Abstract
Aeroallergy results from maladaptive immune responses to ubiquitous, otherwise innocuous environmental proteins1. While the proteins so targeted represent a tiny fraction of the airborne proteins humans are exposed to, allergenicity is a quite public phenomenon—the same proteins typically behave as aeroallergens across the human population. Why particular proteins tend to act as allergens in susceptible hosts is a fundamental mechanistic question that remains largely unanswered. The major house dust mite allergen, Der p 2, has structural homology with MD-2, the lipopolysaccharide (LPS)-binding component of the Toll-like receptor (TLR)4 signalling complex2–4. Here we show that Der p 2 has functional homology as well, facilitating signalling through direct interactions with the TLR4 complex, and reconstituting LPS-driven TLR4 signalling in the absence of MD-2. Mirroring this, airway sensitization and challenge with Der p 2 led to experimental allergic asthma in wild type and MD-2-deficient, but not TLR4-deficient, mice. Our results suggest that Der p 2 tends to be targeted by adaptive immune responses because of its auto-adjuvant properties. The fact that other members of the MD-2-like lipid binding family are allergens, and that a majority of defined major allergens are thought to be lipid-binding proteins5, suggests that intrinsic adjuvant activity by such proteins and their accompanying lipid cargo may have some generality as a mechanism underlying the phenomenon of allergenicity.
While the potential biological significance of the fact that several allergens have protease activity has received experimental attention5,6, cogent mechanistic hypotheses for why most aeroallergens have a propensity to generate maladaptive effector T cell responses are lacking. TLRs, receptors for conserved microbial structures, play a critical role in the controlling initiation and class specification of adaptive immune responses by antigen presenting cells (APCs)7. Exogenous antigen presentation by APCs in the absence of direct TLR stimulation generally leads to tolerance8. Moreover, efficient generation of effector T cell responses by APCs is dependent upon the presence of TLR ligands in the phagosome containing the antigen being presented9.
House dust mites are major sources of aeroallergens for patients with allergic asthma10. Concentrated in mite fecal pellets11, the major group 2 allergens, Der p 2 and Der f 2, are highly allergenic; among defined dust mite antigens, they have the highest rates of skin test positivity in atopic patients12. Sequence homology places these allergens in the recently recognized MD-2-related lipid-recognition (ML) domain family of proteins13. Notably, the crystal structures of MD-2 and Der p 2 exhibit structural homology2–4, as previously predicted14, with two anti-parallel β-pleated sheets stabilized by disulfide bonds and enclosing a hydrophobic cavity accessible from one end. Several lines of evidence have linked exposure to LPS with regulation of the development of allergic asthma, as well as with exacerbation of existing asthma15.
