Abstract
The feasibility of a family-based clinic-integrated behavioral intervention to improve family management of type 1 diabetes was evaluated. In each of four clinical sites, 30 to 32 families (122 total) were randomized to intervention or usual care comparison group. The WE*CAN intervention, based on family problem-solving methods, was delivered during 3 routine clinic visits by trained “Health Advisors”. Of eligible families across the four sites, 83% agreed to participate, of whom 96% completed the baseline, mid-term, and post-intervention assessments. Families participated in an average of 2.85 intervention sessions over an 8-month period. The intervention was integrated into the clinic setting without impairing clinic flow, and was implemented with fidelity and consistency across sites by trained non-professionals. The findings provide evidence of the feasibility of conducting a multi-site trial to evaluate the effects of a clinic-integrated problem-solving intervention to improve family management. Many lessons were learned that provide guidance for recruitment, measurement, and intervention for the larger clinical trial.
Keywords: diabetes mellitus, type 1, clinical trial, child, adolescent, family
INTRODUCTION
The management of type 1 diabetes requires daily management behaviors, including frequent blood glucose monitoring, multiple insulin doses, regulation of carbohydrate intake, regular exercise, insulin and carbohydrate adjustments to moderate blood glucose fluctuations, and other lifestyle adaptations to prevent short-term and long-term complications (1). Despite substantial commitment of resources dedicated to clinical management, disease management and control decline during adolescence, increasing the risk of short-term and long-term medical complications (2,3).
Adolescence is often a particularly troublesome period as youth and their parents must deal with developmental issues and hormonal changes that can complicate disease management (4,5). Effective diabetes management depends greatly on family involvement and adaptation to the demands of the disease (6–8). Appropriate parent involvement in disease management is positively associated with adolescent adjustment, adherence, and control (9,10). Difficulties in the transition of responsibility sharing between parents and adolescents can result in poor adherence, conflict, and disease complications (2,3,11,12). Therefore, it is timely to intervene during late childhood and early adolescence to optimize family management practices.
Research on behavioral interventions for youth with type 1 diabetes and their parents has demonstrated improvements in treatment adherence (11), quality of life (9,11,13), coping skills (13–15), parent-adolescent communication (9,10,16), and glycemic control (11,17–19). Much of the research to date has been conducted as single-site trials of approaches that focus on specific sub-populations and/or use intervention methods that are separate from routine clinical care. Such research provides an important foundation; however, there remains a need to develop effective approaches that could be broadly generalized in clinical practice. Such a trial would optimally be conducted in multiple sites, with an intervention approach that is integrated into routine clinical practice (20) and grounded in developmental (12,21) and family (16,19) theories. However, a multi-site study poses a number of difficult challenges, including coordinating within varying busy clinical settings, determining appropriate staffing, training assessment and intervention staff across multiple sites, conducting thorough assessments, developing an intervention approach that can be flexibly applied depending on families varying needs, and achieving consistency in implementation across differing clinical environments.
Given these substantial challenges, the investigators determined that a feasibility study would provide crucial data to inform the development of a subsequent larger and longer duration multi-site clinical trial evaluating a clinic-integrated, family-focused behavioral intervention to improve family management of type 1 diabetes during late childhood and early adolescence. The purpose of this paper is to report the methods and lessons learned from this pilot study, conducted to determine the feasibility of the Family Management of Childhood Diabetes (FMOD) multi-site clinical trial. Findings are presented regarding the feasibility of (1) recruitment; (2) measurement; and (3) intervention.
METHODS
Study Design and Participant Recruitment
At each of four major medical centers, 30 to 32 families (total of 122) meeting the eligibility criteria were recruited and randomized into intervention or usual care groups. Patient eligibility requirements included the following: age 9.0 to 14.5 years; diagnosed with type 1 diabetes at least 1 year requiring an insulin dose of > 0.5 u/kg/day with an A1c of less than 13.0%; no other major chronic diseases or psychological problems; able to read and write in English; not involved in competing trials; residing within a 90-minute drive of the clinic; and having one adult caregiver, not currently under treatment for substance abuse or hospitalized for psychological problems in the past six months, who agreed to participate. Parents provided consent and youth provided assent according to approved human subject procedures. Survey and interview data were collected during home visits at baseline and post-intervention and by telephone at mid-point. Parents and children were each provided incentives of $25 for each home assessment and $10 for the telephone assessment, $5 per visit for bringing their blood glucose meter(s), appointment making assistance and appointment reminders, and parking vouchers (in sites with paid parking). The intervention was to be delivered at 3 routine clinic visits over a maximum of 12 months.
Treatment Conditions
The goal was to develop an intervention based on behavioral principles that would be delivered with fidelity by well-trained college-educated research assistants, implemented with consistency across multiple sites and interventionists coincident with routine diabetes clinic visits, and experienced by families as a positive experience.
WE*CAN Intervention
The development of the intervention was guided by the recognition that diabetes outcomes (glycemic control, treatment adherence, quality of life, and mental health) are influenced in part by both parent and child factors. Accordingly, the goal was to develop an intervention that could be integrated into routine clinic visits and that would facilitate appropriate family responsibility sharing, minimize conflict, and improve problem solving.
Problem solving approaches have been usefully employed to facilitate resolution of a range of complex life issues. Problem solving for goal attainment is central to social cognitive theory (22), which posits that goal attainment is determined in part by outcome and efficacy expectations and can be modified through skill development, experience, and reinforcement. Because consistent diabetes management behavior does not always result in optimal glycemic control, outcome and efficacy expectations can decline and undermine motivation and behavior. Further, many normal life issues during the transition to adolescence create obstacles to optimal management. Problem solving provides a useful structure for facilitating self-regulatory behavior (23), helping families identify the barriers and facilitators that influence day-to-day diabetes management, enhancing motivation, and increasing skills in overcoming barriers (24).
