Abstract
Lamellar ichthyosis (LI) is a genetically heterogeneous, severe genodermatosis showing widespread hyperkeratosis of the skin. Transglutaminase 1 (TGase1) deficiency by TGase1 gene (TGM1) mutations is the most prevalent cause of LI. Screening of TGase1 deficiency in skin is essential to facilitate the molecular diagnosis of LI. However, cadaverine, the most widely used substrate for TGase activity assay, is not isozyme specific. Recently, a human TGase1–specific highly preferred substrate peptide K5 (pepK5) was generated. To evaluate its potential as a diagnostic tool for LI, we performed pepK5 labeling of TGase1 activity in normal human and LI skin. Ca2+-dependent labeling of FITC-pepK5 was clearly seen in the upper spinous and granular layers of normal human skin where it precisely overlapped with TGase1 immunostaining. Both specificity and sensitivity of FITC-pepK5 labeling for TGase1 activity were higher than those of FITC-cadaverine labeling. FITC-pepK5 labeling colocalized with involucrin and loricrin immunostaining at cornified cell envelope forming sites. FITC-pepK5 labeling was negative in LI patients carrying TGM1 truncation mutations and partially abolished in the other LI patients harboring missense mutations. The present results clearly indicate that pepK5 is a powerful tool for screening LI patient TGase1 deficiency when we make molecular diagnosis of LI.
One of the essential events during terminal differentiation of epidermal keratinocytes and skin barrier formation is the production of a 15-nm-thick layer of protein on the inner surface of the keratinocyte cell membrane, termed the cornified cell envelope (CCE). The CCE is assembled by the accumulation of several precursor proteins including involucrin and loricrin.1 It is known that the precursor proteins are cross-linked together by the formation of Nε-(γ-glutamyl) lysine isodipeptide bonds catalyzed by the action of transglutaminase isoforms. Transglutaminase 1 (TGase1) is a key enzyme in CCE formation in the epidermis.
Lamellar ichthyosis (LI) is a major subtype of autosomal recessive congenital ichthyosis and clinically characterized by large, thick, dark scales over the entire body without serious background erythroderma.2 Since the identification of TGase1 gene (TGM1) mutations in a number of families with LI in 1995,3,4 more than one hundred TGM1 mutations have been reported in LI families. TGase1 deficiency attributable to TGM1 mutations is a major underlying causative factor in LI patients,5,6 although LI is thought to be a genetically heterogeneous disorder and several causative molecules including TGase1 have been identified.3,4,7,8,9,10,11 Although genotype/phenotype correlations in autosomal recessive congenital ichthyosis including LI with TGM1 mutations have been studied for years, the exact nature of the relationship has yet to be fully elucidated.5,6,12,13,14,15 Thus, it is difficult to know whether a causative gene is TGM1 or not in each LI patient from each patient’s clinical features alone.
To date, to facilitate molecular diagnosis in LI patients with TGM1 mutations, in situ transglutaminase (TGase) activity assays have been performed using cadaverine as a substrate to detect TGase1 activity in the patients’ skin,16,17,18,19,20 despite the fact that cadaverine is not an isozyme-specific probe, and detects total TGase activity in the epidermis. Recently, a human TGase1 specific, highly preferred substrate peptide K5 (pepK5) was generated.21 We hypothesized that, as previously shown in mouse skin, pepK5 would detect in situ TGase1 activity with high specificity and sensitivity in the human epidermis. If it is the case, pepK5 can be a useful tool to detect TGase1 deficiency in LI patients with TGM1 mutations.
In the present study, we demonstrated that pepK5 can be used as an efficient probe to detect TGase1 activity in the human epidermis. In addition, we performed in situ TGase1 activity assay using pepK5 in skin specimens from LI patients with TGM1 mutations and clearly revealed that this preferred substrate for TGase1, pepK5 is a powerful tool for evaluation of TGase1 activity in LI patients and for molecular diagnosis of LI.
