Abstract
Hypothesis
The physiological increase in muscle protein anabolism induced by insulin is blunted in healthy, glucose tolerant older adults. We hypothesized that the age-related defect in muscle protein anabolism is a true insulin resistance state and can be overridden by supraphysiological hyperinsulinemia.
Methods
We used dye dilution, stable isotopic and immunoblotting techniques to measure leg blood flow, muscle protein synthesis, Akt/mTOR signaling, and amino acid kinetics in 14 healthy, glucose tolerant older subjects at baseline, and during an insulin infusion at postprandial (PD, 0.15 mU·min-1·100 ml-1) or supraphysiological high (HD, 0.30 mU·min-1·100 ml-1) doses.
Results
Leg blood flow, muscle protein synthesis, and Akt/mTOR signaling were not different at baseline. During hyperinsulinemia leg blood flow (P<0.01) and muscle protein synthesis increased only in the HD group only (PD: 0.063±0.006 to 0.060±0.005; HD: 0.061±0.007 to 0.098±0.007; %·h-1; P<0.01). Muscle Akt phosphorylation increased in both groups, but tended to be larger in HD (P=0.07). S6K1 phosphorylation increased only in the HD group (P<0.05). Amino acid net balance across the leg improved in both groups, but a net anabolic effect was observed only in HD (P<0.05).
Conclusion
We conclude that supraphysiological hyperinsulinemia is necessary to stimulate muscle protein synthesis and anabolic signaling in healthy older individuals, suggesting the existence of a true age-related insulin resistance of muscle protein metabolism.
Keywords: Aging, Sarcopenia, Insulin, Insulin Resistance, Muscle Protein Synthesis
Introduction
Physiological hyperinsulinemia stimulates muscle protein synthesis and anabolism in young subjects regardless of glucose tolerance as long as amino acid delivery to the muscle is not decreased (1-9). However, aging is associated with an inability of insulin to stimulate muscle protein synthesis and net amino acid uptake in healthy, glucose tolerant persons (8;10). This defect is associated with endothelial dysfunction and a blunted mammalian target of rapamycin (mTOR) signaling, which limit muscle perfusion and protein anabolism during the hyperinsulinemic stimulus in older adults (8;10;11). The decline in the muscle protein anabolic response to insulin is likely responsible for the observed reduction in muscle protein anabolism following mixed feeding in elders (12;13), thus contributing to the development of muscle loss of aging (sarcopenia). Sarcopenia, in turn, is an important contributor to frailty and disability of aging (14), and it has been recently estimated that its healthcare cost may be comparable to that of osteoporosis (15).
We have recently demonstrated that an insulin-sensitizing intervention, aerobic exercise, can acutely restore a normal protein anabolic response to physiological hyperinsulinemia in older individuals with normal glucose tolerance (11), suggesting that this age-related defect shares at least some features with the classical insulin resistance of type 2 diabetes. However, the inability of muscle protein metabolism to mount an anabolic response during physiological hyperinsulinemia in otherwise healthy elders cannot be defined as an insulin resistance state unless it is demonstrated that insulin concentrations above the physiologic range can produce a skeletal muscle anabolic effect.
The purpose of this study was to determine if exposure of skeletal muscle to insulin concentrations above the physiologic postprandial range could induce a protein anabolic response in healthy older adults.
Methods
Participants
We studied 14 older subjects (11 men and 3 women) from the greater Galveston/Houston area and the Los Angeles metropolitan area. All volunteers provided informed written consent before participating in the study, which was approved by the Institutional Review Boards of the University of Texas Medical Branch at Galveston (UTMB) and the University of Southern California, Los Angeles (USC). All volunteers were healthy and independent (no impairments in the activities of daily living or instrumental activities of daily living), but were not engaged in an exercise training program. Screening was performed with clinical history, physical examination and number of laboratory tests including a 2-hour 75 g oral glucose tolerance test. Only volunteers with screening results within normal limits were randomly assigned to one of two groups receiving either a postprandial (PD) or high-dose (HD) insulin infusion. Characteristics of enrolled volunteers are summarized in Table 1. This experiment was part of a larger randomized study in which young and older individuals were assigned to various treatments. Data from six volunteers in the PD group have been included in ref. (10). Primary outcome data (blood flow and muscle protein synthesis) obtained in young, body mass index (BMI)-matched volunteers (n=7, age 31±2 years, BMI 26.2±1.4 kg/m2, P=NS vs. older volunteers by one way ANOVA) undergoing the same postprandial insulin infusion experiment as the PD group are also reported.
Table 1.
Characteristics of the older volunteers undergoing a local insulin infusion in one leg at postprandial or high dose.