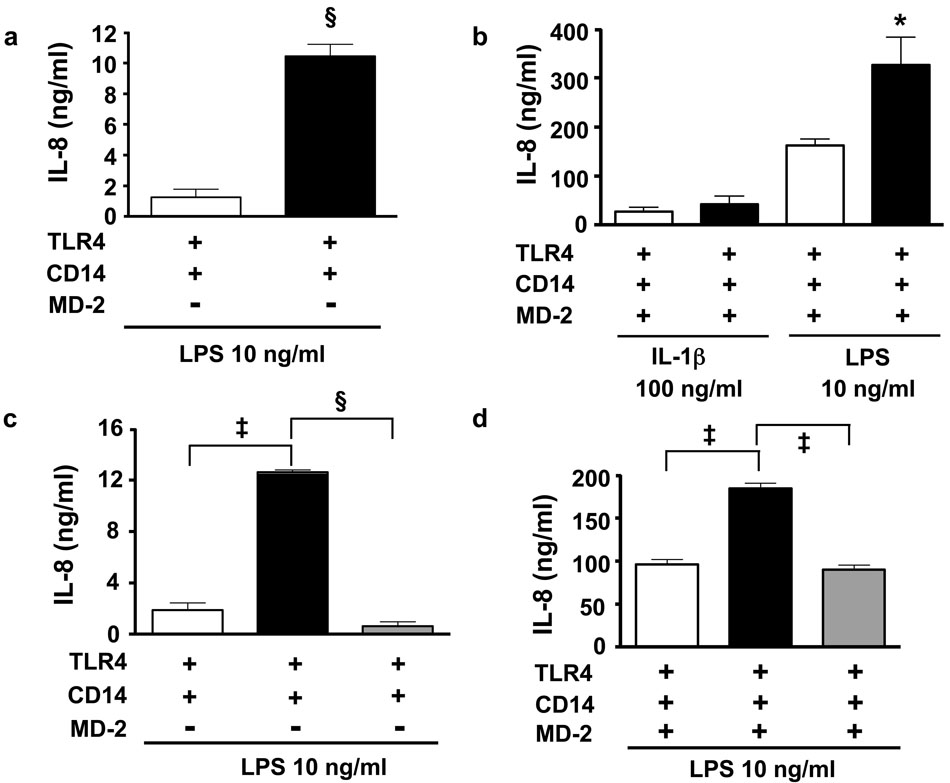
These considerations suggested the possibility of functional homology between MD-2 and Der p 2 as well. We examined the effect of Der p 2 on TLR4 signalling in HEK293 cells, cells lacking endogenous TLR4 and MD-2, but with fully functional TLR signalling machinery. Der p 2 reconstituted LPS-driven TLR4 signalling and IL-8 production in HEK293 cells in the absence of MD-2 (Fig. 1a). Furthermore, Der p 2 augmented LPS-driven IL-8 production in HEK293 cells co-expressing TLR4 and MD-2 (Fig. 1b). It will be noted that LPS-driven TLR4 signalling was more robust in the presence of MD-2 alone, compared with Der p 2 alone. The effects of Der p 2 on IL-8 production by HEK293 cells were TLR4- and LPS-dependent (data not shown). In contrast, Der p 2 did not increase IL-1-driven IL-8 production (Fig. 1b), suggesting specific effects of Der p 2 on TLR4 activation and not on the signalling pathways shared with the IL-1 receptor. RP105/MD-1, a cell surface heterodimer structurally related to TLR4/MD-2, inhibits TLR4 signalling in myeloid and HEK293 cells; the interaction of RP105/MD-1 with TLR4/MD-2 reduces the ability of the latter complex to bind LPS16. Der p 2 blunted the decrease in LPS-driven IL-8 production observed in HEK293 cells expressing RP105/MD-1 as well as TLR4/MD-2 (Suppl. Fig. 1).
Figure 1. Der p 2 reconstitutes and amplifies TLR4 signalling in the absence and presence of MD-2, respectively.
a, HEK293 cells expressing CD14 and TLR4 (HEK293/CD14/TLR4) were transfected with Der p 2 (black bars) or empty vector (EV, white bars) and stimulated (or mock stimulated) with LPS (10 ng/ml). b, HEK293/CD14/TLR4 cells transfected with MD-2 along with Der p 2 (black bars) or EV (white bars) were stimulated (or mock stimulated) with LPS or IL-1β (100 ng/ml). c and d, HEK293/CD14/TLR4 cells transfected with Der p 2 (black bars), Der p 2-Y91A (grey bars) or EV (white bars), in the absence (c) or presence (d) of MD-2, were stimulated (or mock stimulated) with LPS. Data represent means ± S.E. of cultures (stimulated - mock-stimulated) in a single experiment, representative of an experimental n of 3–8. *P < 0.05; ‡P < 0.005; §P < 0.001; unpaired t-test.
All sequenced MD-2 species contain a conserved tyrosine (Y102) at a site functionally important for TLR4 signaling4,17 (Suppl. Fig. 2). This tyrosine, which resides in a loop formed between cysteines of a disulfide bridge (Cys95—Cys105 in MD-2; Cys89—Cys94 in Der p 2), is absent in MD-1 but conserved in Der p 2 (Y91). Mutation of this tyrosine to alanine (Y91A) ablated the ability of Der p 2 to reconstitute LPS-driven IL-8 production in HEK293 cells expressing TLR4 without MD-2 (Fig. 1c), or to augment LPS-driven IL-8 production in HEK293 cells co-expressing TLR4 and MD-2 (Fig. 1d). Thus, in HEK293 cells, Der p 2 promotes LPS-induced TLR4 signalling, in a manner that appears to depend on Der p 2/TLR4 interactions.