Materials and Methods
Synthesis of Transglutaminase Substrate Peptides
PepK5, peptide K5QN (pepK5QN), and peptide form T26 (pepT26) were synthesized as previously described.21,22 Briefly, a phage-displayed random peptide library was used to screen primary amino acid sequences that are preferentially selected by human TGase1. The peptides selected as glutamine donor substrate exhibited a marked tendency in primary structure, conforming to the sequence: QxK/RψxxxWP (where x and ψ represent nonconserved and hydrophobic amino acids, respectively). Using glutathione S-transferase (GST) fusion proteins of the selected peptides, several sequences were identified as preferred substrates and confirmed that they were isozyme-specific. The 12-aa peptide pepK5 (YEQHKLPSSWPF) was synthesized. Even in peptide form, K5 appeared to have high and specific reactivity as substrate. In addition, a mutant peptide in which glutamine was substituted by asparagine was also synthesized as pepK5QN (YENHKLPSSWPF). pepT26 (HQSYVDPWMLDH) was synthesized as the transglutaminase 2 (TGase2) preferred substrate peptide for comparison.22 Finally, these synthesized peptides were conjugated with FITC.21
In Situ TGase1 Activity Assay
Skin sections were prepared from skin biopsy patient specimens and normal control specimens using standard methods.21,23 The frozen sections were dissected into 6-μm slices and stored frozen at −80°C until use.
Sections were dried and then blocked with 1% BSA in NaCl/Pi at room temperature. The sections were incubated for 90 minutes with a solution containing 100 mmol/L Tris/HCl pH 8.0, 5 mmol/L CaCl2 or 1 mmol/L EDTA, and 1 mmol/L dithiothreitol, in the presence of 5 μmol/L (or other concentrations) of FITC-labeled substrate peptide or FITC-cadaverine (Sigma-Aldrich, St. Louis, MO). This in situ TGase1 activity assay works by measuring the fluorescence of fluorescein isothiocyanate (FITC)-labeled substrate peptide incorporated into cellular proteins by cross-linking catalyzed by TGase1. After washing with NaCl/Pi three times for 5 minutes, antifading solution was added to the sections, which were then sealed with a cover glass and mountant. In addition, we performed the above-mentioned pepK5 labeling using normal human skin specimens and LI patients’ skin samples under various incubation conditions (pH 7.4, 8.0 and 8.4; temperature 25°C, 33°C and 37°C).
Double Labeling for in Situ TGase1 Assay and Immunofluorescence Staining
For double labeling (in situ TGase1 activity assay and immunofluorescence), at first, we performed in situ TGase1 activity assay as described above, then the sections were labeled with immunofluorescence methods below. Immunofluorescence labeling was performed as described previously.23 Primary antibodies used in this study were as follows: mouse monoclonal anti-TGase 1 antibody (B.C1; Biomedical Technologies, Inc., Stoughton, MA), rabbit polyclonal anti-TGase1 antibody (Novus Biologicals, LLC, Littleton, CO), anti-loricrin antibody (Covance Lab., Richmond, CA), and anti-involucrin antibody (Biomedical Technologies, Inc., Stoughton, MA). We used FITC-conjugated or tetramethylrhodamine-isothiocyanate (TRITC)-conjugated rabbit anti-mouse immunoglobulin (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA) or donkey anti-rabbit immunoglobulins (DAKO, Glostrup, Denmark), as secondary antibodies.
Ichthyosis Patients Involved in the Present Study
In total, four unrelated LI patients with TGM1 mutations were included in this study. Patient 1 was a recently examined LI case and the other three patients were reported previously.6,20,24 As controls, two TGM1-unrelated autosomal recessive congenital ichthyosis patients harboring ABCA12 mutations25 were also included in the present study.
Fully informed consent was obtained from the participants or their legal guardians for this study. This study had been previously evaluated and approved by the ethics committee at Hokkaido University Graduate School of Medicine and was conducted according to the Declaration of Helsinki Principles.
Mutation Search
TGM1 mutation search was performed as previously reported.19 Briefly, genomic DNA isolated from peripheral blood was subjected to polymerase chain reaction amplification, followed by direct automated sequencing and verification of the mutation by restriction enzyme digestions. Most oligonucleotide primers used for amplification of all 15 exons of TGM1 have been reported elsewhere12 and partially modified for the present study.19 The entire coding regions of TGM1 including the exon/intron boundaries were sequenced using genomic DNA samples from patients and their family members. One hundred normal alleles (50 unrelated, healthy Japanese individuals) were sequenced as normal controls.