| Subject characteristics | Postprandial Dose | High Dose | P |
|---|---|---|---|
| Sex | 6M, 2F | 5M, 1F | 0.70 |
| Age (years) | 69 ± 1 | 72 ± 2 | 0.14 |
| Weight (kg) | 83 ± 3 | 86 ± 4 | 0.56 |
| Height (cm) | 176 ± 4 | 168 ± 3 | 0.14 |
| BMI (kg/m2) | 27 ± 1 | 30 ± 1 | 0.06 |
| Lean Body Mass (kg) | 54 ± 3 | 56 ± 2 | 0.56 |
| Fat Mass (kg) | 21 ± 2 | 26 ± 3 | 0.17 |
| Leg volume (liters) | 9.3 ± 0.4 | 9.0 ± 0.7 | 0.63 |
Values are the mean ± SE
Study design
We measured blood flow, muscle protein, amino acid and glucose kinetics, and components of the Akt/mTOR signaling pathway in the post-absorptive basal state (0-5 hours) and during insulin infusion (5-8 hours). The afternoon prior to the infusion study, each subject was admitted to the UTMB or USC General Clinical Research Center. At 1830 hr, all volunteers were fed a standardized dinner (1/3 of their estimated daily energy requirements; 60% carbohydrate, 20% protein and 20% fat), and given a snack at 2200 hr after which they were allowed only water ad libitum until the end of the experiment. The next morning, catheters were inserted into a forearm vein for tracer and dextrose infusion, in a contralateral hand vein for arterialized blood sampling, and in the femoral artery and vein of one leg for blood sampling. The arterial catheter was also used for infusion of insulin (Humulin R, Eli Lilly, Indianapolis, IN) and indocyanine green (ICG, Akorn Inc., Buffalo Grove, IL).
At 0730 hr, after drawing a blood sample to measure background phenylalanine enrichment and ICG concentration, a primed (2 μmol/kg) continuous infusion of L-[ring-13C6]phenylalanine (0.05 μmol·kg-1·min-1, Cambridge Isotope Laboratories, Andover, MA) was started and maintained until the end of the experiment. Two hours after initiation of the stable isotope infusion, we took the first muscle biopsy from the lateral portion of the vastus lateralis of the leg bearing the femoral catheters, using a 5 mm Bergström biopsy needle, aseptic procedure and local anesthesia with 1% lidocaine injected subcutaneously and on the muscle fascia. The 150-400 mg muscle sample was quickly rinsed with ice-cold saline, blotted, and immediately frozen in liquid nitrogen and stored at -80°C until analysis. During the 5th hour of tracer infusion, ICG was infused in the femoral artery (0.5 mg/min); four blood samples were taken at 10 min interval from femoral and hand vein to measure ICG concentration, after which another series of four blood samples were taken from femoral artery and vein to measure glucose concentrations and phenylalanine concentrations and enrichments. Four additional samples were also drawn from hand and femoral vein to measure systemic and femoral insulin concentrations, respectively. A second muscle biopsy was taken at the end of the 5th hour as previously described.
Immediately after the second biopsy, an insulin infusion was started directly into the femoral artery at the following infusion rates: PD = 0.15 mU·min-1·100ml-1 (∼0.2 mU·kg-1·min-1); HD = 0.30 mU·min-1·100ml-1 (∼0.4 mU·kg-1·min-1). This technique allowed us to avoid the remarkable reduction in blood amino acid concentrations typically seen during systemic insulin infusion (1;16). A euglycemic clamp was performed infusing 20% dextrose at a variable rate, which was adjusted based on blood glucose concentrations measured every 5-10 min.
During the 8th and last hour of the experiment, ICG was infused again to measure leg blood flow, and blood samples were taken to measure ICG concentrations, phenylalanine and glucose enrichments and concentrations, and insulin concentrations, as described above for the basal period. At 480 min, before stopping the insulin and tracer infusion, a third muscle biopsy was taken as described above.
Analytical Methods
Plasma glucose concentration was measured using an automated enzymatic method (YSI, Yellow Springs, OH), and serum ICG concentration was measured spectrophotometrically at λ=805 nm (Beckman Coulter, Fullerton, CA) (17;18).
Blood phenylalanine concentrations and enrichments were determined by gas chromatography-mass spectrometry (GCMS, Agilent Technologies, Palo Alto, CA) as previously described (19). Phenylalanine concentration was used as a predictor of insulin-induced changes in the concentration of all essential amino acids (20).
Muscle tissue free amino acids and proteins were extracted as previously described (19), and intracellular free phenylalanine concentrations and enrichments of were determined by GCMS as previously described (19). Mixed muscle protein-bound phenylalanine enrichment was analyzed by GCMS after protein hydrolysis and amino acid extraction (19) using the external standard curve approach (21).
Immunoblotting was performed on ∼50 mg of frozen tissue after homogenization (22). Total protein concentration was determined using the Bradford assay (Smartspec Plus, BioRad, Hercules, CA). The supernatant was diluted (1:1) in a sample buffer mixture then boiled for 3 min at 100°C, except 4E-BP1. Samples were separated by electrophoresis (Criterion, BioRad, Hercules, CA); gels contained an internal loading control (rodent skeletal muscle) and molecular weight ladder (Precision Plus, BioRad, Hercules, CA). Proteins were then transferred to a polyvinylidene difluoride membrane (BioRad, Hercules, CA) and incubated in primary antibody overnight at 4°C. The next morning, blots were incubated in secondary antibody for 1h. Chemiluminescent solution (ECL plus, Amersham BioSciences, Piscataway, NJ) was applied to each blot and optical density measurements were obtained (ChemiDoc, BioRad, Hercules, CA). Densitometric analysis was performed using Quantity One 4.5.2 software (BioRad, Hercules, CA). Membranes were stripped using Restore Western blot Stripping Buffer (Pierce Biotechnology, CA) and reprobed for total protein. Density values were normalized to the internal loading control and data was expressed as phospho divided by total protein. Primary antibodies were purchased from Cell Signaling (Beverly, MA). Phospho antibody concentrations were: 1:1000 for Akt (Ser473), mTOR (Ser2448), 4E-BP1 (Thr37/46), eukaryotic elongation factor 2 (eEF2) (Thr56); and 1:500 for phospho-p70 S6K1 (Thr389). Antibody dilutions for all total proteins were 1:1000. Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from Amersham Bioscience (1:2000).
