Abstract
Background
Despite a concerted effort from many laboratories, the critical subunits that participate in vascular smooth muscle cell (VSMC) NADPH oxidase function have yet to be elucidated. Given the potential therapeutic importance of cell-specific inhibition of NADPH oxidase, we investigated the role of Nox activator 1 (NoxA1), a homologue of p67phox, in VSMC NADPH oxidase function, and atherosclerosis.
Methods and Results
The presence of NoxA1 in mouse aortic VSMC was confirmed by RT-PCR and sequencing. NoxA1/p47phox interaction, following thrombin treatment, was observed by immunoprecipitation/Western analysis of lysates from p47phox−/− VSMC transfected with adenoviral HA-NoxA1 and Myc-p47phox. Infection with AdNoxA1 significantly enhanced thrombin-induced ROS generation in wild-type but not in p47phox−/− and Nox1−/− VSMC. Thrombin-induced ROS production and VSMC proliferation were significantly reduced following downregulation of NoxA1 using shRNA. Infection with NoxA1 shRNA but not scrambled shRNA significantly decreased thrombin-induced activation of redox-sensitive protein kinases―Janus kinase 2, Akt and p38 mitogen-activated protein kinase―in VSMC. Adenovirus-mediated overexpression of NoxA1 in guidewire-injured mouse carotid arteries significantly increased superoxide production in medial VSMC and enhanced neointimal hyperplasia. NoxA1 expression was significantly increased in aortas and atherosclerotic lesions of ApoE−/− mice compared with their age-matched wild-type mice. Further, in contrast to p67phox, immunoreactive NoxA1 is present in intimal and medial SMC of human early carotid atherosclerotic lesions.
Conclusions
NoxA1 is the functional homologue of p67phox in VSMC and regulates redox signaling and VSMC phenotype. These findings support the potential for modulation of NoxA1 expression as a viable approach for the treatment of vascular diseases.
Keywords: atherosclerosis, restenosis, neointimal hyperplasia, NADPH oxidase, thrombin, signal transduction
INTRODUCTION
NADPH oxidases are the most important of the superoxide (O2.−) producing enzymes for vascular pathologies such as hypertension and atherosclerosis.1 Vessel wall NADPH oxidases are similar to the well-characterized neutrophil enzyme active in host defense but vary kinetically and in subunit composition, with differences reported among cell types and vascular beds.2
Given the large number of potential combinations of subunits, NADPH oxidases are categorized based upon the catalytic subunit, the Nox protein, and which of the five homologues of Nox is present in a particular complex. At least four Nox homologues are present in one or another vessel wall cell type. Rodent aortic vascular smooth muscle cells (VSMC) contain Nox1 and Nox4,3,4 which directly interact with p22phox, a subunit common to all vascular oxidases,5 to form a functional NADPH oxidase. Nox1 is inactive under basal conditions,6 whereas Nox4 is constitutively active.7,8 Nox2 is expressed at very low levels in rodent VSMC4 and targeted deletion of NOX2 gene had no effect on agonist-induced O2.− generation in these cells.9 Very recently, it has been reported that four isoforms of Nox5 are expressed in human aortic smooth muscle cells and Nox5 shRNA significantly inhibited platelet-derived growth factor stimulated ROS production and cell proliferation.10
The Nox1-based NADPH oxidase contains regulatory cytosolic components p47phox, NoxA1 and Rac1, in addition to Nox1 and p22phox which form the membrane bound cytochrome b558. Both p22phox and p47phox have been shown to be essential for NADPH oxidase activity in VSMC. Transfection of VSMC with anitsense p22phox cDNA significantly inhibits agonist-stimulated NADH/NADPH-dependent O2.− production.11 We and others have previously demonstrated that genetic deletion of p47phox inhibits agonist-induced O2.− production in VSMC.9,12,13 Both p22phox and p47phox are widely expressed in nonvascular cells. For this reason, a strategy of targeting either p22phox or p47phox is a non-selective means to inhibiting vascular wall NADPH oxidase activity. The Nox2-based NADPH oxidase also contains p47phox and Rac1 in addition to Nox2. In this complex, p67phox is a necessary subunit for activation.14
Whether p67phox is a necessary component of the VSMC NADPH oxidase, and if not what subunit substitutes for p67phox, remains controversial. We reported that p67phox was not expressed in rat aortic VSMC.15 However, p67phox mRNA was reported in VSMC from human resistance arteries.16 The report of novel human and mouse homologues of p47phox (NoxO1, Nox organizer 1) and p67phox (NoxA1, Nox activator 1) raised the question of the subunit composition of NADPH oxidases in vascular cells.17,18 The question of the role of p47phox and p67phox versus NoxO1 and NoxA1 is complicated by reports of their interdependence in some systems. Expression of Nox1 with NoxO1 and NoxA1 produced stimulus-independent generation of O2.− in HEK293 cells and the constitutive activation of Nox1 was lost when NoxO1 was replaced with p47phox.18 This is because NoxO1 lacks the autoinhibitory region present in p47phox and therefore can localize to cell membrane under basal conditions.19 However, this interdependence may not exist in VSMC. NoxA1 is expressed in the cytoplasm of VSMC and treatment of cells with epidermal growth factor induces the translocation of NoxA1 and p47phox to the cell membrane.20 In addition, antisense inhibition of NoxA1 attenuates agonist-induced O2.− production in mouse VSMC. Rac1 is directly involved in the activation of Nox1, perhaps via its interaction with NoxA1.21,22
The present study was designed to determine whether NoxA1 was an important functional component of the VSMC NADPH oxidase and, if so, whether it might represent an appropriate target for therapeutic intervention. We examined the expression of NoxA1 and its interaction with p47phox in mouse aortic VSMC. Suppression of NoxA1 expression decreased whereas overexpression increased thrombin-induced O2.− production. Localized overexpression of NoxA1 significantly enhanced neointimal hyperplasia in injured mouse carotid arteries. NoxA1 expression is increased in aortas and atherosclerotic lesions from ApoE−/− mice. Further, NoxA1 expression is correlated with the progression of atherosclerotic lesions whereas both NoxA1 and p67phox were present in advanced atherosclerotic lesions of human carotid arteries. These data suggest that it may be possible to reduce vascular lesion formation and its complications by targeting NoxA1 with specific therapeutic interventions.
Materials and Methods
Cell Culture
Mouse aortic VSMC were isolated from 4-month old male C57BL/6J mice as previously described23 (see supplemental materials).
Reverse transcriptase-Polymerase Chain Reaction (RT-PCR) and Sequencing of NoxA1
The first-strand cDNA synthesis was carried out from total RNA isolated from mouse aortic VSMC using SuperScript II reverse transcriptase (Invitrogen) as per manufacturer’s instructions. PCR was performed with primers generated from the mouse NoxA1 sequence (5′-TTCCAGCTGCAGAGGTTCCAG-3′ as forward primer and 5′-AAGTCCAAATCCTCCGGTCT-3′ as reverse primer) to amplify a 923-bp fragment (247–1169 bp) of the NoxA1 gene. RT-PCR to determine the expression of p67phox in mouse VSMC was performed with primers generated from the mouse p67phox sequence (5′-CTACCTGGAGCCAGTTGAGC-3′ as forward primer and 5′- GGGCAGCCTCATAACTGAAG-3′ as reverse primer) to amplify a 636-bp fragment (876–1411 bp) of the p67phox gene (see supplemental materials for details).
Retroviral Vector Construction and Infection of Mouse Aortic VSMC
A double-stranded hairpin oligonucleotide designed to target the mouse NoxA1 cDNA nucleotides 376–394 (5′-TACAACATGGCATCAGCAC-3′) of NoxA1 gene was cloned into the BglII/HindIII site of pSUPER.retro.puro vector to generate NoxA1 shRNA (see supplemental materials for details).
Adenovirus Construction and Infection of Mouse Aortic VSMC
Adenoviral constructs encoding HA-NoxA1 and Myc-p47phox and infection of VSMC were performed as described in the supplemental materials.
Measurement of Intracellular Reactive Oxygen Species
Intracellular hydrogen peroxide levels in VSMC were measured using 2',7'-dichlorofluorescein diacetate as described previously.24 Superoxide levels in mouse VSMC25 and in the aortas9 were measured by staining with dihydroethidium. Superoxide anion generation in thrombin-treated mouse VSMC suspension was measured using chemiluminescence enhancer L012 [8-amino-5-chloro-7-phenylpyridol[3,4-d]pyridazine-1,4(2H,3H)dione] (see supplemental materials for details).
Immunoprecipitation and Western Blot Analysis
Immunoprecipitation and Western blotting were preformed as described26 (see supplemental materials).
