Abstract
Context
While maternal smoking has been associated with child emotional and behavioral problems, to our knowledge, no study has evaluated the association between overall household smoking and such problems.
Objectives
To investigate whether children who live with smokers are more likely than children who do not live with smokers to have emotional or behavioral problems and to explore this association in households with non-smoking mothers.
Design, Setting, and Participants
Nationally representative data from the 2000–2004 Medical Expenditure Panel Surveys, involving 30,668 children aged 5–17 years, were utilized. Associations between child emotional or behavioral problems and household smoking, and child, maternal and family characteristics, were examined. SUDAAN software was used to adjust for complex sampling design.
Main Outcome Measures
Overall score on the Columbia Impairment Scale (CIS), a 13 item parent-report measure of child emotional or behavioral functioning (range, 0–52, ≥16 indicates a child with such problems).
Results
Children in smoking vs. non-smoking households were significantly more likely to have behavioral problems (17.39% vs 9.29%, P<0.001). After adjusting for all covariates, male sex, older age of child, younger age of mother, unmarried mother, maternal depression, below average maternal physical and mental health, each were independently associated with increased likelihood of emotional and behavioral problems, as was the presence of one or more adult smokers in the household (Adjusted OR 1.42; 95% CI:1.26–1.60). The odds of CIS≥16 increased with increasing number of smokers in the household, even among children whose mothers did not smoke.
Conclusion
Children living with smokers are at increased risk for emotional or behavioral problems, and rates of such problems increase with increasing numbers of smokers in the household, even in the absence of maternal smoking.
According to the 2006 U.S. Surgeon General's Report, there is no safe level of tobacco exposure, yet more than 30% of children live in households with at least one adult smoker, and nearly 60% have evidence of recent exposure to secondhand smoke (SHS)1–3. SHS exposure, either in utero or during childhood, has been causally linked to numerous adverse health outcomes, and is a leading preventable cause of both low birth weight and Sudden Infant Death Syndrome, and a major contributor to increased rates of lower respiratory infections and otitis media, and increased asthma severity1, 4–7. Recently, associations between SHS exposure and other childhood problems including increased rates of dental caries8, 9 and the metabolic syndrome10 have been identified, as well as the finding that there are increased rates of food insecurity among children and adults in households where adults smoke, probably through the mechanism of decreased money available for food when money is spent on cigarettes.11
Studies also indicate that prenatal tobacco and childhood SHS exposure are associated with child behavioral problems, including internalizing and externalizing behaviors12–24, ADHD17, 25–36, and conduct disorder.19, 37–39 These observational human epidemiologic studies usually can not distinguish between prenatal tobacco and postnatal secondhand smoke exposure and their potential associations with negative behavioral or emotional outcomes as it is the very rare individual who smokes during pregnancy but not after pregnancy. Similarly, in the absence of biomeasures of smoking exposure, it is not possible to distinguish between children's exposure to secondhand smoke and exposure to smokers, who themselves are more likely to be poor, have less education and higher rates of depression and anxiety disorders than do non-smokers. This growing body of epidemiologic work is complemented by findings from experimental animal studies. Animal models have demonstrated that in utero nicotine exposure is associated with impairment of coordination and motor activity, as well as increased rates of hyperactivity, inattention, and learning and memory problems40–45, and a recent study reported that primate postnatal tobacco smoke exposure leads to changes in brain cell development similar to prenatal nicotine exposure in both rodents and monkeys.46
Human studies have focused on behavioral problems in children exposed to tobacco or SHS from prenatal and/or postnatal maternal smoking, but to our knowledge, no study has examined the association between overall household adult smoking and child emotional or behavioral problems.
The purpose of this study was to utilize a nationally representative sample to investigate whether children who live in households with smokers are more likely than children who do not live with smokers to have emotional or behavioral problems, and to explore this association in households with non-smoking mothers.
METHODS
Data Source
Concatenated data from the 2000–2004 Medical Expenditure Panel Survey (MEPS), a large and nationally representative survey of the US civilian and non-institutionalized population, co-sponsored by the Agency for Healthcare Research and Quality (AHRQ) and the National Center for Health Statistics, were used to investigate the association between adult household smoking and emotional or behavioral problems among 30 668 children aged 5–17 years. The MEPS has been conducted annually since 1996, and information on smoking status has been collected since 2000.
The sample of households participating in the MEPS was selected from the previous year's respondents to the National Health Interview Survey. For each participating household, five rounds of interviews were conducted over two calendar years to collect a set of data from that family. The interviews were conducted in the home by a trained interviewer using computer assisted personal interviewing technology. Typically, during each round, the same adult respondent, most often the individual most knowledgeable about the household's health and health care, was interviewed and reported on behalf of all individuals in that household. Sociodemographic and health-related information collected during these interviews comprised the Household Component of the MEPS, which was the main source of data for this study47–49. Data were also abstracted from supplemental sections in the MEPS. All data were weighted and annualized to produce national estimates. For a detailed description of the MEPS, further information is available at http://www.meps.ahrq.gov/.