Calculations
Mixed muscle protein fractional synthetic rate (FSR) was calculated using the precursor-product model to calculate the synthesis rate as follows (23):
where ΔEP is the increment in protein-bound phenylalanine enrichment between two sequential biopsies, t is the time between the two sequential biopsies, EM(1) and EM(2) are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsies. Data are expressed as percent per hour.
Muscle phenylalanine kinetic parameters were calculated using both the two- and the three-pool arteriovenous balance models since each model provides unique information regarding leg plasma (two-pool) and intracellular (three-pool) phenylalanine kinetics (1). The models' assumptions and validation are extensively reviewed in ref.(24). The two- and three-pool model parameters were calculated as previously described (11). Phenylalanine utilization for protein synthesis and release from breakdown during hyperinsulinemia were corrected by changes in phenylalanine intracellular concentration as previously described (25).
Leg glucose utilization was calculated as net glucose uptake across the leg:
Changes in blood flow can affect insulin concentration during exogenous infusion. Moreover, insulin recycling from the systemic circulation can increase insulin delivery to the leg. Thus, we calculated the total insulin delivery rate to the leg (10) as follows:
We used the femoral insulin concentration (InsFV) in the calculation because arterial insulin concentration was not measurable due to utilization of the arterial catheter for insulin infusion. Because some insulin is cleared by the muscle tissue (26), this method may slightly underestimate the total insulin delivery rate. Nonetheless it provides an additional means to evaluate the degree of muscle insulinization.
Statistical analysis
Statistical analysis was performed using SigmaStat ver. 3.5. Primary outcome measures were blood flow and FSR. Sample size was calculated to detect between group differences of 50% and 20% in the change from baseline of FSR and blood flow, respectively, with α=0.05 and β=0.80. The Kolmogorov-Smirnov test (with Lilliefors' correction) was used for normality testing. Volunteers' characteristics and baseline values for all measured variables were analyzed using the Student's t-test or the Rank Sum test when data failed the normality test. The effect of the insulin dose on the response variables was analyzed using ANOVA for repeated measures, the main effects being group (PD, HD) and time (basal, insulin infusion). If the data failed the normality test, log transformation was performed before conducting ANOVA in order to achieve reasonably normal distribution.. If the baseline values were significantly different between groups, we performed ANCOVA, using the baseline value as covariate. Pairwise multiple comparisons were performed using the Tukey-Kramer test. Differences were considered significant at P<0.05. P for trend was set at P<0.10. Data are the mean ± SE.
Results
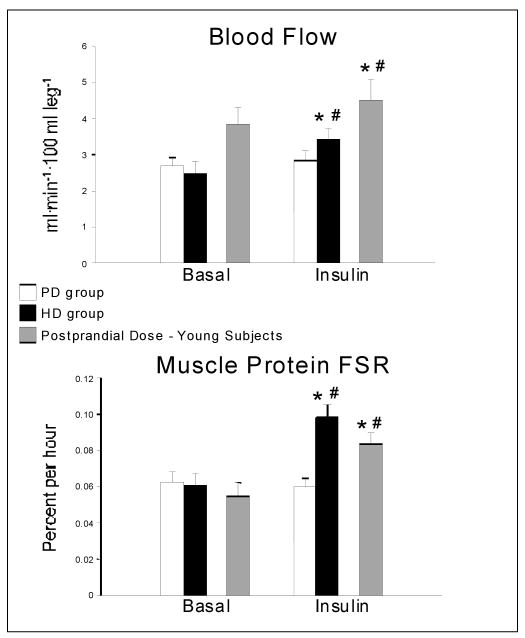
Blood flow (Figure 1)
Figure 1.
Blood flow and muscle protein fractional synthetic rate (FSR) in older volunteers at baseline and during the intra-arterial infusion of insulin at two different rates, which were chosen to increase the femoral vein insulin concentrations to either a postprandial level (PD: n=8) or a supraphysiological high level (HD: n=6). Data from a group of younger (n=7, age 31 ± 2), BMI-matched volunteers undergoing an insulin infusion at postprandial dose are also reported. Values are the mean±SE. *P<0.05 vs. Basal; #P<0.01 vs. PD
Leg blood flow was not different between groups at baseline and increased significantly only in the HD group (P<0.01) with a power of 0.86. The change in blood flow in the HD group was comparable to that observed in younger volunteers during a postprandial insulin infusion.
Insulin and glucose (Table 2)
Table 2.
