Abstract
The influence of hormone treatment on brain and cognition in postmenopausal women has been a controversial topic. Contradictory patterns of results have prompted speculation that a critical period, or a limited window of opportunity, exists for hormone treatment to protect against cognitive and neural decline in older women. Consistent with this hypothesis, studies in both humans and rodents indicate that the latency between the time of menopause and the initiation of hormone treatment is an important factor in determining whether hormone treatment will prevent or exacerbate cognitive impairment. In this cross-sectional study of 102 postmenopausal women, we examined whether hippocampal, amygdala, or caudate nucleus volumes and spatial memory performance were related to the interval between menopause and the initiation of hormone treatment. Consistent with a critical period hypothesis, we found that shorter intervals between menopause and the initiation of hormone treatment, as determined by self-report, were associated with larger hippocampal volumes compared with longer intervals between menopause and treatment initiation. Initiation of hormone treatment at the time of menopause was also associated with larger hippocampal volumes when compared to peers who had never used hormone treatment. Furthermore, these effects were independent from potentially confounding factors such as age, years of education, the duration of hormone treatment, current or past use of hormone therapy, the type of therapy, and the age at menopause. Larger hippocampal volumes in women who initiated hormone treatment at the time of menopause failed to translate to improved spatial memory performance. There was no relationship between the timing of hormone initiation, spatial memory performance, and amygdala or caudate nucleus volume. Our results provide support for the idea that there is a limited window of opportunity at the time of menopause for hormone treatment to influence hippocampal volume, yet the degree to which these effects translate to improved memory performance is uncertain.
Cross-sectional studies and meta-analyses have promoted the idea that hormone treatment (HT) might enhance verbal memory performance and decrease the risk for developing dementia in postmenopausal women (Maki et al., 2001; Miller et al., 2001; Sherwin, 2003). A number of small randomized clinical trials have also reported protective effects of HT on verbal memory performance (Sherwin et al., 1988; Phillips & Sherwin, 1992; Joffe et al., 2006). Consistent with this view, estrogen administration augments cholinergic transmission (Luine, 1985; Gibbs & Aggarwal, 1998), improves spatial memory performance (Korol & Kolo, 2002), and enhances dendritic spine formation in the hippocampus of rodents (Liu et al., 2008). These results support the idea that HT is a potential preventive measure against cognitive decline and dementia in postmenopausal women. However, results from large randomized clinical trials, most notably the Women’s Health Initiative (WHI) have challenged this conclusion with reports that HT use increases the risk for developing dementia and impairs memory performance (Rapp et al., 2003; Resnick et al., 2006a; 2006b, Shumaker et al., 2003; 2004).
One explanation for the contradictory findings is that a critical period, or window of opportunity, exists between menopause and the initiation of HT (Henderson, 2006; Maki, 2006; Sherwin, 2006; Sherwin & Henry, 2008). Initiation of HT at or near the time of the menopause might be protective against cognitive impairment, whereas initiation of HT many years after the menopausal transition might result in either no cognitive benefits or increased deficits (Sherwin & Henry, 2008; Henderson, 2006). In the WHI, volunteers were between 65 and 79 years of age and had been postmenopausal for about 21 years at the time of recruitment (Shumaker et al., 2004), whereas other smaller randomized clinical trials (Phillips & Sherwin, 1992) and cross-sectional studies (Maki et al., 2001) usually recruit women who initiate HT near the time of menopause (Sherwin & Henry, 2008). This source of variation could be a significant factor in explaining the contradictory findings among previous studies.
In support of this hypothesis, a study from the WHI reported that the risk for cardiovascular disease increases with an increase in years between menopause and the initiation of HT (Rossouw et a., 2007). Longitudinal studies have also reported that women who initiated HT near the time of menopause were protected against cognitive decline, whereas those women who were older when they initiated HT were not protected against cognitive decline (Matthews et al., 1999). Similarly, in the Cache County longitudinal study, women who had initiated HT soon after menopause were protected against developing Alzheimer’s disease (Zandi et al., 2002). Rodent studies also support this critical period hypothesis. For example, in aged rats, when estrogen was administered within 3-months of ovariectomy animals performed better on a hippocampal-dependent spatial memory task than control rats, but spatial memory was not enhanced when aged rats received estrogen after a 10-month delay (Gibbs, 2000; Daniel et al., 2006). In sum, evidence from both behavioral neuroscience and human cognitive studies argue that a critical period exists for HT to exert beneficial or protective effects on brain and cognition.
The enhancing effect of estrogen administration on the cellular architecture of the hippocampal formation and spatial-relational memory function in rodents is well documented (Daniel, 2006). In humans, the hippocampus atrophies at an annual rate of one to two percent per decade in late life for non-demented individuals (Raz et al., 2004a; 2005) and between a three and five percent annual decline in individuals with mild cognitive impairment and Alzheimer’s disease (Jack et al., 1998), and this deterioration is related to memory impairment (Kramer et al., 2007). It is therefore scientifically and socially important to determine whether HT might prevent or reduce volumetric decline in the hippocampus of older women. Using high-resolution magnetic resonance imaging techniques, some studies have demonstrated that HT use is associated with larger hippocampal volumes in postmenopausal women (Boccardi et al., 2006; Eberling et al., 2003; Erickson et al., 2005; Hu et al., 2006; Lord et al., 2008), however an equal number of studies have reported that hippocampal volume does not differ as a function of HT use (Eberling et al., 2004; Low et al., 2006; Raz et al., 2004b; 2004c; Sullivan et al., 2005). In one recent study, hippocampal volumes were smaller in women (mean age=77.5 years) that had been randomly assigned to receive HT compared to women who received the placebo (Resnick et al., 2009; Coker et al., 2009). This effect may have been related to the advanced age of the sample and the probable gap between menopause and hormone treatment initiation (Resnick et al., 2009). Thus, it has been speculated that this variation and inconsistency among results might be related to unaccounted individual variation in the interval between menopause and treatment initiation (Erickson & Korol, in press; Resnick et al., 2009). It has been reported that longer exposures to HT are associated with smaller prefrontal, parahippocampal, and hippocampal structures (Erickson et al., 2007; Lord et al., 2008). However, whether hippocampal volume in postmenopausal women varies as a function of the interval between menopause and HT initiation has not been examined.