Study Variables
Behavioral and Emotional Problems
Children's scores on the Columbia Impairment Scale (CIS), a 13 item structured parent interview that measures overall emotional and behavioral functioning, was the outcome measure (Table 1). The CIS is administered by a lay interviewer and provides a respondent-based rating with good reliability, validity and correlation with clinicians' scores on the Children's Global Assessment Scale, school difficulties, and mental health services referrals50–52. As part of the Child Preventive Health Supplement section of the MEPS, the CIS was administered to adult household respondents for all children aged 5–17 years living in the respondent's home. Respondents were asked, “In general, how much of a problem do you think (person) has with” [each of the 13 items on the CIS]. Response to each item ranged from 0 (“No problem”) to 4 (“A Very Big Problem”), with the summated score ranging from 0 to 52, with a CIS score of ≥16 indicating that the child has a behavioral or emotional problem53, 54. All 13 items on the CIS were answered in 86.7% of cases; in 10.7% of cases one item was not answered, in 2.1% of cases 2 items were unanswered, and in 0.5% 3–8 items were unanswered. Consistent with previous studies, imputed mean scores were used to assign values to missing CIS items50, and a validated cutoff score of ≥16 to indicate emotional or behavioral problems was employed51. The CIS measures the presence of either behavioral or emotional problems, or both together in a particular child, but due to its unifactorial nature, it can not discriminate between emotional and behavioral problems and thus throughout this paper elevated CIS scores are indicated as reflecting “emotional or behavioral problems” of children and adolescents, whereas in actuality the elevated CIS score may reflect elevated emotional and behavioral problems in some cases.
Table 1.
The Columbia Impairment Scale (CIS)
| How much of a problem does child have with: |
| 1) getting along with mother |
| 2) getting along with father |
| 3) feeling unhappy or sad |
| 4) (his/her) behavior at school |
| 5) having fun |
| 6) getting along with adults |
| 7) feeling nervous or afraid |
| 8) getting along with brothers and sisters |
| 9) getting along with other kids |
| 10) getting involved in activities like sports or hobbies |
| 11) (his/her) schoolwork |
| 12) (his/her) behavior at home |
| 13) staying out of trouble |
In the Child Health Supplement to the Medical Expenditure Panel Survey, respondents were asked to rate on a scale from 0 (“No Problem”) to 4 (“A Very Big Problem”), how much of a problem the child has with thirteen specified activities. Questions not applicable for a specific child were coded to “Asked, but Inapplicable” (99). A key reference for the Columbia Impairment Scale is Bird HR et al 199651.
Adult Household smoking
Information on household smoking was derived from the Adult Self Administered Questionnaire section of the MEPS. All individuals in the household ≥18 years of age were given a mail-in questionnaire, which included the question “Do you currently smoke?” Households were dichotomized as having no adult smokers vs. those with one or more adult smokers, and were additionally categorized as having 0, 1, 2, and 3 or more adult smokers. For adults for whom data on smoking status was not reported (12% in 2004), imputations were conducted using a 2-step model. The first step targeted adults for whom smoking status was reported in only one of the two years of MEPS participation. Based on the finding that 6.5% of participants in the 2003 and 2004 MEPS who reported smoking status for both years reported a change in smoking status between the two years, with the percent who started (3.0%) and quit (3.4%) smoking balanced, reported smoking status in one year was assigned to the year not reported. In the second step, which targeted adults missing data from both years, an algorithm developed by AHRQ, was applied2. No information was available on amounts smoked by each adult household smoker, whether they smoked in the home or automobile, or whether the household had rules prohibiting smoking indoors or in automobiles.
Child and Family Characteristics
Based on previously described associations with child behavioral problems, covariates and potential confounders, including child age, sex, race/ethnicity (per respondent report), number of adults in the household, poverty status, and maternal characteristics such as age at child's birth, education, marital status, depression, physical, and mental health, were chosen15, 55–64. These data were available in the MEPS household file and supplemental sections. As detailed in Table 2, child age was dichotomized into younger (5–11 years) and older (12–17 years), and maternal marital status was dichotomized into married vs. not-married (single, divorced, widowed). Household income was divided into 5 categories (poor, near poor [100<125% poverty], low income [125<200% poverty], middle income [200<400% poverty], and high income ≥ 400% poverty]65. Child race/ethnicity, number of adults in the household, and maternal education were categorized. Maternal age at child's birth was calculated using maternal age and child age at time of interview.
Table 2.
Factors Associated with Children's Emotional and Behavioral Problems (CIS≥16)a Medical Expenditure Panel Surveys 2000–2004, N=30,668b
| Variable | unweighted (%) | Bivariate Analyses |
Logistic Regression |
|||
|---|---|---|---|---|---|---|
| CIS≥16 | P | Adjusted OR | 95%CI | P | ||
| Age | ||||||
| 5–11 years | 52.90 | 9.32 | <0.001 | * | * | * |
| 12–17 years | 47.10 | 13.69 | 1.61 | 1.42–1.79 | <0.001 | |
|
| ||||||
| Gender | ||||||
| Male | 51.20 | 12.86 | <0.001 | 1.41 | 1.27–1.56 | <0.001 |
| Female | 48.80 | 9.83 | * | * | * | |
|
| ||||||
| Race/Ethnicity | ||||||