Insulin and glucose concentrations and leg kinetics in two groups of healthy older volunteers undergoing a local insulin infusion in one leg at postprandial or high dose.
| Postprandial Dose | High Dose | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Basal | Insulin | Basal | Insulin | Group | Time | Interaction | |
| Insulin | |||||||
| Systemic concentration (pmol/L) | 53 ± 9 | 149 ± 23 | 37 ± 9 | 190 ± 21 | 0.57 | <0.0001 | 0.07 |
| Femoral vein concentration (pmol/L) | 53 ± 9 | 552 ± 42 | 37 ± 9 | 920 ± 155 | 0.03 | <0.0001 | 0.02 |
| Delivery to the leg (pmol ·min-1·100 ml leg-1) | 134 ± 24 | 1,493 ± 61 | 90 ± 23 | 3,085 ± 535 | <0.01 | <0.0001 | <0.01 |
| Glucose | |||||||
| Arterial concentration (mmol/L) | 4.8 ± 0.2 | 4.6 ± 0.2 | 5.2 ± 0.2 | 5.0 ± 0.2 | 0.28 | 0.18 | 0.54 |
| Infusion rate (μmol·kg·-1min-1) | 11.5 ± 1.0 | 16.9 ± 1.3 | <0.01 | ||||
| Leg uptake (μmol·min-1·100 mL leg-1) | 1.8 ± 0.4 | 3.5 ± 0.5 | 0.01 | ||||
Data are the mean ± SE. Boldface values indicate statistical significance
Systemic and femoral vein insulin concentrations were not different at baseline and significantly increased in both groups during insulin infusion (P<0.0001), but the change was larger in the HD group (P<0.05). Insulin delivery to the leg was not different between groups at baseline and it increased significantly during insulin infusion in both groups (P<0.0001), with a larger effect (P<0.01) in the HD group.
Plasma arterial glucose concentrations were not different between groups at baseline and did not change during insulin infusion with euglycemic clamp. However, the exogenous glucose infusion rate and leg glucose uptake during insulin infusion were significantly higher in the HD group (P<0.05).
Phenylalanine concentrations and enrichments (Table 3)
Table 3.
Phenylalanine concentrations and enrichments at baseline and during insulin infusion in two groups of healthy older volunteers undergoing a local insulin infusion in one leg at postprandial or high dose.
| Postprandial Dose | High Dose | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Basal | Insulin | Basal | Insulin | Group | Time | Interaction | |
| Concentrations (μmol/L) | |||||||
| Femoral Artery | 60 ± 2 | 55 ± 1 | 61 ± 3 | 57 ± 4 | 0.68 | <0.001 | 0.39 |
| Femoral Vein | 66 ± 2 | 55 ± 1 | 68 ± 3 | 55 ± 4 | 0.81 | <0.0001 | 0.12 |
| Muscle Tissue | 88 ± 6 | 66 ± 6 | 81 ± 5 | 72 ± 4 | 0.90 | <0.01 | 0.22 |
| Enrichments (tracer tracee ratio %) | |||||||
| Femoral Artery | 9.2 ± 0.2 | 10.3 ± 0.2 | 9.7 ± 0.2 | 10.4 ± 0.2 | 0.70 | <0.001 | 0.33 |
| Femoral Vein | 6.7 ± 0.1 | 8.1 ± 0.1 | 7.0 ± 0.1 | 8.5 ± 0.1 | 0.64 | <0.01 | 0.77 |
| Muscle Tissue | 5.2 ± 0.3 | 6.2 ± 0.3 | 5.9 ± 0.4 | 6.7 ± 0.4 | 0.27 | <0.05 | 0.81 |
Units are μmol/L. Values are the mean ± SE. Boldface values indicate statistical significance.
Phenylalanine concentrations in the artery, in the vein and in the muscle were not different at baseline, and slightly but significantly decreased in both groups (P<0.05) with no differences between groups. Phenylalanine enrichments in the femoral artery and vein and in the muscle tissue were not different between groups in the basal period, and were at steady state during both sampling periods (data not shown). Phenylalanine enrichment in the femoral artery, vein and muscle increased slightly but significantly (P<0.05) during insulin infusion in both groups, with no group differences.
Mixed muscle FSR (Figure 1)
Mixed muscle protein FSR (%/hr) was not different between groups in the basal state and significantly increased in the HD group only (P<0.05) with a power of 0.83. The change in mixed muscle FSR in the HD group was comparable to that observed in younger volunteers during a postprandial insulin infusion.
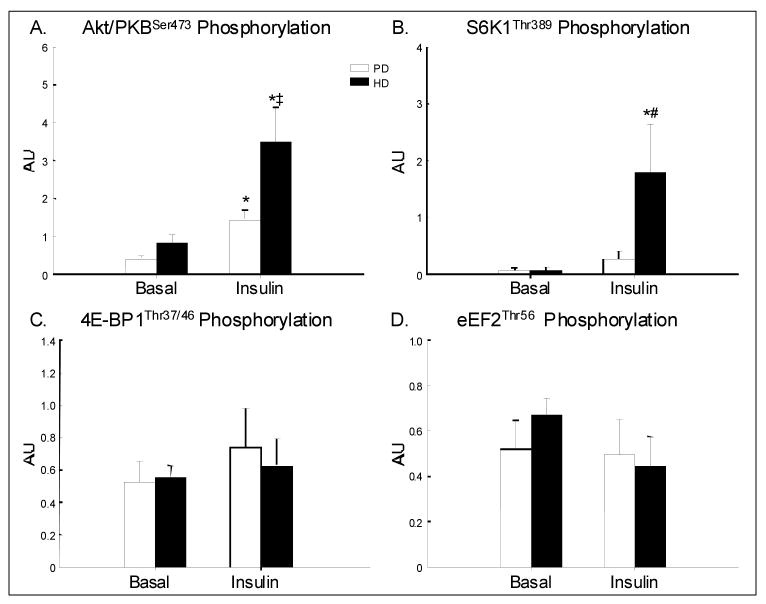
Akt and mTOR Signaling (Figure 2)
Figure 2.
