Abstract
Purpose
We undertook this study to determine 1) the frequency with which spilled tumor cells of favorable histology produced intra-abdominal disease in patients treated with differing chemotherapy regimens and abdominal radiation therapy (RT) and 2) the patterns of relapse and outcomes in such patients.
Materials and Methods
The influence of RT dose (0, 10 and 20 Gy), RT fields (flank, Whole abdomen) and chemotherapy with dactinomycin and vincristine (2 drugs) vs. added DOX (3 drugs) on intra-abdominal tumor recurrence rates was analyzed by logistic regression in 450 patients. Each patient was considered at risk for two types of failure: flank and sub-diaphragmatic beyond-flank recurrence, with the correlation between the two outcomes accounted for in the analyses.
Results
The crude odds ratio for the risk of recurrence relative to no RT was 0.35 (0.15–0.78) for 10Gy and 0.08 (0.01–0.58) for 20Gy. The odds ratio for the risk of recurrence for DOX relative to 2 drugs after adjusting for RT was not significant. For stage II patients (NWTS-4), the 8 yr. event rates with and without spillage respectively were 79% and 87% for relapse-free survival (p=0.07), and 90% and 95% for overall survival (p=0.04).
Conclusions
Irradiation (10 Gy or 20 Gy) reduced abdominal tumor recurrence rates following tumor spillage. Tumor spillage in stage II patients reduced relapse-free survival and overall survival, but only the latter was of statistical significance. These data provide a basis for assessing the risks versus benefits when considering treatment for children with favorable histology Wilms tumor and surgical spillage.
Keywords: Wilms tumor, radiation therapy, tumor spillage, local recurrence, survival
INTRODUCTION
Spillage of tumor cells during abdominal surgery for Wilms tumor has in the past been associated with an increased risk of tumor recurrence but has not affected overall survival (1, 2). In the National Wilms Tumor Study (NWTS) –1 and -2, the Wilms tumor grouping system was utilized, and patients with tumor spillage were classified as having group III disease (3, 4).
The staging system in NWTS-3 and -4 has been detailed elsewhere (4). In brief, Stage II tumors were those that penetrated the capsule but were totally excised. Stage III implied any of the following alone or in combination: positive lymph nodes, pre- or intra-operative gross spillage of tumor cells, and residual microscopic or gross disease.
An attempt was made in NWTS -3 and -4 to discriminate between gross peritoneal contamination and more confined spillage. The definitions adopted were “local” tumor spillage when the spillage was confined to the flank, or “diffuse” tumor spillage when there was contamination of the entire peritoneal cavity following tumor rupture (1, 5). In NWTS-3 and -4, patients with “local” tumor spillage were down-staged to stage II, while those with “diffuse” tumor spillage were retained in the stage III category (6). In NWTS – 4, there was a three-fold increase in abdominal tumor recurrence rates among stage II patients of all histologies with tumor spillage compared to patients without any tumor spillage (1).
These analyses were undertaken to determine 1) the frequency with which spilled tumor cells produced clinically detectable intra-abdominal disease and the effect of treatment with chemotherapy regimens that did or did not include doxorubicin (DOX) and different abdominal radiation therapy doses and volumes on this frequency; and 2) the patterns of recurrent disease in such patients and their outcomes.
METHODS AND MATERIALS
Between May 1979 and May 1985, 992 patients with stage II–IV FH Wilms tumor (excluding focal or diffuse anaplasia, clear cell sarcoma of kidney and rhabdoid tumors) were entered in the randomized or followed categories of NWTS-3 (5). Between August 1986 and September 1994, 1,318 patients with stage II–IV favorable histology Wilms tumor were entered in the randomized or followed categories of NWTS-4 (7). Surgical tumor spillage was identified in 515 of the 2,310 patients. Of these 515, 42 did not receive treatment with drugs and RT appropriate for their study and tumor stage. Another 23 patients were excluded as they had gross peritoneal tumor implants. The outcomes of the remaining 450 patients form the basis of this report.