Brain regions other than the hippocampus are also affected by estrogen. For example, the amygdala contains a wealth of estrogen receptors and is sensitive to estrogen administration. Estrogen administration enhances cued fear conditioning (Jasnow et al., 2006) and might elevate cell proliferation through a synergistic relationship with neurotrophin factors (Fowler et al., 2005). The caudate nucleus is another brain region that is influenced by estrogen administration. Estrogen can impair striatum-dependent response learning strategies (Korol & Kolo, 2002; Zurkovsky et al., 2007) and can augment and reduce striatal neurotransmitter function including dopamine and cholinergic release and receptor binding properties (Daniel, 2006). However, in human neuroimaging studies, neither the volume of the amygdala (Low et al., 2006; Lord et al., 2008) nor the caudate nucleus (Greenberg et al., 2006) varies as a function of HT; further, whether the volume of these structures is related to the timing of treatment initiation remains unknown.
In this study, we used a highly robust automated segmentation algorithm to measure hippocampal, amygdala, and caudate nucleus volume from high-resolution magnetic resonance images (MRI) in a cross-sectional sample of 102 postmenopausal women between 59 and 81 years of age who had either never initiated HT, initiated HT at or near the time of menopause, or had initiated HT 1–18 years post-menopause. Participants also performed a spatial memory paradigm that has been previously associated with aerobic fitness and hippocampal volume in a sample of 165 older adults (Erickson et al., in press). We examined whether hippocampal, caudate nucleus, or amygdala volume, and spatial memory performance, varied as a function of the interval between the age at menopause and the age of HT initiation. We predicted that HT initiated at or near the time of menopause would be associated with larger hippocampi and better spatial memory performance than HT initiated between 1–18 years post-menopause after controlling for confounding variables including age, years of education, hormone status, hormone type, treatment duration, and age at menopause.
Experimental Procedures
Participants
One-hundred and two postmenopausal women between 59 and 81 years of age participated in the study (mean age = 66.84; SD = 5.7). Participants were recruited from the Champaign-Urbana community from advertisements in the local newspaper, television, local radio stations, newsletters, and through family members and friends of participants. All participants were screened for dementia by the revised and modified Mini-Mental Status Examination (Stern et al., 1987) and were excluded from participation if they did not reach the required cut-off of 51 (high score of 57; 89% correct). Studies using the traditional MMSE often use a cut-off of 27 or 28 out of 30 possible points (90% cut-off) for healthy or high-functioning adults, which is in the same range as the cut-off for the modified MMSE score used in this manuscript. All participants met or surpassed all criteria for participating in a magnetic resonance imaging study including no previous head trauma, no previous head or neck surgery, no diagnosis of diabetes, no neuropsychiatric or neurological condition including brain tumors, and no metallic implants that could interfere with or cause injury due to the magnetic field. In addition, none of the women were currently receiving psychotropic medications such as anti-depressants or anxiolytics that could influence cognitive or brain function and all women had normal blood pressure. All participants were highly educated (mean years of education = 15.34; SD = 2.67). All participants signed an informed consent approved by the University of Illinois.
Hormone replacement therapy information
Thirty-seven of the participants reported never using HT (mean age = 69.46; SD = 6.39), 46 participants reported previously using HT to reduce menopausal symptoms (mean age = 65.32; SD = 4.83), and 19 participants reported currently using HT (mean age = 65.42; SD = 4.51). There were a total of 65 women who had used HT at some point in their lives. These women were included in the critical period analysis and were compared with the women who had never used HT. Age at menopause, age at which the treatment was begun, the duration of treatment, the type of treatment, and the reason for beginning HT was collected from each of the participants Age at menopause was defined for all women as 12-months after the age of their last menstrual period or the age at which their surgery took place (see Table 1). Some women began receiving HT during the perimenopausal period. If women reported receiving HT during the 12-months after the last menstrual cycle, this was categorized as receiving HT coincidentally with menopause. Thus, age at menopause could be defined by three events: (a) 12-months of amenorrhea, (b) age at HT initiation for women who had not yet experienced amenorrhea, and (c) age at bilateral oophorectomy or hysterectomy.
Table 1.