[3H]Thymidine Incorporation Assay
[3H] Thymidine incorporation into DNA of VSMC treated with or without thrombin were performed as described26 (see supplemental materials).
VSMC Migration Assay
VSMC migration was determined in a scratch wound assay (see supplemental materials).
Real-time RT-PCR to Measure NoxA1 Expression
Detailed information regarding RNA extraction and real-time RT-PCR for NoxA1 expression is available in the supplemental materials.
Mice and Diet
Wild-type and apoE−/− mice were purchased from Jackson Laboratory. Mice were maintained at 22°C with 12-hour light/dark cycle and given free access to food and water. Mice on high-fat diet were maintained on standard rodent chow until 4 weeks of age and then fed a Western-type diet (Harlan Teklad / TD88137) for 12 weeks. All procedures involving the experimental animals were performed in accordance with protocols approved by UNC IACUC according to NIH guidelines.
Carotid Artery Injury and NoxA1 Overexpression
The right external and common carotid arteries were surgically exposed in 10-week old, anesthetized wild-type mice. Angioplasty guide wire, introduced through an arteriotomy made in external carotid artery, was used to denude the endothelium in the artery. The denuded artery was flushed with PBS and a 1 cm segment isolated with ligatures and a 50 µl PBS or PBS containing AdNoxA1 or AdGFP vector (1×109 pfu) was injected through a catheter and incubated for 30 min. After removal of this solution, the external carotid artery was ligated and blood flow to the internal carotid artery was restored. The arteriotomy site was ligated by tying the previously placed suture and skin incision was closed with 7–0 sutures (see supplemental materials).
Human Carotid Arteries
Fresh frozen human carotid arteries, collected during autopsy performed 10–12 hours after death, were obtained from National Disease Research Interchange (Philadelphia, PA). Carotid artery endarterectomy samples were collected at St Thomas’ Hospital, London under informed consent. The use of human specimens was approved by UNC IRB. Carotid atherosclerotic lesions were graded as normal ― no lesions, Type I ― very initial lesions, II ― first grossly visible lesions characterized by foam cells and lipid droplets in intimal SMC and droplets of extracellular lipid and III ― intermediate lesions according to AHA Atherosclerotic Plaque Classification.27
Histology and Morphometry
Carotid artery sections were stained with combined Masson’s trichrome elastic stain or oil red O and morphometric analysis was performed (see supplemental materials).
Immunohistochemistry
NoxA1 staining of carotid artery and aortic sections was described online in the supplemental materials.
Statistical Analysis
Data are presented as mean ± SEM. All data were analyzed using one-way ANOVA followed by Newman-Keuls multiple comparison test. Differences were considered significant at P<0.05.
Results
Confirmation of NoxA1 Expression in Mouse Aortic VSMC
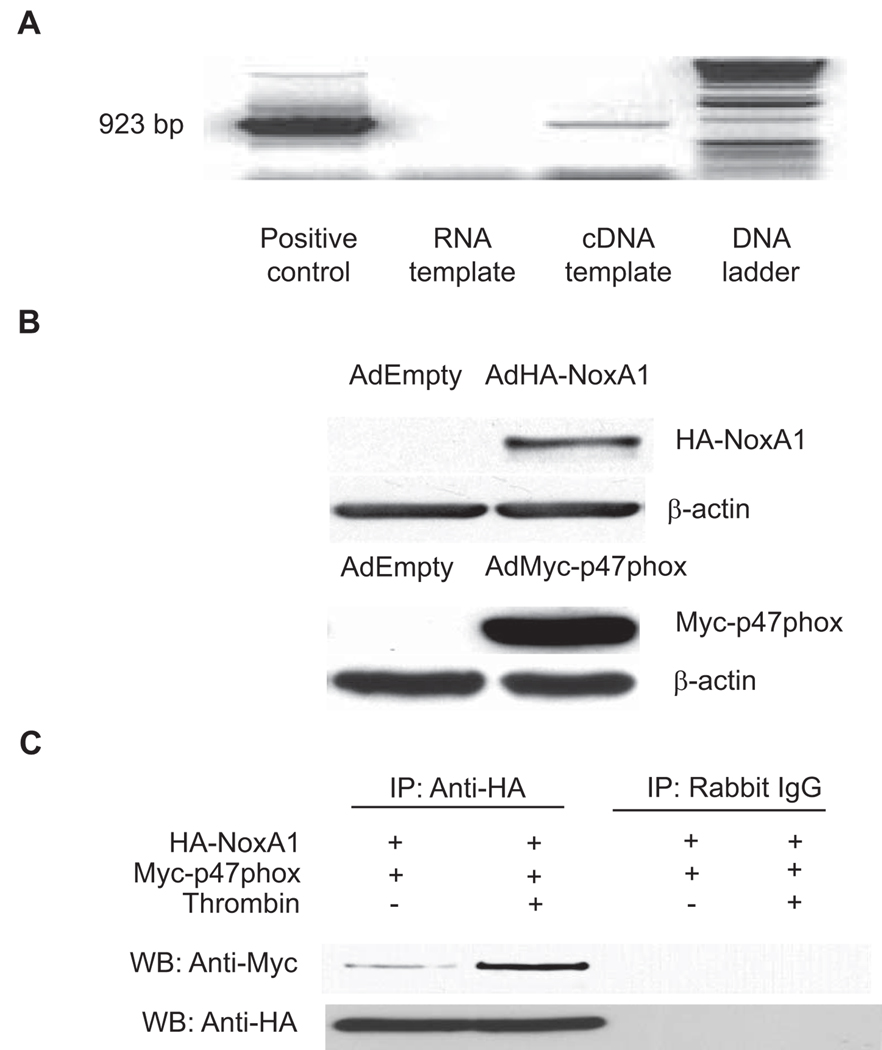
The molecular composition of vascular wall NADPH oxidases continues to be investigated because of its complexity. Multiple possible subunit combinations, variability between cell types, in the same cell type from different species and even in a single cell type (VSMC) from different anatomic locations have all been demonstrated.28 An important remaining question is whether one or both activating subunits, p67phox or NoxA1, are functionally important. We began by investigating expression levels and previously reported that p67phox was not detectable in human and rat aortic VSMC.15 Ambasta et al.20 recently reported the expression of NoxA1, a p67phox homologue, in mouse aortic VSMC by Western analysis. To determine whether NoxA1 is expressed in mouse VSMC, we performed RT-PCR amplifying a region from 247–1169 bp of NoxA1 coding sequence. We were able to amplify the expected (923 bp) fragment using NoxA1 cDNA, but not cellular RNA as the template, indicating that the PCR product was not amplified from the contaminating genomic DNA present in the RNA preparation (Figure 1A). Sequence analysis of the PCR product confirmed the NoxA1 expression in mouse aortic VSMC. As with human and rat aortic VSMC, p67phox protein was not expressed in mouse aortic VSMC as well (data not shown). These data indicate that NoxA1 is the p67phox homologue expressed in mouse aortic VSMC.
Figure 1.
NoxA1 is expressed and interacts with p47phox in mouse aortic VSMC. A, The cDNA generated from VSMC was used as a template for PCR with NoxA1-specific primers to amplify a 923-bp NoxA1 coding sequence. pcDNA3-NoxA1 was used as a positive control and total RNA as a negative control. B, p47phox−/− VSMC were infected with adenoviruses encoding either HA-NoxA1 or Myc-p47phox and Western blot analysis was performed with anti-Myc or anti-HA antibodies. C, p47phox−/− VSMC infected with adenoviruses encoding HA-NoxA1 and Myc-p47phox were quiesced for 72 hours and then treated without or with thrombin (1 U/mL) for 10 minutes. Cell lysates were immunoprecipitated (IP) with either anti-HA antibody or non-immune Rabbit IgG and Western blot (WB) analysis was preformed with anti-Myc or anti-HA antibody.
NoxA1 Interacts with p47phox
It was previously demonstrated that fluorescent fusion proteins of NoxA1 and p47phox, when expressed in mouse VSMC, are translocated from cytosol to the membrane and cytoskeleton after stimulation with epidermal growth factor.20 This is consistent with the hypothesis that p47phox binds NoxA1 and forms a functional NADPH oxidase at the membrane in an analogous manner to association of the complex in phagocytes. We used immunoprecipitation/Western analysis to address the interaction of NoxA1 with p47phox and determine if physiologic factors that modulate ROS production, such as thrombin, also effect the NoxA1-p47phox interaction. Because NoxA1 specific antibodies were not available at this time, we infected p47phox- deficient mouse VSMC with adenoviruses encoding myc-tagged p47phox and HA-tagged NoxA1 (Figure 1B). Cells were treated with or without thrombin for 10 minutes and immunoprecipitation was performed with anti-HA antibody. Western blot analysis with anti-myc antibody showed a significant increase in the interaction of NoxA1 with p47phox after thrombin stimulation (Figure 1C). These results confirm that interaction of NoxA1 with p47phox is an essential initial event in the assembly of a functional NADPH oxidase upon agonist stimulation in mouse VSMC.
