Abstract
The rapid elevation in rat brain temperature achieveable with focused beam microwave irradiation (FBMI) leads to a permanent inactivation of enzymes, thereby minimizing enzyme-dependent post mortem metabolic changes. An additional characteristic of FBMI is that the NMR properties of the tissue are close to those of the in vivo condition and remain so for at least 12 hours. These features create an opportunity to develop MRI and MRS on microwave-irradiated samples into a technique with a resolution, coverage and sensitivity superior to any experiment performed directly in vivo. Furthermore, when combined with pre-FBMI infusion of 13C-labeled substrates, like [1-13C]-glucose, the technique can generate maps of metabolic fluxes, like the TCA and glutamate-glutamine neurotransmitter cycle fluxes at an unprecedented spatial resolution.
Keywords: Microwave fixation, in situ NMR spectroscopy, rat brain
INTRODUCTION
The spatial resolution of in vivo 1H NMR spectroscopy has dramatically increased with higher magnetic fields, improved RF coils and optimized acquisition strategies. However, even on state-of-the-art MR systems, the spatial resolution of in vivo NMR spectroscopy studies on animal brain is still relatively coarse (> 10 µL) compared to typical anatomical structures, leading to significant partial volume effects. For low-concentration metabolites like γ-amino butyric acid (GABA), the spatial resolution is reduced even further in order to compensate for the reduced amount of signal. The sensitivity and hence spatial resolution at high magnetic fields can essentially only be increased by (1) increased measurement time (averages) or (2) improved RF coil design. Surface coil reception can locally improve sensitivity at the cost of reduced sample coverage. While this effect can partially be mitigated by phased-array coils, the obtainable in vivo sensitivity is ultimately limited by the physiological (i.e. temporal) stability of the animal.
Many neurochemicals, like cyclic nucleotides, ATP, glucose, lactate and certain neurotransmitters, like GABA, undergo rapid post mortem alterations due to on-going metabolism, thereby giving a distorted characterization of in vivo metabolism. These alterations can be minimized by methods that reduce or even completely halt post mortem metabolism rapidly. In rodent studies the most commonly used methods of enzyme inactivation involves rapid in situ freezing with liquid nitrogen, such as freeze-blowing (Veech et al. 1973), freeze-clamping (Bolwig and Quistorff 1973), or funnel-freezing (Ponten et al. 1973). However, these methods suffer from limited control of region specificity, reactivation of enzymes upon thawing and poorer preservation of deeper brain regions due to the finite speed of the freezing front before blood flow ceases. Obtaining tissue from well-defined brain regions from the frozen cranium is both laborious and challenging, requiring the use of a cryostat for dissection of non-superficial cortical regions.
Since the early 1970s focused beam microwave irradiation (FBMI) has been used as an alternative for the preparation of post mortem tissue samples on small laboratory animals (Stavinoha et al. 1970). Using a dedicated FBMI device with 5 – 10 kW output power, the technique rapidly (0.5 – 1.5 s) elevates brain temperature to 80 – 90 °C. This leads to a permanent inactivation of enzymes, thereby minimizing enzyme-dependent post mortem metabolic changes.
Comparisons following FBMI and freezing methods have been reported for several compounds in brain tissues that are characterized by rapid post-mortem concentration changes, including acetylcholine (Stavinoha et al. 1973), ATP (Delaney and Geiger 1996), cyclic AMP (Schmidt et al. 1971; Balcom et al. 1975), GABA (Balcom et al. 1975; Elekes et al. 1986; Miller et al. 1990), glutathione (Miller et al. 1990), glycogen (Kong et al. 2002; Morgenthaler et al. 2006) and neuropeptides (Parkin et al. 2005). In all cases the metabolite levels found with FBMI were much closer to the known or suspected in vivo levels than with the freezing methods, supporting the contention that FBMI minimizes post mortem neurochemical alterations. It should be noted that many of the mentioned metabolites, like cyclic AMP, are not NMR observable due to their low concentration or their relaxation properties.
Here we explore the use of in situ NMR on microwave-irradiated samples to improve the sensitivity/resolution by both mechanisms, while maintaining the metabolic profile and structural tissue integrity of the in vivo situation. Besides the minimal alteration of post mortem metabolite levels, two additional benefits of FBMI are that the inactivation of enzymes is permanent (i.e. enzymes can not be re-activated) and that the NMR properties of the tissue are close to those of the in vivo condition (i.e. the tissue is not frozen). These two additional features create an opportunity to develop NMR spectroscopy on microwave-irradiated samples into a technique that can generate spatial maps of in vivo metabolism with a resolution, coverage and sensitivity superior to any NMR experiment performed directly in vivo. Presented results will demonstrate that the microwave-irradiated samples are metabolically and structurally stabile for at least 12 hours, providing a large time-window in which to acquire high-resolution maps of in vivo metabolism.
MATERIALS AND METHODS
All in vivo and in situ experiments were performed on a 11.74 T Magnex magnet (Magnex Scientific Ltd, Oxford, UK) interfaced to a Bruker Avance Spectrometer (Bruker Instruments, Billerica, MA) equipped with 9.0 cm diameter Magnex gradients capable of switching 395 mT/m in 180 µs. For in vivo experiments RF transmission and reception was performed with a 14 mm diameter surface coil tuned to the proton NMR frequency (499.8 MHz). For all in situ experiments RF transmission and reception was achieved with a four-turn, 25 mm diameter solenoidal coil of 45 mm length. Heteronuclear spectral editing and broadband decoupling was performed with a 28 mm diameter Helmholtz coil (29 mm coil separation) tuned to the carbon-13 NMR frequency (125.7 MHz) and placed outside of the proton NMR coil.