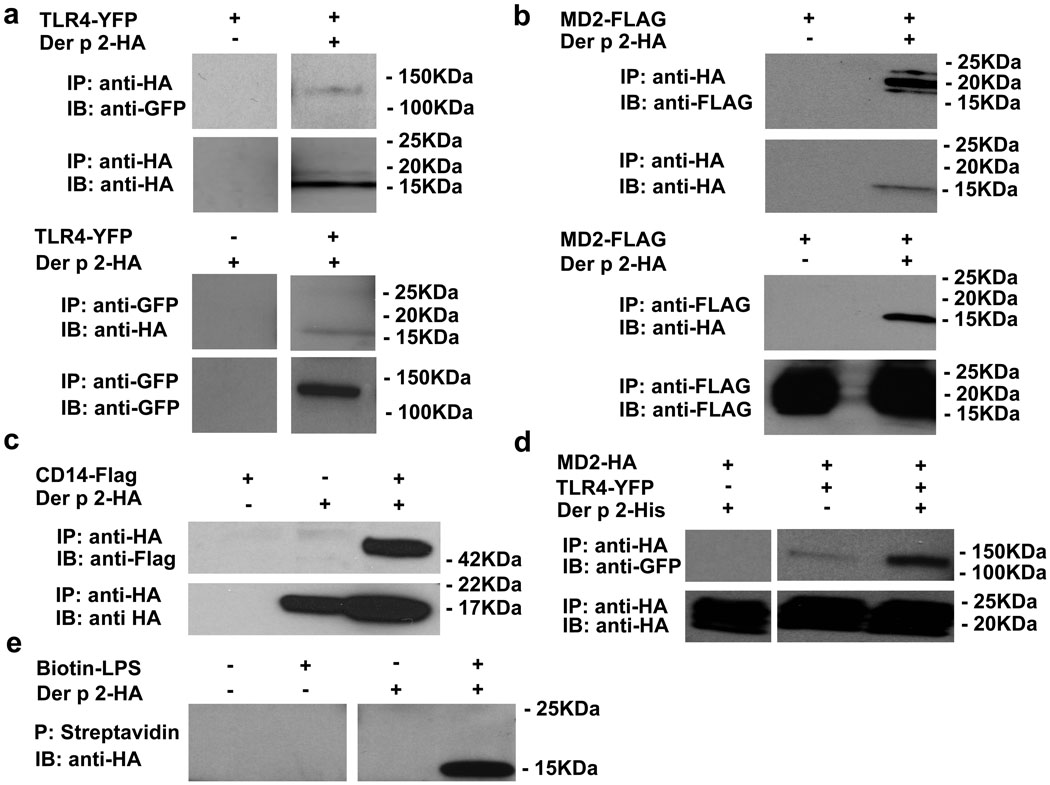
The direct association of Der p 2 with TLR4 was demonstrated by co-immunoprecipitation in lysates of cells co-expressing epitope-tagged Der p 2 and TLR4 (Fig. 2a). Interaction of Der p 2 with the ectodomain of TLR4 (TLR4ECD) was shown by co-capture of Der p 2 by protein G beads after incubation of Der p 2 with a TLR4ECD-Fc fusion protein (Suppl. Fig. 3). Like MD-2, rDer p 2 forms disulfide-linked aggregates (Suppl. Fig. 4). In a further parallel with MD-2, it is the monomeric form of Der p 2 that co-immunoprecipitates with TLR4 and with MD-2 (data not shown). Direct association of Der p 2-HA and MD-2-FLAG was shown by co-immunoprecipitation in cell lysates using antibodies to HA and FLAG (Fig. 2b). Like MD-2, Der p 2 also associated with CD14 (Fig. 2c). Of interest, while immunoprecipitation analysis of Der p 2 (Y91A) suggested that this non-functional mutant interacts with TLR4 and MD-2 in a manner similar to that of native Der p 2 (data not shown), Der p 2 (Y91A) exhibited increased binding to CD14 in such assays (Suppl. Fig. 5). The ability of Der p 2 (Y91A) to bind TLR4 is consistent with crystallographic data that does not show direct involvement of MD-2 Tyr102 in the TLR4 interaction4. The observed increased affinity of Der p 2 (Y91A) for CD14 suggests that this mutation may interfere with TLR4 signalling by altering the dynamics of CD14 engagement and/or LPS transfer. Notably, co-expression of Der p 2 with MD-2 and TLR4 increased the co-immunoprecipitation of TLR4 with MD-2 (Fig. 2d), suggesting interactions between TLR4/MD-2 and TLR4/Der p 2 heterodimers that could contribute to the increased TLR4-dependent activation by LPS observed when cells co-expressing TLR4 and MD-2 also express Der p 2 (Fig. 1b and 1d). Similarly, Der p 2 reduced co-immunoprecipitation of MD-2 with MD-1 (Suppl. Fig. 6), suggesting that inhibition of RP105/MD-1—MD-2/TLR4 interactions by Der p 2 contributes to the increased activation by LPS of cells co-expressing TLR4/MD-2 and RP105/MD-1 when Der p 2 is present (Suppl. Fig. 1). Finally, like MD-2 (but not MD-1)16, Der p 2 binds LPS, as demonstrated by co-precipitation of Der p 2-HA with biotinylated LPS captured by streptavidin beads (Fig. 2e). Thus, Der p 2 can bind TLR4, MD-2, CD14 and LPS. Taken together, these data suggest that Der p 2 facilitates the aggregation of TLR4 needed for receptor activation by promoting TLR4/MD-2—Der p 2/TLR4 interactions in the presence, and TLR4/Der p 2—Der p 2/TLR4 interactions in the absence, of MD-2.