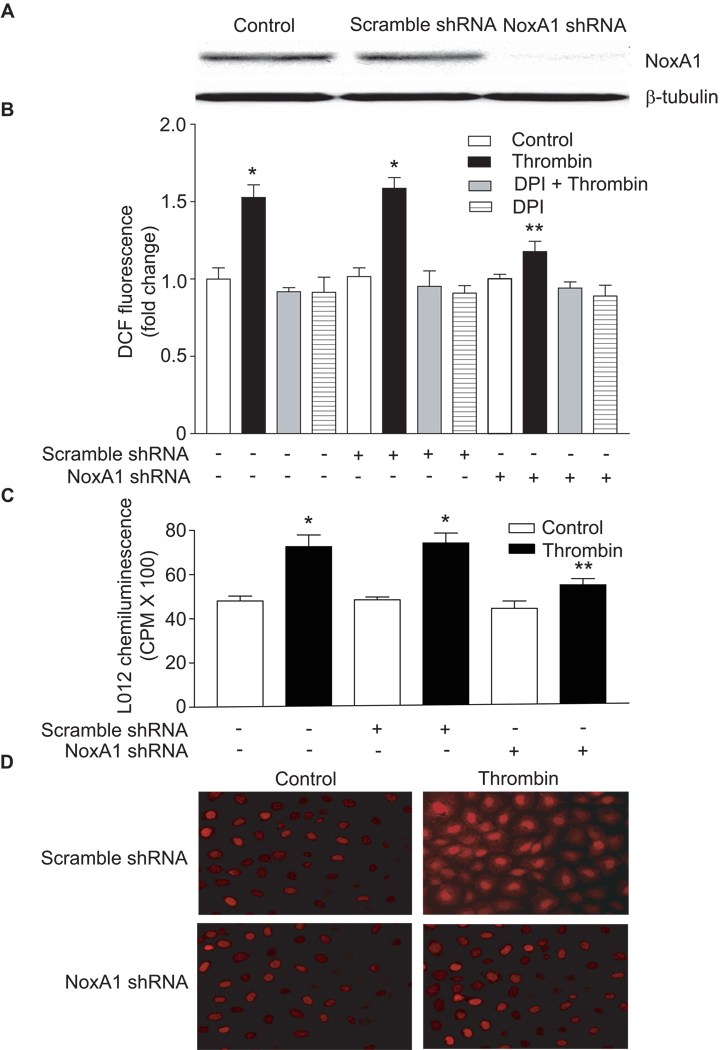
NoxA1 is Essential for Thrombin-induced ROS Production in Mouse VSMC
To determine whether NoxA1 is required for ROS production, wild-type mouse aortic VSMC were infected with retroviruses encoding either NoxA1-specific shRNA or scrambled shRNA (Figure 2A). NoxA1-specific shRNA infection resulted in approx. 90% decrease in the expression of a 51-kDa band recognized by the NoxA1 antibodies confirming the effectiveness of shRNA in regulating NoxA1 expression. In addition, regulation of NoxA1 expression using retroviral NoxA1 shRNA or adenoviral NoxA1 constructs had no effect on Nox1 expression as measured by real-time RT-PCR (Supplemental Figure IA). The manipulation of NoxA1 expression by these methods did not cause cellular toxicity (data not shown) or activation of cellular stress response as measured by HSP70 levels (Supplemental Figure IB). In cells not infected with NoxA1 shRNA, treatment with thrombin for 10 minutes significantly increased (P<0.001) ROS production as measured by the oxidation of H2DCF-DA (Figure 2B) and by L012 chemiluminescence (Figure 2C). Thrombin-induced ROS generation was largely inhibited by pretreatment with diphenyleneiodonium (DPI), a flavoprotein inhibitor. Suppression of NoxA1 by infection of VSMC with retrovirus encoding NoxA1 shRNA had no significant effect on basal ROS production. However, thrombin-induced ROS generation in mouse VSMC expressing NoxA1 shRNA was significantly (P<0.01), but not completely inhibited. Infection of VSMC with retrovirus encoding scrambled shRNA had no effect on thrombin-induced ROS generation (Figures 2B & 2C). Consistent with these observations, conversion of DHE to ethidium, a sensitive marker for O2.− generation, was markedly attenuated in thrombin-treated VSMC expressing NoxA1 shRNA, but not scrambled shRNA (Figure 2D). Together, these data indicate that NoxA1 is necessary for agonist-induced increases in ROS production in mouse VSMC. Further, these data are consistent with the notion that either low levels of NoxA1 (or an undetectable level of p67phox) are sufficient for basal ROS production.
Figure 2.
Suppression of NoxA1 expression decreases thrombin-induced ROS production. A, Wild-type mouse aortic VSMC were infected with retroviruses encoding either scrambled shRNA or NoxA1 shRNA. NoxA1 protein levels were determined by Western blot analysis using anti-NoxA1 antibody. β-tubulin levels were used as loading controls. B, VSMC expressing scrambled or NoxA1 shRNA were treated without or with thrombin (1 U/mL) after pretreatment with DPI. ROS levels were determined by measuring DCF fluorescence (mean ± SEM, n=4; *P<0.001 vs respective controls; **P<0.01 vs thrombin-treated scrambled shRNA expressing cells). C, ROS levels in the above mentioned VSMC were determined by measuring L012 chemiluminescence (mean ± SEM, n=4; *P<0.001 vs respective controls; **P<0.01 vs thrombin-treated scrambled shRNA expressing cells). D, VSMC expressing scrambled or NoxA1 shRNA were treated with or without thrombin and ROS production was detected by DHE staining.
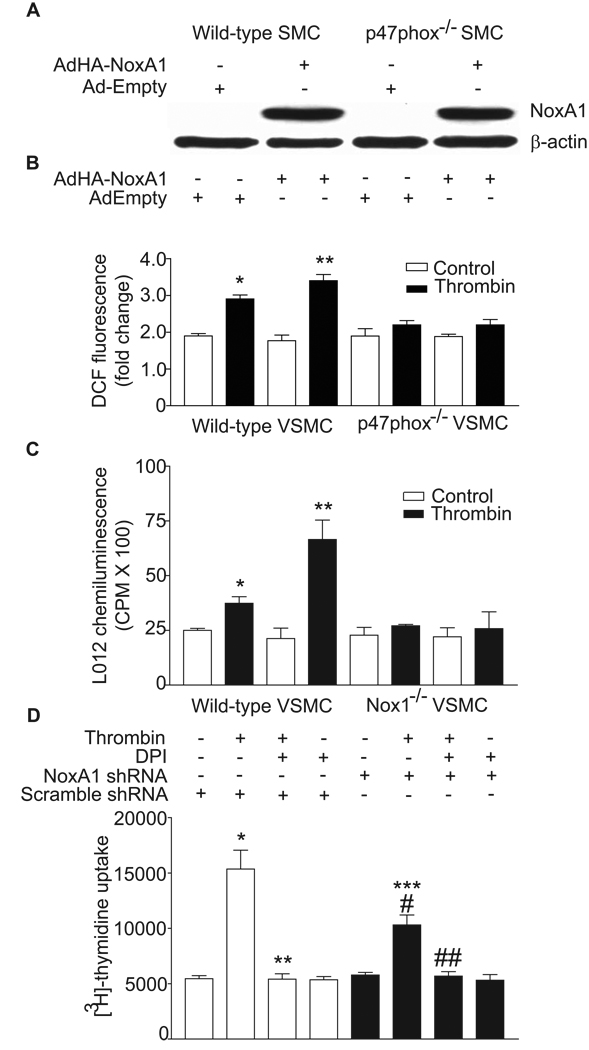
Overexpression of NoxA1 Increases ROS Production in Wild-type, But Not in p47phox−/− and Nox1−/− VSMC
It has been reported that in endothelial dysfunction and atherosclerosis increased ROS production is associated with enhanced expression of NoxA1 homologue, p67phox.29,30 Similarly, increased p67phox and other NADPH oxidase subunit gene expression was observed in vascular cells in response to pathophysiological agonists31 and is correlated with increased ROS production.32 To determine the effect of increased expression of NoxA1 on thrombin-induced ROS generation, wild-type and p47phox knockout mouse aortic VSMC infected with control adenovirus or adenovirus expressing HA-tagged NoxA1 were quiesced for 72 hours and then left untreated or treated with thrombin for 10 minutes. Infection with HA-tagged NoxA1 adenovirus caused a marked increase in the expression of NoxA1 in both wild-type and p47phox knockout VSMC (Figure 3A). As previously reported,25 thrombin significantly increased ROS production in wild-type (P<0.05) but not in p47phox−/− (Figure 3B) and Nox1−/− (Figure 3C) VSMC . NoxA1 overexpression potentiated thrombin-induced ROS generation in wild-type VSMC (P<0.001) but had no effect on ROS generation in p47phox−/− (Figure 3B) and Nox1−/− (Figure 3C) cells. These data confirm that interaction of p47phox with NoxA1 is necessary for agonist-induced ROS generation and suggest that modulation of NoxA1 under pathophysiological conditions would increase oxidative stress in the vasculature.