For the in situ studies ten male Sprague-Dawley rats (206 ± 11 g, mean SD) were euthanized by microwave irradiation using the commercially available 10 kW Muromachi Microwave Fixation System (Stoelting Co, Wood Dale, IL) in accordance to the guidelines established by the Yale Animal Care and Use Committee. Under light halothane anesthesia the animals were place in dedicated animal holders to ensure proper positioning within the device. The head of the animal was constrained by a narrow, water-cooled area with dimensions similar to a rat head of size 180 – 220 g. In order to prevent the animal from pulling out of the device, the body was blocked at the tail end. The optimal fixation power and duration represents a compromise between stopping metabolism and the generation of microscopic air bubbles that severely compromise the B0 magnetic field homogeneity (see Results). The current group of animals were irradiated at 4.5 kW in 1.2 s, which essentially halted cerebral metabolism for at least 12 hours (see Results), while generating only a small number of air bubbles. Following microwave fixation all extracranial tissues were removed after which the skull and brain were immersed in deuterium oxide (17 mm diameter, 55 mm long tube) to minimize magnetic susceptibility artifacts created at air-brain interfaces. Since brain water exchanges with deuterium oxide over the span of hours, in later studies the deuterium oxide was replaced with Fluorinert FC-43 (3M, St. Paul, MN), an inert, clear fluid with a magnetic susceptibility close to water (Olson et al. 1995). The sample tube fitted snugly into the proton solenoidal coil for optimal NMR sensitivity.
For in vivo studies three male Sprague-Dawley rats (210 ± 13 g, mean ± SD) were prepared in accordance to the guidelines established by the Yale Animal Care and Use Committee. The animals were tracheotomized and ventilated with a mixture of 70 % nitrous oxide and 28.5 % oxygen under 1.5 % halothane anesthesia. A femoral artery was cannulated for monitoring of blood gases (pO2, pCO2), pH and blood pressure. Physiological variables were maintained within normal limits by small adjustments in ventilation (pCO2 = 33–45 mm Hg; pO2 > 120 mm Hg; pH = 7.20–7.38; blood pressure = 90–110 mm Hg). After all surgery was completed, anesthesia was maintained by 0.3 – 0.7 % halothane in combination with 70 % nitrous oxide. During NMR experiments animals were restrained in a head holder, while additional immobilization was obtained with d-tubocurarine chloride (0.5 mg/kg/40 mins, i.p.). The core temperature was measured with a rectal thermosensor and was maintained at 37 ± 1 °C by means of a heated water pad.
The magnetic field homogeneity was optimized through MRI-based B0 mapping (Koch et al. 2006). Briefly, four gradient-echo images (TR = 1000 ms, 128 × 64 over 38.4 × 19.2 mm, 30 slices over 18.0 mm) with different echo-time TE + τ (with τ = 0, 0.33, 1.0 and 3.0 ms) were acquired. The phase of each image was determined relative to the first image. Temporal phase-unwrapping, under the assumption that the phase in the second image is within the range [−π, +π], yielded a linear (phase, time τ) curve. The slope, obtained by linear regression, is then proportional to the magnetic field offset ΔB0. The measured ΔB0 map was fitted with first and second spherical harmonics across the entire brain after which the shims could be updated.
Single volume 1H NMR spectroscopy was performed with LASER (Slotboom et al. 1991; Garwood and DelaBarre 2001) with TR/TE = 4000/14 ms on a 16 µL volume positioned in the cerebral cortex. Water suppression was achieved with SWAMP (de Graaf and Nicolay 1998), an adiabatic analog of CHESS (Haase et al. 1985).
Multi-slice 1H MRSI (TR/TE = 3500/10 ms) was performed with a double spin-echo sequence consisting of a 1 ms Shinnar-Le Roux optimized excitation pulse (Pauly et al. 1991) and a pair of 1 ms adiabatic full passage refocusing pulses. 11 slices (1 mm thickness) were selected and encoded over a circular k-space sampling grid (284 total phase-encoding steps, FOV = 20 × 20 mm), giving a nominal MRSI voxel size of 1 µL. Since all extracranial tissues were removed, no outer volume suppression was required.