On NWTS-3, patients with stage II disease were randomized to receive 0 or 20 Gy to the operative bed (flank). The flank irradiation (RT) portal was designed to include the volume of the affected kidney on preoperative CT scan or excretory urogram with a margin of 1 cm. The medial margin of the RT portal extended across the midline to include all of the vertebral bodies at the levels concerned (Fig 1). Patients with stage III disease were randomized to receive 10 or 20 Gy, and all patients with stage IV disease received 20 Gy to the tumor bed (5). On NWTS-4, patients with stage II disease did not receive flank RT. Patients with stage III and stage IV disease received 10 Gy to the flank or whole abdomen (WA) (7). In this report, patients with stage IV tumors were analyzed according to the stage and treatment of their abdominal disease (stage II or III). In NWTS-3 and 4, patients with “diffuse” tumor spillage received whole abdominal (WA) RT. This portal extended from the diaphragmatic domes superiorly to the bottom of the obturator foramen inferiorly. Thus the entire peritoneal cavity including the flank was included in the irradiated volume. The whole abdomen RT dose was either 10 Gy or 20 Gy in NWTS-3 (5) and 10 Gy for patients enrolled in NWTS-4 (7). In NWTS-3, among patients randomized to receive 20 Gy to the WA, the dose to the remaining kidney was limited to < 15 Gy by the use of kidney shielding (5).
Figure 1.
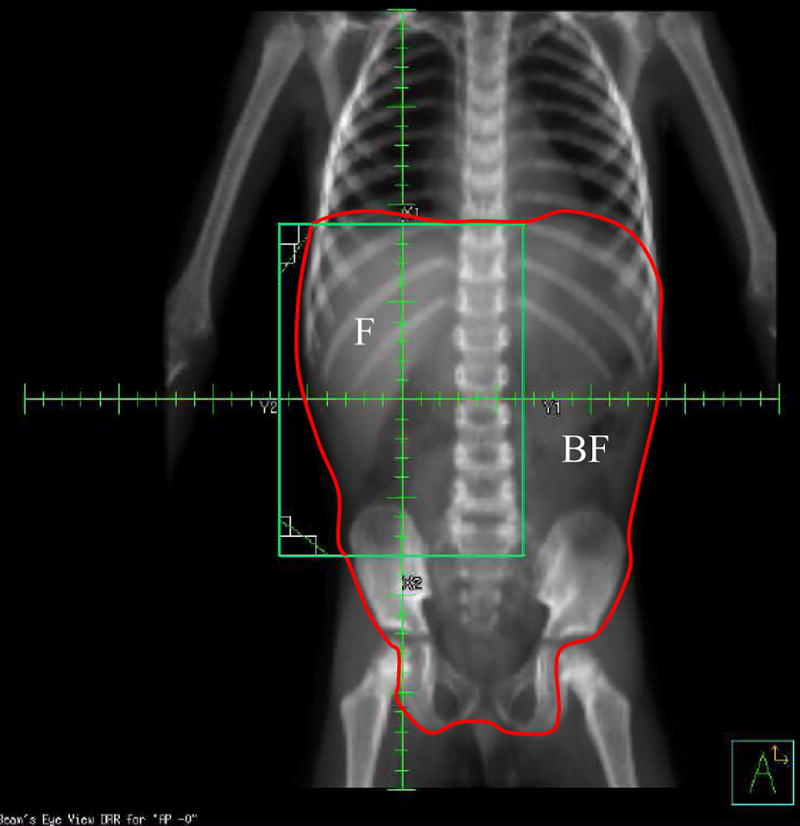
Digitally Reconstructed Radiograph (DRR) of a child receiving flank irradiation (RT) for a right-sided Wilms tumor. The regions at risk for relapse after spillage; flank (F) and beyond flank (BF), as defined by RT fields are shown.
RT was to be started within 11 days of surgery, and that criterion was met in most cases. The mean delay for all patients in the 2 studies was 10.9 days (8). While the assigned RT dosages were either 10 Gy or 20 Gy, the delivered RT doses could range from 10–10.8 Gy or 20–21.6 Gy respectively, depending on the 150, 180 or 200 cGy dose/fraction that was utilized. Information regarding RT doses and fields was gathered from patient charts and was reviewed by the NWTSG radiation oncologists.