Age, years of education, duration of treatment, and age at menopause broken down by never users, and the interval between menopause and hormone initiation (D = age at hormone initiation – age at menopause). All four of these factors were included as covariates in a standard regression model to test for an association between the timing of hormone initiation and regional brain volume. Standard deviations are in parentheses and the range of values are in square brackets.
| Age | Yrs education |
Age at menopause |
Duration of treatment (yrs) |
|
|---|---|---|---|---|
| Never users | 69.46 (6.25) [59–81] |
15.23 (2.39) [12–20] |
50.67 (6.31) [35–61] |
-- |
|
HT initiation coincident with menopause (D = 0) |
64.10 (3.89) [59–74] |
14.95 (2.52) [12–20] |
47.54 (7.46) [32–60] |
14.26 (10.05) |
|
HT initiation after menopause (D = 1–18) |
67.18 (5.34) [60–78] |
16.17 (2.91) [12–24] |
45.50 (5.83) [34–61] |
8.1 (4.34) |
Twelve percent of the women with a history of hormone use had surgical menopause, while 10% of the women with no history of hormone use had surgical menopause. Of the women receiving HT, 63% received unopposed conjugated equine estrogens (CEE), 29% received CEE in combination with medroxyprogesterone acetate (MPA), and the remainder received phytoestrogens (PE). All women receiving HT reported receiving treatment to alleviate menopausal symptoms based on physician suggestion. All information pertaining to HT treatment was acquired through self-report by a questionnaire and interactions with an experimenter.
MR imaging protocol and image processing
For all participants, high-resolution (1.3 mm × 1.3 mm × 1.3 mm) T1-weighted brain images were acquired using a 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo Imaging) protocol with 144 contiguous slices collected in an ascending fashion. All images were collected on a 3T Siemens Allegra scanner with an echo time (TE) = 3.87 ms, repetition time (TR) = 1800 ms, field of view (FOV) = 256 mm, an acquisition matrix of 192 mm × 192 mm, and a flip angle of 8 degrees.
Segmentation and volumetric analysis of the left and right hippocampus, amygdala, and caudate nucleus was performed using FMRIB’s Integrated Registration and Segmentation Tool (FIRST) in FMRIB’s Software Library (FSL) version 4.0. FIRST is a semi-automated model-based subcortical segmentation tool utilizing a Bayesian framework from shape and appearance models obtained from manually segmented images from the Center for Morphometric Analysis, Massachusetts General Hospital, Boston. Subcortical structure and landmarks from 317 manually segmented and labeled T1 weighted brain images from a number of populations including children, adults and pathological populations (including schizophrenia and Alzheimer’s disease) were modeled as a point distribution model in which the geometry and variation of the shape of the structure are submitted as priors. Volumetric labels are parameterized by a 3D deformation of a surface model based on multivariate Gaussian assumptions. FIRST then searches through linear combinations of shape modes of variation for the most probable shape given the intensity distribution in the T1 weighted image (see Patenaude et al., 2007a, 2007b for further description of this method).
This method runs a two-stage affine registration of the images in native space to a standard space template (MNI space) with 1 mm resolution using 12-degrees of freedom and a mask to exclude voxels outside the subcortical regions. Next, the left and right hippocampus, left and right amygdala, and left and right caudate nucleus are segmented with 30, 50, and 30 modes of variation respectively in native space to obtain volumetric measures for each structure without warping to standard space. The modes of variation are optimized based on leave-one-out cross-validation on the training set and increases the robustness and reliability of the results (Patenaude et al., 2007b). Finally, boundary correction takes place for each structure that classifies the boundary voxels as belonging to the structure or not, based on a statistical probability (z-score > 3.00; p<.001). The volume of each structure is measured as cm3 to describe subcortical volume. The hippocampus volume comprised the dentate gyrus, the ammonic subfields (CA1-4), the prosubiculum, and the subiculum and did not include the fimbria/fornix behind the posterior commissure. The caudate nucleus volume comprised only the head of the structure. The amygdala included the central, medial, basomedial, basolateral, lateral, and cortical sections. Segmentations from each participant were visibly checked for any significant error that could have occurred during the segmentation process. No errors were noted.
Intracranial volume (ICV) is often used to adjust regional brain volume measures for sex and height (e.g. Raz et al., 2005). Here, we calculated ICV as the sum of gray, white, and cerebrospinal fluid and adjusted the hippocampal, amygdala, and caudate nucleus regions by this measure using FMRIB’s automated segmentation tool in FSL version 4.0. (Zhang et al., 2001; Smith et al. 2004). In accordance with other volumetric analyses, adjustment was performed for each region by an analysis of covariance approach: adjusted volume = raw volume – b × (ICV – mean ICV), where b is the slope of a regression of an ROI volume on ICV (Raz et al., 2005; Kennedy et al., 2008). Adjusted volume was used as a dependent variable for all analyses described in this manuscript. Previous studies have utilized similar semi-automated subcortical segmentation routines to discriminate hippocampal volumes in individuals with AD, MCI, and normal aging (e.g., Colliot et al., 2008).
Spatial memory task
We employed a spatial memory paradigm in which performance has been found to vary as a function of aging and a genetic predisposition for AD, which is associated with increased hippocampal atrophy (Greenwood et al., 2005). The spatial memory task was administered in a quiet room approximately one-week before the MRI session. First, a black fixation crosshair appeared for 1 second on white background. Participants were instructed to keep their eyes on the crosshair. Following the fixation, one, two, or three black dots appeared at random locations on the screen for 500 milliseconds. The dots were removed from the display and the fixation cross reappeared on the screen for 3 seconds. During this time, participants were instructed to try and remember the locations of the previously presented black dots. At the end of the 3-second delay, a red dot appeared on the screen in either one of the same locations as the target dots (match condition) or at a different location (nonmatch condition). Participants had 2 seconds to respond to the red dot by pressing one of two keys on a standard keyboard – the ‘x’ key for a nonmatch trial, and the ‘m’ key for a match trial. Forty trials were presented for each set size (1, 2, or 3 locations), with 20 trials as match trials and 20 trials as nonmatch trials. Participants were instructed to respond as quickly and accurately as possible. Several practice trials were performed before the task began in order to acquaint the participants with the task instructions and responses.