RESULTS
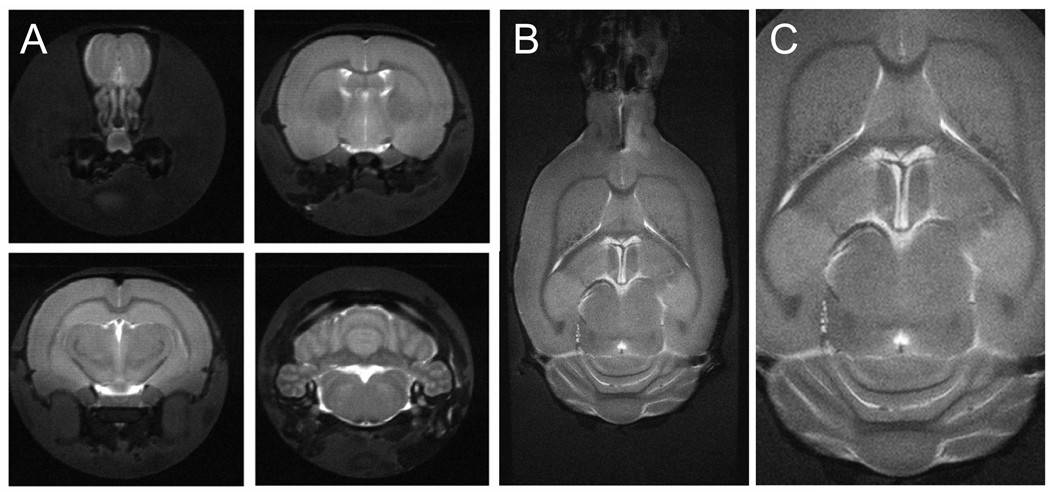
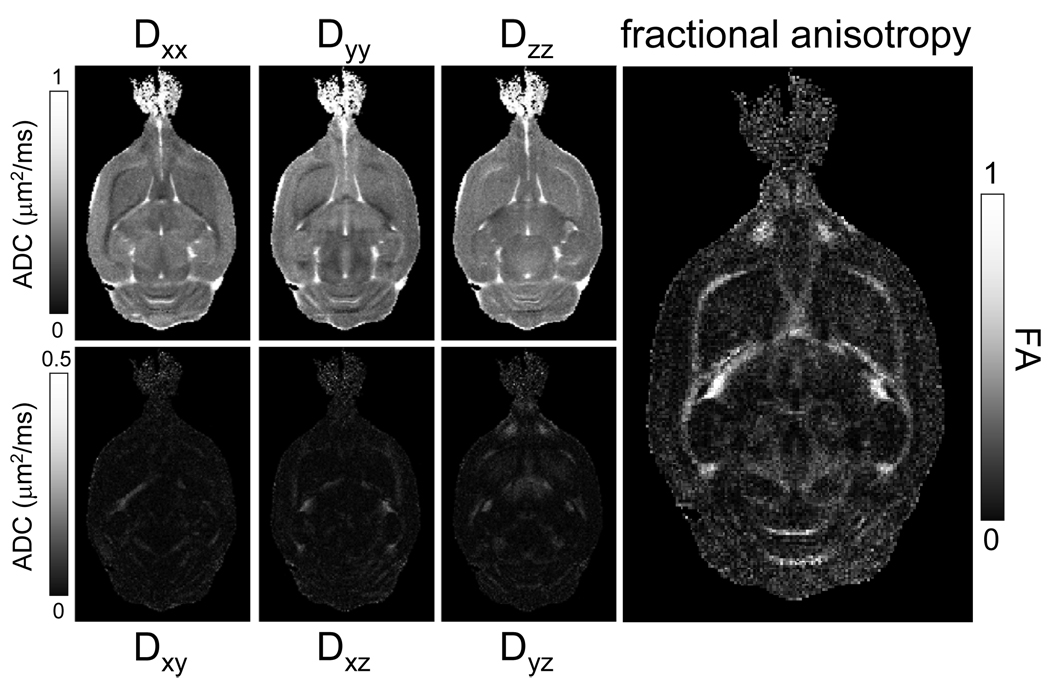
One of the requirements for meaningful in situ NMR of microwave fixed brains is structural integrity of the tissue. T2-weighted MRI is sensitive to the formation of edema, whereas diffusion tensor imaging (DTI) can be used to assess integrity of white matter fiber tracts. Figure 1 shows high-resolution (75 µm and 60 µm in-plane resolution for A and B/C, respectively) T2-weighted images (TE = 35 ms) of microwave-irradiated rat brain. Excellent soft-tissue contrast was apparent without any noticeable signs of edema formation or tissue degradation. This is essential for a variety of high-resolution MRI applications to characterize disease progression, like plaque formation in Alzheimer’s disease (Jack et al. 2005). Figure 2 shows a typical DTI data set, which reveals strong white matter anisotropy (fractional anisotropy = 0.7 – 0.8), mild and uniform anisotropy in gray matter (FA = 0.15 – 0.25), and essentially isotropic diffusion in CSF (FA ~ 0). These diffusion characteristics are identical to those found in rat brain in vivo, thereby further supporting the notion that FBMI does not significantly affect tissue structure.
Figure 1.
Anatomical MR imaging on microwave-fixated rat brain. (A) Axial and (B/C) coronal T2-weighted, multi-slice, multi-shot RARE images (TR/TE = 3500/35 ms, 32 segments) of microwave-irradiated rat brain acquired over 750 µm and 600 µm thick slices, respectively. 256 × 256 and 640 × 320 data matrices were acquired for A and B/C, over 19.2 × 19.2 and 38.4 × 19.2 mm FOVs, respectively. Excellent soft-tissue contrast partially demonstrates structural integrity of microwave-irradiated samples. Data were acquired in 40 min (A) and 90 min (B/C). (C) represents an expanded view of (B).
Figure 2.
Diffusion tensor imaging (DTI) on microwave-fixated rat brain. Data was acquired at a 120 × 120 µm in-plane resolution over 1000 µm thick slices. Diffusion maps were calculated using a SVD over 14 experiments (13 directions, b-value = 1.4 ms/µm2 and 1 image with b = 0 ms/µm2). White matter anisotropy is clearly visible in all six directional diffusion maps. This is further emphasized in the fractional anisotropy (FA) map, calculated from the three eigenvalues of the diffusion tensor. Excellent contrast between gray matter (FA ~ 0.2) and white matter (FA > 0.6) is visible.