Based on these concepts, the WE*CAN structure (Table 1) was developed: W - Work together to set goals; E - Explore possible barriers and solutions; C - Choose the best solutions; A - Act on your plan; N - Note the results. The goal of WE*CAN is to improve family management of diabetes, including domains of blood sugar monitoring, insulin administration, diet, physical activity, and management of blood sugar excursions. The specific objectives of the intervention are to (1) improve disease management problem solving; (2) improve parent-child cooperation and communication and reduce conflict regarding disease management; and (3) facilitate appropriate sharing of disease management responsibility. WE*CAN provides a simple structure with wide applicability to many diabetes management issues, and allows for a flexible, individualized approach because the problem-solving process can be applied to the area(s) most pertinent to each family. It facilitates effective family collaboration to identify difficulties, develop and evaluate solutions, examine the results of their behavior, and revise future actions to obtain better outcomes.
Table 1.
WE*CAN intervention structure and materials
| WE*CAN PROBLEM SOLVING ACRONYM | |
|
| |
| W | Work together to set goals |
| E | Explore possible barriers and solutions |
| C | Choose the best solutions |
| A | Act on your plan |
| N | Note the results |
|
| |
| WE*CAN INTERVENTION PROCESS | |
|
| |
| Phase | Activities |
|
| |
| Preparation | HA calls family
|
|
| |
| Action | During clinic visit, HA works with parent and youth together on the development and implementation of their diabetes management plan
|
|
| |
| Follow Up | HA contacts family 2 and 6 weeks after clinic visit via mail, telephone, or internet
|
|
| |
| WE*CAN HANDOUTS | |
|
| |
| WE*CAN Problem Solving (description of the steps of the WE*CAN problem solving process) | |
|
| |
| “Blood Sugar Monitoring: A Tool, Not a Test” (effective use of blood glucose monitoring) | |
|
| |
| “Family Communication” (communicating about diabetes management and blood sugars) | |
|
| |
| “Ups and Downs” (parent-child communication around out of range blood sugars) | |
|
| |
| “Sharing the Load” (developmentally appropriate sharing of responsibility) | |
|
| |
| “Who’s Business Is It?”(managing child desire for autonomy; determining responsibility) | |
|
| |
| “Making Sure Helping is Helpful” (avoiding miscarried helping) | |
|
| |
| “Managing Conflict” (using WE*CAN to manage conflict) | |
|
| |
| “Stress Management” (understanding and using WE*CAN to deal with stress) | |
|
| |
| “Managing Diabetes Burnout” (understanding and using WE*CAN to deal with burnout) | |
|
| |
| “Diabetes and School” (obtaining needed support at school) | |
|
| |
| “Checking Your Blood Sugar Before Taking Insulin” | |
|
| |
| “Best Timing of Shots or Boluses” | |
Intervention Delivery
Health Advisors (HA), specially trained college graduates, were responsible for delivery of the three WE*CAN components of preparation, action, and follow up, outlined in Table 2. Provisions of the intervention by persons not currently part of the health care team allowed implementation of the intervention to be free from influence by previous interactions or perceptions, and guarded against potential contamination that could occur if existing providers acted as interventionists. Reliance on non-mental health professionals was guided by a desire to maximize feasibility and minimize costs of intervention delivery. Preparation – a week prior to the clinic visit the HA contacted the family by telephone, reminded them of their clinic appointment, and assisted them in preparing for the scheduled visit. Action – during the clinic visit the HA met with the parent and child to (1) identify areas of difficulty or conflicts with respect to diabetes management and set a specific goal to improve management; (2) facilitate family motivation to address the targeted area of difficulty; (3) facilitate adaptive communication, problem solving, and developmentally appropriate sharing of diabetes responsibility; and (4) develop a plan to be implemented over the next several months. Families determined the area of diabetes management most salient for current problem solving efforts. The HA facilitated family discussions about goal selection and provided guidance through the steps of the problem-solving process, using worksheets designed for this purpose. Supplementary handouts addressing common issues such as communication and conflict were employed as needed. At the first intervention clinic visit, families were encouraged to select a relatively simple goal, such as carrying fast-acting carbohydrates, in order to learn the problem solving process, and then move to more difficult goals in subsequent sessions. However, each family was free to choose the goal area they most wanted to address at each visit. Follow up –the HA contacted the families via telephone 2 weeks and 6 weeks after the clinic visit to discuss and facilitate progress on their plan, identify issues or barriers, provide suggestions and encouragement, and facilitate revision of the plan if needed. HAs received both local and central training, and participated in monthly conference calls led by the investigators designed to resolve intervention issues and improve fidelity to the intervention. Each HA was responsible for administering the study protocol to a minimum of 15 families.
Table 2.