Figure 3.
NoxA1 expression levels affect thrombin-induced ROS generation and VSMC proliferation. A, Wild-type and p47phox−/− VSMC were infected with either HA-NoxA1-encoding adenovirus or empty adenovirus and Western analysis was performed with either anti-HA or anti-β-actin antibodies. B, Wild-type and p47phox−/− VSMC, infected with either HA-NoxA1-encoding adenovirus or empty adenovirus, were quiesced and then treated with thrombin (1 U/mL) for 10 minutes. ROS levels were determined by measuring DCF fluorescence (mean ± SEM, n=4; *P<0.05 vs control; **P<0.001 vs thrombin-treated, empty adenovirus infected cells). C, Wild-type and Nox1−/− VSMC, infected with either HA-NoxA1-encoding adenovirus or empty adenovirus, were quiesced and then treated with thrombin (1 U/mL) for 10 minutes. ROS levels were determined by measuring L012 chemiluminescence (mean ± SEM, n=4; *P<0.05 vs control; **P<0.05 vs thrombin-treated, empty adenovirus infected cells). D, Wild-type mouse aortic VSMC infected with retroviruses encoding either scrambled shRNA or NoxA1 shRNA were quiesced, pretreated with DPI for 1 hour prior to treatment with or without thrombin (1 U/mL) for 16 hours. Cells were incubated with [3H]-thymidine for the last 3 hours. Data presented are mean ± SEM (n=3) of three separate experiments (*P<0.001 vs respective control; **P<0.001 vs thrombin-treated scrambled shRNA expressing cells; #P<0.001 vs respective control; ##P<0.001 vs thrombin-treated NoxA1 shRNA expressing cells; ***P<0.001 vs thrombin-treated, scrambled shRNA expressing cells).
Downregulation of NoxA1 Expression Decreases Thrombin-induced Proliferation of VSMC
We have previously shown that thrombin promotes VSMC proliferation in a ROS-dependent manner through the activation of NADPH oxidase.9,15 So we next examined the role of NoxA1 in thrombin-induced VSMC proliferation (Figure 3D). Growth-arrested mouse aortic VSMC, infected with retroviruses encoding either NoxA1-specific shRNA or scrambled shRNA, were treated with or without thrombin in presence and absence of DPI. Thrombin induced a 2.8-fold increase in DNA synthesis in VSMC expressing scrambled shRNA, as measured by thymidine uptake, an effect that was significantly inhibited by DPI (P < 0.001). Thrombin-induced DNA synthesis increased only 1.8-fold in VSMC expressing NoxA1 shRNA compared to the respective control, but was significantly less than that in cells infected with scrambled shRNA (P<0.001). Importantly, DPI pretreatment reduced thrombin-induced increases in DNA synthesis in cells expressing NoxA1 shRNA. This suggests that the residual increase in ROS levels in these cells contributes to increased thymidine uptake. DPI had no effect on basal thymidine uptake in VSMC expressing either NoxA1 shRNA or scrambled shRNA. Together these data indicate that NoxA1 contributes to agonist-induced proliferative response of VSMC.
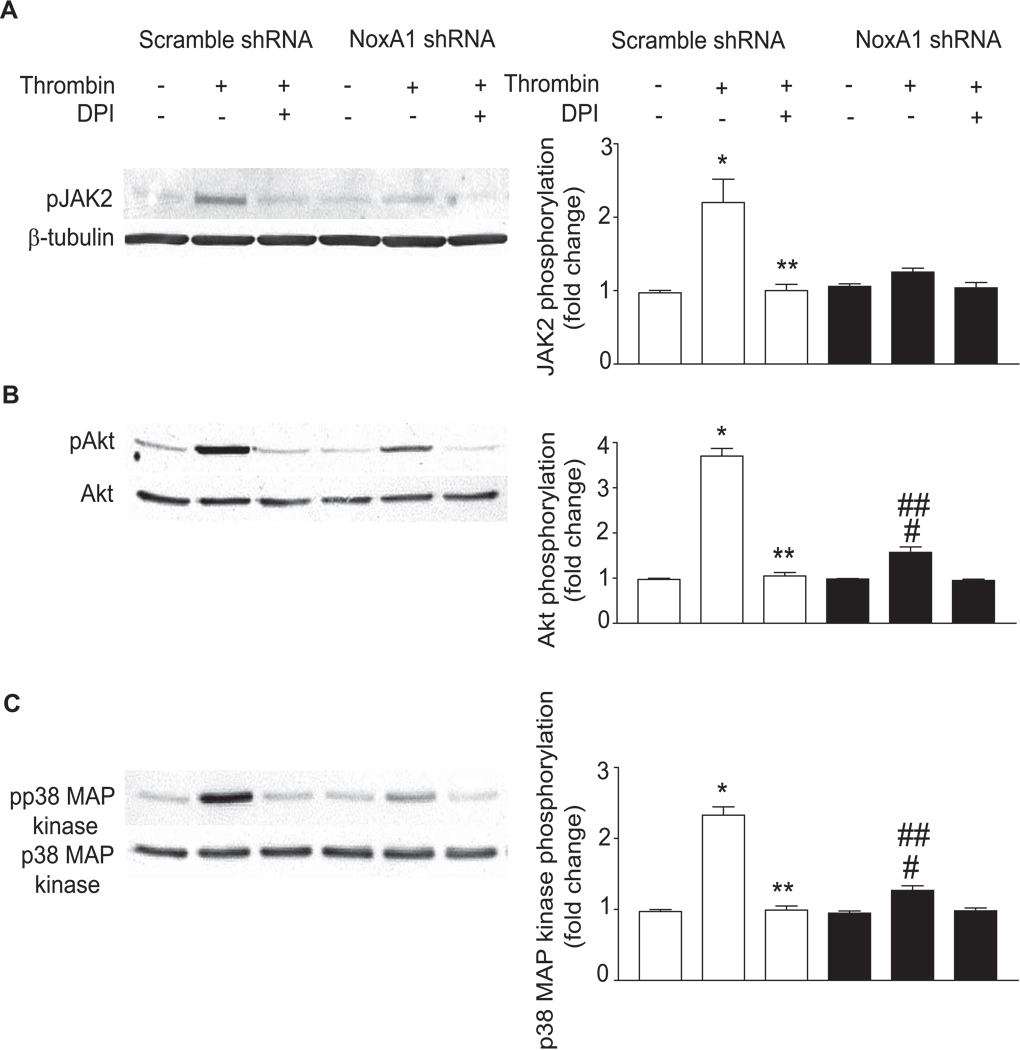
Suppression of NoxA1 Expression Decreases Thrombin-induced Activation of Protein Kinases in Mouse Aortic VSMC
ROS derived from vascular NADPH oxidases serve as second messengers to activate multiple intracellular proteins that play an important role in the physiology and pathophysiology of vascular cells.33 To determine the role of NoxA1-containing NADPH oxidases in thrombin-induced VSMC proliferation, we examined the activation of redox-sensitive protein kinases such as Janus kinase 2 (JAK2), Akt/protein kinase B and p38 mitogen-activated protein kinase (p38 MAP kinase) in NoxA1 shRNA and scrambled shRNA expressing wild-type VSMC treated with thrombin (Figure 4). Western blot analysis of thrombin-stimulated VSMC lysates with a phospho-specific JAK2 antibody showed a significant increase in JAK2 tyrosine phosphorylation at 10 minutes in scrambled shRNA expressing cells (2.20 ± 0.55 fold increase, P<0.001), which was abrogated by pretreatment with DPI (P<0.001) (Figure 4A). In contrast, no such increase in JAK2 phosphorylation in response to thrombin treatment was observed in VSMC expressing NoxA1 shRNA. Thrombin treatment significantly stimulated Akt/PKB phosphorylation at 10 minutes in both scrambled shRNA (3.70 ± 0.30 fold increase, P<0.001) and NoxA1 shRNA expressing VSMC (1.57 ± 0.21 fold increase, P<0.01) (Figure 4B). However, Akt/PKB phosphorylation in NoxA1 shRNA expressing cells was significantly less than that in scrambled shRNA expressing cells (P<0.001) and was completely abrogated by pretreatment with DPI in both the cells.