| White (non-Hispanic) | 63.33 | 12.27 | <0.001 | * | * | * |
| Black (non-Hispanic) | 15.02 | 12.76 | 0.84 | 0.70–1.01 | 0.065 | |
| Hispanic | 17.57 | 8.45 | 0.58 | 0.48–0.69 | <0.001 | |
| Asian/ PI (non-Hispanic) | 4.08 | 5.17 | 0.43 | 0.29–0.65 | <0.001 | |
|
| ||||||
| Adults in Household | ||||||
| 1 | 13.29 | 17.01 | <0.001 | NS | NS | NS |
| 2 | 63.13 | 10.66 | * | * | * | |
| ≥3 | 23.58 | 10.13 | 0.77 | 0.66–0.90 | 0.001 | |
|
| ||||||
| Family income as a percentage of poverty | ||||||
| Poor/negative, <100% | 25.41 | 16.46 | <0.001 | NS | NS | NS |
| Near poor, 100~<125% | 7.91 | 13.77 | NS | NS | NS | |
| Low income, 125~<200% | 19.65 | 12.96 | NS | NS | NS | |
| Middle income, 200~<400% | 28.56 | 11.33 | * | * | * | |
| High income, >=400% | 18.47 | 8.98 | NS | NS | NS | |
|
| ||||||
| Smoker in Householdc | ||||||
| Yes | 33.96 | 15.45 | <0.001 | 1.40 | 1.23–1.60 | <0.001 |
| No | 66.04 | 9.29 | * | * | * | |
|
| ||||||
| Smokers in Household | ||||||
| 0 | 66.04 | 9.29 | <0.001 | * | * | * |
| 1 | 20.28 | 14.72 | 1.34 | 1.15–1.55 | <0.001 | |
| 2 | 11.03 | 15.99 | 1.48 | 1.24–1.78 | <0.001 | |
| ≥3 | 2.66 | 18.77 | 1.78 | 1.28–2.48 | <0.001 | |
|
| ||||||
| Maternal Smokingc | ||||||
| Yes | 20.82 | 17.39 | <0.001 | 1.33 | 1.14–1.55 | <0.001 |
| No | 79.18 | 9.80 | * | * | * | |
|
| ||||||
| Maternal Age at Child's Birth | ||||||
| <20 years | 8.83 | 15.94 | <0.001 | 1.29 | 1.08–1.53 | 0.005 |
| 20–34 years | 78.52 | 11.01 | * | * | * | |
| ≥35 years | 12.65 | 10.51 | NS | NS | NS | |
|
| ||||||
| Maternal Education | ||||||
| <HS degree | 29.68 | 14.23 | <0.001 | NS | NS | NS |
| HS degree/GED | 32.08 | 12.23 | * | * | * | |
| Bachelor degree | 32.76 | 10.27 | NS | NS | NS | |
| Above bachelor degree | 5.48 | 7.67 | 0.74 | 0.57–0.96 | 0.02 | |
|
| ||||||
| Maternal Marital Status | ||||||
| Married | 74.77 | 9.50 | <0.001 | * | * | * |
| Not Married | 25.23 | 16.96 | 1.53 | 1.31–1.77 | <0.001 | |
|
| ||||||
| Maternal Depression | ||||||
| Yes | 9.84 | 24.31 | <0.001 | 1.74 | 1.47–2.07 | <0.001 |
| No | 90.16 | 9.97 | * | * | * | |
|
| ||||||
| Maternal PCS Score | ||||||
| Average and above | 65.79 | 9.31 | <0.001 | * | * | * |
| Within 1SD below average | 19.16 | 13.63 | 1.40 | 1.22–1.60 | <0.001 | |
| 1–2 SD below average | 8.19 | 17.90 | 1.39 | 1.15–1.70 | <0.001 | |
| ≥ 2 SD below average | 6.86 | 17.83 | 1.46 | 1.15–1.86 | <0.001 | |
|
| ||||||
| Maternal MCS Score | ||||||
| Average and above | 61.71 | 7.37 | <0.001 | * | * | * |
| Within 1SD below average | 22.35 | 14.73 | 1.94 | 1.70–2.22 | <0.001 | |
| 1–2 SD below average | 10.44 | 20.60 | 2.56 | 2.14–3.07 | <0.001 | |
| ≥ 2 SD below average | 5.51 | 26.02 | 2.64 | 1.48–4.71 | <0.001 | |
Abbreviations: CIS, Columbia Impairment Scale; PI, Pacific Islander; HS, High School; PCS, Physical Component Scale; MCS, Mental Component Scale; SD, standard deviation; OR, odds ratio; CI, confidence interval; NS, non-significant
The Columbia Impairment Scale (CIS) is a 13 item parent-report measure of child emotional and behavioral functioning. Each item is scored from 0 (“No Problem”) to 4 (“A Very Big Problem”). Summated scores range from 0–52, with higher scores indicating worse emotional and behavioral functioning. CIS score≥16 indicates emotional and behavioral problems.
N=47 243 296 weighted
Analyzed in separate logistic regression models adjusting for all covariates
Reference values
Maternal Physical and Mental Health
Scores on the Short Form-12 (SF-12) Physical Component Scale (PCS) and Mental Component Scale (MCS) were used as measures of maternal physical and mental health. The SF-12 is a validated, 12 item self- report health measure that has been well studied in the general population and in populations with specific physical (e.g. arthritis, diabetes, MI) and mental health conditions (e.g. depression, anxiety)66. PCS and MCS scores range from 0–100, with lower scores indicating poorer health. PCS and MCS scores were available in the Self Administered Questionnaire supplement. Mean scores and standard deviations were calculated and scores were categorized for analyses (Table 2).
Sample Selection
Of the 34,681 children ages 5–17 years who participated in the MEPS in 2000–2004, 808 children were excluded because of missing scores for all CIS items. 2267 children were excluded because of missing maternal data, primarily for households in which a biological, adoptive or step mother was not present. While children who were not living with their biological parents were not specifically excluded, the exclusion of children for whom some maternal data were missing probably increased the likelihood of excluding children who were not living with their biological parents. Due to a taxonomy change between the 2002 and 2003 MEPS, 938 children designated as “multiple” or “other” races were also excluded. Total sample size was N=30,668 children.
Analyses
Mean total CIS scores and mean scores on each of the 13 CIS items were calculated and compared, using t-tests, for children living in smoking vs. non-smoking households. The associations between emotional or behavioral problems (CIS≥16) and smoking in the household, number of smokers in the household, maternal smoking, and other child, maternal and family characteristics were examined in bivariate analyses using chi square tests. All variables associated with elevated CIS scores (i.e. >16) in bivariate analyses at p<0.10 were entered into the multivariate analyses. Logistic regression models were constructed to explore independent relationships between CIS ≥16 and household smoking, as well as CIS≥16 and number of household smokers, adjusting for (Table 2). Similar logistic regression analyses were conducted exclusively for children whose mothers were not current smokers. SUDAAN software was used to adjust for complex sample design.
