Summary
Inositol 1,4,5-trisphosphate (IP3) selectively evokes an inward (excitatory) current in cultured lobster olfactory receptor neurons (ORNs) and directly activates two types of channels in cell-free patches of plasma membrane from the ORNs. The IP3-activated channels have kinetic properties of odor-activated channels in the ORNs and pharmacological properties of intracellular IP3-activated channels in other systems. An antibody directed against an intracellular, cerebellar IP3, receptor recognizes a protein with a molecular weight similar to the mammalian receptor in the ORNs. The antibody selectively increases odor-evoked inward currents and IP3-activated unitary currents in the ORNs. The data provide further evidence for IP3 as an olfactory second messenger and implicate at least one and possibly two novel plasma membrane IP3 receptors in olfactory transduction.
Introduction
Adenosine 3′,5′-cyclic monophosphate (cAMP) is now well established as a second messenger in olfactory transduction (for reviews see Anholt, 1991; Firestein, 1991). Since odors rapidly and transiently elevate levels of inositol 1,4,5-trisphosphate (IP3) in the cilia/outer dendritic membranes of olfactory receptor neurons (ORNs) in fish (Huque and Bruch, 1986), rats, and insects (Breer et al., 1990). IP3 must also be considered as an olfactory second messenger.
The relationship between phospholipid and cyclic nucleotide second messengers in olfactory transduction is still obscure. Odors that elevate IP3 in ciliary membrane preparations of rat ORNs fail to elevate cAMP and vice versa (Boekhoff et al., 1990; Breer and Boekhoff, 1991), suggesting that the two second messengers mediate different, odor-specific transduction pathways. Indeed, two distinct transduction pathways can be predicted in lobster and amphibian ORNs, where odors have been shown to suppress as well as excite the cells via separate conductances (McClintock and Ache, 1989; Michel et al., 1991; Dionne, 1992). In lobster ORNs, cAMP mediates an inhibitory transduction pathway that suppresses the output of the cell (Michel and Ache, 1992). Given that IP3 has been implicated as an olfactory second messenger in at least one other species of arthropod (Breer et al., 1990). IP3 is a logical candidate to mediate excitation in the lobster, but the excitatory transduction pathway in lobster ORNs is unknown.
IP3 is known to release Ca2+ from nonmitochondrial intracellular stores by binding to a receptor protein that contains both an IP3 recognition site and a Ca2+ channel (for review see Ferris and Snyder, 1992). It is unclear whether such IP3 receptors are associated with the plasma membrane in neurons (Worley et al., 1987; Maeda et al., 1989, 1991; Mignery et al., 1989; Ross et al., 1989), although IP3 receptors occur in the plasma membrane of lymphocytes (Kuno and Gardener, 1987; Khan et al., 1992) and mast cells (Penner et al., 1988) and in transverse tubules (Viven and Coronado, 1988). Evidence is beginning to implicate what is perhaps a novel type of IP3 receptor in the plasma membrane of ORNs. IP3 activates a channel reconstituted from the cilia of catfish ORNs (Restrepo et al., 1990). The cilia are enriched in a 107 kd protein that binds radiolabeled IP3, but whose molecular weight and affinity for IP3 are less than those reported for intracellular cerebellar IP3 receptors (Kalinoski et al., 1992). Preliminary evidence localizes immunoreactivity of an antibody directed against cerebellar IP3 receptors to the cilia of rat ORNs (Cunningham et al, 1992, Chem. Senses, abstract). As the cilia of ORNs are devoid of organelles, it could be assumed that the target of this second messenger in olfactory neurons is a plasma membrane IP3 receptor.
Here, we report that IP3 mediates excitation in cultured lobster ORNs by directly gating ion channels in the plasma membrane. The study provides functional evidence for channels activated by IP3 in the plasma membrane of neurons.
Results
Macroscopic Currents
Introducing 2.4 × 10−5 M IP3 into the cells through the patch pipette evoked a prolonged, inward current in 17 of 41 (42%) cells, with an average peak amplitude of 35.1 ± 10.4 pA (Figure 1A). Without IP3 in the pipette, the cells held a steady baseline over the test interval of 4 min. These particular cells were not tested for their ability to respond to odors, but the most effective odor we have been able to test, an extract of fish food (TET [TetraMarin]), excites approximately 37% of cultured ORNs (Fadool et al., 1993). The percentage of cells activated by introducing IP3 through the pipette, therefore, is consistent with the percentage of cells that would be expected to be excited by odors.
Figure 1. IP3- and Odor-Evoked Macroscopic Currents in Voltage-Clamped Cultured Lobster ORNs.
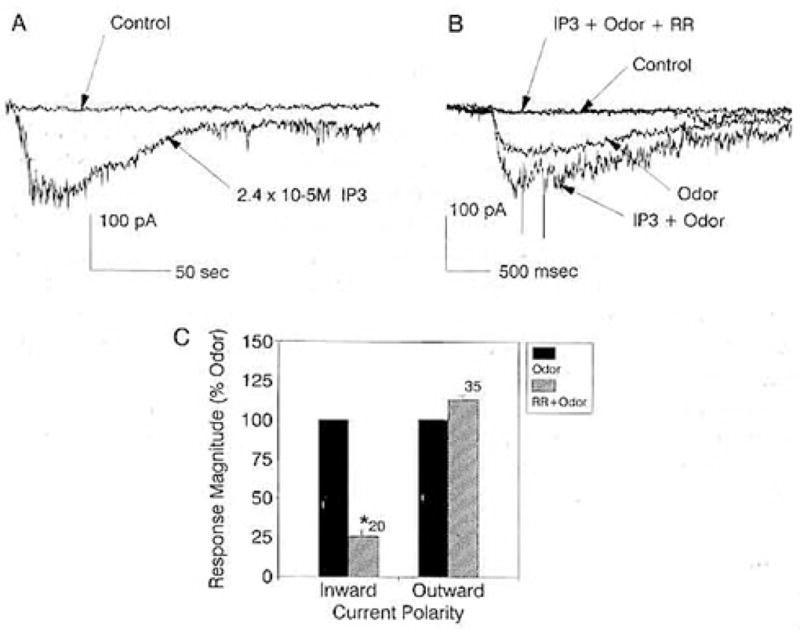
(A) Whole-cell recording from an ORN that was sequentially patched with normal patch solution and then IP3 in the patch pipette. Holding potentials, −60 mV.
(B) Whole-cell recording from an ORN that was sequentially patched with normal patch solution and then IP3 in the patch pipette and then “spritzed” in each instance with odors. First patch: Control, response to recording media. Odor, response to TET. Second patch: IP3 + Odor, response to TET with 2.4 × 10−3 M IP3 in the patch pipette. IP3 + Odor + RR, response to TET with IP3 in the patch pipette while bathing the cell in 10 μM RR. Holding potentials, −60 mV.
(C) Plot of the mean peak amplitude of odor-evoked inward (n = 20) and outward (n = 35) currents in the absence (closed bar) and presence (striped bar) of 10 μM RR in the bath. Response magnitudes in the absence of RR were normalized to 100%. Asterisk indicates significant difference at p ≤ 0.05, paired t test. Holding potential, −60 mV.
The polarity of the IP3-induced current matched the polarity of the current induced by TET (Figure 1B). Introducing 2.4 × 10−5 M IP3 through the patch pipette increased the magnitude of the TET-evoked inward current to 188% ± 12% of that evoked by TET without IP3 in the pipette (n = 4 sequentially patched cells) (Figure 1B). It was not determined whether higher concentrations of IP3 could saturate the odor-evoked inward current. The inward current induced by TET + IP3 in all four instances was substantially blocked by bathing the cells with 10 μM ruthenium red (RR, a drug reported to block some IP3-gated conductances; Ehrlich and Watras, 1988; Berridge, 1989), supporting a common origin of the IP3- and odor-induced currents (Figure 1B).