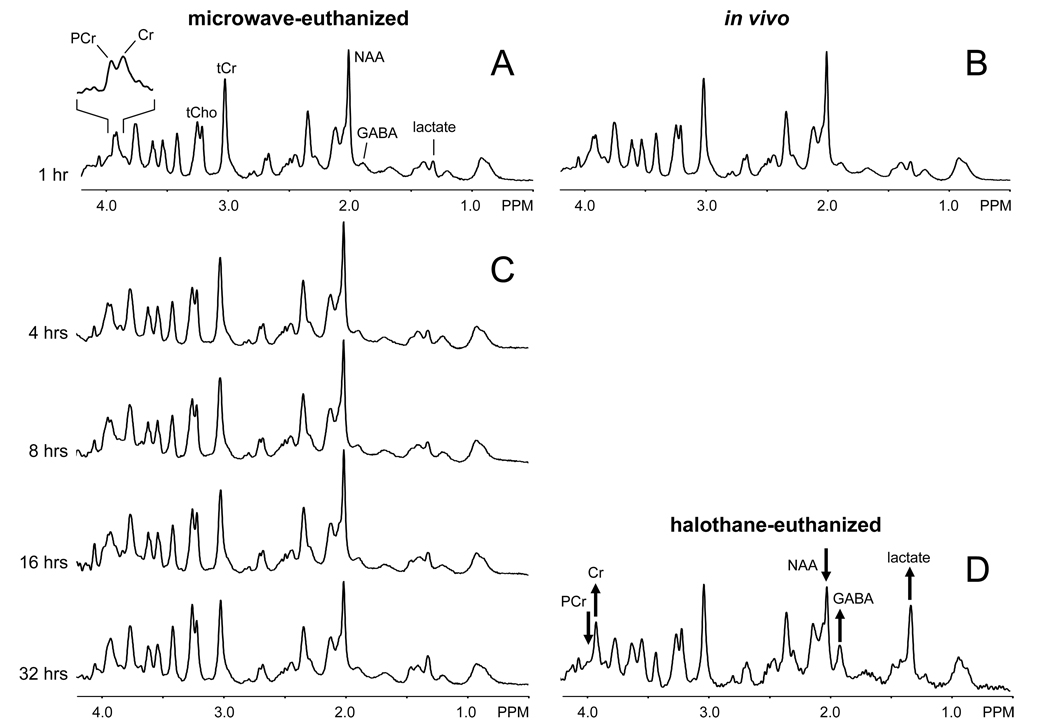
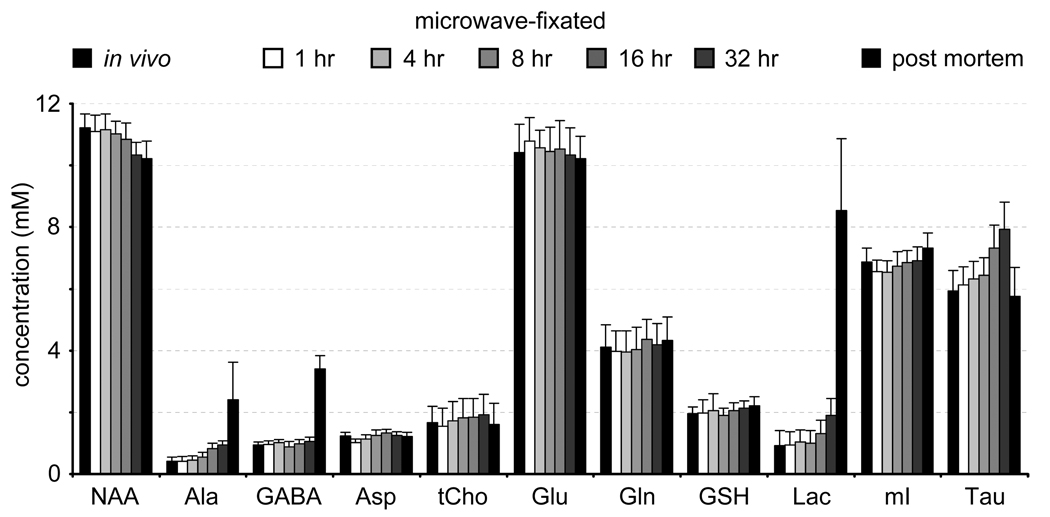
A prime concern in the development of in situ NMR on microwave irradiated samples is the temporal stability of the samples. Fig. 3A shows a localized 1H-NMR spectrum from rat brain, 60 min following microwave irradiation. The spectrum closely resembles an in vivo 1H brain spectrum (Fig. 3B). Low levels of lactate and GABA, high levels of phosphocreatine and common ratios of N-acetyl aspartate (NAA), total creatine and total choline are all indicative of minimal post mortem changes in metabolite levels. A 1H NMR spectrum of halothane-euthanized rat brain is shown in Fig. 3D for comparison. The 1H NMR spectra from microwave-irradiated rat brain show remarkably little change in the first 12–16 hours (Fig. 3C). After 16 hours, the total choline, taurine, lactate, and alanine resonances increase, indicating that not all enzymatic activity has been completely halted. However, a time window of 16 hours typically represents a maximum measurement time due to limited MR system availability and diminishing S/N returns. Figure 4 presents the absolute metabolite concentrations of the different spectra using LCmodel spectral fitting and assuming a 10 mM concentration for total creatine.
Figure 3.