Schedule of assessment and treatment group contacts
| Time Frame | Assessment | Control Contacts | Intervention Contacts |
|---|---|---|---|
| Within 4 weeks prior to first study clinic visit | Baseline in-home assessment | ||
|
| |||
| Within 1 week prior to clinic visit 1 | Pre-visit telephone 1 | Pre-visit telephone 1 | |
| Family’s regularly- scheduled clinic visit | Clinic visit 1 | Clinic Visit 1 | |
| 2 to 3 weeks after clinic visit 1 | 2-week follow-up telephone 1 | ||
| 6 to 8 weeks after clinic visit 1 | 6-week follow-up telephone 1 | ||
|
| |||
| Within 1 week prior to clinic visit 2 | Pre-visit telephone 2 | Pre-visit telephone 2 | |
| Next scheduled clinic visit* | Clinic visit 2 | Clinic Visit 2 | |
| 2 to 3 weeks after clinic visit 2 | 2-week follow-up telephone 2 | ||
| 6 to 8 weeks after clinic visit 2 | 6-week follow-up telephone 2 | ||
|
| |||
| Within 4 weeks after clinic visit 2 | Mid-point telephone assessment* | ||
|
| |||
| Within 1 week prior to clinic visit 3 | Pre-visit telephone 3 | Pre-visit telephone 3 | |
| Next scheduled clinic visit | Clinic visit 3 | Clinic Visit 3 | |
| 2 to 3 weeks after clinic visit 3 | 2-week follow-up telephone 3 | ||
| 6 to 8 weeks after clinic visit 3 | 6-week follow-up telephone 3 | ||
|
| |||
| Within 3 weeks after clinic visit 3 | Follow-up in-home assessment | ||
If participants did not attend a second clinic visit within 7 months of their first study clinic visit, they were considered to have “missed” clinic visit 2 and mid-point telephone assessment; their subsequent clinic visit was considered clinic visit 3 and followed by the in-home assessment.
Usual Care Comparison
Families assigned to the usual care group received standard medical care, participated in measurement, and received clinic preparation and administrative assistance and attention from the HAs, as shown in Table 2. HAs contacted the usual care group during a pre-clinic visit telephone call to remind them about their appointment and met with the family during the clinic visit to give incentive items and address any study-related administrative issues. Following completion of the pilot study, families received a notebook containing the WE*CAN intervention materials and a summary of the problem-solving process.
Measurement
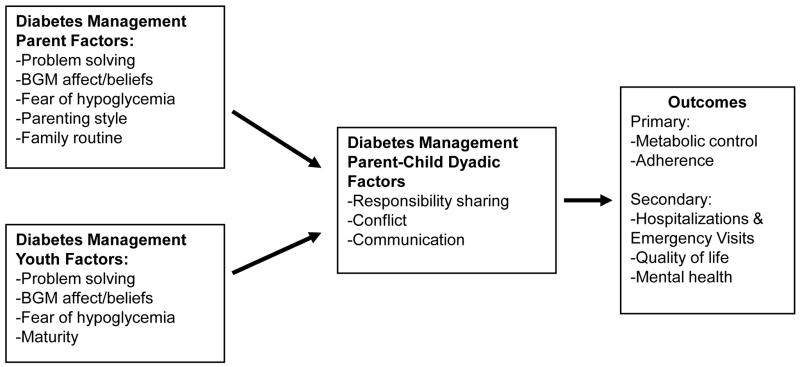
The selection of measures was based on the study goals, as shown in Figure 1. Accordingly, the diabetes outcomes of metabolic control, adherence, quality of life, mental health, and medical events would be expected to improve as a function of favorable parent-child collaboration and communication around diabetes management. These dyadic factors are the product of individual parent and youth behaviors, beliefs, attitudes, and capabilities.
Figure 1.
FMOD study conceptualization of parent and child diabetes management factors in relationship to outcomes
Except for biomedical data, which was obtained from medical records reviews and by interview during clinic visits, data collection occurred at home visits at baseline and follow-up by trained interviewers not employed by the clinic. An abbreviated telephone assessment consisting of measures of adherence, conflict, and family responsibility was conducted at study mid-point. During the study, some scales were eliminated from the home assessment to reduce participant burden and shorten the length of assessment.
Contextual Variables
Relevant demographic variables including age, grade, sex, age of diagnosis, family composition, parental education, household income, race, and ethnicity were collected at baseline.
Patient Records/Biomedical Data
Blood samples were obtained by finger-stick and shipped to a central laboratory for A1c assay (Tosoh Medics, Foster City, CA, reference range 4–6%). Additional biomedical data were collected at each clinic visit from medical records and interviews with families, including diabetes management regimen (e.g., insulin delivery modality, insulin types, doses, and schedule), hospitalizations, ER visits, and episodes of hypoglycemia requiring treatment assistance.
Behavioral Data
Adherence was assessed by the modified 24-item versions of the Diabetes Self Management Profile (DSMP; 25), with separate versions for children using conventional fixed dose or flexible regimens (26). At baseline and each quarterly clinic visit, the past two weeks of data from patients’ home glucose meter were downloaded to determine frequency of blood glucose monitoring per day. Children and parents completed the PedsQL Core Generic Module and Diabetes Module (27,28), Diabetes Family Responsibility Questionnaire (9), and Diabetes Family Conflict Scale (29). Additional measures employed, but not reported in this analysis include measures of each of the parent and youth factors depicted in Figure 1, as well as a videotaped family communication sample. Initially, the depression subscale of the Beck Youth Inventory (30) was administered (n=66); however some of the younger participants experienced difficulties with comprehension of the measure; and the investigators decided to change the protocol to use of the Children’s Depression Inventory (31) to determine whether this measure should be used in the main study. Due to this change, however, findings related to child depression cannot be analyzed for this study. Scores over the clinically-indicated cut-points for either instrument resulted in provision of a mental health referral (n=20); referred families remained in the study.
Process Measures
Process data were collected on the extent of intervention delivery. Intervention sessions were audio taped and coded to assess protocol adherence. One investigator at each site listened to a sample of four audio-taped sessions and used a standard form to evaluate the fidelity of intervention delivery across 21 session content and interaction domains, with each domain rated as not completed, partially completed, or fully completed. Records of the content and issues or problems associated with each intervention contact were recorded by the HAs, along with a subjective rating of the family’s level of engagement in the session. Intervention group participants completed measures of satisfaction with the intervention.