The effect of RR was selective for the inward current (Figure 1C). Bathing the cells in 10 μM RR significantly reduced the peak amplitude of the inward current evoked by TET, proline, or betaine from an average of 19.1 ± 4.0 pA to 4.8 ± 4.3 pA (n = 20, paired t test). The drug, however, had no significant effect on the peak amplitude of the outward current evoked by proline, betaine, glycine, or taurine, which averaged 16.4 ± 2.6 pA before and 15.5 ± 6.9 pA after bathing the cells in 10 μM RR (n = 35, paired t test).
Unitary Currents
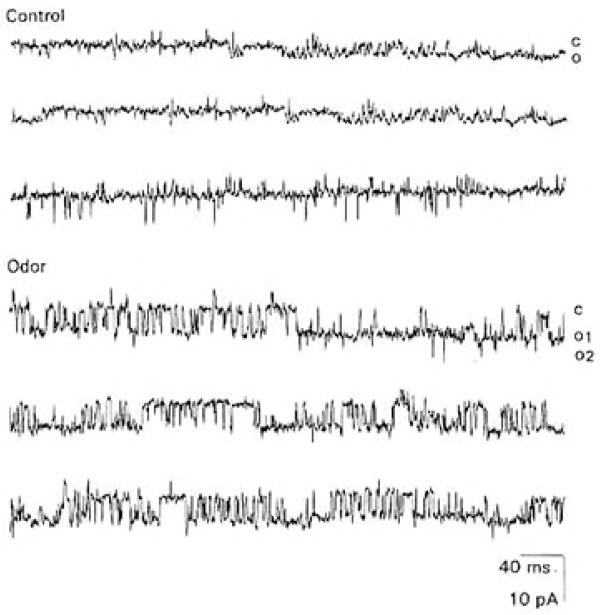
TET transiently activated unitary currents in 4 of 21 cell-attached recordings (Figure 2). The mean estimated chord conductance for the 4 channels was 86.7 ± 17.1 pS (Table 1). The probability of being open (Propen) of the 4 odor-activated channels increased from an average of 0.02 ± 0.001 to 0.11 ± 0.03. The channels characteristically had “flickery” kinetics and opened in long bursts averaging 72.2 ± 53.6 ms. The mean open (to) and closed (tc) times for the 4 odor-activated channels were best fit by double exponentials (Table 1).
Figure 2. Odor-Evoked Unitary Currents in a Cell-Attached Patch from a Cultured Lobster ORN.
Control: Unitary currents recorded in response to “spritzing” an ORN with normal patch solution to depolarize it.
Odor: Unitary currents recorded in response to “spritzing” an ORN with the odor TET. In both upper and lower traces, membrane potential, −60 mV. Records filtered at 2 kHz. In this and subsequent figures, “C” denotes closed state of the channel, whereas “O” denotes open state of the channel. For the open-channel state, superpositions in multiple channels in a patch are numbered in subscript.
Table 1.
Properties of Odor- and IP3-Activated Channels in Cultured Lobster ORNs
| Property | Large IP3 Channel | Small IP3 Channel | Odor Channel |
|---|---|---|---|
| Conductance | 73.7 ± 5.7 pS | 30.0 ± 1.6 pS | 86.7 ± 17.1 pSa |
| to | |||
| τ1 | 0.38 ± 0.07 ms | 0.49 ± 0.06 ms | 0.43 ± 0.05 ms |
| τ2 | 2.52 ± 0.43 ms | 6.33 ± 1.64 ms | 5.18 ± 1.39 ms |
| tc | |||
| τ1 | 2.64 ± 0.19 ms | 1.25 ± 0.24 ms | 2.85 ± 0.37 ms |
| τ2 | 36.49 ± 6.80 ms | 42.64 ± 15.90 ms | 35.78 ± 1.34 ms |
Denotes estimated chord conductance rather than slope conductance. See text for sample size of each measure.
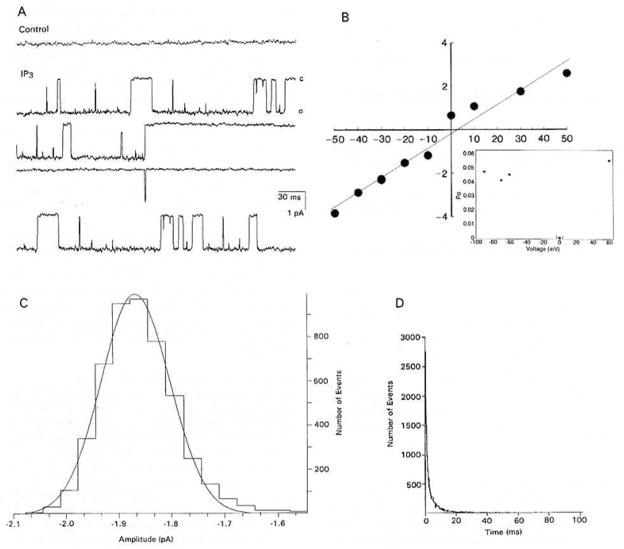
Applying 10−7 M IP3 to the inside face of 86 cell-free patches activated unitary currents in 63 of the patches within 100 ms of application. The patches typically contained 1–3 channels, although 4 and 5 channels could be resolved in 2 of the patches, respectively. The channels were of two different types; in only one instance were both types observed in a single patch of membrane. One type of channel (Figure 3A) had a mean slope conductance of 73.7 ± 5.7 pS and reversed polarity at 2.4 ± 2.2 mV in symmetrical solutions (n = 12) (Figure 3B). The open probability function closely followed a Gaussian distribution (Figure 3C). The Propen was between 0.04 and 0.05 from −90 mV to +60 mV (Figure 3B, inset). The to and tc values were best fit by double exponentials (n = 20) (Figure 3D; Table 1).
Figure 3. IP3-Evoked, 74 pS Unitary Currents in Inside-Out Patches of Membrane from Cultured Lobster ORNs.
(A) Basal current prior to (control) and after (IP3) “spritzing” 10−7 M IP3 on the internal face of a patch. Membrane potential, −30 mV. Records filtered at 2 kHz. “C” and “O” as defined in Figure 2.
(B) Plot of the current-voltage relation of the channel shown in (A). The current reversed near zero in symmetrical solutions with a mean slope conductance of 73.7 ± 5.7 pS (n = 12). Voltage in mV on the abscissa and current in pA on the ordinate. Inset: Propen of the channel in (A) at different membrane potentials.
(C) Amplitude histogram of 9965 open-time events from the membrane patch in (A), fit by a Gaussian distribution with mean and standard deviation of −1.87 ± 0.07 pA.
(D) Plot of the open-time distribution of 2735 events of an IP3-activated channel fit by a double exponential. τ1 = 0.38 ± 0.07 ms, τ2 = 2.52 ± 0.43 ms (n = 14). Closed-time distribution not shown.
