Abstract
Approximately 25% of elderly patients scheduled for carotid endarterectomy (CEA) develop post-operative cognitive dysfunction (CD). We tested the hypothesis that the plasma levels of matrix metalloproteinase 9 (MMP-9) are predictive of moderate to severe CD after CEA. A total of 73 patients were prospectively enrolled in this Institutional Review Board approved study. Plasma samples were obtained at baseline and day 1 post-surgery. We measured the plasma concentrations of both MMP-9 and its inhibitor, tissue inhibitor of metalloproteinases 1 (TIMP-1). We estimated the MMP-9 activity by calculating the MMP-9:TIMP-1 ratio. The cognitive performance day 1 post-surgery was quantified with z-scores, using a control group who were undergoing spinal surgery. The criteria used to define CD was performance of ≥ 1.5 standard deviations worse than the control group; approximately 19% of eligible patients developed CD. Compared to patients without CD, this group had both higher total (81.66 ± 12.25 ng/mL versus [vs.] 43.18 ± 4.44 ng/mL, p = 0.005) and activity (0.88 ± 0.24 ng/mL vs. 0.54 ± 0.06 ng/mL, p = 0.003) MMP-9 levels at baseline. All of the results were adjusted for age, diabetes and neurovascular symptoms.
Keywords: Aged 60 and over, Cognition disorders, Endarterectomy, carotid, Matrix metalloproteinase 9, Post-operative complications
1. Introduction
Approximately 20% to 25% of patients undergoing carotid endarterectomy (CEA) develop cognitive dysfunction (CD) within the first month post-procedure.1 Although the mechanisms underlying CD are likely to be subtle and multifaceted, features of carotid atheromatous disease and blood–brain barrier dysfunction, two conditions dependent on inflammatory activity, are believed to influence cognitive outcome.2,3
Carotid artery plaque instability has previously been associated with neuroinflammation and a greater risk of cognitive decline.4 While numerous biomarkers have been implicated in the assessment of atheromatous plaque stability, inflammatory factors such as matrix metalloproteinases (MMPs) and the macrophages secreting them have emerged as significant markers of plaque vulnerability.5,6 More specifically, the proteolytic enzyme matrix metalloproteinase 9 (MMP-9) has been found at significantly increased concentrations and expression levels in unstable carotid plaques.7 Matrix metalloproteinases also contribute to blood brain barrier degradation and vasogenic edema following cerebral ischemia.8–10 The objective of our study was to compare baseline and postoperative MMP-9 plasma levels in patients with and without CD following CEA. We hypothesized that MMP-9 plasma levels would be predictive of cognitive performance following CEA.
2. Methods
This study, an analysis of 73 consecutive patients who, between 2005 and 2007, underwent elective CEA under general anesthesia, was approved by the Columbia University Medical Center Institutional Review Board (Clinical Trial Registration: NCT 00597883). All participants provided written informed consent. These patients were initially prospectively recruited as participants of an ongoing, observational study of cognitive change following CEA.1 Serum samples were obtained at the time of surgery (baseline). As part of this protocol, only patients ≥ 60 years old and who were able to perform neuropsychometric (NP) evaluations in English were included. Patients were excluded from the study at the first follow-up examination if they had: (i) a permanent neurological deficit, or (ii) post-operative stroke or pain scores of > 4.
2.1. Neuropsychometric evaluations
All patients underwent assessment using a brief battery of six NP tests (Controlled Oral Word Association; Boston Naming; Hopkins Verbal Learning; Rey Complex Figures; Halstead-Reitan Trails A and B; and Grooved Pegboard) that were chosen to represent a range of cognitive domains. Under the supervision of a neuropsychologist, the tests were administered by trained research assistants at least 3hours after any analgesic or sedative medication had been taken.
For each NP test, the change in performance from baseline to day 1 post-procedure was converted to a z-score.11 The z-scores were relative to the mean performance change within a control group of patients undergoing spinal surgery using a similar anesthetic protocol. A negative z-score value indicated cognitive performance worse than the mean of the control group. Control patients reporting pain levels of ≥ 5/10 on a verbal numeric scale were excluded, as this has been shown to negatively influence cognitive performance following surgery.12 As a global measure of cognitive performance, we calculated an average z-score by dividing the sum of all available z-scores by the total number of completed tests. Patients with an average z-score of ≤−1.5 were considered to have moderate to severe CD.