Analyses
The goal of the pilot study was to assess feasibility of the research methods and intervention approach across centers; we did not hypothesize differences in outcomes between treatment groups given the abbreviated nature of the intervention provided. Thus, the analyses describe the following: (1) recruitment and retention; (2) data collection and measurement; (3) the extent and quality of intervention implementation; (4) family satisfaction with the intervention; (5) performance of study outcomes; and (6) diabetes-related events.
RESULTS
Recruitment and Retention
Of 328 families reviewed for recruitment, 135 did not meet eligibility criteria, primarily due to being recently diagnosed, requiring insufficient insulin dose, less than 2 visits in the past 12 months, parent not literate in English, child not in a geographically stable home, presence of another major health problem, or diabetes diagnosis not definitively type 1. Of 193 eligible families, 167 were approached; 35 declined and 132 (83%) consented. The 35 who declined were not significantly different from those who agreed to participate on HbA1c, age, gender, ethnicity, diabetes regimen, or duration of diabetes.
Of the 132 who consented, 122 (92%) completed baseline assessments (91%–97%/site); of these, 119 (97.5%) completed the mid-point telephone assessment, and 117 (95.9%) completed the final home visit. Fifty-eight of the 60 (96.7%) families randomized to WE*CAN and 58 of 62 (93.5%) randomized to the usual care condition completed all assessments.
There were no significant differences between the intervention and comparison groups on any of the primary demographic variables or on baseline HbA1c. For the total sample, the average age was 11.5 years, age at diagnosis was 6.7 years, and mean A1c was 8.43%. The study participants were 71.1% white, 9.9% Hispanic, 11.6% Black, and 7.4% other race. Most participants (91.1%) were from families with two or more adults in the home, 45.4% of parents had a college degree, 77.4% of families reported an annual income was of $50,000 or greater.
Measurement Feasibility
The time required for parents and children to complete the baseline assessment was 124 (range = 75 to 225) minutes. The assessment battery was determined to be too lengthy, and modifications were made for subsequent assessments. The mid-point telephone assessment was 26 minutes (range = 16- to 45) and the final home assessment 103 minutes (64 to 176). Interviewer assessments during home visits of child performance regarding ability to understand questions; cooperation, and interest were generally high, ranging from 5.7 to 6.8 on a 7.0 point scale, with average standard deviations of about 1.0 in all cases.
Data from blood glucose meters were obtained from 87.6% of participants at the first clinic visit, 95.8% at the second visit, and 93.0% at the third visit. Children for whom meter data were not obtained demonstrated significantly higher HbA1c values than those for whom data were obtained (visit 1 8.3% versus 9.4%, p=.003; visit 2 8.4% versus 10.3%, p=.004; visit 3 8.5% versus 10.4%, p=.001).
Intervention Implementation and Fidelity
During the study, the usual care group participants averaged 2.70 clinic visits and the intervention group participants averaged 2.85 visits (p=ns). HA ratings of participant involvement in the sessions indicated that participating caregivers were “completely” or “somewhat” involved, except one caregiver at one session who appeared distracted and inattentive. The most frequently chosen goals were blood sugar monitoring (25%), healthy eating (23%), and remembering to carry fast acting carbohydrates (20%). Older children, those with higher A1C values (HbA1C > 8.3%), and those reporting greater conflict at baseline chose blood sugar monitoring most frequently. Evaluation of 16 audio-taped sessions indicated that HA’s either fully completed or partially completed each of the 21 specified session content and interaction domains excepting 3 sessions in which no supplementary handout was provided. (However, handouts were considered optional, to be used as applicable.)
Satisfaction with intervention
Satisfaction with participation in WE*CAN is reported in Table 3. Over 91% of youth and 97.7% of parents agreed or strongly agreed that the HA “helped us learn new ways to solve problems; 88.8% of youth and 95.5% of parents agreed or strongly agreed that “… the solutions we came up with worked well”; 97.7% of youth and 93.4% of parents agreed or strongly agreed with the statement, “overall, I liked being in WE*CAN”; 93.2% of youth and 82.2 of parents disagreed or strongly disagreed with the statement, “This program took too much of our time”.
Table 3.
Satisfaction with intervention reported by child and parent after final assessment (n= 48)
| Questionnaire Item | Child Percent (%) | Parent Percent (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| SD | DA | A | SA | SD | DA | A | SA | |
| 1. The telephone calls from my Health Advisor before our diabetes clinic visits helped me to prepare for the visit. | 2.2 | 4.4 | 75.6 | 17.8 | 0.0 | 6.7 | 73.3 | 20.0 |
| 2. The calls and/or emails between visits helped me remember appointments and other issues. | 4.4 | 6.7 | 66.7 | 22.2 | 0.0 | 4.6 | 72.7 | 22.7 |
| 3. Completing the WE*CAN worksheet was helpful | 2.3 | 4.6 | 63.6 | 29.6 | 0.0 | 4.4 | 64.4 | 31.1 |
| 4. The WE*CAN steps were easy to remember | 0.0 | 11.1 | 64.4 | 24.4 | 0.0 | 8.9 | 71.1 | 20.0 |
| 5. The goals we worked on were important to me | 0.0 | 2.2 | 62.2 | 35.6 | 2.2 | 2.2 | 57.8 | 37.8 |
| 6. My Health Advisor listened to what I had to say | 0.0 | 2.2 | 44.4 | 53.3 | 0.0 | 0.0 | 46.7 | 53.3 |
| 7. The WE*CAN program did not teach me anything new. | 31.1 | 44.4 | 22.2 | 2.2 | 17.8 | 66.7 | 8.9 | 6.7 |
| 8. The Health Advisor made sure I got a chance to talk during our sessions. | 0.0 | 0.0 | 51.1 | 48.9 | 0.0 | 0.0 | 57.8 | 42.2 |
| 9. The Health Advisor helped us learn new ways to solve problems. | 0.0 | 2.2 | 64.4 | 33.3 | 0.0 | 8.9 | 64.4 | 26.7 |
| 10. The solutions we came up with during the clinic visit worked well | 0.0 | 4.4 | 73.3 | 22.2 | 0.0 | 11.1 | 64.4 | 24.4 |
| 11. This WE*CAN program has helped our family handle diabetes issues better | 0.0 | 6.7 | 64.4 | 28.9 | 0.0 | 11.1 | 75.6 | 13.3 |
| 12. At home, we often use things that we learned in the sessions | 0.0 | 11.1 | 66.7 | 22.2 | 2.3 | 9.1 | 72.7 | 15.9 |
| 13. This program took too much of our time. | 40.0 | 42.2 | 13.3 | 4.4 | 20.5 | 72.7 | 2.3 | 4.6 |
| 14. Overall, I liked being in WE*CAN. | 0.0 | 6.7 | 57.8 | 35.6 | 0.0 | 2.3 | 56.8 | 40.9 |
SD = strongly disagree; DA = disagree; A = Agree; SA = strongly agree.