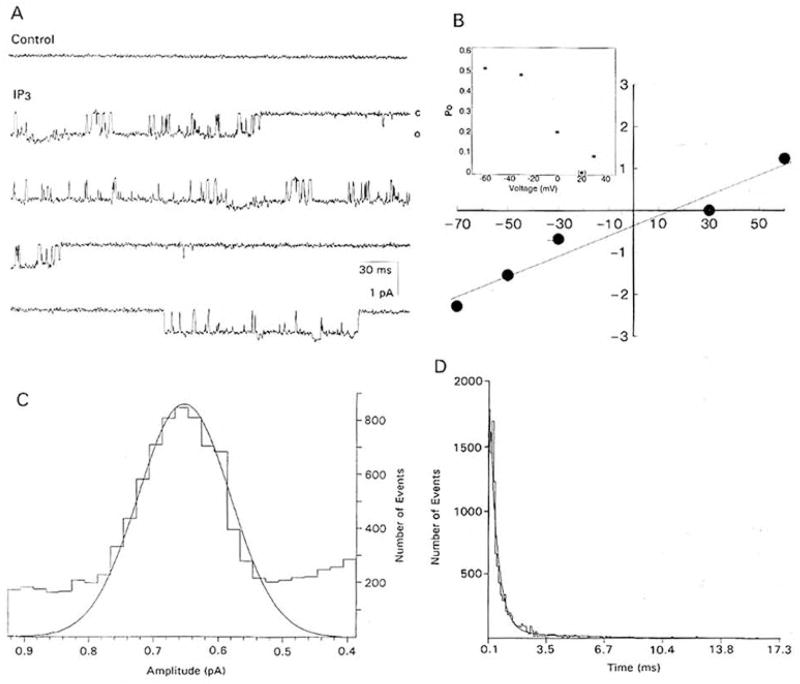
The second type of channel (Figure 4A) had a mean slope conductance of 30.0 ± 1.6 pS and reversed polarity at 30.2 ± 1.5 mV in symmetrical solutions (n = 16) (Figure 4B). The open probability function followed a Gaussian distribution (Figure 4C), but less closely than that of the larger conductance channel The Propen of the channel was voltage dependent and decreased from 0.5 to 0.1 between −40 and +40 mV (Figure 4B, inset). The to and tc were best fit by double exponentials (n = 32) (Figure 4D; Table 1).
Figure 4. IP3-Evoked, 30 pS Unitary Currents in Inside-Out Patches of Membrane from Cultured Lobster ORNs.
(A) Basal currents prior to (control) and after (IP3) “spritzing” 10−7 M IP3 on the internal face of a patch. Membrane potential, −30 mV. Records filtered at 2 kHz. “C” and “O” as defined in Figure 2.
(B) Plot of the current-voltage relation of the unitary currents shown in (A). The current reversed between 20 and 30 mV in symmetrical solutions with a mean slope conductance of 30.0 ± 1.6 pS (n = 16). Voltage in mV on the abscissa and current in pA on the ordinate. Inset: Propen of the channel in (A) over different membrane potentials.
(C) Amplitude histogram of 3699 open-time events from the membrane patch in (A), fit by a Gaussian distribution with mean and standard deviation of 0.65 ± 0.02 pA.
(D) Plot of the open-time distribution of 1737 events of an IP3–activated channel fit by a double exponential, τ1 = 0.49 ± 0.06 ms, τ2 = 6.33 ± 1.64 ms (n = 15). Closed-time distribution not shown.
There was no significant difference in the distribution of to for the two types of IP3-activated channels and the odor-activated channels (one-way analysis of variance, ANOVA). However, there was a significant difference in the distribution of tc for the two types of IP3-activated channels compared with that of the odor-activated channels, which could be attributed to the second exponent (τ2) of the smaller conductance channel (ANOVA, Student-Newman-Keuls).
The Propen of both types of IP3-activated channels (n = 5, each type) increased with the concentration of IP3 applied to the patch, as would be expected if IP3 functioned as a second messenger. The Propen for the larger conductance channel increased from 0.04 ± 0.02 at 10−7 M to 0.25 ± 0.03 at 10−5 M, while the same parameter for the smaller conductance channel increased from 0.06 ± 0.02 at 10−7 M to 0.28 ± 0.13 at 10−5 M. Concentrations outside of this range were not tested. When membrane patches (n = 5) containing the larger conductance channel were stimulated repeatedly with 10−5 M IP3, so as to expose the channel continuously to the ligand for 50 s, the channel remained in the open state 42% ± 12% of the time and failed to desensitize. The smaller conductance channel was not tested with continuous stimulation.
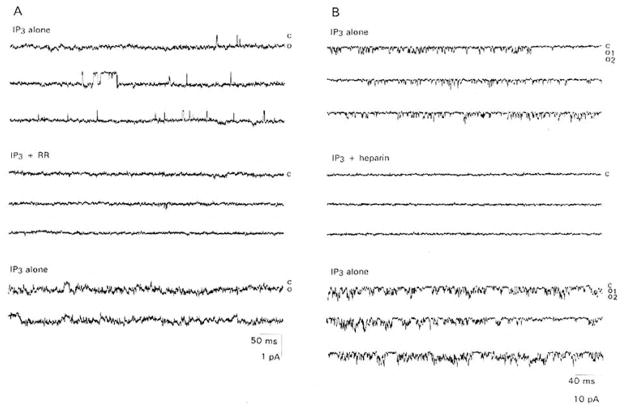
Both the large (n = 2) and the small (n = 2) conductance channels were blocked by 10 μM RR, consistent with the pharmacology of the macroscopic current (Figure 5A). The blockade was partially reversible within 5 min of washout of the drug. Although not tested on the macroscopic current, both the larger (n = 3) and the smaller (n = 2) conductance channels were also blocked by 2.5 μM heparin, a drug known to block intracellular IP3 receptors (Supattapone et al., 1988; Frank and Fein, 1991) (Figure 5B). Heparin blockade was fully reversible upon washout.
Figure 5. RR and Heparin Block of IP3-Activated Channels in Inside-Out Patches from Cultured Lobster ORNs.
(A) Activity of a channel of the type shown in Figure 4 induced by “spritzing” 10−7 M IP3 on the inside face of the patch (upper three traces) is abolished when 10 μM RR is copresented with IP3 (middle three traces) and partially recovers (lower two traces) when RR is rinsed and IP3 re-presented. Membrane potential, −60 mV. Records filtered at 2 kHz. “C” and “O” as defined in Figure 2.
(B) Activity of a channel of the type shown in Figure 3 induced by “spritzing” 10−7 M IP3 on the inside face of the patch (upper three traces) is abolished when 2.5 μM heparin is copresented with IP3 (middle three traces) and recovers (lower three traces) when heparin is rinsed and IP3 re-presented. Membrane potential, −60 mV. Records were filtered at 2 kHz. “C” and “O” as defined in Figure 2.
Channels of both types were insensitive to modulation by ATP, unlike the 2- to 4-fold increases in Propen reported for some IP3 receptors (Ehrlich and Watras, 1988). Copresenting up to 50 mM ATP with 10−7 M IP3 failed to alter the Propen (as reported above) of either the larger (n = 3) or the smaller (n = 4) conductance channels (signed rank test).