2.2. Biomarkers
For all of the CEA patients, 5 mL to 10 mL of blood was drawn from a radial arterial line into untreated tubes just after induction (baseline) and then 20 to 24 hours after surgery. Blood samples were centrifuged, the supernatants extracted and the plasma stored at −80°C. The total concentrations of MMP-9 were measured in the plasma using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA). Given that MMP-9 is secreted as an inactive enzyme, we used the same ELISA kit to measure the plasma levels of tissue inhibitor of metalloproteinases 1 (TIMP-1) and to estimate MMP-9 activity by calculating the MMP-9:TIMP-1 ratio. We also measured MMP-9 activity using enzyme-linked immunofluorescence (R&D Systems).
2.3. Surgical and anesthesia procedures
Patients were considered for CEA if they presented with symptomatic or asymptomatic carotid artery stenosis of ≥ 70%. All of the patients who underwent this procedure were placed in a supine position, with the head slightly rotated to allow optimal access to the vascular structures. A complete description of the surgical technique has been presented previously.13 Shunting was performed whenever significant changes were observed on the electroencephalogram performed after carotid artery cross-clamping.
All of the patients underwent general anesthesia with endotracheal intubation and standard monitoring. In those patients who were scheduled for CEA, a radial artery catheter, electroencephalography with 18 electrodes positioned according to the International 10–20 system, and transcranial Doppler were also used to monitor cerebral blood flow velocity, the presence of emboli and cerebral ischemia. Propofol or etomidate, succinylcholine or vecuronium, and fentanyl were used for induction of anesthesia. Isoflurane or sevoflurane and a nitrous oxide in oxygen (2:1) gas mixture were administered for anesthetic maintenance. All of the patients received neostigmine to reverse paralysis and glycopyrrolate to prevent bradycardia before emergence from anesthesia. They were then extubated in the operating room and recoveredfor 1 to 3 hours in a postoperative care unit (spine patients) or in the neuro-intensive care unit (carotid patients). All of the patients remained inthe hospital for a further 1 to 3 days for post-operative pain managementand NP testing.
2.4. Statistical analysis
The statistical analyses were performed (by JGG) using both Excel (Microsoft, Redmond, WA, USA) and JMP 7 software (SAS Institute, Cary, NC, USA). Results of all statistical tests were considered significant if the probability of a type I error (p value) was less than 0.05. Demographic, surgical and anesthetic variables in patients with and without CD were compared using non-parametric tests (Mann–Whitney U-test for continuous variables, Fisher’s exact test for categorical variables) to identify any potential confounders. We conducted an event-rate analysis by comparing either MMP-9 total levels or MMP-9 activity levels in patients with and without CD. Using multivariate regression models, all of the results were adjusted for known and potential confounders of cognitive function following carotid surgery (e.g. age, diabetes mellitus and symptomatic neurological disease).
3. Results
We prospectively collected plasma samples from 73 consecutive patients who were scheduled for elective CEA. A total of nine patients were then excluded–four due to incomplete cognitive testing and five due to significant electroencephalographic changes after cross-clamping of the carotid artery that required intraoperative shunting. This subgroup of five patients performed significantly worse than those patients who did not require intraoperative shunting (data not shown; the MMP-9 total levels as well as the MMP-9 activity levels were similar in patients who did and did not require intraoperative shunting).
3.1. Cognitive function
Twelve of the remaining 64 (19%) participants developed moderate to severe CD day 1 post-procedure. Viewed collectively, patients with CD tended to be older and were more likely to present with symptomatic carotid artery disease and neurological symptoms (Table 1). These two factors have previously been associated with a higher risk of CD following CEA.14 Age being a well-established risk factor for CD in and surgical and non-surgical patients with vascular disease,15 we found that the baseline cognitive performance was significantly worse in patients who developed post-operative CD. For these reasons, all of the analyses were statistically adjusted for both age and the presence of neurological symptoms at baseline.
Table 1.