The details of the chemotherapy regimens used in NWTS-3 and –4 for stage II–IV disease have been published earlier (5, 7). For the purpose of this analysis these regimens have been classified as either two drugs (vincristine and dactinomycin) or three drugs (vincristine, dactinomycin and DOX).
Surgical spillage classified as “local” or “diffuse” by the operating surgeon was reviewed by the surgical committee of the NWTSG. The reviewers often found it difficult, however, to establish whether spillage categorized as “local” or “diffuse” by the operating surgeons fulfilled the protocol criteria for this distinction. Since no anatomical barriers separate the flank (operative bed) from other areas of the peritoneal cavity, even a “locally” spilled tumor cell has the potential to be dispersed into the entire peritoneal cavity. To evaluate the efficacy of RT in destroying spilled tumor cells, the peritoneal cavity was divided into two sites (regions) that may have received different amounts of RT albeit each was at risk for spilled cell implantation: “flank” (F) denotes the region covered by the standard flank RT portal; “beyond-flank” (BF) denotes the remainder of the peritoneal cavity (Fig. 1). The site(s) of abdominal tumor recurrence were determined from surgical notes and diagnostic imaging reports and classified (JAK and GJD) as ‘F’ or ‘BF’ if only a single site was involved, or as ‘F+BF’ when both were involved. Recurrence in either site at any time was recorded whether preceded by, concurrent with, or after recurrent disease elsewhere; e.g., the lung or liver.
Statistics
The RT dose to each of the two possible sites of recurrence was determined by the total RT dose and the type of RT field (flank only or whole abdomen). For example, in a patient who received 10 Gy to the flank, the RT doses to the flank and beyond-flank sites were recorded as 10 Gy and 0 Gy, respectively. If the patient received 10 Gy to the whole abdomen, by contrast, the RT doses to the flank and beyond-flank sites were both 10 Gy. The aim of the statistical analysis was to estimate the impact of the different RT doses (0, 10 Gy, 20 Gy) and DOX in preventing tumor recurrence in the flank and beyond-flank sites in the abdomen after tumor spillage in abdominal stage II and stage III patients. Each patient contributed two data records to the logistic regression analysis, one for the flank and one for beyond flank. The odds ratios (OR) for tumor recurrence in relation to RT dose and DOX were adjusted for each other, for stage of disease and for recurrence site (F vs. BF). The correlation between the two treatment outcomes (flank, beyond-flank) in the same patient was accounted for by using robust standard errors based on a statistical procedure known as generalized estimating equations (GEE) (9,10). Estimation and comparison of relapse-free survival (RFS) and overall survival (OS) rates were conducted using the Kaplan-Meier (11) and log-rank (12) procedures.
RESULTS
Thirty-three among 450 patients (7.3%) in whom there had been operative spillage developed recurrent disease in the abdominal cavity [11 flank only, 13-beyond-flank (BF) only, and 9-both]. The flank was the most common site of relapse (20/33, 61%). The frequency of abdominal recurrence after treatment with two- or three-drug chemotherapy and no RT among stage II patients was 13.3% (14/105). Despite the entire peritoneal cavity being at risk for tumor recurrence from spilled cells, the flank was the most common site of recurrence in unirradiated stage II patients: the flank alone was a site of recurrence in 8 of the 14 unirradiated patients (57%), beyond-flank alone in 1 and both sites in 5 patients. The frequency of tumor development from spilled cells after treatment for all irradiated patients was 5.5% (19/345). (Table 1).
Table 1.
Abdominal tumor recurrence by study, stage, RT dose and chemotherapy for stage II–IV patients with tumor spillage.