Performance of Outcome Measures
Table 4 shows the baseline and follow up data for the outcome measures and other key variables by treatment group. Mean HbA1c increased from baseline to final assessment by an average of 0.3% in both groups. There were also minor declines in the frequency of blood sugar monitoring, conflict, and family responsibility sharing. No treatment group differences were observed; however, none were expected because the study purpose was to test feasibility, the intervention was in development, and a non-therapeutic dose of the intervention was delivered.
Table 4.
Child and parent outcome measure baseline properties, and means and standard deviations at baseline and follow-up by group (n=117)
| Variable | Control Baseline Mean (SD) | Control Follow up Mean (SD) | Treatment Baseline Mean (SD) | Treatment Follow up Mean (SD) |
|---|---|---|---|---|
| CHILD | ||||
| HbA1c from Central Lab | 8.3 (1.3) | 8.6 (1.2) | 8.5 (1.4) | 8.8 (1.9) |
| Adherence DSMP | 59.6 (8.5) | 60.9 (9.3) | 60.8 (10.7) | 61.1 (10.7) |
| Adherence DSMP – Conventional (n= 34, items= 25) α = 0.68, x= 57.2, SD= 9.96 | 56.4 (9.3) | 57.6 (8.5) | 58 (10.9) | 57.9 (8.4) |
| Adherence DSMP – Flexible (n= 71, items=25) α = 0.68, x=61.6, SD= 9.19 | 61.2 (7.8) | 61.8 (9.4) | 62 (10.5) | 62.1 (11.2) |
| BGM Frequency (n= 102) x= 4.52, SD=1.87 | 4.7 (2.0) | 4.0 (1.9) | 4.4 (1.7) | 4.1 (1.6) |
| Generic QOL (n= 121, items=23), α =0.88, x= 79.7, SD= 12.1 | 79.8 (12.8) | 81.2 (12.2) | 79.5 (11.4) | 81.9 (12.7) |
| Diabetes-Specific QOL (n= 121, items=11) α = 0.75, x= 62.8, SD= 13.2 | 60.7 (13.2) | 61.4 (10) | 64.9 (13) | 63.1 (14.3) |
| Parent-Child Conflict (n= 120, items=15) α = 0.95, x= 26.7, SD= 13.7 | 27.8 (14.7) | 26 (13.2) | 25.6 (12.6) | 22.4 (7.8) |
| Family Responsibility Sharing (n= 121, items=17) α = 0.76, x= 33.8, SD= 4.89 | 33.4 (4.8) | 32.7 (4.7) | 34.1 (5) | 33.2 (4.3) |
| PARENT | ||||
| Adherence DSMP | 59.9 (11.7) | 61 (11.7) | 60.4 (10.4) | 59.9 (11.5) |
| Adherence DSMP - Conventional (n= 40, items=25) α = 0.66, x= 57.9, SD= 10.1 | 57.7 (9.5) | 59.7 (10.9) | 58.1 (10.9) | 54.5 (12.1) |
| Adherence DSMP - Flexible (n= 81, items=25) α = 0.76, x= 61.3, SD= 11.4 | 61 (12.6) | 61.4 (12) | 61.6 (10.1) | 61.7 (10.9) |
| Diabetes-Specific QOL (n= 121, items=11 items) α = 0.83, x= 62.5, SD= 13.3 | 62.1 (12.5) | 62.5 (10.7) | 63 (14.1) | 63.6 (13.5) |
| Generic QOL (n= 121, items=23) α =0.88, x= 76.3, SD= 11.8 | 75 (12.2) | 75.5 (11.1) | 77.5 (11.2) | 78.6 (13.1) |
| Parent-Child Conflict (n= 121, items=15) α = 0.90, x= 24.6, SD= 8.45 | 24.9 (9.1) | 25.6 (8.8) | 24.4 (7.8) | 25 (8.3) |
| Family Responsibility Sharing (n= 121, items=17) α = 0.67, x= 37.0, SD= 3.61 | 36.5 (3.9) | 35.5 (4.7) | 37.6 (3.2) | 35.8 (3.6) |
DSMP = Diabetes Self Management Profile; BSM= blood sugar monitoring; QOL=quality of life; α = Cronbach alpha coefficients for each instrument
Diabetes-Related Events
Few acute diabetes-related medical events requiring emergency room (ER) visits and/or hospitalizations were reported by participants in either treatment group. The incidence rate (IR) of ER visits was about 15 to 20 events per 100-pt-yrs, IR of hospitalizations was 7 to 8 events per 100-pt-yrs, and IR of severe hypoglycemia was 2 to 3 events per 100-pt-yrs (requiring parenteral therapy) and 18 to 25 events per 100-pt-yrs (requiring assistance with oral therapy). This rate of acute events reported is similar to the rates commonly reported in this population (32–34). A total of 20 mental health referrals were provided at baseline due to scores on the depression measure (17 from the Beck Youth Inventory and 3 from the Children’s Depression Inventory); 8 referrals were provided at follow-up (2 of which were previously identified at baseline).