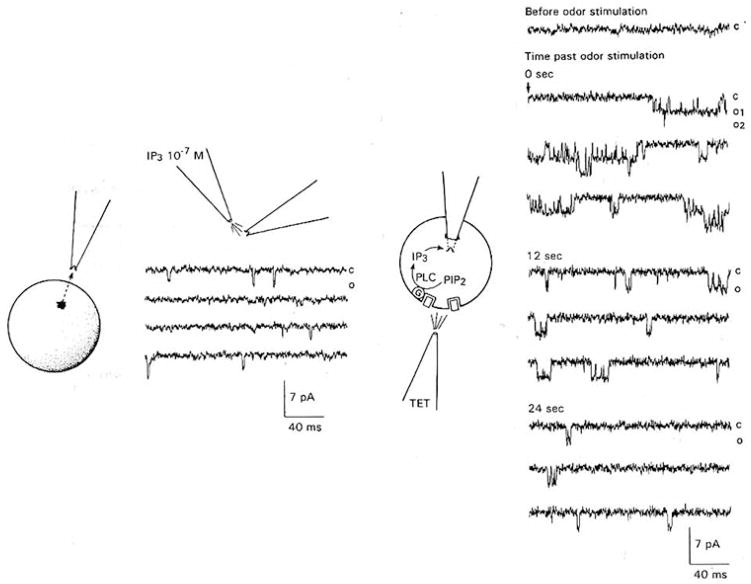
In one trial, it was possible to insert successfully a pipette with an inside-out patch containing the larger conductance channel taken from one cell into a second cell (Figure 6). “Spritzing” the odor TET on the second, recipient cell induced channel activity in the patch, presumably, although not necessarily, due to elevation of intracellular IP3, as illustrated in Figure 6. The activity of the channel decreased over the subsequent 24 s.
Figure 6. Odors Activate an IP3-Gated Channel inserted into a Cultured Lobster.
Left: An inside-out patch taken from 1 ORN was confirmed to contain an IP3-gated channel of the type shown in figure 3 by “spritzing” 10−7 M IP3 on the inner face of the patch. IP3 was applied at the beginning of the trace shown.
Right: The patch was subsequently “crammed” into a second ORN, which was subsequently “spritzed” with TET (arrow). Application of the odor activates 2 superimposed channels in the patch, suggesting that the odor elevates the intracellular concentration of IP3. The peak concentration of IP3 in the cell is presumably greater than 10−7 M, since it elicited superimposed channel openings; 10−7 M IP3 elicited only single channel openings in the calibration trial (left). Activity of the channels declines over the subsequent 24 s following odor stimulation. Cartoon depicts the hypothesized mechanism of action: odors (solid square) bind to cell surface receptors and activate the enzyme phospholipase C (PLC) through G protein (G) mediation to convert phosphatidylinositol 4,5-bisphosphate (PIP2) to IP3, which is detected by the IP3-gated channels in the patch. No channel activity was observed prior to odor stimulation. Membrane potential, −60 mV. Records filtered at 2 kHz. “C” and “O” as defined in Figure 2.
Immunochemistry and Related Physiology
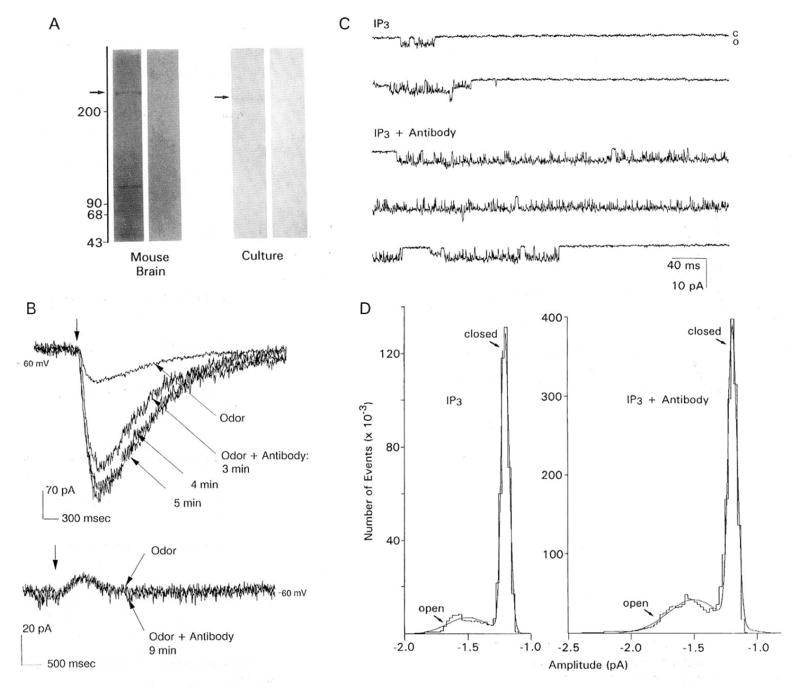
A polyclonal antibody raised against the 19 C-terminal amino acids of a cDNA clone of a mouse cerebellar IP3 receptor (Mignery et al., 1989; kindly supplied by Dr. P. DeCamilli) immunolabeled a band greater than 200 kd, as well as several lower molecular weight bands (Figure 7A, left panel, left column). Only the immunoreactivity of the greater than 200 kd band was incrementally blocked by preabsorbing the antibody with increasing concentrations of a synthetic peptide patterned after the original antigen PCD6 (data not shown). The antibody also labeled a band greater than 200 kd in membranes isolated from cultured lobster ORNs (Figure 7A, right panel, left column). No bands were observed in either the mouse or lobster membrane preparation when the primary antibody was replaced with nonimmune rabbit serum (Figure 7A, both panels, right columns). It was not possible to test preimmune rabbit serum.
Figure 7. Localization and Functional Selectivity of an IP3 Receptor in Cultured Lobster ORNs.
(A) Immunoreactivity of membrane fractions from mouse cerebellum and cultured lobster ORNs (culture) to an antibody directed against a cDNA clone of a mammalian IP3 receptor (first lane of each pair) and to nonimmune rabbit immnunoglobulin G (second lane of each pair). Molecular weight standards as indicated. Arrow denotes antibody cross-reactivity with a greater than 200 kd protein in each preparation.
(B) Upper traces: Whole-cell recording from a voltage-clamped cultured lobster ORN that was sequentially patched to determine the amplitude of inward current evoked by the odor TET (on at arrow) with (odor + antibody) and without (odor) the antibody in (A) in the patch pipette. The current increased over time (as indicated) with antibody in the pipette. Membrane potential, −60 mV. Lower traces: Same protocol applied to an outward current evoked by proline (on at arrow).
(C) Unitary currents recorded in an inside-out patch, activated by “spritzing” 10−7 M IP3 on the inner face of the patch (IP3), are increased when the same concentration of IP3 is copresented with the antibody in (A) (IP3 + Antibody). Both traces are representative records of channel activity several seconds after “spritzing.” Membrane potential, −60 mV. Records filtered at 2 kHz. “C” and “O” as defined in Figure 2.
(D) Amplitude histogram of the unitary currents recorded in (C), activated by “spritzing” 10−7 M IP3 on the inner face of the patch in the absence (IP3) and presence (IP3 + Antibody) of the antibody in (A). Propen, but not the conductance, increases in the presence of the antibody. “Closed-” and “open-” state current distributions of the channel are indicated with arrows.