Figure 4.
Depletion of NoxA1 expression attenuates redox-sensitive cellular signaling pathways. Retrovirus-infected VSMC were quiesced, pretreated with DPI for 1 hour prior to treatment without or with thrombin (1 U/mL) for 10 min. A, Representative Western blots of cell lysates using anti-phosphotyrosine-specific JAK2 and anti-β-tubulin antibodies (left panel). Densitometry analysis of JAK2 tyrosine phosphorylation (mean ± SEM, n=3; right panel). *P<0.001 vs respective control; **P<0.001 vs thrombin-treated scrambled shRNA expressing cells. B, Representative Western blots using anti-phosphospecific-Akt/PKB and anti-Akt/PKB antibodies (left panel). Densitometric analysis of Akt phosphorylation (mean ± SEM, n=3; right panel). *P<0.001 vs respective control; **P<0.001 vs respective thrombin-treated cells; #P<0.01 vs respective control; ##P<0.001 vs thrombin-treated scrambled shRNA expressing cells. C, Representative Western blots using anti-phosphospecific-p38 MAPK and anti-p38 MAPK antibodies (left panel). Densitometric analysis of p38 MAP kinase phosphorylation (mean ± SEM, n=3; right panel). *P<0.001 vs respective control; **P<0.001 vs respective thrombin-treated cells; #P<0.05 vs respective control; ##P<0.001 vs thrombin-treated scrambled shRNA expressing cells.
Phosphorylation of p38 MAPK increased significantly in scrambled shRNA (2.33 ± 0.20 fold increase, P<0.001) and in NoxA1 shRNA expressing VSMC (1.27 ± 0.11 fold increase, P<0.01) treated with thrombin (Figure 4C). Similar to its effect on Akt/PKB phosphorylation, p38 MAP kinase phosphorylation was significantly decreased in NoxA1 shRNA expressing VSMC compared with scrambled shRNA expressing VSMC (P<0.001). Thrombin-induced p38 MAP kinase activation in scrambled shRNA expressing VSMC was abrogated by DPI pretreatment. These data indicate that NoxA1-containing NADPH oxidase contributes to VSMC proliferation by the activation of mitogenic intracellular signaling pathways.
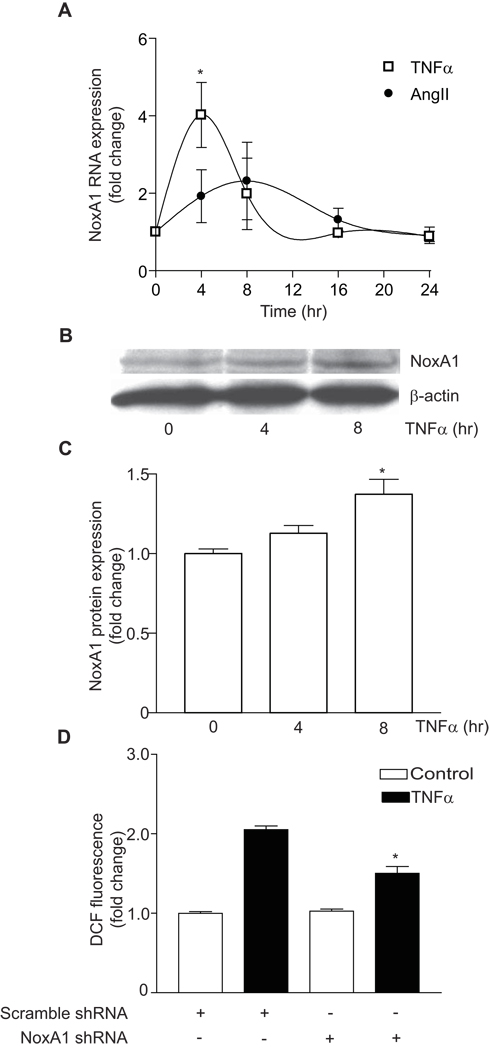
TNFα, Not Thrombin, Increased the Expression of NoxA1, and NoxA1 shRNA Inhibited TNFα-induced ROS Production in VSMC
We next examined the effect of thrombin on NoxA1 expression by RT-PCR. Although NoxA1 is essential for thrombin-induced ROS generation, thrombin (1 U/mL) per se did not induce NoxA1 expression in mouse VSMC (data not shown). However, treatment with tumor necrosis factor-α (TNFα, 20 ng/mL) for 4 hours significantly increased NoxA1 expression (4.35 ± 1.70 fold increase, P<0.05) in mouse VSMC (Figure 5A). Angiotensin II (100 nmol/L) was a weak inducer of NoxA1 expression (1.92 ± 0.68 fold increase at 4 hours treatment) (Figure 5A). In contrast to the report of Honjo et al.34 in human umbilical vein endothelial cell line, oxidized low-density lipoprotein (30 µg/mL or 60 µg/mL) had no stimulatory effect on NoxA1 expression in mouse VSMC for up to 24 hours of treatment (data not shown).
Figure 5.
TNFα induces NoxA1 expression and TNFα-induced ROS production was inhibited in NoxA1 shRNA expressing mouse VSMC. A, Real-time RT-PCR analysis of NoxA1 expression in VSMC treated with 20 ng/mL TNFα or 100 nmol/L angiotensin II. Data expressed as fold change relative to untreated control (mean ± SEM, n=3; *P<0.05 vs untreated cells). B, Western analysis of NoxA1 protein expression in lysates from VSMC treated with 20 ng/ mL TNFα. C, Densitometric analysis of NoxA1 protein (mean ± SEM, n=4; *P<0.05 vs untreated cells). D, ROS production, as measured by DCF fluorescence, in VSMC expressing scrambled or NoxA1 shRNA and treated with 20 ng/mL TNFα (mean ± SEM, n=4; *P<0.001 vs TNFα-treated scrambled shRNA expressing cells).
Consistent with real-time RT-PCR data, we observed a significant increase in NoxA1 protein expression in VSMC treated with TNFα for 8 hours (P<0.05) (Figure 5B and 5C). As in the case of thrombin, NoxA1 shRNA expression significantly attenuated TNFα-induced ROS generation in mouse VSMC (Figure 5D). These data indicate that thrombin regulates ROS generation without affecting NoxA1 expression levels, whereas other agonists might enhance ROS production by modulating NoxA1 expression.