Figure 2. Der p 2 interacts directly with the TLR4 complex and with LPS.
a, Co-immunoprecipitation (co-IP) of Der p 2 with TLR4 in lysates of HEK293FT cells transfected with the indicated constructs or EV controls (−). b, Co-IP of Der p 2 with MD-2. c, Co-IP of Der p 2 with CD14. d, Effect of Der p 2 on co-IP of MD-2 with TLR4. e, Supernatants from HEK293FT cells transfected with HA-tagged Der p 2 (or EV) underwent incubation with biotinylated LPS, precipitation (P) with streptavidin beads, and immunoblotting (IB) with HA Ab. Similar results were seen with immunoaffinity-purified Der p 2 and Der p 2 mAb. Data are representative of an experimental n = 2–8.
To model the interactions of exogenously delivered Der p 2 with host cells important in inducing adaptive immune responses, we tested the effect of Der p 2 on primary APC. Der p 2, immunoaffinity-purified from house dust mites, induced TNF-α secretion by mouse bone marrow-derived dendritic cells [DCs] (Suppl. Fig. 7). Like LPS-induced signalling in these cells, Der p 2-triggered activation was TLR4-dependent (Suppl. Fig. 7b), but not TLR2-dependent (Suppl. Fig. 7d). In contrast to LPS, Der p 2 could also activate DCs from MD-2-null mice (Suppl. Fig. 7c), mimicking the TLR4-activating properties of LPS:MD-2 complexes18,19. The immunoaffinity-purified Der p 2 was indeed contaminated with endotoxin-like activity (equivalent to 0.4 ng E. coli LPS/µg Der p 2), suggesting that the observed TLR4-dependent activation of DCs by Der p 2 was mediated by endotoxin:Der p 2 complexes.
To test this more directly, we generated recombinant Der p 2 in a baculovirus expression system. At physiological temperatures, MD-2 secreted into serum-free media rapidly loses biological activity in the absence of LPS18. Similarly, rDer p 2 secreted in the absence of LPS was inactive in functional assays (data not shown). Thus, we generated and purified rDer p 2 in the presence of defined amounts of E. coli LPS. rDer p 2 recovered under these conditions stimulated TLR4-dependent production of TNF-α, IL-12/23p40 and IL-6 by mouse peritoneal macrophages, in the presence and in the absence of MD-2 (Suppl. Fig. 8). The amount of LPS co-purified with Der p 2 (60 pg LPS/100 µg Der p 2) was sub-stoichiometric, and insufficient to activate the macrophages in the absence of Der p 2 (Suppl. Fig. 8a and 8c). Thus, exogenously delivered Der p 2 facilitates LPS signalling in primary APCs, with or without MD-2 being present.
Taken together, these data suggest that the propensity of Der p 2 to be a target of adaptive immune responses is due to the fact that Der p 2 promotes TLR4 signalling and thus has auto-adjuvant activity. This suggested a mechanism for allergenicity as well. High levels of exposure to bacterial products such as LPS in early life are inversely correlated with the development of atopy and allergic disease20–22. Usually interpreted in the context of the hygiene hypothesis, it is thought that such exposures lead to the development of robust counter-regulatory responses23. However, epidemiological and human challenge data indicate that LPS exposure can exacerbate established asthma, likely by stimulating pro-inflammatory responses in the airway20,24.
In addition to confirming the ability of LPS to exacerbate established asthma25, mouse models have provided mechanistic insight into the role of LPS in regulating the development of allergic asthma. Consonant with the hygiene hypothesis, a key variable appears to be LPS dose. Whereas airway sensitization with a model antigen (ovalbumin; OVA) along with very low-dose (< 1 ng) LPS induces tolerance, airway sensitization with OVA along with low-dose (100 ng) LPS drives Th2 immune responses and allergic asthma in a TLR4-dependent fashion26. On the other hand, airway sensitization with OVA along with high-dose (100 µg) LPS drives a Th1 response, likely along with a robust regulatory response as well26,27.