| Abdominal recurrence | |||||||
|---|---|---|---|---|---|---|---|
| NWT Study | Stage | RT (Gy) | Drugs | Total # patients | #F Only | #BF only | #F + BF |
| 3 | II | 0 | 2 | 24 | 1 | 0 | 2 |
| 0 | 3 | 11 | 1 | 0 | 0 | ||
| 20 Flank | 2 | 11 | 0 | 0 | 0 | ||
| 20 Flank | 3 | 17 | 0 | 1 | 0 | ||
| III | 10 Flank | 2 | 1 | 0 | 0 | 0 | |
| 10 WART | 2 | 11 | 0 | 0 | 1 | ||
| 10 Flank | 3 | 17 | 0 | 0 | 0 | ||
| 10 WART | 3 | 17 | 0 | 0 | 0 | ||
| 20 Flank | 2 | 10 | 0 | 1 | 0 | ||
| 20 Flank 10 WART | 2 | 2 | 0 | 1 | 0 | ||
| 20 WART | 2 | 11 | 0 | 0 | 0 | ||
| 20 Flank | 3 | 11 | 0 | 1 | 0 | ||
| 20 Flank 10 WART | 3 | 1 | 0 | 0 | 0 | ||
| 20 WART | 3 | 21 | 0 | 1 | 0 | ||
| IV | 20 Flank | 3 | 23 | 0 | 1 | 0 | |
| 20 Flank 10 WART | 3 | 1 | 0 | 0 | 0 | ||
| 20 WART | 3 | 6 | 0 | 0 | 0 | ||
| 4 | II | 0 | 2 | 70 | 6 | 1 | 3 |
| III | 10 Flank | 3 | 62 | 1 | 3 | 1 | |
| 10 WART | 3 | 75 | 1 | 0 | 1 | ||
| IV | 10 Flank | 3 | 35 | 0 | 3 | 1 | |
| 10 WART | 3 | 13 | 1 | 0 | 0 | ||
| Total | 450 | 11 | 13 | 9 | |||
Effect of RT on flank and beyond-flank recurrence in stage II and III patients (Tables 2, 3)
Table 2.
Outcomes by site of recurrence and RT dose in abdominal stage II and III patients: each patient evaluated for both sites of potential recurrence.
| Flank | Beyond - Flank | |||
|---|---|---|---|---|
| RT dose | # Evaluated | # Events (%) | # Evaluated | # Events(%) |
| No RT | 105 | 13(12.4) | 292 | 18(6.2) |
| 10 Gy | 231 | 7(3.0) | 120 | 3(2.5) |
| 20 Gy | 114 | 0(0.0) | 38 | 1(2.6) |
| Total | 450 | 20(4.4) | 450 | 22(4.9) |
Table 3.
Multiple logistic regression analysis of recurrence on site, RT dose and tumor stage.
| Odds ratio (OR) | *95% CI | * P-value | |
|---|---|---|---|
| Trial | |||
| Flank | 1.0 | ------ | |
| Beyond flank | 0.56 | (0.24–1.25) | 0.16 |
| RT dose | |||
| 0 Gy | 1.0 | ------ | |
| 10 Gy | 0.26 | (0.09–0.79) | 0.02 |
| 20 Gy | 0.06 | (0.01–0.53) | 0.01 |
| Stage | |||
| II | 1.0 | ------ | |
| III | 1.04 | (0.43–2.52) | 0.93 |
| IV | 1.26 | (0.44–3.56) | 0.66 |
Estimated by GEE, p-value of test for trend with dose 0.002
The outcomes by site of recurrence and RT dose for flank and beyond-flank recurrences in Stage II and III patients are summarized in Table 2 and Fig. 1. The flank relapse rates for 0 Gy, 10 Gy and 20 Gy were 12.4%, 3% and 0% respectively. The beyond-flank relapse rates for 0Gy, 10Gy and 20Gy were 6.2%, 2.5% and 2.6% respectively. After adjustment for site of recurrence and institutional stage of disease (Table 3), the odds ratio (OR) for the risk of tumor recurrence relative to no RT was 0.26 for 10 Gy (95% CI 0.09–0.79, p-value 0.02) and 0.06 for 20 Gy (95% CI 0.01–0.53, p-value 0.01). The p-value of the adjusted test of trend for relapse with dose of RT was 0.002.
Effect of RT on flank and beyond-flank recurrence in stage II and only beyond-flank recurrence in stage III patients (Tables 4, 5)
Table 4.
Outcomes by RT dose for flank recurrence in abdominal stage II and beyond-flank recurrence in abdominal stage II–III patients.
| Flank | Beyond Flank | |||
|---|---|---|---|---|
| RT dose | # Evaluated | # Events (%) | # Evaluated | # Events (%) |
| No RT | 105 | 13(12.4) | 292 | 18(6.2) |
| 10 Gy | 0 | 0(0.0) | 120 | 3(2.5) |
| 20 Gy | 28 | 0(0.0) | 38 | 1(2.6) |
| Total | 133 | 13(9.8) | 450 | 22(4.9) |
Table 5.