DISCUSSION
This pilot study was designed to determine the feasibility of conducting a multi-site clinical trial and to obtain information about how best to design and conduct such a trial. The pilot provided essential experience with recruitment, measurement, and intervention, informing development and conduct of the larger multi-site clinical trial to follow.
Recruitment, Sample, and Participation
The pilot demonstrated that it was feasible to recruit and retain families for a behavioral intervention study, despite the demands diabetes management places on families and the competition for patients from other clinical trials at the study sites. Participant maintenance was very high in both groups, suggesting that the intervention did not place an undue burden on families and that those in the comparison group were willing to continue participation in return for modest attention from the HAs and minor incentives, including assistance with appointment making and parking.
While the study population was mainly white, its racial and ethnic composition was consistent with that of the patients in the participating clinics. The exclusion criteria, which were selected to minimize potential confounding, excluded many families, with over half (193 of 328) not meeting the eligibility criteria. While the impact of these exclusion on external validity must be considered, it is notable that many of the ineligible patients were in the early stages of the disease and its management, representing a different clinical population. Also, more families than anticipated lived too far from the clinic for the study to practically reach them for home assessments. These findings indicate an added consideration challenge in the use of home assessments; but not with the design of the intervention itself.
Despite the number of families not meeting eligibility criteria, there was no indication that most of the eligibility criteria should be relaxed. However, with the greater numbers afforded by the main trial, allowing for potential subgroup analyses, we determined that it would be feasible to include recently diagnosed participants. The full participation of nearly all eligible families suggests a desire by families for greater assistance with diabetes management. In addition, the equivalent retention of families in the WE*CAN and control groups underscores the families’ commitment to their child’s care and to participation in diabetes research.
Measurement
The high retention rates, with only five participants assessed at baseline failing to complete the post assessment, suggest that the burden imposed by measurement did not unduly discourage participation in either treatment condition. Home assessment at baseline and follow-up was selected because the battery of measures was too extensive to complete at the clinic. Fortunately, the concern that families would not be willing to participate in lengthy home visits was not realized. At baseline, 9 families completed the assessment at the clinic or a public place other than home due to family request or concerns about safety; 7 families did so at follow-up also. The telephone interviews went smoothly and were completed within the expected periods, although many calls were required to reach some families. The concern that younger participants might not be able to complete some measures proved not to be a problem. The high compliance with bringing meters suggests the feasibility of obtaining meter download data; however it is notable that families who did not bring their meters were in significantly poorer control, suggesting the importance of efforts to minimize this bias when collecting meter data.
The analysis of outcome measures at baseline and follow up confirmed the relevance of these variables. The decline in these measures over time was consistent with expectations and previous findings and although the study duration was briefer, the data nonetheless provided information useful for determining the power needed for the main trial. The small number of diabetes-related events were reasonably distributed over time and similar in the two groups, suggesting no adverse effects. Given families’ willingness to participate in the assessment, the investigators determined that it would be feasible to invite both caregivers in 2-caregiver families to participate in the full multi-site trial. Considering the current lack of data on second caregivers (usually fathers), the additional assessment should provide an important scientific contribution.
Intervention
Ratings of the delivery of intervention components suggested that the HAs conducted the intervention with adequate fidelity and consistency, although only a subsample of intervention sessions were rated. According to the evaluations, HAs were able to complete the session activities fully or partially. Feedback from the satisfaction measure indicated that the HAs appeared to be well-liked and respected by the families. Importantly, families did not confuse the role and expertise of the HA with that of their health care providers. Despite potential barriers to clinic-based intervention, surprisingly few problems were encountered conducting the intervention as designed and no conflicts with diabetes management staff or interference with clinic flow were encountered, as previously experienced at a single site (9,19).
HAs had difficulty reaching some families for the telephone follow up calls and it became necessary to set a limit of 6 calls to each family. At follow-up, parents and children almost uniformly reported enthusiasm for the process and utility of the intervention. Because only 2 to 3 intervention sessions per family were delivered, the sustainability of the intervention could not be determined. It was expected that the brief intervention and follow up period would not yield significant treatment group effects. Our goal was to develop an intervention approach with a preventive focus that is integrated into clinical care. Accordingly, it was designed to be of low intensity delivered over many clinic visits across the developmental time period in which parent-child issues in diabetes management are particularly salient. As such, the duration of this feasibility study was insufficient to deliver an intervention dose with a reasonable likelihood of impacting the target behaviors. Since developmental transitions relevant to diabetes management occur across a protracted time period, it seems reasonable to anticipate that support designed to improve family problem-solving across this transition be provided for an extended period of time. Consequently, in the main study, the authors anticipate testing the intervention approach for 18 to 24 months.