Medical and demographic characteristics of patients with and without cognitive dysfunction (CD) following carotid endarterectomy.
| Patients with CD (n = 12) | Patients without CD (n = 52) | p value | |
|---|---|---|---|
| Age (IQR) | 75 (72–78) | 71 (63–77) | 0.06 |
| Male (%) | 11/12 (91) | 36/52 (69) | 0.16 |
| BMI (IQR) | 27.3 (25.1–29.4) | 26.9 (24.0–30.4) | 0.77 |
| Years of education (IQR) | 16 (13–18) | 16 (13–18) | 0.91 |
| Active smoking (%) | 11/12 (91) | 35/52 (67) | 0.15 |
| Hypertension (%) | 10/12 (83) | 40/52 (77) | 1.00 |
| Diabetes mellitus (%) | 3/12 (25) | 8/52 (15) | 0.42 |
| Dyslipidemia (%) | 11/12 (91) | 42/52 (81) | 0.67 |
| Neurological symptoms (%) | 4/12 (33) | 5/52 (9) | 0.06 |
| Cross-clamping duration (IQR) | 40 (31–62) | 36 (29–53) | 0.33 |
BMI = body mass index, CD = cognitive dysfunction, IQR = interquartile range
3.2. MMP-9 total and activity levels in plasma
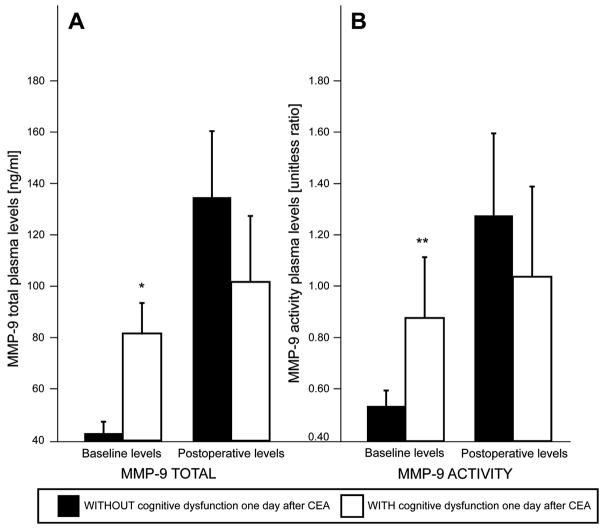
The median baseline and day 1 post-surgery MMP-9 total and activity levels are presented in Figure 1. Plasma levels for both total and activity MMP-9 increased in all patients following surgery, suggesting that surgical stress not only stimulated MMP-9 release, but also triggered enzymatic activation. In patients who had moderate to severe post-operative CD, at baseline the median MMP-9 total levels (Fig. 1A: 81.66 ± 12.25 ng/mL vs. 43.18 ± 4.44 ng/mL) and activity levels (Fig. 1B: 0.88 ± 0.24 ng/mL vs. 0.54 ± 0.06 ng/mL) were significantly higher than those patients who did not have CD. The p values were adjusted for pre-operative levels: MMP-9 total, p = 0.005; MMP-9 activity, p = 0.003. However, after surgery total median MMP-9 concentrations (Fig. 1A: 101.86 ± 25.70 ng/mL vs. 135.31 ± 26.34 ng/mL) and activity levels (Fig. 1B: 1.04 ± 0.35 ng/mL vs. 1.28 ± 0.32 ng/mL) were similar between the two groups.
Fig. 1.
Presentation of the matrix metalloproteinase 9 (MMP-9) plasma concentrations in patients with and without cognitive dysfunction (CD) 1 day following carotid endarterectomy showing that patients who have early post-operative CD have higher total MMP-9 levels and activity levels at baseline compared to patients without early postoperative CD., Post-operatively the total MMP-9 and activity levels were similar between the two groups. * Adjusted to pre-operative levels p = 0.005; ** adjusted, p = 0.003.
We indirectly measured MMP-9 activity by calculating the ratio of MMP-9 to its inhibitor, TIMP-1. Results obtained with this accepted measure of enzymatic activity significantly correlated with the measures of MMP-9 activity based on enzyme-linked immunofluorescence assay (R&D Systems) (Fig. 2).
Fig. 2.