Multiple logistic regression analysis of recurrence on site and RT dose limited to patients/events shown in Table 3.
| OR | *95% CI | * P-value | |
|---|---|---|---|
| Trial | |||
| Flank | 1.0 | ------ | |
| Beyond flank | 0.51 | (0.24–1.08) | 0.08 |
| RT dose | |||
| 0 Gy | 1.0 | ------ | |
| 10 Gy | 0.38 | (0.11–1.30) | 0.12 |
| 20 Gy | 0.16 | (0.02–1.26) | 0.08 |
Estimated by GEE, p-value for trend with dose 0.02.
This analysis was performed excluding flank relapses in stage III patients because not only spilled cells but also other factors inherent in the definition of stage III such as positive surgical margins, lymph node invasion etc. can also contribute to flank recurrences.
The outcomes by RT dose for flank recurrence in stage II and beyond-flank recurrence in Stage II–III patients are summarized in Table 4. The flank relapse rates for 0 Gy and 20Gy were 12.4% and 0% respectively. None of the patients with stage II tumors received 10Gy in NWTS-3,4. After adjustment for the site of recurrence, the odds ratio (OR) for the risk of tumor recurrence relative to no RT was 0.38 for 10 Gy (95% CI 0.11–1.30, p-value 0.12) and 0.16 for 20 Gy (95% CI 0.02–1.26, p-value 0.08) (Table 5). The p-value of the adjusted test of trend for relapse with dose of RT was 0.02.
Effect of doxorubicin on tumor recurrence following spillage
The odds ratio (OR) for the risk of local recurrence following the use of DOX in addition to vincristine and dactinomycin (3 drugs) relative to 2 drugs only (vincristine and dactinomycin) was not significant after adjustment for site of relapse and RT dose: adjusted OR was 0.64 (95%CI 0.24–1.50, p = 0.30).
Site and timing of recurrent disease and outcomes in unirradiated Stage II patients who relapsed below the diaphragm
These analyses were undertaken to determine the frequency with which spilled tumor cells produced clinically detectable intra-abdominal disease and the effects of treatment with chemotherapy regimens that did or did not include DOX and different abdominal radiation therapy doses. Therefore, the sequence of recurrent abdominal disease, whether it was before, concurrent with or after relapse elsewhere was evaluated in the 14 children who experienced abdominal relapse.
Seven of the 14 had relapsed earlier in the thorax (6) or liver (1) before abdominal relapse, and 5 of these 7 (71.4%) were alive. Five of the remaining 7 who relapsed first in the flank (one with simultaneous liver metastases) died of recurrent tumor in sites beyond the flank, 3 with liver and/or lung metastases as well as infra-diaphragmatic tumor. Thus, none of the 7 children died solely of progressive disease in the tumor bed. Finally, seven of the 14 patients (50%) were alive for periods ranging from 6 to 22 years after salvage therapies.
Outcomes in unirradiated stage II NWTS-4 patients treated with two drugs with or without intraoperative tumor spillage
The 8 yr. relapse-free (RFS) rates for stage II patients in NWTS-4 available for analysis were 79% following tumor spillage (103 children, 21 relapses observed vs. 14.9 expected) and 87% in the absence of spillage (310 patients, 40 relapses vs. 46.1 expected, p=0.07). The 8 yr. overall survival rates were 90% and 95% respectively in patients with (11 deaths observed vs. 6.5 expected) and without spillage (16 deaths observed vs. 20.5 expected, p=0.04).
DISCUSSION
The likelihood that unirradiated spilled tumor cells will produce clinically detectable intra-abdominal disease after two-drug chemotherapy is less than 15%.
The analysis of abdominal relapse rates by conventional methods does not give an accurate assessment of RT effects since beyond-flank recurrences at sites that did not receive RT would be counted as relapses against flank-only RT fields. In this analysis, therefore, flank and beyond-flank relapses were analyzed separately using GEE (9, 10). The addition of 10 Gy or 20 Gy decreased the likelihood of local treatment failure 3 and 12 fold respectively. These results are consistent with the data reported by Breslow et al. who found similar statistically significant differences in relapse rates according to the RT dose category among more than 3500 Wilms tumor patients (13).