Introducing the antibody into the cell through the patch pipette enhanced the odor-(TET) evoked inward current (Figure 7B, upper traces). The peak amplitude of the odor-evoked current increased an average of 427% ± 48% within 3 min after breakthrough over that evoked without the antibody in the pipette (5 of 5 sequentially patched cells). The effect of the antibody increased slightly over the 30 min following the second breakthrough, presumably reflecting further diffusion of the antibody from the pipette. The effect of the antibody was selective for the inward current (Figure 7B, lower traces). Introducing the antibody into the cell failed to alter the odor- (proline, taurine, alanine) evoked outward current visibly (9 of 9 sequentially patched cells). Without antibody in the pipette, odor- (TET, proline, taurine, betaine) evoked currents of both polarities decreased to as much as 43% of their initial magnitude over the 20–30 min following breakthrough. To determine whether the effect on the inward current was specific for the antibody, another group of cells was single patched with pipettes that were tip filled with normal patch solution and backfitted with either heat-inactivated antibody (8 cells), rabbit serum (7 cells), or an antibody raised against Goff (16 cells; a generous gift of R. Bruch; this antibody does not recognize any protein in lobster ORNs by Western blot analysis, unpublished data), and the protein was allowed to diffuse into the cell for up to 30 min. All three control proteins failed to alter visibly the magnitude of the odor-evoked current of either polarity from that observed without antibody in the pipette (arcsin transformation of percentage data followed by Student’s t test). Using this same technique, but backfilling with the antibody, enhanced the odor- (TET) evoked inward current to approximately the same extent observed in the sequentially patched cells (375% ± 29%, 4 cells), suggesting that the control proteins were able to diffuse into the cells during the experimental interval. In the absence of odor stimulation, the antibody had no effect on the basal current (data not shown).
The antibody increased the ability of 10−7 M IP3 to activate unitary currents in 4 of 5 cell-free patches (Figure 7C). The antibody increased the Propen of all channels in the 4 patches (n = 2 small and 5 large) from an average of 0.11 ± 0.06 to 0.41 ± 0.07 (Figure 7D). In 1 patch that contained multiple channels (4 large), the channels showed a marked increase in superposition in the presence of the antibody: from 1–2 of the channels being open 78% of the time to 3–4 of the channels being open 65% of the time. The antibody selectively increased the duration of the τ2 of to from 0.84 ± 0.17 ms to 19.06 ± 9.66 ms; the other kinetic parameters and the conductance were unaltered (n = 4). In the fifth patch, the antibody first decreased and then blocked the activity of a large conductance channel.
Discussion
The ability of odors to increase channel activity in cell-attached recordings argues strongly for the involvement of a diffusible second messenger in the excitatory transduction pathway and minimizes the possibility that odors directly gate ion channels in these cells. Second messenger mediation is also consistent with evidence that the odor-evoked inward current in lobster ORNs is affected by probes directed against GTP-dependent proteins (Fadool et al., 1991, Chem. Senses, abstract) that presumably would link receptor activation to second messenger production.
Several findings argue that IP3 is the excitatory second messenger. First, IP3 selectively evokes macroscopic inward currents, which would be expected to depolarize (excite) the cells. Second, RR blocks both the odor-evoked macroscopic current and the IP3-gated unitary current. Third, an odor (TET) activates an IP3-gated channel inserted into the cell, as would be expected if odor binding elevated the intracellular concentration of IP3. Although, in the latter instance we cannot exclude that the odor altered the intracellular environment in some way other than elevating IP3, preliminary findings suggest that altering intracellular pH from 5–9 and elevating the intracellular Ca2+ concentration up to 30 mM fail to initiate channel activity in cell-free patches (unpublished data).
The ability of IP3 to elicit channel activity in cell-free patches argues that IP3 acts directly on the channels and not through activation of a protein kinase or some other additional step in the transduction cascade, since any soluble enzymes or substrates required for activation would presumably be diluted below threshold concentrations in the bath shortly after patch excision. We cannot conclude directly that the IP3-gated channels observed in cell-free patches are the same as the channels activated by odors in cell-attached recordings. Our findings that the unitary currents in both recording configurations have similar to values and that both the odor-evoked macroscopic current and the IP3-gated channels observed in cell-free patches can be blocked by RR argue that the IP3-gated channels observed in cell-free patches are a component of an IP3-mediated excitatory transduction cascade.
Finding channels on the soma of cultured ORNs that presumably function on the dendritic processes of these cells in situ would be consistent with the discovery that cAMP-gated channels that mediate excitation in the cilia of dissociated vertebrate ORNs also occur in low density on the dendrite and soma (Nakamura and Gold, 1987; Firestein et al., 1991). Whether the IP3-activated channels normally occur on the soma of lobster ORNs or are inserted there prior to relocation to the dendritic processes during neurite outgrowth in culture remains to be tested.
We conclude that IP3 gates 2 different channels rather than 1 channel with two different subconductance states, as has been reported for IP3-activated channels in canine cerebellum or aortic smooth muscle (Watras et al., 1991; Mayrleitner et al., 1991). Save for 1 patch, the large and small conductance channels occurred in different patches of membrane. Differences in the voltage dependencies and reversal potentials in symmetrical ionic conditions further support the conclusion that IP3 gates 2 different channels. Since two variably spliced mRNAs code for intracellular IP3 receptors (eg., mouse cerebellar IP3 receptor; Nakagawa et al., 1991), it would be consistent to have two subtypes of IP3-activated channels expressed in lobster ORNs.
The lack of identical recording conditions makes it difficult to compare the lobster olfactory channels with other reported IP3-activated channels based on their respective conductances (Ehrlich and Watras, 1988; Mayrleitner et al., 1991; Restrepo et al., 1990; Maeda et al., 1991), but the ability of RR and heparin to block the IP3-gated channels in lobster ORNs is similar to the pharmacological properties of Intracellular IP3 receptors. RR inhibits IP3-activated channels in skeletal muscle sarcoplasmic reticulum but not in aortic smooth muscle (Ehrlich and Watras, 1988). Heparin, which is thought to block the IP3-binding site (Supattapone et al., 1988), acts in a variety of cell types (Worley et al., 1987; Hill et al., 1987; Ghosh et al., 1988; Kobayashi et al., 1988; Komori and Bolton, 1990; Frank and Fein, 1991; Fisher et al., 1992). The reversibility of the heparin block that we observed with washing has also been reported in smooth muscle (Mayrleitner et al., 1991) and demonstrates that heparin, which is known to be cytotoxic at high concentrations (Frank and Fein, 1991), did not damage the membrane.
The IP3-activated channels in lobster ORNs differ from intracellular IP3 receptors in their ATP dependency. While ATP failed to alter the conductance or the Propen of the channels in lobster ORNs, it increases IP3-induced macroscopic currents in aortic smooth muscle sarcoplasmic reticulum (Mayrleitner et al., 1991) and the Propen of intracellular IP3-gated channels (Mayrleitner et al., 1991; Ehrlich and Watras, 1988). The ATP-driven Ca2+ pump postulated to be associated with the IP3 release mechanism in other, nonsensory cells (Ferris et al., 1990) may not be functional in sensory transduction and therefore not present or active in the lobster cells. Danoff et al. (1991) report mRNA coding for IP3 receptors in rat and human that lack the SII region of the channel protein that contains the ATP-binding sites. From our electrophysiological data alone, we cannot assign the apparent lack of modulation by ATP to protein variation, species differences, or olfaction.
The IP3-activated channels in lobster ORNs presumably share some structural homology with mammalian IP3 receptors, since an antibody raised against a cDNA clone of a mammalian IP3 receptor recognized a protein of similar size in the lobster. The molecular weight (>200K) of the immunolabeled band in lobster ORNs is consistent with the size of intracellular IP3 receptors, which are proposed to be a tetramer of noncovalently bound isomers with a characteristic M, of 260K by SDS-polyacrylamide gel electrophoresis analysis (Mignery et al., 1989; Fisher et al., 1992). That the antibody is binding to a functionally relevant molecule is suggested by the ability of the antibody to increase odor-evoked inward currents selectively. The ability of the antibody to increase the Propen and to of IP3-activated channels selectively argues further that the antibody targets the channel to augment the macroscopic current The antibody would not necessarily be expected to target the transmembrane pore and block the ion flow, as it was directed against the 19 amino acid carboxyl terminus of the receptor that DeCamilli et al. (1990) postulate is involved in Ca2+ sequestration. Indeed, a monoclonal antibody targeting a different epitope of the C-terminus has been shown recently to block IP3- and Ca2+-induced release of Ca2+ in fertilized hamster eggs, suggesting that the C-terminus can regulate channel gating (Miyazaki et al., 1992). Independent of mechanism, the ability of the antibody to selectively enhance odor-evoked inward currents independently of outward currents provides further support that IP3 is the excitatory second messenger.