Statistical analyses
We examined whether adjusted left or right hippocampal volume, left or right amygdala volume, or left or right caudate nucleus volume varied as a function of the length of the interval between age at menopause and the initiation of HT (the critical period hypothesis) using multiple regression. The difference (D) between age at menopause and the age at treatment initiation was calculated to provide a measure of the critical period.
Age, years of education, age at menopause, duration of HT, and D were entered into a multiple regression model as continuous variables and current or past hormone therapy use and type of treatment (unopposed CEE, CEE with MPA, or PE) were entered as categorical variables. Age, years of education, age at menopause, duration of hormone therapy, hormone type, and hormone status (current or past use) were entered first followed by D, thereby isolating the variance of hippocampal, amygdala, or caudate nucleus volume associated with D. T-scores and standardized betas (β) from these regression analyses are presented in the Results section.
In a secondary analysis, we examined whether hippocampal, caudate nucleus, or amygdala volume differed between those that never used HT versus those that initiated HT near the time of menopause and those that initiated HT 1–18 years post-menopause. We assessed this using an independent samples t-test between each of the groups with age, years of education, and age at menopause as covariates.
Spatial memory performance measures (%correct) were examined in relation to both D and regional brain volume through a series of multiple regression analyses in which age, years of education, hormone status, hormone type, duration of treatment, and age at menopause were considered as covariates and brain volume for each region or D were factors of interest. In these analyses, spatial memory performance (% correct) for each set-size (1, 2, or 3-items) was the dependent variable.
Results
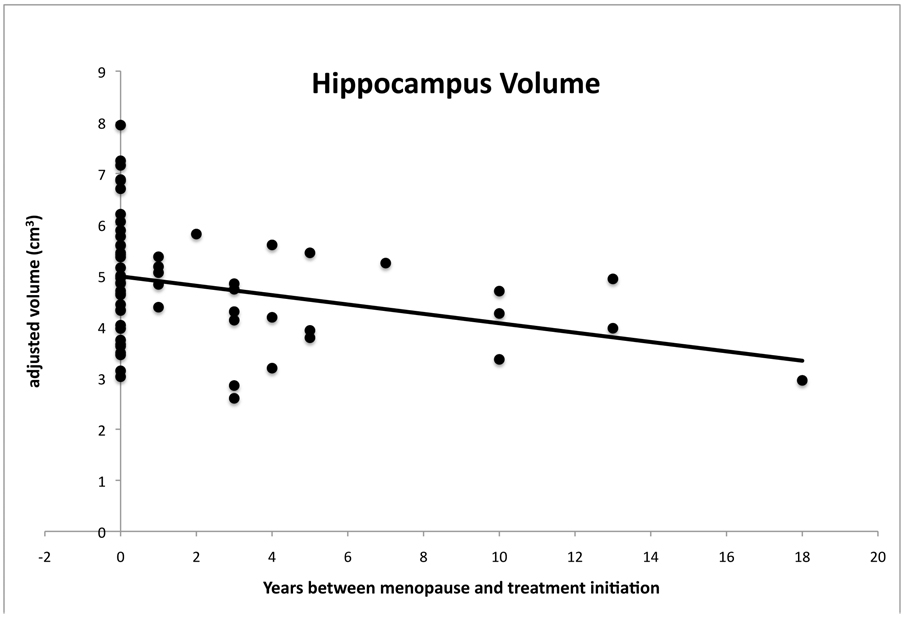
Three individuals were excluded due to artifacts in the MRI data. This left a sample size of 62 individuals who had used HT. In a multiple regression analysis with age, years of education, hormone status (current or past hormone use), hormone type (unopposed CEE, CEE with MPA, or PE), age at menopause, duration of therapy, and D entered, the overall ANOVA model was significant for the left (F (7,54) = 2.43; p<.03) and marginally significant for the right (F (7,54) = 2.10; p<.06) hippocampus. As predicted, there was a significant negative relationship between the interval between menopause and hormone initiation (D) and hippocampal volume for both left (T=−2.33; β=−.32; p<.02) and right hemispheres (T=−2.14; β=−.31; p<.03), after controlling for the variance in hippocampal volume associated with age, years of education, hormone status (current or prior), type of hormone, duration of therapy, and age at menopause. Thus, our effect can be considered statistically independent from these confounding factors. This result is clearly in line with a window of opportunity hypothesis and indicates that shorter intervals between menopause and hormone initiation are associated with larger hippocampi (see Figure 1). There were non-significant trends for age, years of education, and age at menopause in relation to hippocampal volume (p<.20) while hormone status (current, past), hormone type, and duration of therapy were unrelated to volume (both p>.60). Furthermore, we removed the one individual with the longest period between hormone initiation and menopause from the analysis to examine whether one individual was driving the observed effect. With this one individual removed, all of the effects of D as described above remained significant.
Figure 1.