Phosphorylation of Akt/PKB, S6K1, 4E-BP1 and eEF2 in older volunteers at baseline and during the intra-arterial infusion of insulin at two different rates, chosen to increase the femoral vein insulin concentrations to either a postprandial level (PD: n=6 for Akt/PKB and S6K1; n=5 for 4E-BP1 and eEF2) or a supraphysiological high level (HD: n=6 for all proteins). Values are the mean ± SE. *P < 0.05 vs. Basal; ‡P = 0.07 vs. PD; #P<0.05 vs. PD
For all five selected proteins, the baseline phosphorylation status was not different between groups and the total protein content did not change over time in either group. Akt/PKBSer473 phosphorylation increased significantly with insulin (time effect P<0.01), but the response tended to be greater in the HD group (P=0.07). mTORSer2448 phosphorylation did not increase with insulin (P=0.12) with no significant group effect or interaction. S6K1Thr389 phosphorylation increased significantly with insulin only in the HD group (P<0.05) with a significant difference between groups (P<0.05). 4EBP1Thr37/46 phosphorylation and eEF2Thr56 phosphorylation did not change with insulin in either group, with no difference between groups.
Phenylalanine kinetics (Table 4)
Table 4.
Phenylalanine kinetics at baseline and during insulin infusion in two groups of healthy older volunteers undergoing a local insulin infusion in one leg at postprandial or high dose.
| Postprandial Dose | High Dose | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Basal | Insulin | Basal | Insulin | Group | Time | Interaction | |
| Delivery to the leg | 160 ± 12 | 154 ± 12 | 150 ± 17 | 195 ± 21 | 0.39 | 0.03 | 0.02 |
| Release from the leg | 175 ± 13 | 155 ± 11 | 167 ± 16 | 186 ± 20 | 0.50 | 0.95 | 0.05 |
| Net balance across the leg | -15 ± 2 | -1 ± 3 | -17 ± 2 | 9 ± 4 | 0.17 | <0.0001 | 0.04 |
| Release in blood from proteolysis | 62 ± 8 | 46 ± 8 | 55 ± 3 | 46 ± 7 | 0.80 | <0.01 | 0.47 |
| Disappearance from the blood | 46 ± 8 | 45 ± 8 | 38 ± 5 | 55 ± 7 | 0.88 | 0.10 | 0.06 |
| Transport into muscle free pool | 115 ± 17 | 88 ± 15 | 103 ± 10 | 115 ± 20 | 0.45 | 0.80 | 0.26 |
| Transport from muscle free pool | 130 ± 18 | 88 ± 13 | 120 ± 8 | 106 ± 22 | 0.70 | 0.02 | 0.23 |
| Release from muscle proteolysis | 70 ± 8 | 61 ± 12 | 62 ± 5 | 61 ± 10 | 0.76 | 0.50 | 0.57 |
| Utilization for muscle protein synthesis | 55 ± 7 | 59 ± 11 | 45 ± 7 | 71 ± 11 | 0.94 | 0.04 | 0.05 |
| Intracellular availability | 185 ± 23 | 148 ± 23 | 166 ± 12 | 176 ± 26 | 0.75 | 0.48 | 0.16 |
Units are nmol·min-1·100 ml leg-1. Values are the mean ± SE. Boldface values indicate statistical significance
All phenylalanine kinetic parameters were not significantly different between groups in the basal period. With insulin infusion, phenylalanine delivery to and release from the leg increased in the HD group only (P<0.05). A significant interaction was found for phenylalanine release from the leg, which tended to increase in the HD group while decreasing from basal in the PD group.
There was a time effect (P<0.05) for phenylalanine release in blood from proteolysis (leg Ra) due to a significant decrease in PD (P<0.05) with no change in the HD group. In contrast, there was a trend (P=0.06) for a time by group interaction for phenylalanine disappearance from the blood (leg Rd), an index of protein synthesis, due to significant increase in the HD group only (P<0.05).
Phenylalanine transport into the muscle free pool and intracellular availability did not change significantly with insulin, whereas phenylalanine transport from the muscle free pool into the venous blood decreased in both groups (P<0.05). Phenylalanine release from muscle proteolysis did not change significantly with insulin in either group. There was a significant time effect and time by group interaction for phenylalanine utilization for muscle protein synthesis, which increased significantly during insulin infusion only in the HD group (P<0.05). Phenylalanine net balance across the leg improved in both groups in response to insulin (P<0.0001), but the increase was significantly larger in HD such that net balance became positive only in the HD group (P<0.01), indicating net muscle protein anabolism.
Discussion
The results of this study indicate for the first time that skeletal muscle protein metabolism is truly insulin resistant in healthy, glucose tolerant, older adults. Specifically, we found in healthy older volunteers that supraphysiological hyperinsulinemia was necessary to stimulate mixed muscle protein synthesis and net muscle protein anabolism, while, consistent with previously published data (8;10;11), hyperinsulinemia within the physiological postprandial range had only minor effects on muscle protein metabolism. Improvements in muscle protein anabolism observed with supraphysiological hyperinsulinemia were associated with enhanced amino acid delivery, induced by increased leg blood flow, and enhanced Akt/PKB and S6K1 signaling. When we compare the effects of the two insulin doses on protein synthesis and blood flow in our elderly individuals to those obtained in BMI-matched healthy younger volunteers with physiological hyperinsulinemia (Figure 1), it is clear that healthy older adults require double the insulin dose in order to increase muscle protein synthesis and vasodilatation to levels normally achieved by younger individuals during postprandial hyperinsulinemia. A fascinating aspect of these results is that despite some analogies with the classic insulin resistance of glucose metabolism as observed in type 2 diabetes, the mechanisms underlying insulin resistance of muscle protein metabolism with aging are probably different.