Proton NMR spectroscopy on microwave-fixated rat brain in situ and rat brain in vivo and post mortem. (A) Localized 1H NMR spectrum (16 µL, 10 min) of rat brain acquired 1 hour following 1.2 s, 4.5 kW microwave irradiation. (B) Localized 1H NMR spectrum (24 µL, 5 min) of rat brain in vivo and (D) acquired 1 hour following euthanasia with 5% halothane. The microwave-irradiated 1H NMR spectrum closely resembles the in vivo 1H NMR spectrum. The hallmarks for post-mortem 1H NMR changes present in (D), i.e. elevated lactate, GABA and creatine (Cr), reduced NAA and missing phosphocreatine (PCr), are noticeably absent from the spectrum in (A). This partially demonstrates the metabolic integrity of brain tissue following microwave irradiation. (C) Acquiring 1H NMR spectra longitudinally over 32 hours shows remarkable stability for 12–16 hours. In the very late time points small changes in taurine/total choline, NAA and lactate become noticeable.
Figure 4.
Absolute metabolite concentrations as obtained from the 1H NMR spectrum acquired on rat brain in vivo (black column, left), on rat brain at 1, 4, 8, 16 and 32 hours following microwave-irradiation and on rat brain post mortem (5% halothane, black column, right). For all conditions an internal total creatine concentration of 10 mM was assumed.
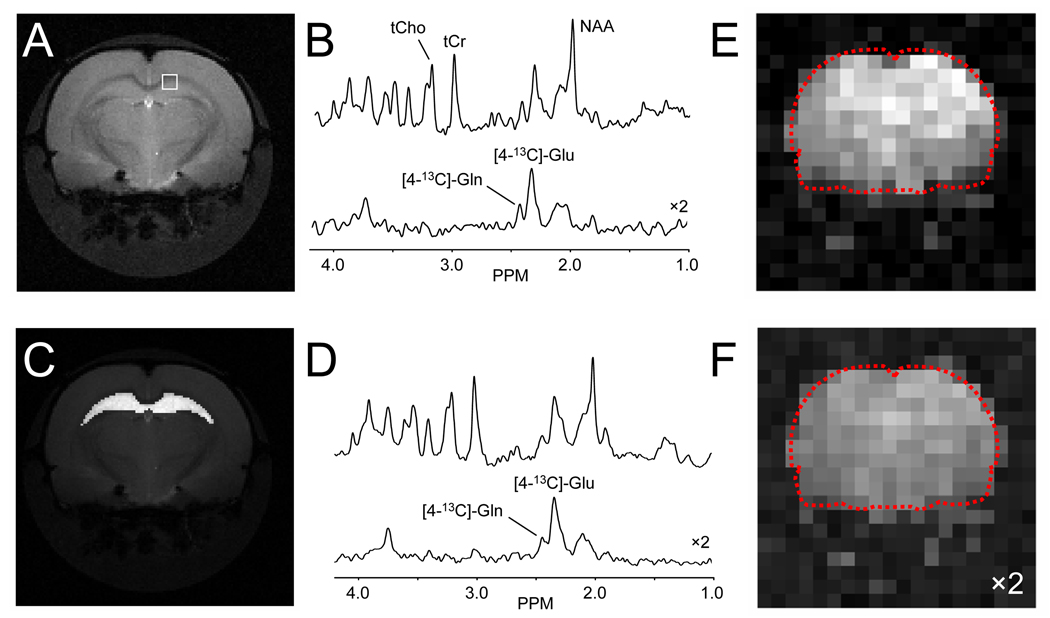
Figure 5 shows one of the metabolic applications whereby the combination of intravenous infusion of 13C-labeled substrates and microwave irradiation can be used to obtain dynamic metabolic information. Figure 5B shows 1H (top) and edited 1H-[13C]-NMR spectra from a 1.0 µL volume extracted from a multi-slice 20 × 20 MRSI data set acquired in circa 6 hours following 2 hours of intravenous infusion of [1,6-13C2]-glucose and subsequent microwave irradiation. The position of the volume is indicated in the MR image shown in Fig. 5A. Despite the small voxel size of 1.0 µL, the sensitivity is adequate to detect the formation of [4-13C]-glutamate, [4-13C]-glutamine and other 13C-labeled products. The high spatial resolution also allows the generation of metabolic maps as shown in Figs. 5E and F for [4-13C]-glutamate and [4-13C]-glutamine. Alternatively spectra from multiple voxels can be co-added to obtain high S/N data as shown in Fig. 5D for 1H and edited 1H-[13C]-NMR spectra of the hippocampus as indicated in Fig. 5C. By repeating the studies shown in Fig. 5 for different infusion times (on different animals), the full dynamic turnover time course can be obtained at a spatial resolution and sensitivity that is several times higher than direct in vivo detection. Combined with mathematical modeling procedures, absolute fluxes can be assessed for a number of important metabolic pathways (e.g. tricarboxylic acid cycle) in neurons and astroglia (de Graaf et al. 2003).
Figure 5.
Multi-slice 1H-[13C]-MRSI on microwave-irradiated rat brain following 2 hours of [1,6-13C2]-glucose infusion. (B) 1 µL volume acquired from the position indicated in (A) showing non-edited (top) and edited 1H-[13C] (bottom) spectra. Regional analysis, e.g. hippocampus as indicated in (C), allows the calculation of high-sensitivity spectra (D) from well-defined regions (see Section C5 for details). (E/F) Spatial map of (E) [4-13C]-glutamate and (F) [4-13C]-glutamine levels (a.u.) obtained by simple (absolute-valued) integration.