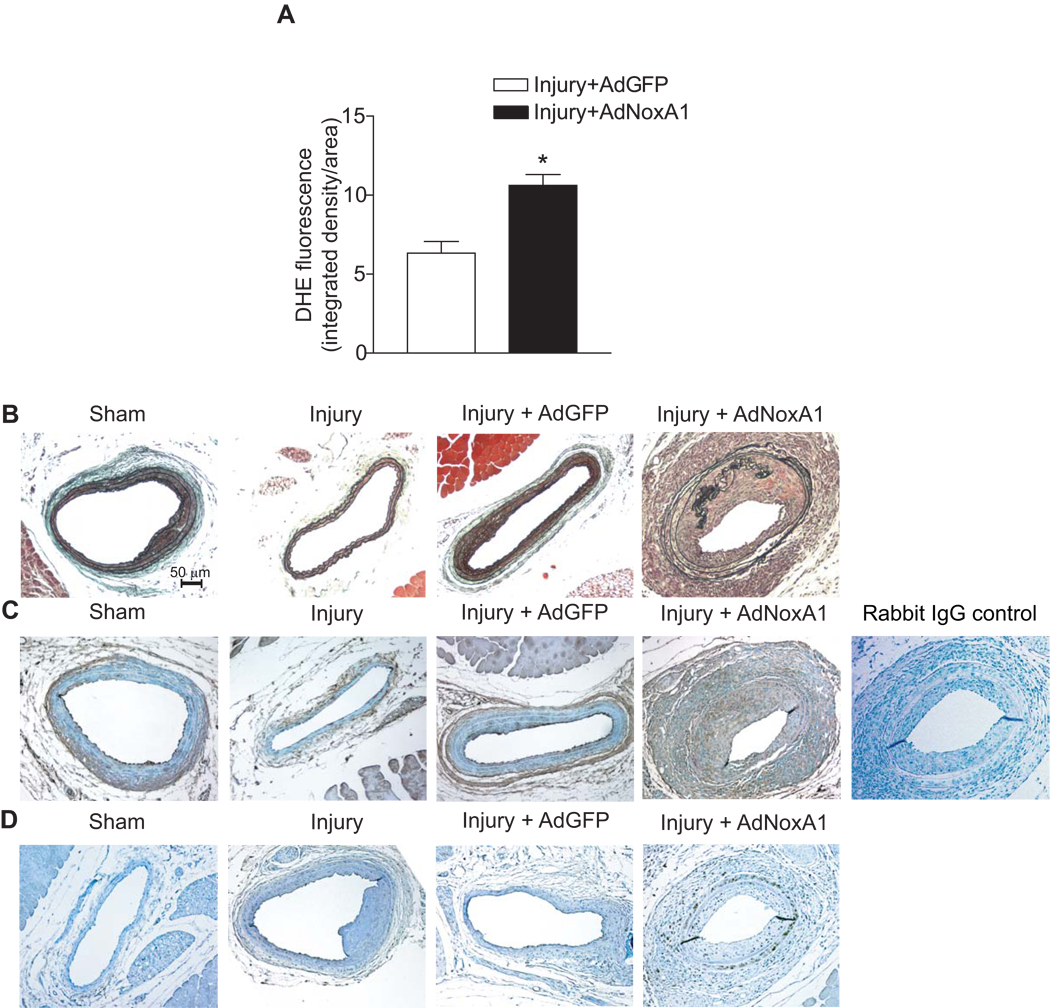
NoxA1 Overexpression Increases Superoxide Production in Injured Carotid Arteries and Enhances Neointimal Hyperplasia
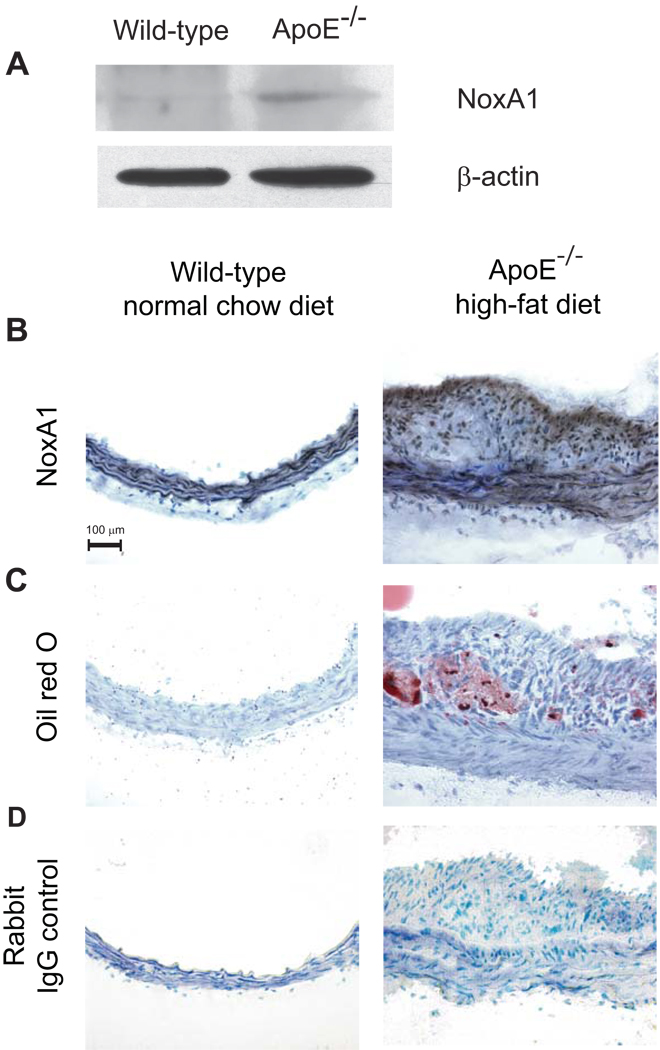
We also examined whether NoxA1-dependent increased production of ROS in VSMC observed in vitro occurs in arterial VSMC in vivo and NoxA1-dependent activation of NADPH oxidase results in enhanced neointimal hyperplasia. Mouse carotid arteries were subjected to guidewire injury and then infected with a recombinant adenovirus encoding NoxA1 and GFP, a control, empty adenovirus expressing only GFP or only treated with PBS. Three days after adenovirus infection, histochemical analysis revealed NoxA1 overexpression in AdNoxA1-infected vessels (Supplemental Figure IIA). DHE staining of injured carotid arteries showed a significant increase (P<0.001) in O2.− levels in the medial VSMC (Supplemental Figure IIB and Figure 6A). Fifteen days after guidewire injury and adenovirus infection, neointimal hyperplasia was assessed morphologically and by morphometric analysis (Figure 6B and Supplemental Figure IIC). Compared with empty adenovirus-infected mice, adenovirus NoxA1 expressing mice had significantly increased (P<0.05) neointimal hyperplasia. Further, immunoreactive NoxA1 expression in neointima 15 days after injury was confined to smooth muscle α-actin positive cells (Figure 6C). Neointimal hyperplasia is correlated with increased intimal SMC proliferation as shown by enhanced immunostaining with proliferating cell nuclear antigen antibody (Figure 6D). We have also observed that NoxA1 overexpression in VSMC results in increased migration as determined by the in vitro scratch assay (Supplemental Figure III). Intriguingly, injury per se had no effect on NoxA1 levels in the carotid artery. However, NoxA1 expression is increased in aortas and atherosclerotic lesions from ApoE−/− mice (Figure 7). Together, these data suggest that in mouse carotid artery injury model, localized overexpression of NoxA1 stimulates O2.− production resulting in increased medial VSMC migration and proliferation and enhanced neointima formation.
Figure 6.
Exogenous NoxA1 overexpression in carotid arteries increases superoxide production and induces neointimal hyperplasia. Quantification of confocal images of DHE-stained fresh-frozen sections of guidewire-injured carotid arteries (A) infected with AdGFP or AdNoxA1 (mean ± SEM, n=8, *p<0.001). Representative sections of carotid arteries, 15 days after sham or guidewire injury and sham infection or infection with AdGFP or AdNoxA1, were stained with combined Masson’s trichrome elastin stain (B) and for NoxA1 (C), or proliferating cell nuclear antigen (D).
Figure 7.
Immunoreactive NoxA1 expression is enhanced in atherosclerotic lesions of apoE−/− mice. Western blot analysis of mouse aortic lysates using anti-NoxA1 or β-actin antibody (A). Representative sections of frozen mouse aortas stained for immunoreactive NoxA1 (B), with oil red O (C) or non-immune rabbit IgG (D).
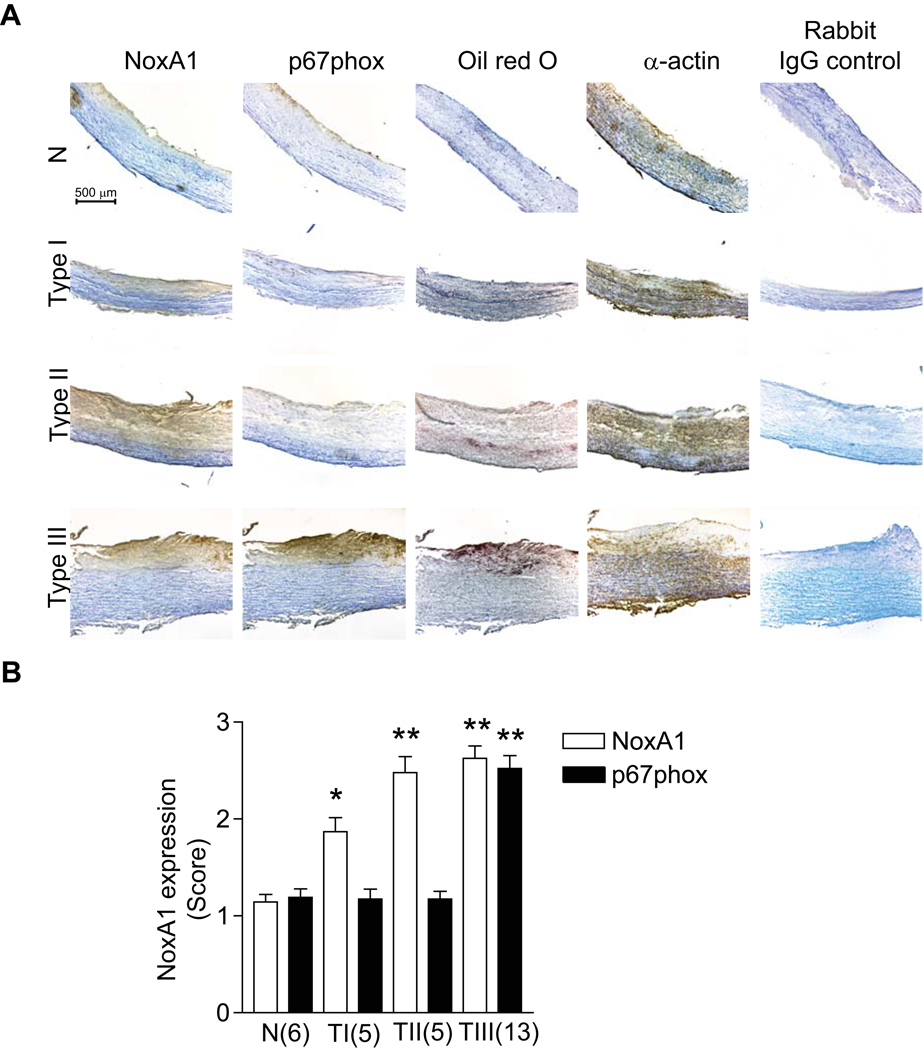
NoxA1 is Expressed in Early Atherosclerotic Lesions of Human Carotid Arteries
To determine the clinical relevance of our NoxA1 data from cell culture experiments and mouse atherosclerosis models, we investigated NoxA1 expression in normal, early and relatively advanced (intermediate) atherosclerotic lesions in human carotid arteries (Figure 8). We analyzed a total of 29 samples of which 22 were male and 7 female. The subjects ranged in age from 21–89 years. NoxA1 expression, which was weak in normal carotid arteries, increased significantly in the intimal and medial SMC of early (Type I and II) atherosclerotic lesions. Expression of p67phox was confined to endothelial layer in both normal and early atherosclerotic arteries. However, the expression of both NoxA1 and p67phox increased significantly in the intimal and medial SMC of early advanced (Type III) atherosclerotic lesions. The presence of NoxA1 and p67phox in VSMC was confirmed by staining with α-actin (Figure 8). Though our small sample size precludes a definitive statement, NoxA1 and p67phox expression in carotid atherosclerotic lesions does not seem influenced by the gender of the subjects. These data suggest that NoxA1 plays a role in the development of human atherosclerosis as well.
