose (with minimal or no side effects) would reduce the dose of epidural bupivacaine if used as an adjuvant for both the initiation and maintenance (as an infusion) of labor analgesia. Without previous data on epidural neostigmine infusion for labor analgesia, we conservatively chose the neostigmine infusion dose and bolus dose to give a cumulative dose within the upper limit of safe administration based on reports of bolus administration, and the result of our phase I safety study. We used PCEA to allow women to titrate epidural study solutions. This method has been used in other studies to assess the local anesthetic-sparing properties of other additives.2,26,27 As expected, the degree of pain relief was similar between groups in the current study, reflecting effective patient titration. However, the number of demands for the bupivacaine-alone group was significantly greater than when neostigmine was added, and the mean hourly bupivacaine dose requirement during labor was less in the neostigmine group. There were no differences at any time in motor block between groups in our study. The current study was not designed or powered to determine whether the reduction in bupivacaine dose resulted in less motor block, but results with fentanyl1 would predict this to be the case. Additionally, we did not examine the dose-response for epidural neostigmine for continuous labor analgesia, but such dose-response studies in comparison to epidural bupivacaine with fentanyl (as control) or other adjuvants are needed.
In conclusion, epidural neostigmine, up to 80μg, does not alter maternal arterial blood pressure, FHR, or produce uterine contractions in non-laboring term, healthy pregnant women. The addition of neostigmine, 4μg/mL, reduces up to 25% of epidural bupivacaine requirement with PCEA during labor. Except for mild sedation during initiation of labor analgesia, no significant adverse effect on progress of labor or on the mother and the fetus was observed with neostigmine doses studied. Epidural neostigmine should be considered an experimental, but possibly promising, alternative to opioids as an adjunct to labor epidural analgesia.
Acknowledgments
Supported in part by grant GM48085 from the National Institutes of Health, Bethesda, MD. There are no disclosures, disclaimers or conflicts of interest.
Footnotes
Implications Statement: Epidural neostigmine, 4μg/mL, reduces the amount of bupivacaine 1.25mg/mL used with patient-controlled epidural analgesia by 25% during labor without causing nausea, suggesting it may be a useful adjunct for obstetric analgesia.
References
- 1.Sharma SK, McIntire DD, Wiley J, Leveno KJ. Labor analgesia and cesarean delivery: an individual patient meta-analysis of nulliparous women. Anesthesiology. 2004;100:142–8. doi: 10.1097/00000542-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Lyak SZ, Eisenach JC, Dobson CE. Patient controlled analgesia during labor. A comparison of three solutions with a continuous infusion control. Anesthesiology. 1990;72:44–49. doi: 10.1097/00000542-199001000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Benhamou D. Are local anesthetics needed for local anesthesia? Anesthesiology. 2004;101:271–2. doi: 10.1097/00000542-200408000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Yaksh TL, Dirksen R, Harty GJ. Antionocieptive effects of intrathecally injected cholinomimetric drugs in rat and cat. Eur Journal of Pharm. 1985;117:81–88. doi: 10.1016/0014-2999(85)90474-1. [DOI] [PubMed] [Google Scholar]
- 5.Yaksh TL, Grafe MR, Malkmus S, Rathbun ML, Eisenach JC. Studies on the safety of chronically administered intrathecal neostigmine methylsulfate in rats and dogs. Anesthesiology. 1995;82:412–427. doi: 10.1097/00000542-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1995;82:331–43. doi: 10.1097/00000542-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Eisenach JC, Hood DD, Curry R. Phase I human safety assessment of intrathecal neostigmine containing methyl-propylparabens. Anesth Analg. 1997;85:842–6. doi: 10.1097/00000539-199710000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Nelson KE, D'Angelo R, Foss ML, Meister GC, Hood DD, Eisenach JC. Intrathecal neostigmine and sufentanil for early labor analgesia. Anesthesiology. 1999;91:1293–98. doi: 10.1097/00000542-199911000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Owen MD, Ozsarac O, Sahin S, Uckunkaya N, Kaplan N, Magunaci I. Low dose clonidine and neostigmine prolong the duration of intrathecal bupivacaine-fentanyl for labor analgesia. Anesthesiology. 2000;92:361–365. doi: 10.1097/00000542-200002000-00016. [DOI] [PubMed] [Google Scholar]