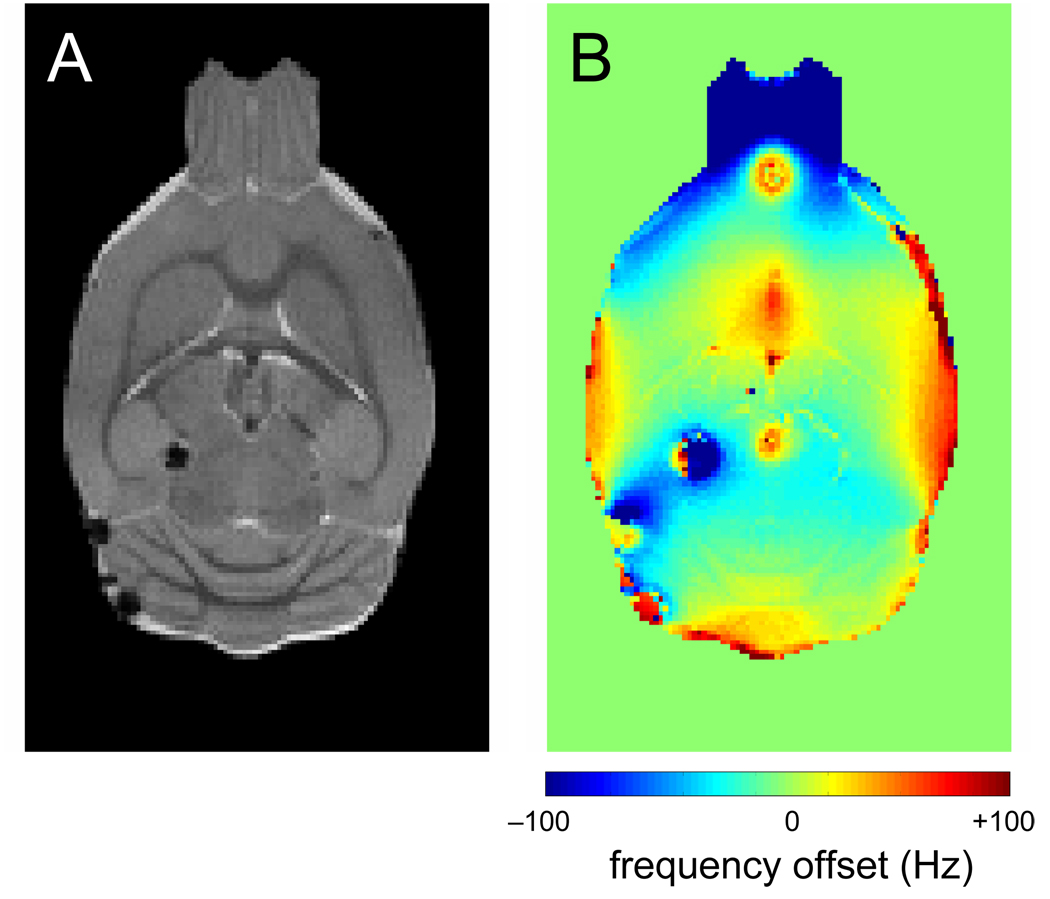
Microwave irradiation generated entrapped air bubbles on a limited number of animals, of which Fig. 6 shows a typical example. When present, the air bubbles typically formed at the boundary of ventricles and brain parenchyma. While air bubbles generally do not prevent high-resolution anatomical MRI (Fig. 6A), they do perturb the magnetic field over a significant area (Fig. 6B). Since 1H and edited 1H-[13C]-NMR spectroscopy rely on a highly homogeneous magnetic field, the presence of air bubbles prevents the acquisition of high quality data in the immediate area surrounding the air bubbles. Generally it was observed that the majority of air bubbles disappear in a matter of hours following microwave irradiation, presumably due to water accumulation or reabsorption.
Figure 6.
The effect of microwave-related air bubbles. (A) Coronal spin-echo MR image and (B) B0 map from microwave-irradiated rat brain. While the spin-echo image is relatively unaffected by small air bubbles formed during FBMI, the magnetic field homogeneity surrounding the bubbles has greatly decreased.
DISCUSSION
Here we have demonstrated that microwave-irradiated rat brain possesses the structural integrity and temporal stability to allow the acquisition of high-resolution in situ MR spectra and images that reflect the in vivo condition. However, with improved RF coil design and greatly increased acquisition times, the in situ results can be obtained at a sensitivity which greatly exceeds that of direct in vivo acquisition.
The 1H NMR spectroscopy results of Fig. 3 and Fig. 4 indicate that FBMI does rapidly inactivate cerebral enzymes. Common post mortem changes observed by 1H NMR (Fig 3D) are a very fast creatine-kinase-catalyzed conversion of phosphocreatine to creatine, a fast accumulation of lactic acid by anaerobic glycolysis and a glutamate-decarboxylase-catalyzed formation of GABA. In the in situ 1H NMR spectra from microwave-irradiated rat brain none of these three processes were observed. However, at the later time points (≥16 hours) some spectral changes were seen, in particular a small elevation in lactate, alanine, and taurine/total choline, and a reduction in NAA. Since the samples were measured at room temperature under non-sterile conditions, the metabolic changes could possibly be attributed to bacterial growth and tissue breakdown. However, the significant increase in taurine after 16 hours is puzzling because previous postmortem measurements of amino acids in rat and human brain tissue maintained at room temperature for up to 4 hours or human brain autopsy specimens stored at 0–4°C for up to 120 hours showed taurine levels to be relatively stable (Perry et al. 1981). Whether potential renaturation of some heat inactivated enzymes could occur is not known, but studies confined to the first 12 hours after microwave irradiation can be expected to be highly stable.