First, our volunteers, although overweight, had a normal glucose tolerance as assessed at screening, and exhibited a leg glucose uptake proportional to the insulin dose and consistent with uptakes previously reported in younger healthy volunteers under comparable experimental conditions (1;10). Moreover, while volunteers in the HD group tended to be heavier as compared to those in the PD group, they positively responded to insulin. Thus, if adiposity had a negative impact on the muscle protein anabolic response to insulin, it would have led to underestimation of the effect of the high insulin dose on skeletal muscle protein anabolism. Furthermore, we have previously shown that type 2 diabetes and excess adiposity do not influence the acute response of skeletal muscle protein synthesis and anabolism in younger individuals (9), and we report here that blood flow and muscle protein synthesis are stimulated by a postprandial insulin dose in younger overweight volunteers. Others have reported that the degree of glucose control does not affect skeletal muscle protein synthesis in type 2 diabetic patients (27). Thus, it is becoming apparent that the age-related insulin resistance of muscle proteins and the classic insulin resistance of glucose metabolism leading to type 2 diabetes are two independent conditions, although they may share some characteristics.
Second, the vasodilatory effect of insulin seems to play a much larger role in the modulation and stimulation of muscle protein anabolism both in young and older volunteers (this study and refs. (1;10;11) as compared to its effect on glucose metabolism, for which it appears to be rate-limiting only when glucose uptake is elevated and the artery-to-vein glucose concentration gradient is high (28-30). An increase in microvascular recruitment increases the amount of tissue perfused and amino acid delivery to the muscle even in the presence of small decreases in amino acid concentrations, as it occurred in the HD group of this study and in previous studies (1;10;11). This interplay between insulin vasodilatation and amino acid availability appears to be an essential component of the overall anabolic effect of insulin. In fact, when profound hypoaminoacidemia is induced with a systemic insulin infusion in the absence of amino acid replacement (7;20;31-33), amino acid delivery to the muscle will be reduced and the insulin anabolic effect will be mainly ascribable to reduced protein breakdown, rather than an increase in synthesis. However, if amino acids concentrations are maintained during a systemic insulin infusion, then insulin has a protein anabolic effect on human skeletal muscle (4;6-8;33;34). On the other hand, if amino acid concentrations are greatly increased to high postprandial levels using mixtures containing large amounts of arginine, the anabolic effect of insulin on protein synthesis may be masked due to significant arginine-induced increases in blood flow (35). Consistent with previous reports (10;11;36), we found that physiological elevations in blood insulin were unable to increase blood flow in elders. However, we also found that supraphysiological hyperinsulinemia induced a significant increase in blood flow, and this effect was associated with stimulation of muscle protein synthesis and net anabolism. It has been shown that the first effect of insulin is a rapid, nitric oxide-dependent microvascular recruitment that precedes increases in total blood flow and the effects on intracellular signaling and glucose uptake in muscle (37). Although we did not directly measure capillary recruitment, it is likely that the differential effects of the two insulin doses on total leg blood flow were accompanied by concomitant differential effects on muscle perfusion, with the higher dose being the only one able to stimulate microvascular recruitment. It is thus conceivable that the age-related endothelial dysfunction may limit the insulin-induced increase in nutrient flow, amino acid uptake and, consequently, muscle protein synthesis. Impaired endothelial-dependent capillary recruitment may also be responsible for the suboptimal anabolic response to mixed feeding in older persons (12), as lack of vasodilation reduces the amount of muscle tissue perfused and, consequently, nutrient and hormone delivery. On the other hand, perfusion alone may not be the only limiting factor. Recent data suggest that insulin transport across the endothelium into the muscle is a saturable process requiring intact insulin signaling, and can therefore be an important regulator of insulin availability and action (26;38).
Third, the muscle cell signaling data also suggest a relative insulin resistance, which may be related to both endothelial dysfunction and anabolic resistance to the insulin stimulus. While the group receiving the postprandial insulin dose was able to increase Akt/PKBSer473 phosphorylation (although to a lesser extent than the group receiving the supraphysiological insulin dose), it was incapable of increasing the phosphorylation, and presumably the activity, of downstream effectors known to regulate translation initiation and protein synthesis in skeletal muscle. In particular, in our older volunteers supraphysiological hyperinsulinemia was capable of increasing the phosphorylation state of S6K1Thr389 whereas, consistent with previous reports (8;11), insulin concentrations in the normal physiological range did not. It should be kept in mind that the phosphorylation status of our selected proteins was measured at baseline and again three hours after the initiation of the insulin clamp. Therefore, because physiological hyperinsulinemia increased Akt/PKBSer473 phosphorylation, it is possible that phosphorylation of mTORSer2448 and S6K1Thr389 increased to some extent at earlier time points during the insulin clamp, but had returned to baseline values when we obtained the last muscle sample. On the other hand, supraphysiological hyperinsulinemia in the older volunteers induced a much larger increase in Akt/PKBSer473 phosphorylation, thus resulting in a sustained phosphorylation of S6K1Thr389 and a significant increase in the rate of muscle protein synthesis. This is consistent with the notion that supraphysiological hyperinsulinemia is capable of overriding the insulin resistance of protein metabolism in older volunteers.