A scatterplot of hippocampal volume averaged across left and right hemispheres for each participant as a function of the number of years between menopause and hormone treatment initiation. Left and right hemisphere volumes were highly correlated (r = .88). Volume values are adjusted for intracranial volume, age, age at menopause, hormone status (current vs. prior), hormone type, duration of hormone use, and years of education. Consistent with a critical period hypothesis, the plots indicate a negative relationship such that longer intervals between menopause and treatment initiation are associated with smaller hippocampal volumes. This relationship remained significant even after removing the individual with the longest interval between menopause and treatment initiation (D=18).
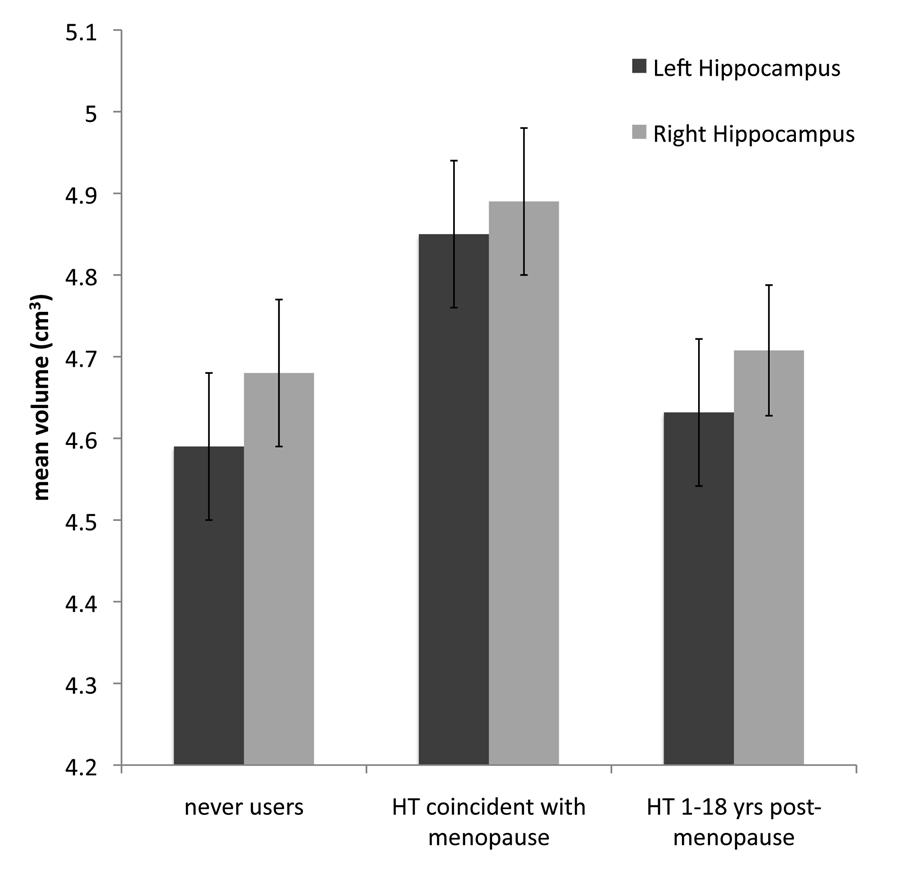
We next examined whether initiating hormone therapy coincidentally with the time of menopause was associated with larger hippocampal volumes compared to women who had never reported taking hormone therapy. For both the left and right hemispheres, those that initiated HT at the time of menopause (N=38) had larger hippocampal volumes than those that had never used HT (N=37; left: T=2.66; p<.009; right: T=2.73; p<.008) suggesting that HT use at or near the time of menopause might be associated with sparing of hippocampal volume (see Figure 2). On the other hand, there were no reliable differences between those that had never used HT and the group that initiated HT between one and eighteen years after menopause (N=24; left: T=.06; p<.95; right: T=.95; p<.34).
Figure 2.
We examined whether those that initiated hormone treatment coincidentally with age at menopause or those that initiated hormone treatment between one and eighteen years post-menopause differed in hippocampal volume from those women who had never reported receiving hormone therapy. We found that women who had initiated HT at the time of menopause had larger left (p<.009) and right (p<.008) hippocampal volumes compared to peers who had never used HT. There were no significant differences between those that had never used HT and those that initiated HT more than 1-year after menopause. Adjusted means and standard errors are represented here for each group for both left and right hippocampus volume.
We applied the regression models used for the hippocampus to assess the left and right amygdala and caudate nucleus volumes. In a multiple regression analysis with age, years of education, hormone status (current or past hormone use), hormone type (unopposed CEE, CEE with MPA, or PE), age at menopause, duration of therapy, and D entered, the overall ANOVA model was not significant for either the left (F (7,54) = 0.44; n.s.) or the right (F (7,54) = 0.36; n.s.) amygdala. Furthermore, the beta coefficients for D were not significant for either the left or right amygdala. The volume of the left (F (7,54) = 1.12; n.s.) and right (F (7,54) = 0.63; n.s.) caudate nucleus was also unrelated to D. Finally, in a secondary analysis of these brain structures when comparing those that had never used HT to those that had initiated HT at the time of menopause or between 1–18 years post-menopause, we found no reliable differences between the groups (all p>.05).
In the analyses described above, we included women in the HT group (N=5) who reported receiving PE as treatment for menopausal symptoms. However, the impact of phytoestrogens on the central nervous system in humans remains a topic of debate. Therefore, to examine whether these five women were influencing the pattern of results described above, we re-ran all analyses while excluding women who had received PE (N=57). When these five women were removed, all results described above remained unchanged.