The addition of DOX to 2 drugs or RT was not shown to add to the protective effects of 10 Gy or 20 Gy. This lack of demonstrated effect by DOX may be due to the fact that RT alone was effective in sterilizing spilled tumor cells or that the degree of overlap between the use of DOX and RT increased the statistical uncertainty to the extent that it precluded the detection of a separate DOX effect.
These analyses demonstrate that two-drug chemotherapy alone without RT in stage II patients with tumor spillage does not completely protect the patient from abdominal tumor recurrence. When the patterns of recurrence in unirradiated Stage II patients were analyzed, the tumor bed (flank) was the predominant site of infra-diaphragmatic relapse. Half the unirradiated children who suffered an abdominal relapse survived. This is less than the 80% 4-year survival recorded in the retrieval study of 58 unirradiated children treated initially with 2 drugs as reported by Green et al (14).
Tumor spillage decreased the relapse-free survival rates and overall survival compared to patients without spillage, but only the latter was of statistical significance. However > 85% of non-irradiated patients in whom spillage occurred did not develop abdominal relapses. Similar findings have been previously reported by the NWTSG and SIOP investigators (1,2,15,16). The Renal Tumor Committee of the Children’s Oncology Group (COG) which is the successor to the NWTSG, has adopted a policy of upstaging patients with tumor spillage to stage III (COG protocol # AREN0533) and treating them with flank RT (10 Gy) and doxorubicin added to vincristine and dactinomycin. The results of these studies will provide a basis for assessing the risk of over treating with abdominal irradiation and DOX for the 85% of children with stage II/FH Wilms tumor and spillage who do not relapse versus the benefit of improving the survival rate and reducing the need for potentially toxic salvage therapy for the 15% of patients who relapse (17). A lower dose of flank RT (10 Gy) with DOX was chosen over 20 Gy for the treatment of these patients as previously published reports from the NWTSG have shown only a minimal impact on growth and development (18,19) after treatment with this regimen and no reports of a second malignant neoplasm in the treated field after RT doses of ≤ 12Gy (20). However adverse consequences on pregnancy outcomes (21) have been reported. The use of doxorubicin has been associated with congestive heart failure, albeit among patients given 150 mg/m2 of doxorubicin no instances of CHF has been reported thus far (22).
CONCLUSIONS
Abdominal recurrence rates following tumor spillage were significantly higher among patients treated with two or three-drug chemotherapy without RT. Irradiation with 10 Gy appeared to be successful in reducing tumor recurrence rates following tumor spillage, and 20 Gy even more so. The addition of DOX to vincristine and dactinomycin did not detectably reduce abdominal tumor recurrence rates. Tumor spillage in stage II patients reduced relapse-free survival and overall survival, but only the latter was of statistical significance. These data provide a basis for assessing the risks versus benefits when considering the management of patients with Wilms tumor and surgical spillage.
Acknowledgments
The authors thank the investigators of the Children’s Oncology Group and the many pathologists, surgeons, pediatricians, radiation oncologists and other health professionals who managed the care of the children entered on the National Wilms Tumor Studies. They are also indebted to the staff of the NWTSG Data and Statistical Center for their patience and dedicated work over many years of collaborative work.