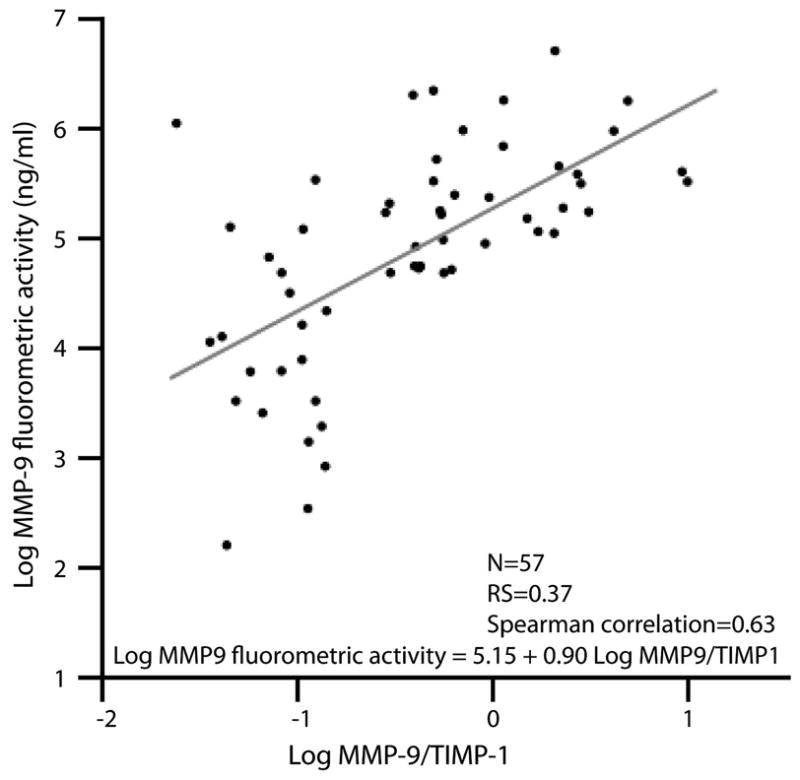
The correlation between the matrix metalloproteinase 9 (MMP-9) to tissue inhibitor of metalloproteinases 1 (TIMP-1) ratio and an immunofluorescent measure of MMP-9 activity. At baseline, the MMP-9:TIMP-1 ratio is linearly associated with MMP-9 activity levels, as measured with a fluorometric assay. Spearman correlation was calculated at 0.63.
4. Discussion
Using a battery of six NP tests to evaluate a range of cognitive domains, we have demonstrated that higher pre-operative MMP-9 total levels, as well as higher pre-operative MMP-9 activity levels, are associated with a worse cognitive outcome one day after CEA.
We used cognitive change from baseline as a measure of cognitive performance, and defined moderate to severe CD as an average z-score of ≤ −1.5 standard deviations worse than the control group of patients who were due to have spinal surgery under general anesthesia. The use of the control group helped to differentiate significant change from practice effects and accounts for the effects of anesthesia. Subclinical cerebral ischemia is likely to play a central role in the development of CD following CEA.16 The mechanisms connecting elevated levels of MMP-9 to CD in this context remain unclear.
MMP-9, one of several proteolytic enzymes secreted by activated macrophages, has a role in the progression and destabilization of atheromatous plaques. Elevated MMP-9 levels may be secondary to an increased systemic and/or local inflammatory state.10,17 Cardiac and neurologic symptoms occur more frequently in presence of an “unstable” plaque, which is characterized by the presence of a large lipid core, a thin fibrous cap and a local inflammatory infiltrate.18,19 Hemodynamic variations encountered during and after CEA, as well as systemic inflammation caused by the surgical procedure, can result in rupture of unstable plaques in large- and medium-size arteries.20 In those patients who have carotid artery disease, elevated levels of MMP-9 are associated with a greater incidence of cerebrovascular episodes.21 MMP-9 has a role in blood brain barrier dysfunction. The blood–brain barrier protects the brain against fluctuations in various ion concentrations, neurotransmitters and growth factors in circulation. Following cerebral ischemia, the absence of an adequately functioning blood brain barrier can lead to abnormal functioning of neuronal and non-neuronal tissues, as well as the formation of edema, all of which are complications that can result in CD.