RESULTS
Table 2 displays the characteristics of the sample and factors associated with children's emotional or behavioral problems (CIS≥16) in bivariate and multivariable analyses. In bivariate analyses, all variables (older age, male gender, Black race, lower number of adults in household, lower family income, the presence of a smoker in the home, increasing number of smokers in the home, maternal smoking, maternal age <20 years at child's birth, lesser maternal education, mother not being married, and worse maternal physical and mental health) were each significantly associated with child emotional or behavioral problems at a P-value of <0.05 As demonstrated in Table 2, 33.96% of all children surveyed live with one or more adult smokers and 20.82% of mothers smoked. Overall, 11.38% of all subjects had CIS ≥16. By contrast, 15.45% of children living in smoking households vs. 9.29% living in non-smoking households had CIS≥16 (P<0.001), 17.39% of children whose mothers smoked had elevated CIS scores as compared to 9.80% of children whose mothers did not smoke (P<0.001), and rates of elevated CIS increased with increasing numbers of adult smokers in the home (9.29% among children living in homes without any adult smokers, 14.72%% for those living with 1 smoker, 15.99% for those living with 2 adult smokers, and 18.77% for those living with 3 or more smokers (P<0.001). Rates of emotional or behavior problems decreased as family income and maternal education increased, and decreased as the number of adults living in the household increased.
After adjustment for all covariates (Table 2), male sex, older age of child (12–17 years), younger age of mother at child's birth (<20 years), unmarried mother, maternal depression, below average maternal physical and mental health each were independently associated with increased rates of emotional and behavioral problems, as was the presence of one or more adult smokers in the household (Adjusted OR [AOR] 1.40; 95% CI: 1.23–1.60), maternal smoking (AOR 1.33, 95% CI 1.14–1.55), and as number of adult smokers in the home increased (1 smoker AOR 1.34, 95% CI 1.15–1.55; 2 smokers AOR 1.48, 95th% 1.24–1.78; 3 or more smokers AOR 1.78, 95th% 1.28–2.48). In contrast, higher family income, higher maternal education, and living with 3 or more adults were each independently associated with lower rates of elevated CIS scores.
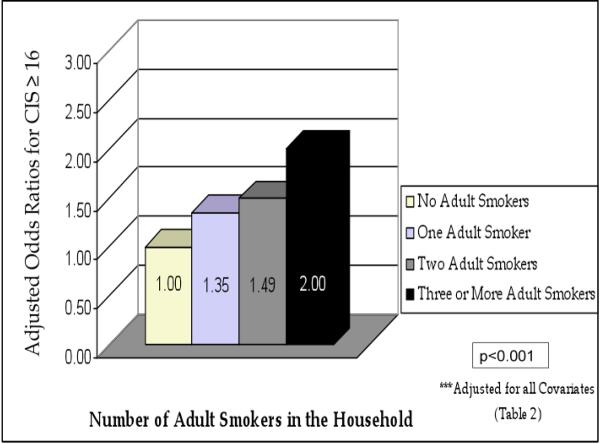
The independent association of number of household smokers and child emotional or behavioral problems is illustrated in graphic form in Figure 1. In a similar analysis including only children for whom the mother was not a smoker, this relationship remained significant (Figure 2), i.e. the percentage of children with elevated CIS scores increased as the number of household smokers increased in multivariate analyses.
Figure 1.
Adjusted Odds Ratios for Emotional and Behavioral Problems (CIS≥16) among Children Living in Households with Varying Numbers of Smokers***
Medical Expenditure Panel Surveys 2000–2004, N=30,668
Figure 2.
Adjusted Odds Ratios for Emotional and Behavioral Problems (CIS≥16) among Children of Non-Smoking Mothers Living in Households with Varying Numbers of Smokers***
Medical Expenditure Panel Surveys 2000–2004, N=24,283
DISCUSSION
These data from a large and nationally representative sample demonstrate an independent association between adult household smoking and child emotional and behavioral problems. After adjustment for numerous potential confounders and well established independent predictors of such problems, children who live with one or more adult smokers are 1.4 times more likely than children who do not live with smokers to have emotional and behavioral problems as shown in Table 2. The likelihood of these problems increases with increasing number of smokers in the household, such that living with 3 or more smokers is independently associated with a a risk that is 1.75 times more than it is among children who are live in households with no smokers.` These findings persist even among children whose mothers are not smokers, suggesting that children exposed to any adult smokers and/or SHS within their homes are at increased risk for emotional or behavioral problems.
Children's involuntary exposure to SHS is a common and profoundly serious problem which has been receiving increasing attention from the pediatric, public health and research communities. The most recent Surgeon General's Report on Secondhand Smoke Exposure mentioned for the first time the association between SHS exposure and cognitive and behavioral problems of children, but included the statement that “the evidence is inadequate to infer the presence or absence of a causal relationship….”1
The human literature on this subject has largely focused on active maternal smoking during pregnancy, and has reported a fairly consistent association between prenatal maternal smoking and child behavioral problems24. The association between postnatal maternal smoking and child behavioral problems is both less well studied and less clear, and as mentioned in the 2006 Surgeon General's Report, disentangling prenatal from postnatal exposure continues to pose a difficult methodological problem1.
As early as 1975, Denson and colleagues reported that for mothers of “hyperkinetic children,” cigarette consumption was 3 times higher than among mothers whose children were not “hyperkinetic”67. That study found no association for paternal smoking, and did not investigate other potential sources of household SHS exposure. Weitzman and colleagues reported that postnatal maternal smoking is independently associated with behavioral problems in children. A dose-response relationship both for children whose mothers smoked during and after pregnancy as well as for those whose mothers smoked only after pregnancy was identified15. Similarly, Williams and colleagues demonstrated a significant association between postnatal maternal smoking at the 5 year visit and externalizing child behavioral problems and suggested “both biological and social explanations, including a direct psychopharmacologic effect of nicotine through passive smoking20.” Additionally, two studies evaluating behavior in 3 year old children demonstrated that current maternal smoking is associated with behavior and attention problems21, 59, and one study reported that 5 year old children exposed to maternal smoking were rated more active by their mothers68. In contrast, in a study of disruptive childhood behaviors (i.e. conduct problems, ADHD), Fergusson and colleagues reported that after adjusting for potential confounders, postnatal maternal smoking alone was not significantly associated with increased behavioral problems16.