To test whether hippocampal volume, amygdala volume, caudate nucleus volume or D was related to spatial memory performance we conducted a series of multiple regression analyses in which age, years of education, hormone status, hormone type, duration of therapy, and age at menopause were entered as covariates of no interest and either brain volume for each region or D as a factor of interest with spatial memory performance (% correct) as a dependent variable. There was no relationship between D and any of the three spatial memory set sizes (all p>.05) indicating that the interval between menopause and initiation of HT was not related to memory performance in this task (see Table 2). However, although not significant, the β values for the comparison between D and spatial memory performance were negative indicating that higher accuracy rates were related to shorter intervals between menopause and HT initiation. Age, years of education, hormone status, hormone type, duration of therapy, and age at menopause were also unrelated to spatial memory performance (all p > .05 for all memory set sizes). In an ANOVA to determine if spatial memory performance differed between those that had never used HT versus women who had initiated hormone use at the time of menopause, we found that there were no differences between the groups for either of the three set-sizes (all p >.05). Finally, neither the hippocampus, amygdala, nor caudate nucleus volumes were related to performance for any of the three memory set sizes (all p >.05).
Table 2.
Summary of spatial memory accuracy rates broken down by the interval between menopause and hormone initiation (D = age at hormone initiation – age at menopause) for each set-size (1-item, 2-item, 3-item), and for never users. There were no statistically significant relationships between spatial memory performance and the time between menopause and hormone initiation or regional brain volume. Standard deviations are in parentheses.
| 1-item accuracy | 2-item accuracy | 3-item accuracy | |
|---|---|---|---|
| Never users | .87 (.04) | .79 (.04) | .72 (.04) |
|
HT initiation coincident with menopause (D = 0) |
.88 (.03) | .82 (.03) | .79 (.03) |
|
HT initiation after menopause (D = 1–18) |
.86 (.03) | .79 (.04) | .76 (.04) |
Discussion
Current theories propose that the time between menopause and hormone initiation is a critical period in which a limited window of opportunity exists for hormone therapy to exert any beneficial effects on cognitive and brain health (Henderson, 2006; Maki, 2006, Sherwin, 2006; Sherwin & Henry, 2008). We, and others, have proposed that a window of opportunity might explain variation in hippocampal volume in postmenopausal women (Erickson & Korol, in press; Resnick et al., 2009). Consistent with this hypothesis, we found that women who began HT at the time of menopause had larger hippocampi than women who initiated HT 1–18 years after menopause. Furthermore, women who initiated HT coincidentally with menopause demonstrated larger hippocampal volumes than their peers who had never taken hormone therapy. Importantly, our effects can be considered independent from the age of the individuals, the number of years of education, the duration of treatment, whether women were current or past users of hormone treatment, the type of treatment they received, or the age at which they went through menopause. Therefore, our inclusion of potentially confounding factors in the statistical model strengthens the argument that there is a limited window of opportunity in which HT might spare hippocampal volume shrinkage. Furthermore, the relation between hippocampal volume and the time between menopause and hormone initiation was unaffected after excluding individuals that received phytoestrogens. Finally, the interval between menopause and treatment initiation did not explain variability in either amygdala or caudate nucleus volume suggesting that there is some regional specificity to these effects.
This result is in line with both human cognitive data (Matthews et al., 1999; Zandi et al., 2002) and rodent data (Daniel et al., 2006; Bohacek et al., 2008) on the effects of timing initiation on regulating estrogenic effects. Although the mechanisms by which the timing of treatment initiation moderates cognitive and brain function remains unknown, one possibility is that long-term estrogen deprivation disrupts cholinergic function that cannot be augmented with delayed administration. A recent study in rodents supports this hypothesis (Bohacek et al., 2008). Specifically, estrogen administration at the time of ovariectomy increased levels of choline acyetyltransferase, a critical enzyme involved in cholinergic function, in the hippocampus but not the prefrontal cortex in both young and middle-aged rodents. However, estrogen administration five-months after ovariectomy failed to increase choline acetyltransferase levels in the hippocampus but reliably increased levels in the prefrontal cortex (Bohacek et al., 2008). This argues that estrogen deprivation for long periods can disrupt cholinergic function, and this disruption cannot be reversed with a delay in estrogen administration. Second, it argues for a reformulation of the critical period hypothesis such that the window of opportunity for experiencing a beneficial effect of estrogen on brain and cognition is dependent on the brain region examined. Such an argument suggests that some cognitive functions might be unrelated or unaffected by the timing of the initiation of treatment, while other functions might be enhanced by a delay in administration. This hypothesis is in line with the results presented in this study as well as other studies in rodents (Korol & Kolo, 2004) and humans (Erickson et al., 2007; Raz et al., 2004b) that argue that HT has regionally specific effects that might be related to estrogen receptor concentration, the rate of age-related brain deterioration, or a host of other factors including aerobic activity levels (Daniel, 2006; Erickson et al., 2007; Erickson & Korol, in press).
It is also important to note that although rodent studies find that estrogen administration enhances spatial memory (Korol & Kolo, 2002), human studies of HT rarely find HT-related enhancements of spatial memory and instead tend to report enhancements of verbal memory (Maki et al., 2001; Sherwin, 2003). Therefore, we can only speculate that a critical window of opportunity in HT might influence verbal memory function differently than spatial memory. It is important for future research to examine this possibility.