Aside from MMP-9, several other risk factors have been associated with post-operative CD after carotid procedures.14 Cognitive impairment is common in older patients who have vascular disease.15 For those who undergo CEA, an older age, a low level of formal educational and/or diabetes mellitus, as well as genotype (presence of an ApoE4 allele of the apolipoprotein E gene, or variants of the inducible nitric oxide synthase gene) have all been associated with a worse postoperative cognitive outcome. In our study, however, patients with and without post-operative CD had the same level of formal education. To account for the potential confounding effect of age, diabetes mellitus and the presence of neurological symptoms before surgery, we adjusted all analyses statistically for these three factors using multivariate regression models. We also used a relative outcome (cognitive change from baseline) rather than absolute post-operative cognitive performance.
Used individually, baseline MMP-9 levels are, however, insufficient predictors of post-operative cognitive performance. With a cut-off value of 50 μg/mL, baseline MMP-9 total levels have 66.6% sensitivity in detecting the event of moderate to severe CD at 1 day post-procedure. In patients who are ≥ 65 years old only, sensitivity of MMP-9 levels of > 50 μg/mL increases to 79.3%. Predictive scales may help identify patients who are at greater risk of developing CD following CEA. As CD is a multifactorial process, such scales require the combination of several pre-operative variables. Pre-operative MMP-9 levels should be considered in the development of future screening tools.
Given that cerebral compensatory mechanisms require more than 24 hours to develop,22 we believe CD 1 day after surgery expresses the true amount of cerebral injury. Patients typically show partial cognitive improvement within the first week following CEA and, in fact, late cognitive performance may reflect how well patients can compensate for early injury. Similarly, early CD may predict long-term cognitive consequences. In a longitudinal assessment of neurocognitive function after coronary artery bypass surgery, Newman et al. showed that cognitive decline immediately after surgery was significantly associated with both the severity of and the incidence of cognitive decline five years later.23 Furthermore, long-term consequences of post-operative CD include increased mortality, risk of leaving the labor market prematurely and dependency on social transfer payments.24
There are several limitations to our study. First, although the use of average z-scores maximizes the number of patients included in the analysis by accounting for any missing tests, it also reduces the effect that a single test has on the overall performance (washout effect). While z-scores are commonly used in cognitive studies, they require the inclusion of a control population to determine the mean and standard deviation changes in performance to account for practice effects. In studies designed to evaluate post-operative cognitive performance, a control population also helps to account for the effects of anesthesia, since all of the control patients undergo surgery using similar anesthetic conditions. Second, we found that patients who developed CD after CEA had worse cognitive performance at baseline on specific tests, these being Hopkins Verbal Learning, Rey Complex Copy, and Halstead-Reitan Trails A and B. Therefore, we cannot exclude the hypothesis that the real relationship is between MMP-9 levels and cognitive performance before surgery. Last, the results obtained from measuring MMP-9 activity indirectly by calculating the ratio of MMP-9 to its inhibitor, TIMP-1 significantly correlated with the measures of MMP-9 activity based on enzyme-linked immunofluorescence.
In conclusion, patients who have early CD following carotid artery endarterectomy have significantly higher MM-9 total and activity levels at baseline, but not after surgery. Elevated MMP-9 levels reflect an increased inflammatory state; they are associated with a higher risk of both atheromatous plaque rupture and blood–brain barrier dysfunction. Measurement of baseline MMP-9 levels, when combined with a systematic screening for other predictors of post-operative cognitive performance, may help identify patients at greater risk of developing post-operative CD.