While these results are very exciting and may add another important piece of understanding to the complex sarcopenia puzzle, generalization to the entire population should be done cautiously. The complexity and invasiveness of the methodologies used to measure muscle protein metabolism is limiting with regards to sample size. For example, only three older women were included in this study. It is still unclear whether sex has a significant effect on muscle protein turnover in young and older adults, since some studies have reported baseline differences (39;40) while others show no effect (41-44). Nonetheless, a recent study suggests that there is no sexual dimorphism in the response of muscle proteins to insulin (44). Thus, while with the current data we cannot draw conclusions regarding the effect of sex on the muscle protein response to insulin in elders, we believe that our results obtained including both men and women are valid.
In conclusion, our data provide for the first time evidence that skeletal muscle protein metabolism is insulin resistant in healthy, non diabetic older persons, as protein anabolism can be elicited only by supraphysiological hyperinsulinemia. This defect appears unrelated to glucose tolerance and associated with endothelial dysfunction and blunted anabolic signaling, although the relative importance of these two complex mechanisms is still unclear and warrants further investigation. Insulin resistance of muscle protein metabolism with aging may be a contributor to the development of sarcopenia in older adults as it may decrease the physiological muscle anabolic response to feeding (12), thereby inducing a slow but progressive decline in muscle protein content. Future mechanistic studies will be necessary to clearly determine the role and contribution of insulin resistance and endothelial dysfunction to muscle loss in elders.
Acknowledgments
We wish to thank the study volunteers for their participation, Ming Zheng for superb technical assistance, and Jerson D. Cadenas and the nurses and personnel of the UTMB and USC General Clinical Research Centers of for their assistance with the clinical conduct of this study. This study was supported by grants # R01 AG18311 and the UTMB Claude D. Pepper Older Americans Independence Center # P30 AG024832 from the National Institute on Aging, NIH; grant # S10 RR16650 from the Shared Instrumentation Grant Program, National Center for Research Resources, NIH; General Clinical Research Center grants # M01 RR00043 (USC) and M01 RR00073 (UTMB), and grant # S10 RR16650 from the National Center for Research Resources, NIH.
Abbreviations
- Akt
protein kinase B
- eEF2
eukaryotic elongation factor 2
- FSR
fractional synthetic rate
- ICG
indocyanine green
- mTOR
mammalian target of rapamycin
- Ra
rate of appearance
- Rd
rate of disappearance
- S6K1
p70 ribosomal S6 kinase 1
- 4E-BP1 4E
binding protein 1
Footnotes
Duality of interest: The authors declare that there is no duality of interest associated with this manuscript
References
- 1.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. The effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745–E754. doi: 10.1152/ajpendo.00271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennet WM, Connacher AA, Scrimgeour CM, Jung RT, Rennie MJ. Euglycemic hyperinsulinemia augments amino acid uptake by leg tissues during hyperaminoacidemia. Am J Physiol. 1990;259:E185–E194. doi: 10.1152/ajpendo.1990.259.2.E185. [DOI] [PubMed] [Google Scholar]
- 3.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995;95:811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman E, Heslin MJ, Wolf RF, Pisters PW, Brennan MF. The effect of systemic hyperinsulinemia with concomitant amino acid infusion on skeletal muscle protein turnover in the human forearm. Metabolism. 1994;43:70–78. doi: 10.1016/0026-0495(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 5.Wolf RF, Heslin MJ, Newman E, Pearlstone DB, Gonenne A, Brennan MF. Growth hormone and insulin combine to improve whole-body and skeletal muscle protein kinetics. Surgery. 1992;112:284–91. discussion 291-2. [PubMed] [Google Scholar]
- 6.Hillier TA, Fryburg DA, Jahn LA, Barrett EJ. Extreme hyperinsulinemia unmasks insulin's effect to stimulate protein synthesis in the human forearm. Am J Physiol. 1998;274:E1067–E1074. doi: 10.1152/ajpendo.1998.274.6.E1067. [DOI] [PubMed] [Google Scholar]
- 7.Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes. 2003;52(6):1377–85. doi: 10.2337/diabetes.52.6.1377. [DOI] [PubMed] [Google Scholar]
- 8.Guillet C, Prod'homme M, Balage M, et al. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB Journal. 2004;18(13):1586–7. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 9.Bell JA, Volpi E, Fujita S, Cadenas JG, Sheffield-Moore M, Rasmussen BB. Skeletal muscle protein anabolic response to increased energy and insulin is preserved in poorly controlled type 2 diabetes. J Nutr. 2006;136:1249–1255. doi: 10.1093/jn/136.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen BB, Fujita S, Wolfe RR, et al. Insulin resistance of muscle protein metabolism in aging. FASEB Journal. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita S, Rasmussen BB, Cadenas JG, et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and akt/mammalian target of rapamycin signaling. Diabetes. 2007;56:1615–1622. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rieu I, Balage M, Sornet C, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol (Lond) 2006;575:1–15. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:e24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 15.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 16.Bell JA, Fujita S, Volpi E, Cadenas JG, Rasmussen BB. Short-term insulin and nutritional energy provision does not stimulate muscle protein synthesis if blood amino acid availability decreases. Am J Physiol Endocrinol Metab. 2005;289:999–1006. doi: 10.1152/ajpendo.00170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorfeldt L, Juhlin-Dannfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism. 1978;27:97–106. doi: 10.1016/0026-0495(78)90128-2. [DOI] [PubMed] [Google Scholar]