Important lessons in the delivery of the intervention were learned. The WE*CAN intervention is individualized to each family, requiring the HA to make decisions about how to proceed during each session, how to help families identify a target topic and goal for the session, how to engage the families in problem solving activities, and how to orient them toward helpful and collaborative diabetes management practices. While most families were able to identify relevant goals, barriers, and solutions, some had difficulty with these activities and the HAs sometimes struggled with how best to facilitate family problem solving. For example, HAs reported difficulty when dealing with families who disagreed on what goal to work on, who selected goals that were likely to be unrealistic, or who demonstrated differential investment in the goal selected. These experiences shaped future training to help HAs respond to these situations in an effective manner. HAs also encountered some families who were satisfied with their current diabetes management and struggled with identifying a diabetes management area to address. To better meet the needs of these families, an approach to the intervention process called the “prevention toolbox” was developed, in which the family problem solving process and relevant handouts were used in a preventive, anticipatory guidance mode.
The length of the initial session, which included introductory information regarding developmental and family issues surrounding diabetes management, proved to be longer and less useful than desirable, requiring modifications to reduce session length, minimize didactic portions, and increase the amount of time in which families were engaged in problem solving. The initial focus on an “easier” goal such as carrying fast-acting carbohydrate proved not to be necessary; families appeared to be better served by choosing the most salient goal at their initial session. HAs reported some difficulty determining how to select the most relevant handouts for families, so additional guidance on the most relevant handouts for common topics was provided. Based on areas identified by the HAs, additional handouts were also developed to meet families’ needs.
More centralized training than originally planned was needed to allow more practice and feedback from experienced investigators who had conceptualized the intervention and developed the protocol. Ongoing training appeared to be particularly valuable because HAs were more able to identify areas of difficulty in intervention delivery based on their experiences and could discuss their needs and concerns with the investigators and with one another. This gave rise to monthly HA conference calls with study investigators. These calls facilitated collaboration, increased uniformity of intervention implementation, and facilitated management and resolution of unanticipated situations. There was only the expected turnover of HAs during the pilot, suggesting the need in a longer trial to provide periodic (at least annual) training.
Conclusion
The study provided evidence that it is possible to recruit and maintain study participants; conduct a clinic-integrated, family-based problem-solving intervention delivered by trained non-professionals in multiple sites; and collect a substantial amount of useful data during home and telephone visits. The challenge remains of conducting and evaluating a multi-site efficacy trial over a longer period of time, with a much larger sample, and with sufficient intensity to reasonably expect improvements in the difficult disease management challenges that children with diabetes and their families deal with daily.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development. The following institutions and investigators comprised the steering committee of the Family Management of Diabetes multi-site trial.
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland: Tonja R. Nansel, PhD, Bruce Simons-Morton, EdD, Ronald J. Iannotti, PhD
Joslin Diabetes Center, Boston, Massachusetts: Lori Laffel, MD MPH, Korey Hood, PhD. Contract N01-HD-4-3364.
Nemours Children’s Clinic, Jacksonville, Florida: Tim Wysocki, PhD, Amanda Lochrie, PhD. Contract N01-HD-4-3361.
Texas Children’s Hospital, Houston, Texas: Barbara Anderson, PhD. Contract N01-HD-4-3362. Children’s Memorial Hospital, Chicago, Illinois: Jill Weissberg-Benchell, PhD, Grayson Holmbeck, PhD. Contract N01-HD-4-3363.
James Bell Associates, Arlington, Virginia; Cheryl McDonnell, PhD, MaryAnn D’Elio, Contract N01-HD-3-3360.
References
- 1.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Journal of Pediatrics. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 2.Bryden KS, Peveler RC, Stein A, Neil A, Mayou RA, Dunger DB. Clinical and psychological course of diabetes from adolescence to young adulthood: a longitudinal cohort study. Diabetes Care. 2001;24:1536–1540. doi: 10.2337/diacare.24.9.1536. [DOI] [PubMed] [Google Scholar]
- 3.Wysocki T, Hough BS, Ward KM, Green LB. Diabetes mellitus in the transition to adulthood: adjustment, self-care, and health status. Developmental and Behavioral Pediatrics. 1992;13:194–201. [PubMed] [Google Scholar]
- 4.Miller VA, Drotar D. Discrepancies between mother and adolescent perceptions of diabetes-related decision-making autonomy and their relationship to diabetes-related conflict and adherence to treatment. J Pediatr Psychol. 2003;28:265–274. doi: 10.1093/jpepsy/jsg014. [DOI] [PubMed] [Google Scholar]
- 5.Palmer DL, Berg C, Wiebe DJ, Beveridge RM, Korbel CD, Upchurch R, Swinyard MT, Lindsay R, Donaldson DL. The role of autonomy and pubertal status in understanding age differences in maternal involvement in diabetes responsibility across adolescence. J Pediatr Psychol. 2004;29:35–46. doi: 10.1093/jpepsy/jsh005. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BJ, Coyne JC. Family context and compliance behavior in chronically ill children. In: Krasnegor NA, Epstein L, Johnson SB, Yaffe SJ, editors. Developmental aspects of health compliance behavior. Hillsdale, NJ: Erlbaum; 1993. pp. 77–8. [Google Scholar]