Figure 8.
NoxA1 vs p67phox expression in different atherosclerotic lesion types in human carotid arteries. A, Representative cross sections of human carotid arteries stained for immunoreactive NoxA1, p67phox, oil red O and smooth muscle α-actin. B, Expression of NoxA1 and p67phox as determined by immunohistochemistry scoring where 1 is weak, 2 moderate, and 3 strong staining. The numerical scoring was confirmed by a second independent observer, blinded to the initial score. Data represent the mean ± SEM (*P<0.01 vs N, **P<0.001 vs respective N).
Discussion
An understanding of the precise molecular composition of vascular NADPH oxidases is essential to elucidate the contribution of cell-specific oxidases in vascular pathology. In this study we confirmed that NoxA1 is a critical component of VSMC NADPH oxidase.20 Downregulation of NoxA1 expression inhibited thrombin-induced O2.− production, mitogenic protein activation and proliferation of VSMC whereas its upregulation increased thrombin-induced O2.− production. Localized overexpression by adenovirus-mediated delivery of NoxA1 enhanced neointimal hyperplasia in the mouse carotid artery injury model indicating that regulation of NoxA1 expression could modulate VSMC NADPH oxidase activity under pathophysiologic conditions.
It is known that rodent VSMC NADPH oxidase has unique composition—presence of Nox1 and Nox4 and the absence of Nox2 isoform— compared to other vascular cells.4,35–37 However, Nox1-containing VSMC NADPH oxidase is similar to other vascular cell and leukocyte NADPH oxidases in that its activity is dependent on other subunits of the enzyme, p22phox, p47phox and Rac.9,11,12,38 Recently Ambasta et al.20 have demonstrated that NoxA1 is the p67phox homologue in functional mouse VSMC NADPH oxidase. In the present study, we confirmed this observation by RT-PCR amplification and sequencing of NoxA1 from mouse VSMC. Our observation that thrombin activates the interaction of p47phox with NoxA1 is consistent with the data of agonist-induced membrane cotranslocation of NoxA1 and p47phox fusion proteins in VSMC.20 Further support for p47phox and NoxA1 interaction in activating VSMC NADPH oxidase is obtained from the data in the current study that NoxA1 overexpression enhances thrombin-induced ROS production in wild-type but not in p47phox−/− VSMC. Activated p47phox binds with NoxA1 and interacts with Nox1 at the membrane to increase O2.− production upon agonist treatment. This is corroborated by the coimmunoprecipitation of NoxA1 with Nox1 in HEK293 cells transfected with Nox1, NoxO1 and NoxA1 fusion proteins.20 Additionally, the critical role of NoxA1 in O2.− production is evident from negligible ROS generation in cells overexpressing Nox1 and NoxO1 in the absence of NoxA1.
NoxA1 plays a critical role in modulating the phenotype of VSMC because depletion of this protein using retroviral shRNA not only inhibits thrombin-induced ROS generation but also significantly attenuates thrombin-stimulated cell proliferation. Our data also indicate that NoxA1 functions in tandem with phosphorylated p47phox in agonist-induced stimulation of Nox1-containing NADPH oxidase because increased ROS generation was observed in NoxA1 overexpressing VSMC treated with thrombin and NoxA1 overexpression per se had no effect on ROS generation.
NoxA1 is not expressed in high abundance in mouse VSMC20 and we did not observe any significant increase in its expression following thrombin treatment. However, a robust increase in NoxA1 expression and NoxA1-dependent NADPH oxidase activation observed in response to TNFα suggests that both the presence of NoxA1 and its expression levels play a regulatory role in VSMC NADPH oxidase activation. In this context, it is worth noting that angiotensin II-induced transcriptional regulation of p67phox enhances NADPH oxidase activity in aortic endothelial cells39 and adventitial fibroblasts.40 Interestingly, p67phox has also been proposed as a putative rate-limiting co-factor in NADPH oxidase activation.41,42
We previously showed that decreased levels of activated mitogenic proteins and attenuated cell proliferation occur downstream of impaired agonist-induced ROS production in p47phox−/− VSMC.9,43 Our current data that depletion of NoxA1 in VSMC significantly decreases thrombin-induced JAK2, Akt and p38 MAPK phosphorylation supports the hypothesis that agonist-induced NADPH oxidase activation plays an important role in VSMC proliferation. These data are also consistent with redox-sensitive regulation of JAK2, Akt and p38 MAP kinase in VSMC.4,13,26,44.
Increased medial O2.− production and enhanced neointimal hyperplasia observed in the present investigation in AdNoxA1-infected injured carotid arteries is consistent with the notion that vascular NADPH oxidase plays a potentially critical role in vascular disease.4,9,43 Further, consistent with the observation of Ambasta et al.20 NoxA1 expression was abundant in proliferating VSMC in injured carotid arteries. The fact that NoxA1 expression is not upregulated by injury per se, but seems to increase in atherosclerotic lesions suggests that NoxA1 expression is fine-tuned to different pathophysiologic stimuli. In this context, it is worth mentioning a recent report by Kim et al45 elucidating a novel mechanism for NoxA1-regulated Nox1 activation in colon cell lines. Protein kinase A-dependent phosphorylation of NoxA1 at Ser172 and Ser461 results in its interaction with 14–3–3ζ and consequent inhibition of Nox1 activity. It would be interesting to see whether a similar mechanism regulates NoxA1-dependent Nox1 activity in vascular cells.
Interestingly, immunoreactive NoxA1 was present in the intimal and medial SMC of early atherosclerotic lesions (Type I and II) whereas NoxA1 as well as p67phox were present in the intimal and medial SMC of early advanced (Type III) atherosclerotic lesions of human carotid arteries. In this context it is noteworthy that Clempus et al. have correlated modulation of rat VSMC phenotypes with relative expression levels of Nox1 and Nox4.35 It would be interesting to see whether a similar phenomenon occurs with regards to NoxA1- and p67phox-dependent NADPH oxidase activity on cellular phenotype in human aortic VSMC.
In conclusion, our results demonstrate that NoxA1 expression regulates NADPH oxidase activity in mouse VSMC. Furthermore, expression of NoxA1 regulates thrombin-induced activation of redox-sensitive mitogenic protein kinases. Increased neointimal hyperplasia in injured mouse carotid arteries following localized overexpression of NoxA1 and the presence of NoxA1 in mouse and human atherosclerotic lesions suggest that NoxA1 is a potential target for therapeutic intervention. We believe that these findings support further investigation of NoxA1 expression in vascular diseases.
Supplementary Material
Acknowledgements
We gratefully acknowledge Dr. Kathy Griendling for providing Nox1−/− VSMC.