While direct 1H NMR spectroscopy demonstrates metabolic stability, the local cellular environment could have significantly changed by the intense heat generated by the microwave irradiation. While DTI provides the first indication that this is not the case, cellular integrity can be investigated further with diffusion-weighted 1H NMR spectroscopy (Nicolay et al. 2001). Diffusion-weighted 1H MRS of intracellular metabolites is sensitive to restrictive barriers, such as cell membranes and sub-cellular organelles and can therefore provide information about cellular and sub-cellular integrity non-invasively. This will be the subject of future studies
The results presented in Fig. 5 demonstrate that high-spatial-resolution information on dynamic metabolic turnover can be obtained when FBMI is combined with in vivo 13C-labeled substrate infusion. Absolute metabolic fluxes, like the TCA cycle flux, can be obtained by either measuring metabolic turnover at an early time point or by measuring multiple time points on multiple animals. In the first case, metabolic fluxes can simply be obtained from linear, initial slope estimates. In the second case it becomes important to either properly co-register between different animals or to perform the analysis on well-defined regions-of-interest.
The main obstacle remaining for consistent whole brain coverage is the formation of small air pockets during microwave irradiation, presumably due to rapid vaporization of tissue water in local hotspots. While these air pockets do not impede conventional processing of microwave-irradiated tissue samples, i.e. tissue dissection, metabolite extraction and subsequent analysis by high-resolution NMR (e.g. (Chowdhury et al. 2008)) or other analytical methods, it can strongly degrade the quality of 1H MRSI data due to the strong disturbance of the local magnetic field homogeneity (see Fig. 6B). The formation of small air pockets is difficult to eliminate completely, as the number and position can vary somewhat despite constant animal body weight and FBMI settings. There is, however, a fairly consistent relationship between the total microwave irradiation power and the number of air pockets. The final setting of the irradiation power (1.2 s, 4.5 kW) was therefore chosen as to minimize the number of air pockets while still halting cerebral metabolism.
In conclusion, FBMI in combination with in situ MRI and MRS is a novel method to obtain high-resolution, high-sensitivity structural and metabolic information from rodent brain with 3-dimensional full brain coverage. Intravenous infusion of 13C-labeled glucose or acetate prior to FBMI, substrates which target neuronal or glial metabolism respectively, can be used to provide cell-specific dynamic rate information on mitochondrial energetics and neuron-glia substrate trafficking at here-to-fore unprecedented spatial resolution. The ability to anatomically register the metabolic images with in situ immunohistochemistry of expressed proteins, phosphoproteins, and neuropeptides, which are preserved by FBMI (Theodorsson et al. 1990; O'Callaghan and Sriram 2004; Che et al. 2005; Hunsucker et al. 2008) and readily measured after the metabolic image is obtained, will add new dimensions to the versatility and information rich potential of this new metabolomics approach.
ACKNOWLEDGEMENTS
This research was supported by NIH grant R21-CA118503 and R01-DK027121. The authors thank Bei Wang for expert animal preparation.
Abbreviations used
- CHESS
chemical shift selective
- FA
fractional anisotropy
- FBMI
focused beam microwave irradiation
- FOV
field-of-view
- LASER
localization by adiabatic spin-echo refocusing
- MRSI
magnetic resonance spectroscopic imaging
- RF
radiofrequency
- SWAMP
selective water suppression with adiabatic modulated pulses
- TE
echo-time
- TCA
tricarboxylic acid
- TR
repetition time
Footnotes
AUTHOR CONTRIBUTIONS
R. A. G. designed the study, performed all in vivo and in situ MR studies, processed all data and wrote the manuscript. G. M. I. C. performed the microwave fixation. P. B. B. designed and created the 1H/13C volume RF coil. D. L. R. and K. L. B. conceived the method and provided valuable inputs throughout the study.
REFERENCES
- Balcom GJ, Lenox RH, Meyerhoff JL. Regional gamma-aminobutyric acid levels in rat brain determined after microwave fixation. J Neurochem. 1975;24:609–613. [PubMed] [Google Scholar]
- Bolwig TG, Quistorff B. In vivo concentration of lactate in the brain of conscious rats before and during seizures: a new ultra-rapid technique for the freeze-sampling of brain tissue. J Neurochem. 1973;21:1345–1348. doi: 10.1111/j.1471-4159.1973.tb07589.x. [DOI] [PubMed] [Google Scholar]
- Che FY, Lim J, Pan H, Biswas R, Fricker LD. Quantitative neuropeptidomics of microwave-irradiated mouse brain and pituitary. Mol Cell Proteomics. 2005;4:1391–1405. doi: 10.1074/mcp.T500010-MCP200. [DOI] [PubMed] [Google Scholar]
- Chowdhury GMI, Banasr M, de Graaf RA, Rothman DL, Behar KL, Sanacora G. Chronic riluzole treatment increases glucose metabolism in rat prefrontal cortex and hippocampus. J Cereb Blood Flow Metab. 2008 doi: 10.1038/jcbfm.2008.78. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf RA, Nicolay K. Adiabatic water suppression using frequency selective excitation. Magn Reson Med. 1998;40:690–696. doi: 10.1002/mrm.1910400508. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, Mason GF, Patel AB, Behar KL, Rothman DL. In vivo1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 2003;16:339–357. doi: 10.1002/nbm.847. [DOI] [PubMed] [Google Scholar]