- 7.Davis CL, Delamater AM, Shaw KH, La Greca AM, Eidson MS, Perez-Rodriguez JE, Nemery R. Brief Report: Parenting styles, regimen adherence, and glycemic control in 4-to 10- year-old children with diabetes. Journal of Pediatric Psychology. 2001;26:123–129. doi: 10.1093/jpepsy/26.2.123. [DOI] [PubMed] [Google Scholar]
- 8.Wysocki T, Greco P. Self-management of childhood diabetes in family context. In: Gochman DS, editor. Handbook of Health Behavior Research II: Provider Determinants. New York: Plenum Press; 1997. pp. 169–187. [Google Scholar]
- 9.Anderson B, Brackett J, Ho J, Laffel L. An office-based intervention to maintain parent-adolescent teamwork in diabetes management: Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22:713–721. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- 10.Wysocki T, Miller KM, Harvey LM, Taylor A, Elder-Danda C, McDonell K, Greco P, Harris MA, White NH. Behavior therapy for families of adolescents with diabetes: effects on directly observed family interactions. Behavior Therapy. 2000;30:507–525. [Google Scholar]
- 11.Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. The Journal of Pediatrics. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- 12.LaGreca AM, Follansbee DM, Skyler JS. Developmental and behavioral aspects of diabetes management in youngsters. Children’s Health Care. 1990;19:132–139. [Google Scholar]
- 13.Grey M, Boland E, Davidson M, Li J, Tamborlane WV. Coping skills training for youth on intensive therapy has long-lasting effects on metabolic control and quality of life. Journal of Pediatrics. 2000;137:107–113. doi: 10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- 14.Greco P, Shroff-Pendley J, McDonell K. A peer group intervention for adolescents with type 1 diabetes and their friends. J Pediatr Psychol. 2001;26:485–490. doi: 10.1093/jpepsy/26.8.485. [DOI] [PubMed] [Google Scholar]
- 15.Satin W, La Greca AM, Zigo MA, Skyler JS. Diabetes in adolescence: Effects of multifamily group intervention and parent simulation of diabetes. Journal of Pediatric Psychology. 1989;14:259–275. doi: 10.1093/jpepsy/14.2.259. [DOI] [PubMed] [Google Scholar]
- 16.Wysocki T, Harris MA, Greco P, Bubb J, Danda CE, Harvey LM, McDonnell K, Taylor A, White NH. Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus. J Pediatr Psychol. 2000;25:23–33. doi: 10.1093/jpepsy/25.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Anderson BJ, Wolf FM, Burkhart MT, Cornell RG, Bacon GE. Effects of peer-group intervention on metabolic control of adolescents with IDDM: Randomized outpatient study. Diabetes Care. 1989;12:179–183. doi: 10.2337/diacare.12.3.179. [DOI] [PubMed] [Google Scholar]
- 18.Delamater AM, Bubb J, Davis SG, Smith JA, Schmidt L, White NH, Santiago JV. Randomized prospective study of self-management training with newly diagnosed diabetic children. Diabetes Care. 1990;13:492–498. doi: 10.2337/diacare.13.5.492. [DOI] [PubMed] [Google Scholar]
- 19.Laffel LM, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. Journal of Pediatrics. 2003;142:409–416. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- 20.Drotar D. Enhancing reviews of psychological treatments with pediatric populations: thoughts on next steps. J Pediatr Psychol. 2002 Mar;27(2):167–176. doi: 10.1093/jpepsy/27.2.167. [DOI] [PubMed] [Google Scholar]
- 21.Iannotti RJ, Bush PJ. Toward a developmental theory of compliance. In: Krasnegor NA, Epstein LH, Johnson SB, Yaffe SJ, editors. Developmental Aspects of Health Compliance Behavior. Hillsdale, NJ: Erlbaum; 1993. pp. 59–76. [Google Scholar]
- 22.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 23.Kazdin AE. Behavior modification in applied settings. Pacific Grove, CA: Brooks/Cole Publishing Company; 1989. [Google Scholar]
- 24.Lask B. Motivating children and adolescents to improve adherence. The Journal of Pediatrics. 2003:430–433. doi: 10.1067/S0022-3476(03)00447-5. [DOI] [PubMed] [Google Scholar]
- 25.Harris MA, Wysocki T, Sadler M, Wilkinson K, Harvey LM, Buckloh LM, Mauras N, White NH. Validation of a structured interview for the assessment of diabetes self-management. Diabetes Care. 2000;23:1301–1304. doi: 10.2337/diacare.23.9.1301. [DOI] [PubMed] [Google Scholar]
- 26.Wysocki T, Xing D, Fiallo-Scharer R, Doyle E, Block J, Tsalikian E, beck R, Ruedy K, Kollman C, Tamborlane W, Harris MA the Diabetes Research in Children Network (DirecNet) Study Group. Diabetes self-management profile-revised for conventional and flexible insulin regimens (Abstract) Diabetes. 2004;53:A436. [Google Scholar]
- 27.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman R, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care. 2003;26:631–637. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- 29.Hood KK, Butler DA, Anderson BJ, Laffel LM. Updated and revised Diabetes Family Conflict Scale. Diabetes Care. 2007;30:1764–1769. doi: 10.2337/dc06-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck J, Beck A, Jolly J. Beck Youth Inventories; manual. San Antonio, Texas: The Psychological Corporation; 2001. [Google Scholar]
- 31.Kovacs M. The Children’s Depression Inventory (CDI) technical manual. Toronto, ON: Multi-Health Systems; 2003. [Google Scholar]
- 32.Levine BS, Anderson BJ, Butler DA, Antisdel JE, Brackett J, Laffel LM. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr. 2001 Aug;139(2):197–203. doi: 10.1067/mpd.2001.116283. [DOI] [PubMed] [Google Scholar]
- 33.Rewers A, Chase HP, Mackenzie T, Walravens P, Roback M, Rewers M, Hamman RF, Klingensmith G. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002 May 15;287(19):2511–2518. doi: 10.1001/jama.287.19.2511. [DOI] [PubMed] [Google Scholar]
- 34.Svoren BM, Volkening LK, Butler DA, Moreland EC, Anderson BJ, Laffel LMB. Temporal trends in the treatment of pediatric type 1 diabetes and impact on acute outcomes. Journal of Pediatrics. 2007;150:279–285. doi: 10.1016/j.jpeds.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]