We conclude that the IP3 receptors in cultured lobster ORNs are in the plasma membrane, since a high percentage of cell-free patches (63 of 86 successful seals) taken from the plasma membrane are directly activated by IP3 and an antibody directed against a known IP3 receptor alters IP3-activated unitary currents in cell-free patches of plasma membrane. It is not likely that organelles closely apposed to the plasma membrane would be frequently drawn into the patch. Even if this occurred, the plasma membrane would need to be disrupted in order to reseal onto the organelle, and the organelle membrane would need to be recorded in the outside-out configuration for IP3 to bind. Earlier reports differ on whether IP3 receptors occur in the plasma membrane of neurons. In studies of IP3 receptors in the cerebellum, Maeda et al. (1989) localized the P400 (IP3) receptor to the endoplasmic reticulum, postsynaptic densities, and the plasma membrane, while Ross et al. (1989) localized the receptor to the endoplasmic reticulum, sub-plasmalemmal cisternae, and nuclear membrane and not to the plasma membrane. Immunogold labeling with the same antibody used in this report failed to reveal an IP3 receptor in the plasma membrane of cerebellar neurons (Mignery et al., 1989). Yet, our conclusion is consistent with evidence emerging from studies of olfactory neurons. Khan et al. (1992) identified a 260 kd protein in the plasma membrane of rat olfactory cilia that stained with antiserum to a cerebellar IP3 receptor in Western blots. Kalinoski et al. (1992) photoaffinity labeled a 107 kd protein in the plasma membrane of catfish olfactory cilia that binds IP3. Restrepo et al. (1990) were able to reconstitute an IP3- activated channel from a membrane preparation of catfish olfactory cilia. To the extent that the membrane preparations used in these studies are purely ciliary, these findings argue for plasma membrane receptors, since the cilia of ORNs are devoid of organelles. Indeed, Cunningham et al. (1992, Chem. Senses, abstract) have preliminary evidence that an immunogold-labeled antibody directed against a cerebellar IP3 receptor labels rat ciliary membrane. The plasma membrane IP3 receptors in olfactory receptor cells may represent a new class of IP3 receptors that shares structural homology with intracellular IP3 receptors, but is localized to the plasma membrane.
Our ability to record IP3-activated unitary currents in native plasma membrane of lobster ORNs and to tie these unitary currents to odor-activated macroscopic inward currents directly implicates plasma membrane IP3 receptors in olfactory transduction. We conclude that odors excite lobster ORNs by increasing intracellular IP3, which activates IP3-gated channels in the plasma membrane of the cell and depolarizes the cell in a concentration-dependent manner. The IP3 mediated transduction pathway presumably coexists in some cells with a second, cAMP-mediated pathway that hyperpolarizes the cell and modulates the magnitude of excitation to reflect the particular ratio of excitatory and inhibitory odor components in an odor blend (Michel et al., 1991; Michel and Ache, 1992). Having two, parallel transduction pathways, one mediated by IP3 and the other by cAMP, allows the lobster ORN to serve as an integrating unit. The ability of olfactory receptor cells with parallel transduction pathways to integrate information about the composition of odor blends may be integral to odor coding, as suggested by Reed (1992), and may explain why odors activate both IP3- and cAMP-mediated second messenger pathways in animals as phylogenetically diverse as arthropods, fish, and mammals, as noted in the Introduction.
Experimental Procedures
Solutions
Panulirus saline (PS) consisted of 458 mM NaCl, 13.4 mM KCl, 9.8 mM MgCl2, 13.6 mM CaCl2, 13.6 mM Na2SO4, 3 mM HEPES, and 2 mM glucose (pH 7.4), Modified L-15 media consisted of 50 ml of Liebowitz L-15 stock, 50 ml of 1.6 times the normal concentration of PS, 0.6 g of dextrose, 0.026 g of L-glutamine, and 0.01% gentamicin. Phosphate sucrose buffer consisted of 10 mM Na2P04 and 250 mM sucrose (pH 7.3). Homogenization buffer consisted of 320 mM sucrose, 10 mM Tris base, 50 mM KCl, and 1 mM EDTA, Tris-buffered saline (TBS) consisted of 50 mM Tris-HCl, 150 mM NaCl (pH 7.5). Tween-TBS was made by adding 0.1% Tween-20 to TBS. Blotting buffer consisted of 25 mM Tris-HCl, 192 mM glycine 0.1% SDS, and 10% methanol, Normal patch solution consisted of 30 mM NaCl, 11 mM EGTA, 10 mM HEPS, 1 mM CaCl2, 180 mM potassium acetate, and 696 mM glucose (pH 7.0). RR (10 μm) and heparin (2.5 μm) were prepared fresh daily.
Odors were an aqueous extract of TET, a commercial flake fish food (Tetra Werke; Melle, FRG), and solutions of D-alanine, betaine, glycine, L-proline, and taurine, compounds known to be adequate stimuli for the chemoreceptors of aquatic organisms such as lobsters (Carr, 1988). A stock extract of TET was made by mixing 2 g of dry flakes in 60 ml of saline, centrifuging the resulting suspension at 1400 × g, filtering it through Whatman #3 filter paper, adjusting it to pH 7.4, and storing it frozen in 5 ml aliquots. The stock extract was thawed and diluted 1000-fold in either PS or culture media, as appropriate, just prior to use and applied at that concentration. The pure compounds were prepared fresh as 10−3 M solutions in either PS or culture media, as appropriate, adjusted to pH 7.4, as needed, and applied at that concentration.
All drugs and chemicals were obtained from Sigma Chemical Co., except for the culture media. Sources for the culture media and supplements are listed in Fadool et al. (1991).
Tissue Culture
Distinct clusters of ORNs were dissected from the olfactory organs (lateral antennular filaments) of adult specimens of the Caribbean spiny lobster Panulirus argus and enzymatically dissociated. The resulting cells were sustained in primary culture as described previously (Fadool et at., 1991). Briefly, the isolated clusters were incubated for 50 min at 80 rpm on an orbital shaker in 10 ml of PS containing 2.5 mg of papain, 12 mg of L-cysteine, 1 % penicillin, streptomycin sulfate, and amphotericin B (GIBCO), which had been 2.0 μm filter-sterilized. Proteolytic digestion was stopped by replacing the enzyme solution with low glucose L-15 medium supplemented with L-glutamine, dextrose, fetal calf serum, and basal minimal essential vitamins. Cells were immediately plated on poly-D-lysine-coated glass coverslips. Cells were maintained at saturation humidity in a modular incubator chamber (Billups-Rothenberg) at 24°C.
Electrophysiology
The cultured cells were viewed at 40x magnification with Hoffman optics for patch-clamp recording (Hamill et al., 1981). Patch electrodes, fabricated from 1.8 mm outer diameter borosilicate glass and fire-polished to a tip diameter of approximately 1.0 μm (bubble number 4.8; Mittman et al, 1987), produced seal resistances between 8 GΩ and 14 GΩ. The electrodes were un-coated. Signals were amplified with an integrating patch amplifier (Dagan 3900).