Site specificity might explain why we failed to find an association between the timing of hormone initiation and spatial memory performance. In a prior study with a larger sample size we demonstrated that better spatial memory performance, for both men and women, on this spatial memory task was related to larger hippocampal volumes (Erickson et al. in press). However, in a mediation analysis we found that the volume of the hippocampus only partially mediated performance, suggesting that performance on this task is dependent upon a network of brain regions that includes, but is not limited to, the hippocampus. Indeed, this task includes working memory, goal maintenance, and executive control processes that are frequently associated with prefrontal cortex function in addition to hippocampal function (D’Esposito et al., 1998). Therefore, it is likely that prefrontal cortical processes that are not moderated by the time of hormone initiation, are additionally mediating performance on this task. It will be important for future studies to examine whether other cognitive processes and brain regions are related to the timing of hormone treatment initiation relative to menopause.
Also consistent with the idea of site specificity of HT, we found that the timing of HT initiation relative to the age at menopause was unrelated to caudate nucleus or amygdala volume, a finding consistent with other MRI studies of HT on the volume of these regions (Low et al., 2006; Lord et al., 2008). This null finding strengthens the claim that HT works in a site-specific fashion in the brain, affecting some regions and not others. Thus, although both the caudate nucleus and amygdala are responsive to estrogen manipulations in rodents, neither HT nor the timing of HT initiation relative to menopause can explain variation in the volume of these regions.
Our results can also be interpreted within the context of an ancillary study of the WHI that examined the effect of CEE with or without MPA treatment on volumetric measurements of the hippocampus and prefrontal cortex in more than 1400 women over 65 years of age (Resnick et al., 2009; Coker et al., 2009). In this large study, women receiving CEE alone or in combination with MPA displayed reduced prefrontal and hippocampal volume. The authors of this study point out that their results may have been influenced by the age at hormone initiation, which in this sample occurred many years post-menopause (Resnick et al., 2009) and suggest that a limited window of opportunity may be an important factor in resolving the apparent discrepancies among studies examining the effects of hormone therapy on brain volume (e.g. Erickson et al., 2005; Low et al, 2006; Raz et al., 2004b; Lord et al., 2008).
The timing of treatment initiation relative to the age of menopause is dependent on the definition of these events. In this study, age at menopause was defined in standard ways, that is – 12-months of amenorrhea, age at surgical menopause, or age at HT initiation if occurring before amenorrhea. Although our effects were statistically independent of age at menopause, it is possible that alternative definitions for these events could influence the pattern of results.
It is important to point out that our study was cross-sectional. Therefore, it is impossible to rule out all possible third variables that may covary with hormone treatment or the timing of hormone treatment initiation. Confounding by indication or severity, selection biases, and a healthy-user bias, may explain some of the relationships described in this manuscript. It is also impossible to determine whether any differences in hippocampal volume pre-existed before the start of HT. In short, it is impossible to determine causality within a cross-sectional design. Longitudinal and clinically randomized trials in which the timing of hormone initiation is manipulated relative to the age at menopause would be nice follow-ups to the current study that would help determine causal relationships between the window of opportunity and hippocampal volume. An additional limitation of our study is that our results were dependent on self-report measurements of when HT was begun, the age at which HT was discontinued, the age of menopause, and the type of HT received. The accuracy of autobiographical memories is always a valid concern in studies that rely on retrospective information and the results from this study are not immune to this issue. However, at least one study has demonstrated a high degree of accuracy in the recall of medical information over a 50-year span (Berney & Blane, 1997). Nonetheless, it will be important for future studies to address whether older post-menopausal women accurately recall certain points in their menopausal transition that may have occurred 20 or more years earlier.
In sum, we demonstrate that hippocampal, but not amygdala or caudate nucleus, volume in postmenopausal women varied as a function of the interval between menopause and HT initiation such that shorter latencies between menopause and HT were associated with larger hippocampal volumes. Postmenopausal women who either never used hormone therapy or initiated hormone therapy at least one year after menopause, failed to show sparing of hippocampal volume. Although these effects did not translate to better spatial memory performance, spared hippocampal volume in an aging population is an important finding given the rate of hippocampal decay in both non-demented (Raz et al., 2004a) and demented elderly individuals (Jacks et al., 1998) and the search for factors that moderate the rate of hippocampal decay.
Acknowledgements
The conduct of this study was supported by grants from the National Institute on Aging (RO1 AG25667 and RO1 AG25302). We would like to thank the following people for their assistance during data collection: Susan Herrel, Nancy Dodge, Holly Tracy, Dawn Epstein, Zuha Warraich, Jennifer Kim, Maritza Alvarado, Heloisa Alves, Edward Malkowski, Susie Heo, and Jason Lewis.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu.