Whereas these studies focused on maternal smoking, this is the first study we are aware of that explores the association between children's exposure to adult household smokers and/or SHS exposure from adult household smokers and child emotional or behavioral problems. A recent study investigating prenatal and postnatal smoking, low level lead and child ADHD, found no association between report of household smoking and rates of childhood ADHD34. That study, however, did not investigate numbers of smokers in the household or the mother's smoking status, and the dependent variable was ADHD, rather than behavioral and emotional problems, as assessed in the current study.
Child, maternal, and family characteristics thought to be potential confounders and independent predictors of child behavioral and emotional problems were included in the logistic regression models. Consistent with previous studies, the majority of these, including: male sex, older age (12–17 years), younger maternal age, unmarried mothers, maternal depression, minority race/ethnicity, or overall mental health problems, and the report of poorer maternal physical heath, were found to be independently associated with increased rates of children's emotional and behavioral problems15, 55–64, 69. After adjustment for all covariates, poverty and maternal education were no longer significantly associated with such problems, except at the highest level of education, which was associated with decreased rates of these problems. This raises the question as to whether the effects of these factors on children's behavioral or emotional health might be mediated through the pathway of SHS exposure or exposure to adult smokers.. Unfortunately, insufficient information regarding the nature of children's exposure (duration, age, and frequency of SHS exposure and inability to disentangle exposure to smokers from exposure to SHS) make it impossible to address this important question.
The mean total Columbia Impairment Scale (CIS) score in this study was 6.47, almost identical to the mean reported by Bird and colleagues in 199651. The CIS is a global measure of impairment52. Because of its unidimensional structure, it can not distinguish emotional from behavioral probems.
There are a number of other limitations to this study. The data did not allow us to investigate the association of prenatal SHS exposure and children's emotional and behavioral problems, and it is possible that findings from this study may in part reflect prenatal exposure. In the present study, smoking status was ascertained by self-report, which has been demonstrated to have high validity in population based studies70, 71 Previous smoking history, however, was not ascertained and therefore could not be included in the analyses, making an underestimation of child SHS exposure more likely37. Data were also lacking as to whether or not smokers were smoking inside the child's home, and information about smoking behaviors of those less than 18 years of age was not available. Because of the cross-sectional nature of the data, it was not possible to determine the child's age at which exposure to SHS began, or the duration of the exposure.
Another limitation is that there is the potential for unmeasured confounders. For example, it is possible that the relationship seen between the number of adult smokers and increased rates of children's emotional/behavioral problems is a result of confounding and actually represents an SES proxy for overcrowding. This appears unlikely, however, as rates of elevated scores on the CIS decreased both in bivariate and multivariate analyses as the number of adults in the household increased, whereas rates of elevated CIS scores increased with increasing numbers of adult smokers in the same analytic models. Similarly, while we were able to adjust for maternal depression and mental health, the data did not allow us to control for other characteristics or behaviors of smoking and non-smoking adults, which may influence the behavior or emotional state of children living in the household. As the Columbia Impairment Scale is highly correlated with the Clinician's Global Assessment Scale, it seems unlikely, however, that the smoking status of the individual rating the child's behavior should directly influence CIS score. In addition, as the Columbia Impairment Scale was administered only for children aged 5–17 years, we were unable to explore behavioral and emotional functioning in children less than 5 years, a group described in the 2006 Surgeon General's report as particularly vulnerable to SHS exposure because of immature and rapidly developing respiratory, immune and nervous systems, and reliance on caregivers1. The sample size was too small to look at children 5–6 separately, to see if the associations of emotional or behavioral problems and living with adult smokers were greater among the youngest children for whom data were available. Also, eliminating children who were of mixed or unknown race and for whom maternal data were missing may have introduced unknown biases. Furthermore, The CIS scale itself has the limitation that it is not standardized for the differences between male and females in its prediction72.
Despite these limitations, this study, which utilizes data from a large and nationally representative sample, uses a well validated epidemiologic measure of behavior or emotional problems, and controls for numerous potential confounders, demonstrates that living with adult smokers, even in the absence of maternal smoking, is independently associated with increased rates of children's behavioral and emotional problems, and that the risk of such problems increases with increasing numbers of household smokers.
While a study such as this adds to the evidence for an association of children's tobacco smoke exposure and increased rates of emotional and behavioral problems, it neither provides evidence for biologic or psychosocial mechanisms underlying this association, nor proves a causal nature of this association. Based on animal models, it is clear that in utero nicotine exposure has profound effects on the developing brain. During childhood, the brain continues to develop, and as demonstrated by studies of exposures to lead and mercury, remains exquisitely sensitive to environmental toxins. As noted previously, a recent study in primates with postnatal SHS exposure demonstrated changes in the brain similar to those observed with prenatal exposure46. As suggested by Williams and colleagues20, we speculate that in children exposed to SHS, it is a combination of the direct effect of this exposure on the developing brain along with other biological and psychosocial factors that ultimately shapes child behavior. Future studies, prospective in design, adjusting for prenatal exposure and using a biological measure of exposure such as cotinine, in addition to self-report, would further elucidate this association and might help disentangle potential neurotoxic effects of SHS from potential untoward consequences of altered home environments and attitudes toward children in homes with adult smokers.