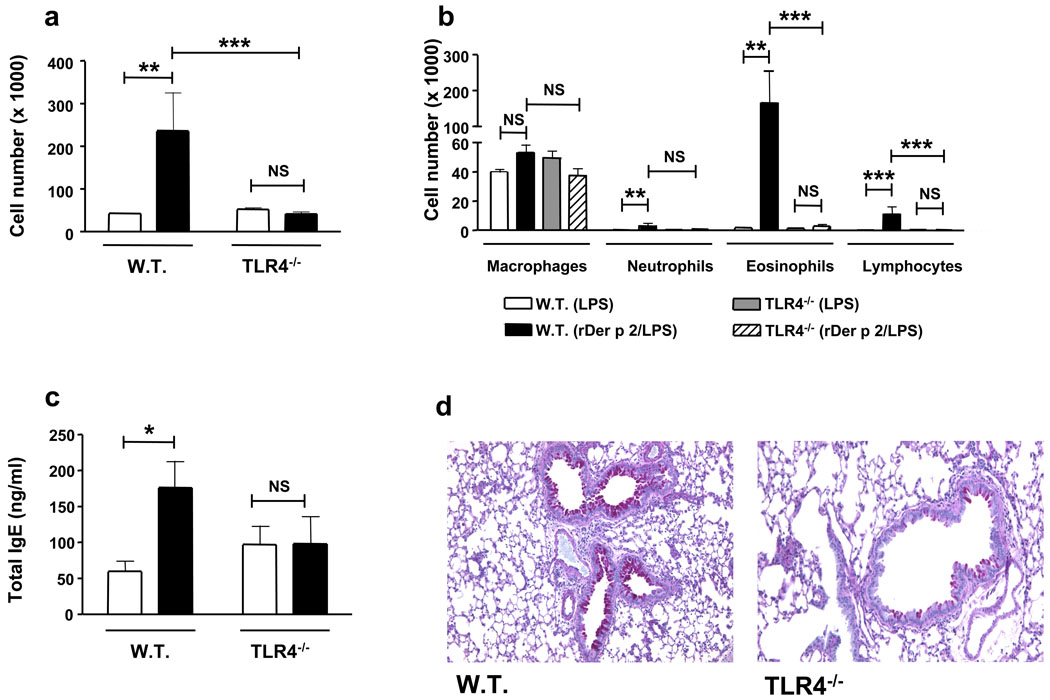
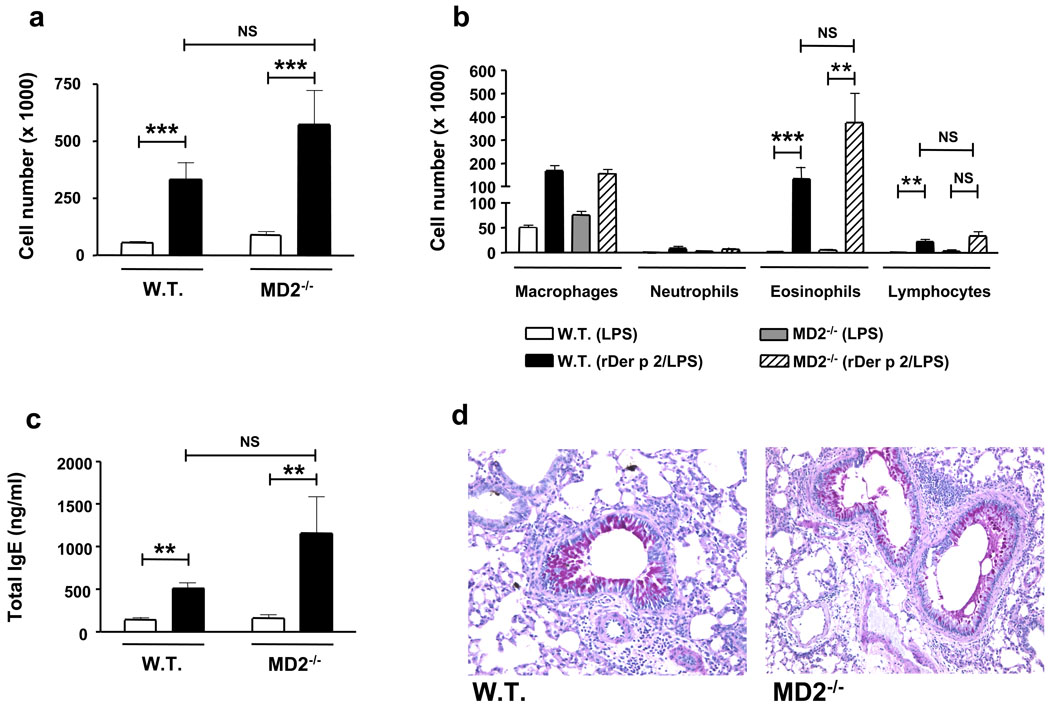
We directly tested the ability of Der p 2 to induce experimental allergic asthma. Sensitization and challenge with Der p 2, along with extremely low-dose (low pg range) LPS, induced robust airway Th2 inflammation—marked by airway eosinophilia and lymphocytosis, mucous metaplasia, and increased plasma IgE concentrations—in wild type mice, but not TLR4-deficient mice (Fig. 3). Notably, Der p 2 similarly induced Th2 inflammation in the airways of MD-2-deficient mice (Fig. 4). On the other hand, mutant (Y91A) Der p 2 failed to induce experimental allergic asthma (Suppl Fig. 9). Thus, in vivo allergenic activity mirrors in vitro functional and biochemical activity: Der p 2 efficiently drives airway Th2 inflammation in vivo in a TLR4-dependent manner, and retains the ability to drive such inflammation in the absence of MD-2.