Footnotes
Presented in part at the annual meeting of the American Society of Therapeutic Radiology and Oncology (ASTRO), Denver, Colorado, October 2005.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shamberger RC, Guthrie KA, Ritchey ML, et al. Surgery-related factors and local recurrence of Wilms tumor in National Wilms Tumor Study 4. Ann Surg. 1999;229:292–297. doi: 10.1097/00000658-199902000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemerle J, Voute PA, Tournade MF, et al. Pre-operative versus postoperative radiotherapy, single versus multiple courses of actinomycin-D in the treatment of Wilms tumors. Preliminary results of a controlled clinical trial conducted by the International Society of Paediatric Oncology (SIOP) Cancer. 1976;38:647–654. doi: 10.1002/1097-0142(197608)38:2<647::aid-cncr2820380204>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.D’Angio GJ, Evans AE, Breslow NE, et al. The treatment of Wilms tumor: Results of the National Wilms’ Tumor Study. Cancer. 1976;38:633–646. doi: 10.1002/1097-0142(197608)38:2<633::aid-cncr2820380203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.D’Angio GJ, Evans AE, Breslow NE, et al. The treatment of Wilms tumor: Results of the Second National Wilms Tumor Study. Cancer. 1981;47:2302–2311. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.D’Angio GJ, Breslow N, Beckwith JB, et al. The treatment of Wilms tumor: Results of the Third National Wilms Tumor Study. Cancer. 1989;64:349–360. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Farewell VT, D’Angio GJ, Breslow N, et al. Retrospective validation of a new staging system for Wilms’ tumor. Cancer Clin Trials. 1981;4:167–171. [PubMed] [Google Scholar]
- 7.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol. 1998;16:237–245. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 8.Kalapurakal JA, Li SM, Breslow NM, et al. Influence of radiation therapy delay on abdominal tumor recurrence in patients with favorable histology Wilms tumor treated on NWTS-3 and NWTS -4. Int J Radiat Oncol. 2003;57:495–499. doi: 10.1016/s0360-3016(03)00598-4. [DOI] [PubMed] [Google Scholar]
- 9.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 10.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:1–10. [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statistical Association. 1958;53:456–481. [Google Scholar]
- 12.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J Royal Statistical Society (Series A) 1972;135:185–206. [Google Scholar]
- 13.Breslow NE, Beckwith JB, Haase GM, et al. Radiation therapy for favorable histology Wilms tumor prevention of flank recurrence did not improve survival on National Wilms Tumor Study -3 and -4. Int J Radiation Oncol Biol Physics. 2006;65:203–209. doi: 10.1016/j.ijrobp.2005.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green DM, Cotton CA, Mologolowkin M, et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine and actinomycin D: A report of the National Wilms Study Group. Pediatr Blood Cancer. 2007;48:493–499. doi: 10.1002/pbc.20822. [DOI] [PubMed] [Google Scholar]
- 15.Grundy P, Breslow N, Green DM, et al. Prognostic factors for children with recurrent Wilms tumor: results from the Second and third National Wilms Tumor Study. J Clin Oncol. 1989;7:638–647. doi: 10.1200/JCO.1989.7.5.638. [DOI] [PubMed] [Google Scholar]
- 16.Thomas PR, Tefft M, Compaan PJ, et al. Results of two radiation therapy randomizations in the third National Wilms Tumor Study. Cancer. 1991;68:1703–1707. doi: 10.1002/1097-0142(19911015)68:8<1703::aid-cncr2820680809>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.D’Angio GJ. Pre- or Postoperative Therapy for Wilms Tumor? J Clin Oncol. 2008;26:4055–4057. doi: 10.1200/JCO.2008.16.5316. [DOI] [PubMed] [Google Scholar]
- 18.D’Angio GJ. Pre- or Post-operative treatment for Wilms tumor? Who, what, where, how and why. Med Pediatr Oncol. 2003;41(6):545–549. doi: 10.1002/mpo.10395. [DOI] [PubMed] [Google Scholar]
- 19.Hogeboom CJ, Grosser SC, Guthrie KA, et al. Stature loss following treatment for Wilms tumor. Med Pediatr Oncol. 2001;36:295–304. doi: 10.1002/1096-911X(20010201)36:2<295::AID-MPO1068>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Breslow NE, Takashima JR, Whitton JA, et al. Second malignant neoplasms following treatment for Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol. 1995;13:1851–1859. doi: 10.1200/JCO.1995.13.8.1851. [DOI] [PubMed] [Google Scholar]
- 21.Green DM, Peabody EM, Nan B, et al. Pregnancy outcome after treatment for Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol. 2002;20:206–2513. doi: 10.1200/JCO.2002.07.159. [DOI] [PubMed] [Google Scholar]
- 22.Green DM, Grigoriev YA, Nan B, et al. Congestive heart failure after treatment for Wilms tumor: A report from the National Wilms Tumor Study Group. J Clin Oncol. 2001;19:1926–1934. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]