It cannot be overstated that exposure to tobacco smoke has numerous deleterious health consequences. In addition to increased risk of SIDS, asthma, respiratory tract infections and otitis media, children living with smokers are also at increased risk for mental health problems. While further studies are necessary to establish causality for the association between household smoking and child emotional and behavioral problems, the findings from this study nonetheless have important implications for primary care and public health. To promote child health and wellbeing, tobacco use for all individuals living in households with children should be assessed, and smoking cessation should be encouraged and facilitated73.
ACKNOWLEDGEMENTS
Funding/Support: This work was made possible by a grant from the Flight Attendant Medical Research Institute and by NIH grant #P60MD000538 from NCMHD.
Role of the Sponsors: None of the sponsors had a role in the design and conduct or the study, in the collection, analysis and interpretation of the data, or in the preparation, review or approval of the manuscript.
Footnotes
Financial Disclosures: The authors have indicated that they have no financial relationships relative to this article to disclose.
Disclaimer: The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the FAMRI or the NCMHD.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. 2006 [PubMed]
- 2.Machlin SR, Hill SC, Liang L. Statistical Brief #147. Agency for Healthcare Research and Qualty; Rockville, Md: Nov, 2006. Children Living with Adult Smokers, United States, 2004. [Google Scholar]
- 3.U.S. Bureau of the Census Center for Disease Control and Prevention, National Center for Health Statistics. 20062006 Unpublished data. [Google Scholar]
- 4.National Cancer Institute . Health effects of exposure to environmental tobacco smoke: The report of the California environmental protection agency. National Cancer Institute, US Department of Health and Human Services; Bethesda, MD: 1999. [Google Scholar]
- 5.American Academy of Pediatrics Commitee on Environmental Health Environmental Tobacco Smoke: a Hazard to Children. Pediatrics. 1997;99(4):639–642. [PubMed] [Google Scholar]
- 6.Cook D, Strachan DP. Health Effects of Passive Smoking-10:Summary of Effects of Paternal Smoking on the Respiratory Health of Children and Implications for Research. Thorax. 1999;54(4):357–366. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004 Apr;113(4 Suppl):1007–1015. [PubMed] [Google Scholar]
- 8.Iida H, Auinger P, Billings RJ, Weitzman M. Association between infant breastfeeding and early childhood caries in the United States. Pediatrics. 2007;120(4):e944–952. doi: 10.1542/peds.2006-0124. [DOI] [PubMed] [Google Scholar]
- 9.Aligne CA, Moss ME, Auinger P, Weitzman M. Association of pediatric dental caries with passive smoking. Jama. 2003 Mar 12;289(10):1258–1264. doi: 10.1001/jama.289.10.1258. [DOI] [PubMed] [Google Scholar]
- 10.Weitzman M, Cook S, Auinger P, et al. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation. 2005 Aug 9;112(6):862–869. doi: 10.1161/CIRCULATIONAHA.104.520650. [DOI] [PubMed] [Google Scholar]
- 11.Cutler CBFG, Miyoshi T, Weitzman M. Increased rates and severity of child and adult food insecurity in households with adult smokers. Arch Ped Adolesc Medicine. doi: 10.1001/archpediatrics.2008.2. in press. [DOI] [PubMed] [Google Scholar]
- 12.Naeye RL, Peters EC. Mental development of children whose mothers smoked during pregnancy. Obstet Gynecol. 1984 Nov;64(5):601–607. [PubMed] [Google Scholar]
- 13.Fried PA, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Dev Behav Pediatr. 1990 Apr;11(2):49–58. [PubMed] [Google Scholar]
- 14.Rantakallio P, Laara E, Isohanni M, Moilanen I. Maternal smoking during pregnancy and delinquency of the offspring: an association without causation? Int J Epidemiol. 1992 Dec;21(6):1106–1113. doi: 10.1093/ije/21.6.1106. [DOI] [PubMed] [Google Scholar]
- 15.Weitzman M, Gortmaker S, Sobol A. Maternal smoking and behavior problems of children. Pediatrics. 1992;90(3):342–349. [PubMed] [Google Scholar]
- 16.Fergusson DM, Horwood LJ, Lynskey MT. Maternal smoking before and after pregnancy: effects on behavioral outcomes in middle childhood. Pediatrics. 1993 Dec;92(6):815–822. [PubMed] [Google Scholar]
- 17.Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am J Psychiatry. 1996 Sep;153(9):1138–1142. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- 18.Olds D. Tobacco exposure and impaired development:a review of the evidence. Mental Retard Dev Disabil Res Rev. 1997;3:257–269. [Google Scholar]
- 19.Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry. 1997 Jul;54(7):670–676. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
- 20.Williams GM, O'Callaghan M, Najman JM, et al. Maternal cigarette smoking and child psychiatric morbidity: a longitudinal study. Pediatrics. 1998 Jul;102(1):e11. doi: 10.1542/peds.102.1.e11. [DOI] [PubMed] [Google Scholar]
- 21.Day NL, Richardson GA, Goldschmidt L, Cornelius MD. Effects of prenatal tobacco exposure on preschoolers' behavior. J Dev Behav Pediatr. 2000 Jun;21(3):180–188. [PubMed] [Google Scholar]
- 22.Hook B, Cederblad M, Berg R. Prenatal and postnatal maternal smoking as risk factors for preschool children's mental health. Acta Paediatr. 2006 Jun;95(6):671–677. doi: 10.1080/08035250500538965. [DOI] [PubMed] [Google Scholar]
- 23.Indredavik MS, Brubakk AM, Romundstad P, Vik T. Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatr. 2007 Mar;96(3):377–382. doi: 10.1111/j.1651-2227.2006.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr Opin Pediatr. 2008 Apr;20(2):184–190. doi: 10.1097/MOP.0b013e3282f56165. [DOI] [PubMed] [Google Scholar]