Figure 3. TLR4-dependent induction of experimental allergic asthma by Der p 2.
Wild type and TLR4-deficient mice were sensitized intranasally on d 0, 1 and 2 with rDer p 2 (0.1 µg)/LPS (0.026 pg) in PBS, or PBS/LPS (0.026 pg), challenged intranasally with 1/4 of the sensitization dose on d 14, 15, 18 and 19, and analyzed on d 21. a, b Inflammatory cells in bronchoalveolar lavage (BAL) fluids. a, Total cell numbers (white bars, PBS/LPS; black bars, Der p 2/LPS). b, Differential cell counts. c, Total serum IgE (bars as in a). d, Representative lung sections stained with periodic acid-Schiff. Data (a–c) represent means ± S.E. of 5–8 animals/group, and are representative of 2 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001; 2-way ANOVA on log-transformed data.
Figure 4. Der p 2 induces experimental allergic asthma in the absence of MD-2.
Wild type and MD-2-deficient mice were sensitized, challenged and analyzed as in Figure 3, except for the use of 10-fold higher doses of Der p 2, given the less robust stimulation of TLR4 by Der p 2 observed in the absence of MD-2. a, b Inflammatory cells in BAL fluids. a, Total cell numbers (white bars, PBS/LPS; black bars, Der p 2/LPS). b, Differential cell counts. c, Total serum IgE (bars as in a). d, Representative lung sections stained with periodic acid-Schiff. Data (a–c) represent means ± S.E. of 5–9 animals/group. *P < 0.05, **P < 0.01, ***P < 0.001; 2-way ANOVA on log-transformed data.
Der p 2-mediated facilitation of TLR4 signalling under conditions of very low ambient LPS exposure, those associated with increasing rates of atopy and aeroallergy, may thus shift the LPS-response curve into the Th2-inducing range. Similarly, Der p 2 is likely to promote LPS-driven exacerbation of established asthma by facilitating TLR4 signalling by airway cells. Here, the ability of Der p 2 to reconstitute TLR4 signalling in the absence of MD-2 may well be of special importance as airway epithelial cells express TLR4, but little or no MD-228.
Although the interaction of Der p 2 with vertebrate TLR4/MD-2 is unlikely to be of biological importance to the mite, it is tempting to speculate that Der p 2 plays a physiological role in the innate immune system of the dust mite gut. Der p 2’s pathophysiological interactions with the vertebrate immune system may be more than a unique oddity. Other ML domain family members are aeroallergens13. More generally, more than 50% of defined major allergens are lipid-binding proteins5. Intrinsic adjuvant activity provided by associated lipids may well underlie the allergenicity of such proteins.
METHODS SUMMARY
Generation of recombinant Der p 2
Recombinant His-tagged Der p 2 (rDer p 2) was generated in baculovirus systems using the pAcGP67A transfer vector (BD Pharmingen) and Sf21 cells, and purification with Ni-NTA agarose beads (Qiagen). rDer p 2 was generated under FCS-free conditions (Baculogold Max-XP media, BD Pharmingen), in the presence of E. coli LPS (100 ng/ml) and LPS-free human serum albumin (HSA) [0.25%] as a carrier and for stability29.
In vitro cell stimulation
HEK293 cell stimulation and analysis was as described16. Bone marrow-derived DCs and thioglycollate-elicited peritoneal macrophages were generated as described16 from wild type, MD-2- (K. Miyake), TLR2- and TLR4-deficient mice (S. Akira)—all on a C57BL/6 background (>10 generations). 24 h after stimulation (or mock stimulation), cell-free supernatants were harvested and cytokine production was quantified by ELISA (BD Pharmingen).