References
- Berney LR, Blane DB. Collecting retrospective data: accuracy of recall after 50 years judged against historical records. Soc Sci Med. 1997;45:1519–1525. doi: 10.1016/s0277-9536(97)00088-9. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Ghidoni R, Govoni S, Testa C, Benussi L, Bonetti M, Binetti G, Frisoni GB. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a voxel-based morphometry study. Menopause. 2006;13:584–591. doi: 10.1097/01.gme.0000196811.88505.10. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–1027. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- Coker LH, Hogan PE, Bryan NR, Kuller LH, Margolis KL, Bettermann K, Wallace RB, Lao Z, Freeman R, Stefanick ML, Shumaker SA. Postmenopausal hormone therapy and subclinical cerebrovascular disease. The WHIMS-MRI Study. Neurology. 2009;72:125–134. doi: 10.1212/01.wnl.0000339036.88842.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colliot O, Chetalat G, Chupin M, Desgranges B, Magnin B, Benali H, Dubois B, Garnero L, Eustache F, Lehericy S. Discrimination between Alzheimer disease, mild cognitive impairment, and normal aging by using automated segmentation of the hippocampus. Radiology. 2008;248:194–201. doi: 10.1148/radiol.2481070876. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Ballard D, Aguirre GK, Zarahn E. Human prefrontal cortex is not specific for working memory: a functional MRI study. Neuroimage. 1998;8:274–282. doi: 10.1006/nimg.1998.0364. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Wu C, Haan MN, Mungas D, Buonocore M, Jagust WJ. Preliminary evidence that estrogen protects against age-related hippocampal atrophy. Neurobiol Aging. 2003;24(5):725–732. doi: 10.1016/s0197-4580(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage. 2004;21(1):364–371. doi: 10.1016/j.neuroimage.2003.08.037. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Raz N, Korol DL, Scalf P, Webb A, Cohen NJ, McAuley E, Kramer AF. Selective sparing of brain tissue in postmenopausal women receiving hormone replacement therapy. Neurobiol Aging. 2005;26(8):1205–1213. doi: 10.1016/j.neurobiolaging.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, Kramer AF. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging. 2007;28(2):179–185. doi: 10.1016/j.neurobiolaging.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Korol DL. The effects of hormone replacement therapy on the brains of postmenopausal women: a review of human neuroimaging studies. In: Chodzko-Zajko W, Kramer AF, editors. Aging, exercise and cognition: The effect of exercise and other interventions on cognition in older adults. (in press) [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. doi: 10.1002/hipo.20547. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles. J Comp Neurol. 2005;489:166–179. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Aggarwal P. Estrogen and basal forebrain cholinergic neurons: implications for brain aging and Alzheimer’s disease-related cognitive decline. Horm Behav. 1998;34:98–111. doi: 10.1006/hbeh.1998.1451. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results from the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DL, Payne ME, MacFall JR, Provenzale JM, Steffens DC, Krishnan RR. Differences in brain volumes among males and female hormone-therapy users and nonusers. Psychiatry Res. 2006;147:127–134. doi: 10.1016/j.pscychresns.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Estrogen-containing hormone therapy and Alzheimer’s disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138(3):1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Hu L, Yue Y, Zuo PP, Jin ZY, Feng F, You H, Li ML, Ge QS. Evaluation of neuroprotective effects of long-term low dose hormone replacement therapy on postmenopausal women brain hippocampus using magnetic resonance scanner. Chin Med Sci J. 2006;21(4):214–218. [PubMed] [Google Scholar]
- Jack CR, Jr, Peterson RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E. Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13(3):411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: A possible window of opportunity effect. Neurobiol Aging. 2008;29:95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Low LF, Anstey KJ, Maller J, Kumar R, Wen W, Lux O, Salonikas C, Naidoo D, Sachdev P. Hormone replacement therapy, brain volumes and white matter in postmenopausal women aged 60–64 years. Neuroreport. 2006;17(1):101–104. doi: 10.1097/01.wnr.0000194385.10622.8e. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psychiatry. 2001;158(2):227–233. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138(3):1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Cauley J, Yaffe K, Zmuda JM. Estrogen replacement therapy and cognitive decline in older community women. J Am Geriatr Soc. 1999;47:518–523. doi: 10.1111/j.1532-5415.1999.tb02563.x. [DOI] [PubMed] [Google Scholar]
- Miller MM, Monjan AA, Buckholtz NS. Estrogen replacement therapy for the potential treatment or prevention of Alzheimer’s disease. Ann NY Acad Sci. 2001;949:223–234. doi: 10.1111/j.1749-6632.2001.tb04025.x. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy D, Jenkinson M. FIRST-FMRIB’s integrated registration and segmentation tool. In Human Brain Mapping Conference, 2007. 2007a [Google Scholar]
- Patenaude B, Smith SM, Kennedy D, Jenkinson M. Technical report TR07BP1. FMRIB Centre – University of Oxford; 2007b. Bayesian shape and appearance models. [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Rapp S, Espeland M, Shumaker S, Henderson V, Brunner R, Manson J, Gass M, Stefanick M, Lane D, Hays J, Johnson K, Coker L, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004a;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Hormone replacement therapy and age-related brain shrinkage: regional effects. Neuroreport. 2004b;15(16):2531–2534. doi: 10.1097/00001756-200411150-00020. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004c;25(3):377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J. Clin. Endocrinol. Metab. 2006a;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women–s Health Initiative Study of Cognitive Aging (WHISCA): A randomized clinical trial of the effects of hormone therapy on age-related cognitive decline. Clinical Trials. 2006b;1:440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Ockene J, Davatzikos C. Postmenopausal hormone therapy and regional brain volumes. Neurology. 2009;72:135–142. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24(2):133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138(3):1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Frontiers in Neuroendocrinology. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Thal L, Wallace R, Ockene J, Hendrix S, Jones B, III, Assaf A, Jackson R, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp S, Thal L, Lane D, Fillit H, Stefanick M, Hendrix S, Lewis CB, Masaki K, Coker L. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Mathews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulson J, Mayeau R. Modified mini-mental state examination: validity and reliability. Neurology. 1987;37:179. [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging. 2005;26(7):1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson ML, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS. Hormone replacment therapy and incidnce of Alzhimer’s disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and expectation maximization algorithm. IEEE Trans on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]