- 10.D'Angelo R, Dean LS, Meister GC, Nelson KE. Neostigmine combined with bupivacaine, clonidine and sufentanil for spinal labor analgesia. Anesth Analg. 2001;93:1560–4. doi: 10.1097/00000539-200112000-00048. [DOI] [PubMed] [Google Scholar]
- 11.Lauretti GR, deOliverira R, Reis MP, Julino MC, Pereira NL. Study of Three different doses of epidural neostigmine co-administered with lidocaine for postoperative analgesia. Anesthesiology. 1999;90:1534–8. doi: 10.1097/00000542-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Lauretti GR, Gomes JMA, Reis MP, Pereira NL. Low dose of epidural ketamine or neostigmine, but not midazolam, improve morphine analgesia in epidural terminal cancer pain therapy. J Clin Anesth. 1999;11:663–68. doi: 10.1016/s0952-8180(99)00122-1. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama M, Ichinose H, Nakabayashi K, Satoh O, Yamamoto S, Namiki A. Analgesic effect of epidural neostigmine after abdominal hysterectomy. J Clin Anesth. 2001;13:86–9. doi: 10.1016/s0952-8180(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 14.Kaya FN, Sahin S, Owen MD, Eisenach JC. Epidural neostigmine produces analgesia but also sedation in women after cesarean section delivery. Anesthesiology. 2004;100:381–5. doi: 10.1097/00000542-200402000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Roelants F, Rizzo M, Lavand'homme P. The effect of epidural neostigmine combined with ropivacaine and sufentanil on neuraxial analgesia during labor. Anesth Analg. 2003;96:1161–6. doi: 10.1213/01.ANE.0000050480.73209.9C. [DOI] [PubMed] [Google Scholar]
- 16.Roelants F, Lavand'homme PM. Epidural neostigmine combined with sufentanil provides balanced and selective analgesia in early labor. Anesthesiology. 2004;101:439–44. doi: 10.1097/00000542-200408000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Clark RB, Brown MA, Lattin DL. Neostigmine, atropine, and glycopyrrolate: Does neostigmine cross the placenta? Anesthesiology. 1996;84:450–53. doi: 10.1097/00000542-199602000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Hotta H, Nakayama H, Suzuki H. Sympathetic and parasympathetic regulation of the uterine blood flow and contraction in the rat. J Auton Nerv Syst. 1996;59:151–58. doi: 10.1016/0165-1838(96)00019-7. [DOI] [PubMed] [Google Scholar]
- 19.Durant PAC, Yaksh TL. Epidural injection of bupivacaine, morphine, fentanyl, lofentanil, and DADL in chronically implanted rats: A pharmacologic and pathologic study. Anesthesiology. 1986;64:43–53. doi: 10.1097/00000542-198601000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi M, Bollen AW, Drasney K. Sodium bisulfite, scapegoat for chloroprocaine neurotoxicity. Anesthesiology. 2004;100:85–91. doi: 10.1097/00000542-200401000-00016. [DOI] [PubMed] [Google Scholar]
- 21.ACOG Practice Bulletin Clinical management guidelines of obstetrician-gyneocologists. Number 70, December 2005 (Replaces Practice Bulletin Number 62, May 2005). Intrapartum fetal heart rate monitoring. Obstet Gynecol. 2005;106:1453–60. doi: 10.1097/00006250-200512000-00053. [DOI] [PubMed] [Google Scholar]
- 22.National Institute of Child Health and Human Development Research Planning Workshop. Electronic fetal heart rate monitoring: Research guidelines for interpretation. Am J Obstet Gynecol. 1997;77:1385–90. [PubMed] [Google Scholar]
- 23.Bromage PR. Epidural Analgesia. Philadelphia, London, Toronto: WB Saunders; 1978. pp. 301–20. [Google Scholar]
- 24.Doufas AG, Wadhwa A, Shah YM, Lin CM, Haugh GS, Sessler Block-dependent sedation during epidural anaesthesia is associated with delayed brainstem conduction. Br J Anaesth. 2004;93:228–34. doi: 10.1093/bja/aeh192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollock JE, Neal JM, Liu SS, Burkhead D, Polissar N. Sedation during spinal anesthesia. Anesthesiology. 2000;93:728–34. doi: 10.1097/00000542-200009000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Eisenach JC, Grice SC, Dean DM. Patient-controlled analgesia following cesarean section; a comparison with epidural and intramuscular narcotics. Anesthesiology. 1988;68:444–48. doi: 10.1097/00000542-198803000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Owen MD, D'Angelo R, Gerancher JC, Thompson JM, Foss ML, Babb JD, Eisenach JC. 0.125% ropivacaine is similar to 0.125% bupivacaine for labor analgesia using patient-controlled epidural infusion. Anesth Analg. 1998;86:527–31. doi: 10.1097/00000539-199803000-00015. [DOI] [PubMed] [Google Scholar]