Experimental allergic asthma model
Ten-twelve wk-old female wild type, MD-2−/− and TLR4−/− mice were sensitized and challenged according to the protocol of Bottomly26, with minor modifications. Briefly, mice anaesthetized with ketamine-xylazine were sensitized intranasally on d 0, 1 and 2 with: (a) rDer p 2 (0.1 µg) in PBS (co-purified with E. coli LPS [0.026 pg] and HSA [0.3 ng]) or (b) PBS (with E. coli LPS [0.026 pg] and HSA [0.3 ng]). Mice were subsequently challenged intranasally with 1/4 of the sensitization dose on d 14, 15, 18 and 19. For experiments with MD-2−/− mice, sensitization and challenge doses of Der p 2/LPS were 10-fold higher. On d 21, mice were sacrificed, and airway inflammation, serum IgE concentrations, and airway mucus metaplasia were assessed as previously described30. Animal care was provided in accordance with NIH guidelines in studies approved by the CCHMC IACUC.
METHODS
Reagents, expression constructs and cell lines
IL-1β was from Peprotech. Re-purified E. coli K235 LPS was from S. Vogel. Pam3Cys was from EMC Microcollections. cDNA encoding Der p 2 was from Heska Corp. Immunoaffinity-purified Der p 2 was from Indoor Biotechnologies. Recombinant His-tagged Der p 2 (rDer p 2) was generated in baculovirus systems using the pAcGP67A transfer vector (BD Pharmingen) and Sf21 cells, and purification with Ni-NTA agarose beads (Qiagen). rDer p2 was generated under FCS-free conditions (Baculogold Max-XP media, BD Pharmingen), in the presence of E. coli LPS (100 ng/ml) and LPS-free human serum albumin (HSA) [0.25%] as a carrier and for stability29. Der p 2 (Y91A) was generated by PCR-mediated mutagenesis. HEK293 cell lines stably expressing CD14–TLR4, and HEK293FT cells, have been described16. All cell lines were Mycoplasma-free. All reagents contacting cultured cells were LPS-free to the limits of detection of the Limulus amebocyte lysate assay (Lonza) at the concentrations employed, unless otherwise stated. Human RP105, MD-1, TLR4 and MD-2 expression constructs were as described16. Der p 2 was cloned into the pCEFL-HA mammalian expression vector (INSERM U482, France). Epitope-tagged constructs were generated by PCR. Transient transfections were performed using PolyFect (Qiagen).
Immunoprecipitation and Western blotting
were performed as described.16
In vitro cell stimulation
One day after transient transfection of HEK293 cells, media was replaced. 72 h later, cells were stimulated (or mock stimulated) for an additional 24 h. Cell-free supernatants were collected and IL-8 production was quantified by ELISA (BD Pharmingen). Bone marrow-derived DCs and thioglycollate-elicited peritoneal macrophages were generated as described16 from wild type, MD-2- (K. Miyake), TLR2- and TLR4-deficient mice (S. Akira)—all on a C57BL/6 background (>10 generations). 24 h after stimulation (or mock stimulation), cell-free supernatants were harvested and TNF-α, IL-12p40/23 and IL-6 production were quantified by ELISA (BD Pharmingen).
Experimental allergic asthma model
Wild type, MD-2−/− and TLR4−/− mice were sensitized and challenged according to the protocol of Bottomly26, with minor modifications. 10–12 wk-old female mice were used in all experiments. Briefly, mice anaesthetized with ketamine-xylazine were sensitized intranasally on d 0, 1 and 2 with: (a) rDer p 2 (0.1 µg) in PBS (co-purified with E. coli LPS [0.026 pg] and HSA [0.3 ng]) or (b) PBS (with E. coli LPS [0.026 pg] and HSA [0.3 ng]). Mice were subsequently challenged intranasally with 1/4 of the sensitization dose on d 14, 15, 18 and 19. For experiments with MD-2−/− mice, sensitization and challenge doses of Der p 2/LPS were 10-fold higher. On d 21, mice were sacrificed, and airway inflammation, total serum IgE concentrations, and airway mucus metaplasia were assessed as previously described30. Animal care was provided in accordance with NIH guidelines; studies were approved by the CCHMC IACUC.
Supplementary Material
Acknowledgements
We thank S. Vogel for re-purified LPS; E. Kurt-Jones and R. Finberg for HEK293 cells expressing TLR4 complex proteins, N. J. Gay for discussions, and L. Flick and J. Bohnert for technical assistance. This work was funded by grants from the Sandler Foundation for Asthma Research (C.L.K.), the National Institute of Allergy and Infectious Diseases (C.L.K., J.P.G.), and the Veteran’s Administration (T.L.G.).