- 25.Milberger S, Biederman J, Faraone SV, Jones J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. J Clin Child Psychol. 1998 Oct;27(3):352–358. doi: 10.1207/s15374424jccp2703_11. [DOI] [PubMed] [Google Scholar]
- 26.Wasserman GA, Liu X, Pine DS, Graziano JH. Contribution of maternal smoking during pregnancy and lead exposure to early child behavior problems. Neurotoxicol Teratol. 2001 Jan–Feb;23(1):13–21. doi: 10.1016/s0892-0362(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 27.Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry. 2002 Apr;41(4):378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Kotimaa AJ, Moilanen I, Taanila A, et al. Maternal smoking and hyperactivity in 8-year-old children. J Am Acad Child Adolesc Psychiatry. 2003 Jul;42(7):826–833. doi: 10.1097/01.CHI.0000046866.56865.A2. [DOI] [PubMed] [Google Scholar]
- 29.Linnet KM, Dalsgaard S, Obel C, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003 Jun;160(6):1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- 30.Thapar A, Fowler T, Rice F, et al. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am J Psychiatry. 2003 Nov;160(11):1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- 31.Knopik VS, Sparrow EP, Madden PA, et al. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol Med. 2005 May;35(5):625–635. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- 32.Langley K, Rice F, van den Bree MB, Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatr. 2005 Dec;57(6):359–371. [PubMed] [Google Scholar]
- 33.Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry. 2005 Mar;46(3):246–254. doi: 10.1111/j.1469-7610.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- 34.Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006 Dec;114(12):1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knopik VS, Heath AC, Jacob T, et al. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychol Med. 2006 Oct;36(10):1461–1471. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz M, Denardin D, Laufer Silva T, et al. Smoking during pregnancy and attention-deficit/hyperactivity disorder, predominantly inattentive type: a case-control study. J Am Acad Child Adolesc Psychiatry. 2006 Nov;45(11):1338–1345. doi: 10.1097/S0890-8567(09)61916-X. [DOI] [PubMed] [Google Scholar]
- 37.Wakschlag LS, Pickett KE, Cook E, Jr., Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am J Public Health. 2002 Jun;92(6):966–974. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakschlag LS, Pickett KE, Kasza KE, Loeber R. Is prenatal smoking associated with a developmental pattern of conduct problems in young boys? J Am Acad Child Adolesc Psychiatry. 2006 Apr;45(4):461–467. doi: 10.1097/01.chi.0000198597.53572.3e. [DOI] [PubMed] [Google Scholar]
- 39.Nigg JT, Breslau N. Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 2007 Mar;46(3):362–369. doi: 10.1097/01.chi.0000246054.76167.44. [DOI] [PubMed] [Google Scholar]
- 40.Yanai J, Pick CG, Rogel-Fuchs Y, Zahalka EA. Alterations in hippocampal cholinergic receptors and hippocampal behaviors after early exposure to nicotine. Brain Res Bull. 1992 Sep–Oct;29(3–4):363–368. doi: 10.1016/0361-9230(92)90069-a. [DOI] [PubMed] [Google Scholar]
- 41.Levin ED, Briggs SJ, Christopher NC, Rose JE. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol Teratol. 1993 Jul–Aug;15(4):251–260. doi: 10.1016/0892-0362(93)90006-a. [DOI] [PubMed] [Google Scholar]
- 42.Richardson SA, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacol Biochem Behav. 1994 Feb;47(2):331–337. doi: 10.1016/0091-3057(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 43.Ajarem JS, Ahmad M. Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol Biochem Behav. 1998 Feb;59(2):313–318. doi: 10.1016/s0091-3057(97)00408-5. [DOI] [PubMed] [Google Scholar]
- 44.Muneoka K, Ogawa T, Kamei K, Mimura Y, Kato H, Takigawa M. Nicotine exposure during pregnancy is a factor which influences serotonin transporter density in the rat brain. Eur J Pharmacol. 2001 Jan 12;411(3):279–282. doi: 10.1016/s0014-2999(00)00925-0. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Wang H. In utero exposure to tobacco and alcohol modifies neurobehavioral development in mice offspring: consideration a role of oxidative stress. Pharmacol Res. 2004 May;49(5):467–473. doi: 10.1016/j.phrs.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Slotkin TA, Pinkerton KE, Seidler FJ. Perinatal environmental tobacco smoke exposure in rhesus monkeys: critical periods and regional selectivity for effects on brain cell development and lipid peroxidation. Environ Health Perspect. 2006 Jan;114(1):34–39. doi: 10.1289/ehp.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen S. MEPS Methodology Report No.1. AHCPR Pub. No. 97-0026. Agency for Health Care Policy and Research; Rockville, Md: 1997. Design and Methods of the Medical Expenditure Panel Survey Household Component. [Google Scholar]
- 48.Cohen S. MEPS Methodology Report No.2. AHCPR Pub. No. 97-0027. Agency for Health Care Policy and Research; Rockville, Md: 1997. Sample Design of the 1996 Medical Expenditure Panel Survey Household Component. [Google Scholar]
- 49.Cohen S. Design Strategies and Innovations in the Medical Expenditure Panel Survey. Medical Care. 2003 July;41(7) doi: 10.1097/01.MLR.0000076048.11549.71. Supplement:III-5–III-12. 2003. [DOI] [PubMed] [Google Scholar]
- 50.Bird HR, Shaffer D, Fisher P, Gould M, Staghezza B. The Columbia Impairment Scale (CIS): Pilot Findings on a Measure of Global Impairment for Children and Adolescents. International Journal of Methods in Psychiatric Research. 1993;3:161–176. [Google Scholar]