- Delaney SM, Geiger JD. Brain regional levels of adenosine and adenosine nucleotides in rats killed by high-energy focused microwave irradiation. J Neurosci Methods. 1996;64:151–156. doi: 10.1016/0165-0270(95)00119-0. [DOI] [PubMed] [Google Scholar]
- Elekes I, Patthy A, Lang T, Palkovits M. Concentrations of GABA and glycine in discrete brain nuclei. Stress-induced changes in the levels of inhibitory amino acids. Neuropharmacology. 1986;25:703–709. doi: 10.1016/0028-3908(86)90085-7. [DOI] [PubMed] [Google Scholar]
- Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153:155–177. doi: 10.1006/jmre.2001.2340. [DOI] [PubMed] [Google Scholar]
- Haase A, Frahm J, Hanicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol. 1985;30:341–344. doi: 10.1088/0031-9155/30/4/008. [DOI] [PubMed] [Google Scholar]
- Hunsucker SW, Solomon B, Gawryluk J, Geiger JD, Vacano GN, Duncan MW, Patterson D. Assessment of post-mortem-induced changes to the mouse brain proteome. J Neurochem. 2008;105:725–737. doi: 10.1111/j.1471-4159.2007.05183.x. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Wengenack TM, Reyes DA, Garwood M, Curran GL, Borowski BJ, Lin J, Preboske GM, Holasek SS, Adriany G, Poduslo JF. In vivo magnetic resonance microimaging of individual amyloid plaques in Alzheimer's transgenic mice. J Neurosci. 2005;25:10041–10048. doi: 10.1523/JNEUROSCI.2588-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KM, McIntyre S, Nixon TW, Rothman DL, de Graaf RA. Dynamic shim updating on the human brain. J Magn Reson. 2006;180:286–296. doi: 10.1016/j.jmr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Jope RS, Ferraro TN, Hare TA. Brain amino acid concentrations in rats killed by decapitation and microwave irradiation. J Neurosci Methods. 1990;31:187–192. doi: 10.1016/0165-0270(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Morgenthaler FD, Koski DM, Kraftsik R, Henry PG, Gruetter R. Biochemical quantification of total brain glycogen concentration in rats under different glycemic states. Neurochem Int. 2006;48:616–622. doi: 10.1016/j.neuint.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay K, Braun KP, de Graaf RA, Dijkhuizen RM, Kruiskamp MJ. Diffusion NMR spectroscopy. NMR Biomed. 2001;14:94–111. doi: 10.1002/nbm.686. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Sriram K. Focused microwave irradiation of the brain preserves in vivo protein phosphorylation: Comparison with other methods of sacrifice and analysis of multiple phosphoproteins. J Neurosci Methods. 2004;135:159–168. doi: 10.1016/j.jneumeth.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Olson DL, Peck TL, Webb AG, Magin RL, Sweedler JV. High-resolution microcoil 1H NMR for mass-limited nanoliter-volume samples. Science. 1995;270:1967–1970. [Google Scholar]
- Parkin MC, Wei H, O'Callaghan JP, Kennedy RT. Sample-dependent effects on the neuropeptidome detected in rat brain tissue preparations by capillary liquid chromatography with tandem mass spectrometry. Anal Chem. 2005;77:6331–6338. doi: 10.1021/ac050712d. [DOI] [PubMed] [Google Scholar]
- Pauly J, Le Roux P, Nishimura D, Macovski A. Parameter relations for the Shinnar-Le Roux selective excitation pulse design algorithm. IEEE Trans Med Imaging. 1991;10:53–65. doi: 10.1109/42.75611. [DOI] [PubMed] [Google Scholar]
- Perry TL, Hansen S, Gandham SS. Postmortem changes of amino compounds in human and rat brain. J Neurochem. 1981;36:406–410. doi: 10.1111/j.1471-4159.1981.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Ponten U, Ratcheson RA, Salford LG, Siesjo BK. Optimal freezing conditions for cerebral metabolites in rats. J Neurochem. 1973;21:1127–1138. doi: 10.1111/j.1471-4159.1973.tb07567.x. [DOI] [PubMed] [Google Scholar]
- Schmidt MJ, Schmidt DE, Robison GA. Cyclic adenosine monophosphate in brain areas: microwave irradiation as a means of tissue fixation. Science. 1971;173:1142–1143. doi: 10.1126/science.173.4002.1142. [DOI] [PubMed] [Google Scholar]
- Slotboom J, Mehlkopf AF, Bovee WM. A single-shot localization pulse sequence suited for coils with inhomogneous RF fields using adiabatic slice-selective RF pulses. J Magn Reson. 1991;95:396–404. [Google Scholar]
- Stavinoha WB, Pepelko B, Smith PW. Microwave irradiation to inactivate cholinesterase in the rat brain prior to analysis for acetylcholine. Pharmacologist. 1970;12:257. [Google Scholar]
- Stavinoha WB, Weintraub ST, Modak AT. The use of microwave heating to inactivate cholinesterase in the rat brain prior to analysis for acetylcholine. J Neurochem. 1973;20:361–371. doi: 10.1111/j.1471-4159.1973.tb12135.x. [DOI] [PubMed] [Google Scholar]
- Theodorsson E, Stenfors C, Mathe AA. Microwave irradiation increases recovery of neuropeptides from brain tissues. Peptides. 1990;11:1191–1197. doi: 10.1016/0196-9781(90)90151-t. [DOI] [PubMed] [Google Scholar]
- Veech RL, Harris RL, Veloso D, Veech EH. Freeze-blowing: a new technique for the study of brain in vivo. J Neurochem. 1973;20:183–188. doi: 10.1111/j.1471-4159.1973.tb12115.x. [DOI] [PubMed] [Google Scholar]