Footnotes
Supplementary information is linked to the online version of the paper at www.nature.com/nature.
Author Information Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Ohto U, et al. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 3.Derewenda U, et al. The crystal structure of a major dust mite allergen Der p 2, and its biological implications. J. Mol. Biol. 2002;318:189–197. doi: 10.1016/S0022-2836(02)00027-X. [DOI] [PubMed] [Google Scholar]
- 4.Kim HM, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Thomas WR, et al. Structural biology of allergens. Curr. Allergy Asthma. Rep. 2005;5:388–393. doi: 10.1007/s11882-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 6.Sokol CL, et al. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 8.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 9.Blander JM, Medzhitov R. Toll dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 10.Maunsell K, et al. Mites and house-dust allergy in bronchial asthma. Lancet. 1968;1:1267–1270. doi: 10.1016/s0140-6736(68)92289-7. [DOI] [PubMed] [Google Scholar]
- 11.Park GM, et al. Localization of a major allergen, Der p 2, in the gut and faecal pellets of Dermatophagoides pteronyssinus. Clin. Exp. Allergy. 2000;30:1293–1297. doi: 10.1046/j.1365-2222.2000.00883.x. [DOI] [PubMed] [Google Scholar]
- 12.Heymann PW, et al. Antigenic and structural analysis of group II allergens (Der f II and Der p II) from house dust mites (Dermatophagoides spp) J. Allergy Clin. Immunol. 1989;83:1055–1067. doi: 10.1016/0091-6749(89)90447-8. [DOI] [PubMed] [Google Scholar]
- 13.Inohara N, Nunez G. ML -- a conserved domain involved in innate immunity and lipid metabolism. Trends Biochem. Sci. 2002;27:219–221. doi: 10.1016/s0968-0004(02)02084-4. [DOI] [PubMed] [Google Scholar]
- 14.Gruber A, et al. Structural model of MD-2 and functional role of its basic amino acid clusters involved in cellular lipopolysaccharide recognition. J. Biol. Chem. 2004;279:28475–28482. doi: 10.1074/jbc.M400993200. [DOI] [PubMed] [Google Scholar]
- 15.Williams LK, et al. The role of endotoxin and its receptors in allergic disease. Ann. Allergy Asthma Immunol. 2005;94:323–332. doi: 10.1016/S1081-1206(10)60983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Divanovic S, et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat. Immunol. 2005;6:571–578. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki K, et al. Identification of mouse MD-2 residues important for forming the cell surface TLR4-MD-2 complex recognized by anti-TLR4-MD-2 antibodies, and for conferring LPS and taxol responsiveness on mouse TLR4 by alanine-scanning mutagenesis. J. Immunol. 2003;170:413–420. doi: 10.4049/jimmunol.170.1.413. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy MN, et al. A complex of soluble MD-2 and lipopolysaccharide serves as an activating ligand for Toll-like receptor 4. J. Biol. Chem. 2004;279:34698–34704. doi: 10.1074/jbc.M405444200. [DOI] [PubMed] [Google Scholar]
- 19.Gioannini TL, et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc. Natl. Acad Sci. U. S. A. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun-Fahrlander C, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 21.Gehring U, et al. House dust endotoxin and allergic sensitization in children. Am. J. Respir. Crit. Care Med. 2002;166:939–944. doi: 10.1164/rccm.200203-256OC. [DOI] [PubMed] [Google Scholar]
- 22.Riedler J, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 23.Wills-Karp M, et al. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 24.Michel O, et al. Effect of inhaled endotoxin on bronchial reactivity in asthmatic and normal subjects. J. Appl. Physiol. 1989;66:1059–1064. doi: 10.1152/jappl.1989.66.3.1059. [DOI] [PubMed] [Google Scholar]
- 25.Tulic MK, et al. Modification of the inflammatory response to allergen challenge after exposure to bacterial lipopolysaccharide. Am. J. Respir. Cell. Mol. Biol. 2000;22:604–612. doi: 10.1165/ajrcmb.22.5.3710. [DOI] [PubMed] [Google Scholar]
- 26.Eisenbarth SC, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nature Rev. Immunol. 2003;3:1–8. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 28.Jia HP, et al. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L428–L437. doi: 10.1152/ajplung.00377.2003. [DOI] [PubMed] [Google Scholar]
- 29.Teghanemt A, et al. Transfer of monomeric endotoxin from MD-2 to CD14: characterization and functional consequences. J. Biol. Chem. 2007;282:36250–36256. doi: 10.1074/jbc.M705995200. [DOI] [PubMed] [Google Scholar]
- 30.Wills-Karp M, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.