Sources of Funding
This work was supported in part by National Institutes of Health grant HL-57352 (M.S.R) and National Institute for Health Research Biomedical Research Center at Guy’s and St Thomas’ National Health Service Foundation Trust (A.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
After decades of investigation, there remains a need for preventive strategies that can reduce atherosclerosis and atherothrombosis, which represent the most common cause of death and disability in the United States. Despite many important advances in the treatment of cardiovascular diseases, elucidation of specific therapies that target vascular wall cells has been elusive. An important limitation of targeting intracellular signaling pathways in dysfunctional vascular cells is that most of them are present ubiquitously and are necessary for normal cellular function. Many investigators have studied the hypothesis that regulated production of reactive oxygen species (ROS) and oxidative stress in vascular cells is important in atherogenesis and that it may be possible to specifically inhibit upregulation of ROS production in vascular cells and in so limit atherogenesis and atherothrombosis. However, the pathways that produce ROS – NADPH oxidase, xanthine oxidase, cyclooxygenase, lipogenase and others – are all necessary for normal cellular function. The experiments described here were designed to test the strategy of targeting a specific component of NADPH oxidase, arguably the most important regulated system for ROS production in vascular cells. To do so, we examined modulation of NoxA1, a NADPH oxidase component necessary for upregulation of superoxide production in vascular smooth muscle cells. Our studies indicate that NoxA1 is critically important in the NADPH oxidase-mediated overexpression of ROS, characteristic of vascular diseases. Although specific inhibitors of NoxA1 are not known, this work suggests that the strategy of cell specific modulation of NADPH oxidase function is a therapeutic approach worthy of further investigation.
Disclosures
None.
References
- 1.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 2.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 3.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 4.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 5.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 6.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 7.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassègue B, Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med. 2008;45:329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 12.Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation. 2001;104:79–84. doi: 10.1161/01.cir.104.1.79. [DOI] [PubMed] [Google Scholar]
- 13.Brandes RP, Miller FJ, Beer S, Haendeler J, Hoffmann J, Ha T, Holland SM, Gorlach A, Busse R. The vascular NADPH oxidase subunit p47phox is involved in redox-mediated gene expression. Free Radic Biol Med. 2002;32:1116–1122. doi: 10.1016/s0891-5849(02)00789-x. [DOI] [PubMed] [Google Scholar]
- 14.Leto TL, Lomax KJ, Volpp BD, Nunoi H, Sechler JM, Nauseef WM, Clark RA, Gallin JI, Malech HL. Cloning of a 67-kD neutrophil oxidase factor with similarity to a noncatalytic region of p60c–src. Science. 1990;248:727–730. doi: 10.1126/science.1692159. [DOI] [PubMed] [Google Scholar]
- 15.Patterson C, Ruef J, Madamanchi NR, Barry-Lane P, Hu Z, Horaist C, Ballinger CA, Brasier AR, Bode C, Runge MS. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47(phox) may participate in forming this oxidase in vitro and in vivo. J Biol Chem. 1999;274:19814–19822. doi: 10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- 16.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 17.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 18.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 19.Cheng G, Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem. 2004;279:4737–4742. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 20.Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free Radic Biol Med. 2006;41:193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- 22.Miyano K, Ueno N, Takeya R, Sumimoto H. Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J Biol Chem. 2006;281:21857–21868. doi: 10.1074/jbc.M513665200. [DOI] [PubMed] [Google Scholar]
- 23.Moon SK, Thompson LJ, Madamanchi N, Ballinger S, Papaconstantinou J, Horaist C, Runge MS, Patterson C. Aging, oxidative responses, and proliferative capacity in cultured mouse aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H2779–H2788. doi: 10.1152/ajpheart.2001.280.6.H2779. [DOI] [PubMed] [Google Scholar]
- 24.Carter WO, Narayanan PK, Robinson JP. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol. 1994;55:253–258. doi: 10.1002/jlb.55.2.253. [DOI] [PubMed] [Google Scholar]
- 25.Vendrov AE, Madamanchi NR, Hakim ZS, Rojas M, Runge MS. Thrombin and NAD(P)H oxidase-mediated regulation of CD44 and BMP4-Id pathway in VSMC, restenosis, and atherosclerosis. Circ Res. 2006;98:1254–1263. doi: 10.1161/01.RES.0000221214.37803.79. [DOI] [PubMed] [Google Scholar]
- 26.Madamanchi NR, Li S, Patterson C, Runge MS. Thrombin regulates vascular smooth muscle cell growth and heat shock proteins via the JAK-STAT pathway. J Biol Chem. 2001;276:18915–18924. doi: 10.1074/jbc.M008802200. [DOI] [PubMed] [Google Scholar]
- 27.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 28.Lassegue B, Griendling KK. Nox is playing with a full deck in vascular smooth muscle, a commentary on "Noxa1 is a central component of the smooth muscle NADPH oxidase in mice". Free Radic Biol Med. 2006;41:185–187. doi: 10.1016/j.freeradbiomed.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Oelze M, Daiber A, Brandes RP, Hortmann M, Wenzel P, Hink U, Schulz E, Mollnau H, von Sandersleben A, Kleschyov AL, Mulsch A, Li H, Forstermann U, Munzel T. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension. 2006;48:677–684. doi: 10.1161/01.HYP.0000239207.82326.29. [DOI] [PubMed] [Google Scholar]
- 30.Guzik TJ, Sadowski J, Kapelak B, Jopek A, Rudzinski P, Pillai R, Korbut R, Channon KM. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arterioscler Thromb Vasc Biol. 2004;24:1614–1620. doi: 10.1161/01.ATV.0000139011.94634.9d. [DOI] [PubMed] [Google Scholar]
- 31.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 32.Teissier E, Nohara A, Chinetti G, Paumelle R, Cariou B, Fruchart JC, Brandes RP, Shah A, Staels B. Peroxisome proliferator-activated receptor alpha induces NADPH oxidase activity in macrophages, leading to the generation of LDL with PPAR-alpha activation properties. Circ Res. 2004;95:1174–1182. doi: 10.1161/01.RES.0000150594.95988.45. [DOI] [PubMed] [Google Scholar]
- 33.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 34.Honjo T, Otsui K, Shiraki R, Kawashima S, Sawamura T, Yokoyama M, Inoue N. Essential role of NOXA1 in generation of reactive oxygen species induced by oxidized low-density lipoprotein in human vascular endothelial cells. Endothelium. 2008;15:137–141. doi: 10.1080/10623320802125433. [DOI] [PubMed] [Google Scholar]
- 35.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 37.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci U S A. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1736–1743. doi: 10.1161/ATVBAHA.107.142117. [DOI] [PubMed] [Google Scholar]
- 39.Rueckschloss U, Quinn MT, Holtz J, Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22:1845–1851. doi: 10.1161/01.atv.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- 40.Pagano PJ, Chanock SJ, Siwik DA, Colucci WS, Clark JK. Angiotensin II induces p67phox mRNA expression and NADPH oxidase superoxide generation in rabbit aortic adventitial fibroblasts. Hypertension. 1998;32:331–337. doi: 10.1161/01.hyp.32.2.331. [DOI] [PubMed] [Google Scholar]
- 41.Freeman JL, Lambeth JD. NADPH oxidase activity is independent of p47phox in vitro. J Biol Chem. 1996;271:22578–22582. doi: 10.1074/jbc.271.37.22578. [DOI] [PubMed] [Google Scholar]
- 42.Paclet MH, Coleman AW, Vergnaud S, Morel F. P67-phox-mediated NADPH oxidase assembly: imaging of cytochrome b558 liposomes by atomic force microscopy. Biochemistry. 2000;39:9302–9310. doi: 10.1021/bi000483j. [DOI] [PubMed] [Google Scholar]
- 43.Vendrov AE, Hakim ZS, Madamanchi NR, Rohas M, Madamanchi C, Runge MS. Atherosclerosis Is Attenuated by Limiting Superoxide Generation in Both Macrophages and Vessel Wall Cells. Arterioscler Thromb Vasc Biol. 2007;27:2714–2721. doi: 10.1161/ATVBAHA.107.152629. [DOI] [PubMed] [Google Scholar]
- 44.Madamanchi NR, Moon SK, Hakim ZS, Clark S, Mehrizi A, Patterson C, Runge MS. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler Thromb Vasc Biol. 2005;25:950–956. doi: 10.1161/01.ATV.0000161050.77646.68. [DOI] [PubMed] [Google Scholar]
- 45.Kim JS, Diebold BA, Babior BM, Knaus UG, Bokoch GM. Regulation of Nox1 activity via PKA-mediated phosphorylation of NoxA1 and 14–3–3 binding. J Biol Chem. 2007;282:34787–34800. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.