- 18.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe RR. Radioactive and stable isotope tracers in biomedicine. Principles and practice of kinetic analysis. 1. Wiley-Liss; New York: 1992. [Google Scholar]
- 20.Denne SC, Liechty EA, Liu YM, Brechtel G, Baron AD. Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. American Journal of Physiology. 1991;261:E809–E814. doi: 10.1152/ajpendo.1991.261.6.E809. [DOI] [PubMed] [Google Scholar]
- 21.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002-0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Sp. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 22.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol (Lond) 2006;576:2–24. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toffolo G, Foster DM, Cobelli C. Estimation of protein fractional synthetic rate from tracer data. Am J Physiol. 1993;264:E128–E135. doi: 10.1152/ajpendo.1993.264.1.E128. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. 2nd. John Wiley & Sons; Hoboken, NJ: 2004. [Google Scholar]
- 25.Katsanos CS, Chinkes DL, Sheffield-Moore M, Aarsland A, Kobayashi H, Wolfe RR. Method for the determination of the arteriovenous muscle protein balance during non-steady-state blood and muscle amino acid concentrations. Am J Physiol Endocrinol Metab. 2005;289:E1064–E1070. doi: 10.1152/ajpendo.00141.2005. [DOI] [PubMed] [Google Scholar]
- 26.Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes. 2007;56:2958–2963. doi: 10.2337/db07-0670. [DOI] [PubMed] [Google Scholar]
- 27.Halvatsiotis P, Short KR, Bigelow M, Nair KS. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes. 2002;51:2395–2404. doi: 10.2337/diabetes.51.8.2395. [DOI] [PubMed] [Google Scholar]
- 28.Baron AD, Tarshoby M, Hook G, et al. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes. 2000;49(5):768–74. doi: 10.2337/diabetes.49.5.768. [DOI] [PubMed] [Google Scholar]
- 29.Vincent MA, Clerk LH, Lindner JR, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 30.Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, Barrett EJ. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes/Metabolism Research Reviews. 2004;20:3–12. doi: 10.1002/dmrr.414. [Review] [70 refs] [DOI] [PubMed] [Google Scholar]
- 31.Tessari P, Inchiostro S, Biolo G, Vincenti E, Sabadin L. Effects of acute systemic hyperinsulinemia on forearm muscle proteolysis in healthy man. J Clin Invest. 1991;88:27–33. doi: 10.1172/JCI115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Feo P, Volpi E, Lucidi P, et al. Physiological increments in plasma insulin concentrations have selective and different effects on synthesis of hepatic proteins in normal humans. Diabetes. 1993;42:995–1002. doi: 10.2337/diab.42.7.995. [DOI] [PubMed] [Google Scholar]
- 33.Moller-Loswick AC, Zachrisson H, Hyltander A, Korner U, Matthews DE, Lundholm K. Insulin selectively attenuates breakdown of nonmyofibrillar proteins in peripheral tissues of normal men. American Journal of Physiology. 1994;266:E645–E652. doi: 10.1152/ajpendo.1994.266.4.E645. [DOI] [PubMed] [Google Scholar]
- 34.Heslin MJ, Newman E, Wolf RF, Pisters PW, Brennan MF. Effect of hyperinsulinemia on whole body and skeletal muscle leucine carbon kinetics in humans. American Journal of Physiology. 1992;262:E911–E918. doi: 10.1152/ajpendo.1992.262.6.E911. [DOI] [PubMed] [Google Scholar]
- 35.Greenhaff PL, Karagounis LG, Peirce N, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meneilly GS, Elliot T, Bryer-Ash M, Floras JS. Insulin-mediated increase in blood flow is impaired in the elderly. J Clin Endocrinol Metab. 1995;80:1899–1903. doi: 10.1210/jcem.80.6.7775638. [DOI] [PubMed] [Google Scholar]
- 37.Vincent MA, Dawson D, Clark AD, et al. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes. 2002;51(1):42–8. doi: 10.2337/diabetes.51.1.42. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Wang AX, Liu Z, Barrett EJ. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes. 2008;57:540–547. doi: 10.2337/db07-0967. [DOI] [PubMed] [Google Scholar]
- 39.Smith GI, Atherton P, Villareal DT, et al. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65-80 year old men and women. PLoS ONE. 2008;3:e1875. doi: 10.1371/journal.pone.0001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson GC, Dhatariya K, Ford GC, et al. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J. 2009;23:631–641. doi: 10.1096/fj.08-117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol. 2001;91:1041–1047. doi: 10.1152/jappl.2001.91.3.1041. [DOI] [PubMed] [Google Scholar]
- 42.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol Endocrinol Metab. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 43.Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab. 2007;292:E77–E83. doi: 10.1152/ajpendo.00173.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith GI, Reeds DN, Mohammed BS, Jaffery H, Mittendorfer B. The basal rate of muscle protein synthesis and its response to hyperinsulinemia-hyperaminoacidemia are the same in young men and women. FASEB J. 2009;23:991.23. Abstract. [Google Scholar]