Acknowledgments
We thank Yaakov Stern for his contribution in selecting and interpreting neuropsychological tests. E.J.H. was supported in part by a grant from the NIA (R01 AG17604). J.G.G. was supported in part by a grant from the NIH (T32 GM008464).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heyer EJ, Sharma R, Rampersad A, et al. A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59(2):217–22. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madl C, Grimm G, Kramer L, et al. Cognitive brain function in non-demented patients with low-grade and high-grade carotid artery stenosis. Eur J Clin Invest. 1994;24(8):559–64. doi: 10.1111/j.1365-2362.1994.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 3.Huber JD. Diabetes, cognitive function, and the blood brain–barrier. Curr Pharm Des. 2008;14(16):1594–600. doi: 10.2174/138161208784705441. [DOI] [PubMed] [Google Scholar]
- 4.Rapp JH, Pan XM, Neumann M, et al. Microemboli composed of cholesterol crystals disrupt the blood–brain barrier and reduce cognition. Stroke. 2008;39(8):2354–61. doi: 10.1161/STROKEAHA.107.496737. [DOI] [PubMed] [Google Scholar]
- 5.Loftus IM, Naylor AR, Goodall S, et al. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke. 2000;31(1):40–7. doi: 10.1161/01.str.31.1.40. [DOI] [PubMed] [Google Scholar]
- 6.Boyle JJ. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol. 2005;3(1):63–8. doi: 10.2174/1570161052773861. [DOI] [PubMed] [Google Scholar]
- 7.Galis ZS, Sukhova GK, Lark MW, et al. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94(6):2493–503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood–brain barrier during inflammation. Am J Physiol. 1998;274(5 Pt 2):R1203–11. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22(5):E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- 10.McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28(38):9451–62. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis MS, Maruff P, Silbert BS, et al. The influence of different error estimates in the detection of postoperative cognitive dysfunction using reliable change indices with correction for practice effects. Arch Clin Neuropsychol. 2007;22(2):249–57. doi: 10.1016/j.acn.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Duggleby W, Lander J. Cognitive status and postoperative pain: older adults. J Pain Symptom Manage. 1994;9(1):19–27. doi: 10.1016/0885-3924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 13.Honeycutt JH, Jr, Loftus CM. Carotid endarterectomy: general principles and surgical technique. Neurosurg Clin N Am. 2000;11(2):279–97. [PubMed] [Google Scholar]
- 14.Mocco J, Wilson DA, Komotar RJ, et al. Predictors of neurocognitive decline after carotid endarterectomy. Neurosurgery. 2006;58(5):844–50. doi: 10.1227/01.NEU.0000209638.62401.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roman GC. Vascular dementia may be the most common form of dementia in the elderly. J Neurol Sci. 2002;203–204:7–10. doi: 10.1016/s0022-510x(02)00252-6. [DOI] [PubMed] [Google Scholar]
- 16.Ogasawara K, Inoue T, Kobayashi M, et al. Cognitive impairment associated with intraoperative and postoperative hypoperfusion without neurologic deficits in a patient undergoing carotid endarterectomy. Surg Neurol. 2006;65(6):577–81. doi: 10.1016/j.surneu.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki M, Saito M, Nagai T, et al. Systemic versus coronary levels of inflammation in acute coronary syndromes. Angiology. 2006;57(4):459–63. doi: 10.1177/0003319706290742. [DOI] [PubMed] [Google Scholar]
- 18.Hennerici MG. The unstable plaque. Cerebrovasc Dis. 2004;17 (Suppl 3):17–22. doi: 10.1159/000075300. [DOI] [PubMed] [Google Scholar]
- 19.Virmani R, Ladich ER, Burke AP, et al. Histopathology of carotid atherosclerotic disease. Neurosurgery. 2006;59(5 Suppl 3):S219–27. doi: 10.1227/01.NEU.0000239895.00373.E4. discussion S3–13. [DOI] [PubMed] [Google Scholar]
- 20.Tang D, Yang C, Zheng J, et al. 3D MRI-based multicomponent FSI models for atherosclerotic plaques. Ann Biomed Eng. 2004;32(7):947–60. doi: 10.1023/b:abme.0000032457.10191.e0. [DOI] [PubMed] [Google Scholar]
- 21.Eldrup N, Gronholdt ML, Sillesen H, et al. Elevated matrix metalloproteinase-9 associated with stroke or cardiovascular death in patients with carotid stenosis. Circulation. 2006;114(17):1847–54. doi: 10.1161/CIRCULATIONAHA.105.593483. [DOI] [PubMed] [Google Scholar]
- 22.van Mook WN, Rennenberg RJ, Schurink GW, et al. Cerebral hyperperfusion syndrome. Lancet Neurol. 2005;4(12):877–88. doi: 10.1016/S1474-4422(05)70251-9. [DOI] [PubMed] [Google Scholar]
- 23.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary artery bypass surgery. N Engl J Med. 2001;344(6):395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 24.Steinmetz J, Christensen KB, Lund T, et al. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548–55. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]