Macroscopic currents were recorded in the whole-cell configuration at a holding potential of −60 mV, unless noted otherwise, using normal patch solution in the pipette. The analog records were filtered at 5 kHz and digitally sampled every 4 ms (every 240 ms, in one noted instance) for subsequent processing on an IBM compatible computer using pCLAMP software (Axon Instruments). The compact, spherical shape of the cultured ceils (see Fadool et al., 1991) facilitated obtaining an effective space clamp on most cells. Odors and membrane permeant probes were “spritzed” on the cells for 120 ms from a six barrel glass micropipette coupled to a pressurized valve system (Picospritzer, General Valve). The concentrations of odors and probes delivered in this manner are reported as the pipette concentration; no attempt was made to correct for dilution. To compare the effect of impermeant probes on cell activity, cells were sequentially patched, first without, then with the probe in the pipette in order to provide an internal control There was no noticeable effect on the response of sequentially patched cells to odor or voltage stimulation in the absence of any experimental manipulation.
Unitary currents were recorded in either the cell-attached or (primarily) the cell-free, inside-out configuration. The normal patch-pipette solution was a potassium acetate patch solution (see solutions); both faces of the patch were exposed to symmetrical solutions. The analog records were filtered at 2 kHz and recorded on videotape. On playback, the records were sampled every 100 μs for processing on an IBM compatible computer, also using pCLAMP software (Axon Instruments). Probes were “spritzed” on the inner face of the patch for 550 ms from the multibarreled pipette mentioned above.
All results are presented as the mean ± standard error of the mean. Statistical significance was calculated at the 95% confidence level.
Immunochemistry
Mouse cerebellum was isolated then diced in chilled phosphate sucrose buffer. Thirty-six hour cultured ORNs were scraped with a rubber policeman, centrifuged below 1,000 rpm for 2 min at 4°C, and removed from media without disrupting the cells. Both tissues were individually homogenized in chilled homogenization buffer, either with 50 strokes by mortar and pestle (Wheaton, size B) or for 2 min by a Kontes tissue grinder in microcentrifuge tubes and then tip sonicated (Heat Systems, Ultrasonics Inc., W-220) three times for 15 s at a setting of 3 while iced. Homogenates were centrifuged twice at 5,000 rpm for 30 min at 4°C. The respective supernatants were combined and centrifuged (Beckman L70 Ultracentrifuge) at 38,000 rpm for 2.5 hr at 4°C. The recovered pellets were resuspended in homogenization buffer by bath sonification (Heat Systems, Ultrasonics Inc., W-225) three times for 15 s at a setting of 5 and frozen at −80°C until use.
Protein was determined by a Bradford photometric assay. Twenty to twenty-five micrograms of the membrane preparation was separated at constant current (25 mA; 3 hr) on a 5%–15% gradient SDS electrophoretic gel (0.55 mm; 29:1 acrylamide:bisa-crylamide). Proteins were transferred to nitrocellulose in a modified blotting buffer at 0.5 mA for 3.5 hr. The nitrocellulose membrane was soaked twice for 10 mm in Tween-TBS. Nonspecific binding was blocked by incubating the membrane for 30 min with 2.5% Carnation dry milk in Tween-TBS. The nitrocellulose membrane was then incubated for 15 hr at 4°C with a polyclonal antibody (1:200) raised against the 19 C-terminal amino acids of a cDNA clone of a mouse cerebellar IP3 receptor (Mignery et al., 1989), washed twice for 10 min in Tween-TBS to remove unbound antibody, and reincubated for 2 hr with a 1:250 dilution of peroxidase-conjugated goat anti-rabbit secondary antibody (Boehringer Mannheim) Gels were visualized with 4-chloro-1-naphthol color reagent, activated by 30% H2O2 in TBS and 20% methanol.
In control experiments, membrane preparations (750 μg of total protein) were separated with a continuous front, 8% gradient SDS electrophoretic gel. Proteins were transferred to nitrocellulose, as done previously, but were subsequently incubated with preabsorbed antibody. The antibody was preabsorbed by incubating it for 30 min on ice with 10:1, 1:1, 0.1:1, and 0.01:1, and 0:1 molar ratios of the peptide PCD6 (Mignery et al., 1989; the last 19 C-terminal amino acids) to antibody. Following incubation, the samples were centrifuged below 1,000 rpm for 30 min at 4°C to remove particulates and antibody-peptide complexes. The respective supernatants were applied as 200 μl samples using a miniblotter.
Acknowledgments
We thank Drs. Judith Drazba, Keith Elmsie, James Fadool, Stuart Firestein, Richard Horn, Steven Jones, Irwin Levitan, and William Michel for much helpful advice; Ms. Leslie Van Ekeris for technical assistance; Ms. Lynn Milstead and Mr. James Netherton for help in preparing the illustrations; and Drs, Barbara-Anne Battelle and Kevin Blair for critically reading the manuscript. We thank Dr. Pietro DeCamilli for supplying the mammalian IP3 receptor antibody and Dr. Richard Bruch for supplying immunopurified Golf antibody. This work was supported by the Office of Naval Research (N0014-90-J-1566), the National Institutes of Health (1 R01 DC01655-01), the National Insitute of Mental Health National Research Service Award (1F31MH10124-01A1), and a scholarship from the Society for General Physiology.