- 51.Bird HR, Andrews H, Schwab-Stone M, et al. Global measure of Impairment for Epidemiologic and Clinical Use with Children and Adolescents. International Journal of Methods in Psychiatric Research. 1996;6:295–307. [Google Scholar]
- 52.Winters NC, Collett Brent R., Myers Kathleen M. Ten-Year Review of Rating Scales, VII: Scales Assessing Functional Impairment. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(4):309–338. doi: 10.1097/01.chi.0000153230.57344.cd. [DOI] [PubMed] [Google Scholar]
- 53.Bird H, Shaffer D, Fisher P, et al. The Columbia Impairment Scale (CIS): Pilot findings on a measure of global impairment for children and adolescents. International Journal of Methods for Psychiatric Research. 1993;3:167–176. [Google Scholar]
- 54.Bird H, Andrews H, Schwab-Stone M, et al. Global measures of impairment for epidemiologic and clinical use with children and adolescents. International Journal of Methods for Psychiatric Research. 1996;6:295–307. [Google Scholar]
- 55.Weissman MM, Gammon GD, John K, et al. Children of depressed parents. Increased psychopathology and early onset of major depression. Archives of General Psychiatry. 1987;44(10):847–853. doi: 10.1001/archpsyc.1987.01800220009002. [DOI] [PubMed] [Google Scholar]
- 56.Korneluk YG, Lee CM. Children's adjustment to parental physical illness. Clin Child Fam Psychol Rev. 1998 Sep;1(3):179–193. doi: 10.1023/a:1022654831666. [DOI] [PubMed] [Google Scholar]
- 57.Uljas H, Rautava P, Helenius H, Sillanpaa M. Behaviour of Finnish 3-year-old children. I: Effects of sociodemographic factors, mother's health, and pregnancy outcome. Developmental Medicine & Child Neurology. 1999;41(6):412–419. doi: 10.1017/s0012162299000882. [DOI] [PubMed] [Google Scholar]
- 58.Najman JM, Williams GM, Nikles J, et al. Bias influencing maternal reports of child behaviour and emotional state. Social Psychiatry & Psychiatric Epidemiology. 2001;36(4):186–194. doi: 10.1007/s001270170062. [DOI] [PubMed] [Google Scholar]
- 59.Kahn RS, Zuckerman B, Bauchner H, Homer CJ, Wise PH. Women's health after pregnancy and child outcomes at age 3 years: a prospective cohort study. Am J Public Health. 2002 Aug;92(8):1312–1318. doi: 10.2105/ajph.92.8.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Visser-Meily A, Post M, Meijer AM, van de Port I, Maas C, Lindeman E. When a parent has a stroke: clinical course and prediction of mood, behavior problems, and health status of their young children. Stroke. 2005;36(11):2436–2440. doi: 10.1161/01.STR.0000185681.33790.0a. [DOI] [PubMed] [Google Scholar]
- 61.Pachter LM, Auinger P, Palmer R, Weitzman M. Do parenting and the home environment, maternal depression, neighborhood, and chronic poverty affect child behavioral problems differently in different racial-ethnic groups? Pediatrics. 2006;117(4):1329–1338. doi: 10.1542/peds.2005-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kahn RS, Wilson K, Wise PH. Intergenerational health disparities: socioeconomic status, women's health conditions, and child behavior problems. Public Health Reports. 2005;120(4):399–408. doi: 10.1177/003335490512000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pilowsky DJ, Wickramaratne PJ, Rush AJ, et al. Children of currently depressed mothers: a STAR*D ancillary study. Journal of Clinical Psychiatry. 2006;67(1):126–136. doi: 10.4088/jcp.v67n0119. [DOI] [PubMed] [Google Scholar]
- 64.Gao W, Paterson J, Abbott M, Carter S, Iusitini L. Maternal mental health and child behaviour problems at 2 years: findings from the Pacific Islands Families Study. Australian & New Zealand Journal of Psychiatry. 2007;41(11):885–895. doi: 10.1080/00048670701634929. [DOI] [PubMed] [Google Scholar]
- 65.Machlin SR, Soget MW. Statistical Brief #64. Agency for Healthcare Research and Quality; Rockville, MD: Jan, 2005. Family Health Care Expenses, by Income Level, 2002. http://www.meps.ahrq.gov/papers/st64/stat64.pdf. [Google Scholar]
- 66.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996 Mar;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Denson R, Nanson JL, McWatters MA. Hyperkinesis and maternal smoking. Can Psychiatr Assoc J. 1975 Apr;20(3):183–187. doi: 10.1177/070674377502000302. [DOI] [PubMed] [Google Scholar]
- 68.Eskenazi B, Trupin LS. Passive and active maternal smoking during pregnancy, as measured by serum cotinine, and postnatal smoke exposure. II. Effects on neurodevelopment at age 5 years. Am J Epidemiol. 1995 Nov 1;142(9 Suppl):S19–29. doi: 10.1093/aje/142.supplement_9.s19. [DOI] [PubMed] [Google Scholar]
- 69.Najman JM, Williams GM, Nikles J, et al. Mothers' mental illness and child behavior problems: cause-effect association or observation bias? Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(5):592–602. doi: 10.1097/00004583-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Rebagliato M. Validation of self reported smoking. J Epidemiol Community Health. 2002 Mar;56(3):163–164. doi: 10.1136/jech.56.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vartiainen E, Seppala T, Lillsunde P, Puska P. Validation of self reported smoking by serum cotinine measurement in a community-based study. J Epidemiol Community Health. 2002 Mar;56(3):167–170. doi: 10.1136/jech.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinhausen H-C, Metzke CW. Global measures of impairment in children and adolescents: results from a Swiss community survey. Australian and New Zealand Journal of Psychiatry. 2001;35(3):282–286. doi: 10.1046/j.1440-1614.2001.00901.x. [DOI] [PubMed] [Google Scholar]
- 73.American Academy of Pediatrics. Committee on Substance Abuse Tobacco's Toll: Implications for the Pediatrician. Pediatrics. 2001;107(4):794–798. [PubMed] [Google Scholar]