References
- Anholt RRH. Odor recognition and olfactory transduction: the new frontier. Chem Senses. 1991;16:421–427. [Google Scholar]
- Berridge MJ. Inositol trisphosphate, calcium, lithium, and cell signaling. JAMA. 1989;262:1834–1841. [PubMed] [Google Scholar]
- Boekhoff I, Tareilus E, Strotmann J, Breer H. Rapid activation of alternative second messenger pathways in olfactory cilia from rats by different odorants. EMBO J. 1990;9:2453–2458. doi: 10.1002/j.1460-2075.1990.tb07422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breer H, Boekhoff I. Odorants of the same odor class activated different second messenger pathways. Chem Senses. 1991;16:19–29. [Google Scholar]
- Breer H, Boekhoff I, Tarelius E. Rapid kinetics of second messenger formation in olfactory transduction. Nature. 1990;344:65–68. doi: 10.1038/345065a0. [DOI] [PubMed] [Google Scholar]
- Carr WES. The molecular nature of chemical stimuli in the aquatic environment. In: Atema J, Fay RR, Popper AN, Tavolga WN, editors. Sensory Biology of Aquatic Animals. New York: Springer-Verlag; 1988. pp. 3–27. [Google Scholar]
- Danoff SK, Ferris CD, Donath C, Fischer GA, Munemitsu S, Ullrich A, Snyder SH, Ross CA. Inositol 1,4,5-trisphosphate receptors: distinct neuronal and nonneuronal forms derived by alternative splicing differ in phosphorylation, Proc Natl. Acad Sci USA. 1991;88:2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCamilli P, Takei K, Mignery GA, Südhof TC. InsP3 receptor turnaround. Nature. 1990;344:495. doi: 10.1038/344495a0. [DOI] [PubMed] [Google Scholar]
- Dionne VE. Chemosensory responses in isolated olfactory receptor neurons from Necturus maculosus, J. Gen. Physiol. 1992;99:415–433. doi: 10.1085/jgp.99.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988;336:583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Fadool DA, Michel WM, Ache BW. Sustained primary culture of lobster (Panulirus argus) olfactory receptor neurons. Tissue Cell. 1991;23:719–732. doi: 10.1016/0040-8166(91)90025-o. [DOI] [PubMed] [Google Scholar]
- Fadool DA, Michel WM, Ache BW. Odor sensitivity of cultured lobster olfactory receptor neurons is independent of process formation. J Exp Biol. 1993 doi: 10.1242/jeb.174.1.215. in press. [DOI] [PubMed] [Google Scholar]
- Ferris CD, Snyder SH. Inositol 1,4,5-trisphosphate-activated calcium channels. Annu Rev Physiol. 1992;54:469–488. doi: 10.1146/annurev.ph.54.030192.002345. [DOI] [PubMed] [Google Scholar]
- Ferris CD, Huganir RL, Snyder SH. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles is allosterically regulated by adenine nucleotides. Proc Natl Acad Sci USA. 1990;87:2147–2151. doi: 10.1073/pnas.87.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S. A noseful of odor receptors. Trends Neurosci. 1991;14:270–272. doi: 10.1016/0166-2236(91)90135-h. [DOI] [PubMed] [Google Scholar]
- Firestein S, Zufall F, Shepherd GM. Single odor-sensitive channels in olfactory receptor neurons are also gated by cyclic nucleotides. J Neurosci. 1991;11:3565–3572. doi: 10.1523/JNEUROSCI.11-11-03565.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SK, Heacock AM, Agranoff BW. Inositol lipids and signal transduction in the nervous system: an update. J Neurochem. 1992;58:18–38. doi: 10.1111/j.1471-4159.1992.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Frank TM, Fein A. The role of the inositol phosphate cascade in visual excitation of invertebrate microvillar photoreceptors. J Gen Physiol. 1991;97:697–723. doi: 10.1085/jgp.97.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh TK, Els PS, Mullaney JM, Ebert CL, Gill DL. Competitive, reversible and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988;263:11075–11079. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth F. Improved patch-clamp techniques for high resolution current recordings from cells and cell free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hill TD, Berggren PO, Boynton AL. Heparin inhibits inositol trisphosphate-induced calcium release from permeabilized rat fiver cells. Biochem Biophys Res Commun. 1987;749:897–901. doi: 10.1016/0006-291x(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Huque T, Bruch RC. Odorant- and guanine nuclcotide-stimulated phosphoinositide turnover in olfactory cilia. Biochem Biophys Res Commun. 1986;137:36–42. doi: 10.1016/0006-291x(86)91172-1. [DOI] [PubMed] [Google Scholar]
- Kalinoski DL, Aldinger SB, Boyle AG, Huque T, Marecek JF, Prestwich GD, Restrepo D. Characterization of a novel inositol 1,4,5-trisphosphate receptor in isolated olfactory cilia. Biochem J. 1992;281:449–456. doi: 10.1042/bj2810449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Steiner JP, Snyder SH. Plasma membrane inositol 1,4,5-trisphosphate receptor of lymphocytes: selective enrichment in sialic acid and unique binding specificity. Proc Natl Acad Sci USA. 1992;89:2849–2853. doi: 10.1073/pnas.89.7.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Somlyo AV, Somlyo AP. Heparin inhibits the inositol 1,4,5-trisphosphate-dependent, but not independent, calcium release induced by guanine nucleotide in vascular smooth muscle. Biochem Biophys Res Commun. 1988;153:625–631. doi: 10.1016/s0006-291x(88)81141-0. [DOI] [PubMed] [Google Scholar]
- Komori S, Bolton TB. Inositol trisphosphate releases stored calcium to block voltage-dependent calcium channels in single smooth muscle cells. Pflügers Arch. 1990;418:437–441. doi: 10.1007/BF00497770. [DOI] [PubMed] [Google Scholar]
- Kuno M, Gardener P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987;326:301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Maeda N, Niinobe M, Inoue Y, Mikoshiba K. Developmental expression and intracellular location of P400 protein characteristic of Purkinje cells in the mouse cerebellum. Dev Biol. 1989;133:67–76. doi: 10.1016/0012-1606(89)90297-2. [DOI] [PubMed] [Google Scholar]
- Maeda N, Kawasaki T, Nakade S, Yokota N, Taguchi T, Kasai M, Mikoshiba K. Structural and functional characterization of inostiol 1,4,5-trisphosphate receptor channel from mouse cerebellum. J Biol Chem. 1991;266:1109–1116. [PubMed] [Google Scholar]
- Mayrleitner M, Chadwick CC, Timerman AP, Fleischer S, Schindler H. Purified IP3 receptor from smooth muscle forms an IP3 gated and heparin sensitive Ca2+ channel in planar bilayers. Cell Calcium. 1991;12:505–514. doi: 10.1016/0143-4160(91)90032-a. [DOI] [PubMed] [Google Scholar]
- McClintock TS, Ache BW. Hyperpolarizing receptor potentials in lobster olfactory receptor cells: Implications for transduction and mixture suppression. Chem Senses. 1999;14:637–647. [Google Scholar]
- Michel WM, Ache BW. Cyclic nucleotides mediate an odor-evoked potassium conductance in lobster olfactory receptor cells. J Neurosci. 1992 doi: 10.1523/JNEUROSCI.12-10-03979.1992. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel WC, McClintock TS, Ache BW. Inhibition of lobster olfactory receptor cells by an odor-activated potassium conductance. J Neurophysiol. 1991;65:446–453. doi: 10.1152/jn.1991.65.3.446. [DOI] [PubMed] [Google Scholar]
- Mignery GA, Südhof TC, Takei K, DeCamilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- Mittman SC, Flaming DG, Copenhagen DR, Belgum JH. Bubble pressure measurement of micropipette tip outer diameter. J Neurosci Meth. 1987;22:161–166. doi: 10.1016/0165-0270(87)90010-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakada S, Mikoshiba K. Block of Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257:251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Okano H, Furuichi T, Aruga J, Mikoshiba K. The subtypes of the mouse inositol 1,4,5-trisphosphate receptor are expressed in a tissue-specific and developmentally specific manner. Proc Natl Acad Sci USA. 1991;88:6244–6248. doi: 10.1073/pnas.88.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Gold GH. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Penner R, Matthews G, Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988;334:499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Reed RR. Signaling pathways in odorant detection. Neuron. 1992;8:205–209. doi: 10.1016/0896-6273(92)90287-n. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Miyamoto T, Bryant BP, Teeter JH. Odor stimuli trigger influx of calcium into olfactory neurons of the channel catfish. Science. 1990;249:1166–1168. doi: 10.1126/science.2168580. [DOI] [PubMed] [Google Scholar]
- Ross CA, Meldolesi J, Milner TA, Satoh T, Supattapone S, Snyder SH. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989;339:468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Supattapone S, Worley PF, Baraban JM, Snyder SH. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988;263:1530–1534. [PubMed] [Google Scholar]
- Viven J, Coronado R. Opening of dihydropyridine calcium channels in skeletal muscle membranes by inositol tris-phosphate. Nature. 1988;336:587–589. doi: 10.1038/336587a0. [DOI] [PubMed] [Google Scholar]
- Watras J, Bezprozvanny I, Ehrlich BE. Inositol 1,4,5-trisphosphate-gated channels in cerebellum: presence of multiple conductance states. J Neurosci. 1991;11:3239–3245. doi: 10.1523/JNEUROSCI.11-10-03239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley PF, Baraban JM, Colvin JS, Snyder SH. Inositol trisphosphate receptor localization in brain: variable stoichiometry with protein kinase C. Nature. 1987;325:159–161. doi: 10.1038/325159a0. [DOI] [PubMed] [Google Scholar]