Results
In Situ Assay Using pepK5 Detected TGase1 Activity with High Specificity and Sensitivity in the Upper Epidermis of Normal Human Skin
With the presence of CaCl2 in the reaction mixture, we detected specific incorporation of FITC-labeled pepK5 (FITC-pepK5; 5 μmol/L) into substrate proteins in the epidermis, mainly at the cell periphery of the upper spinous and granular layers of normal human skin (Figure 1A). No signal was detected in the presence of EDTA (Figure 1B), or when we used FITC-conjugated pepK5QN mutant peptide (FITC-pepK5QN; Figure 1C), which indicated that the cross-linking reaction was catalyzed specifically by TGase1. Using FITC-conjugated pepT26 (FITC-pepT26), a preferable substrate for TGase2, only faint labeling was obtained around the granular layer cells and this labeling was abolished in the presence of EDTA (data not shown). Under various incubation conditions, pH 7.4, 8.0, and 8.4, temperature 25°C, 33°C, and 37°C, no significant difference in the pepK5 labeling intensity was observed in normal human epidermis (data not shown).
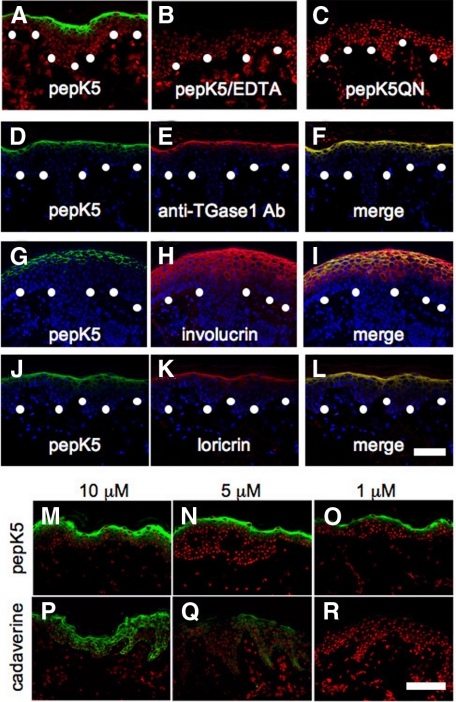
Figure 1.
PepK5 labeling detected in situ TGase1 activity with high specificity and sensitivity at CCE forming sites in normal human skin. A–C: In situ TGase1 activity detected by pepK5 in normal skin. Detection of in situ TGase 1 activity using FITC-labeled pepK5 (5 μmol/L) showed intense membrane-restricted staining within the upper spinous and granular layer keratinocytes of a normal human skin (A). In the presence of EDTA, the pepK5 labeling was completely abolished (B). No labeling was observed with FITC-labeled mutant K5 peptide (pepK5QN; C). Specific labeling, green (FITC); nuclear stain, red (propidium iodide). White dots, basement membrane zone. D–F: Double labeling with pepK5 and anti-TGase1 antibody in normal human skin. Both pepK5 labeling (D, green, FITC) and anti-TGase1 antibody (B.C1) labeling (E, red, TRITC) are seen in the upper epidermis, mainly in the granular layers. The merged image clearly demonstrates that both labeling patterns almost completely overlap (yellow) each other on the cell membrane of the upper epidermal keratinocytes (F). pepK5 labeling, green (FITC), anti-TGase1 antibody labeling, red (TRITC); nuclear stain, blue (TOPRO). White dots, basement membrane zone. G–L: Double labeling with anti-CCE precursor protein antibodies and pepK5 in normal human skin. Anti-involucrin antibody labeling (H, red, TRITC) is seen in the upper half of the epidermis, although pepK5 labeling (G, green, FITC) is observed mainly in the uppermost spinous and granular cell layers. Involucrin and pepK5 labeling overlap each other (yellow) on the cell membrane of the uppermost spinous and granular cell layer keratinocytes in the merged image (I). Both pepK5 labeling (J, green, FITC) and anti-loricrin antibody labeling (K, red, TRITC) are seen mostly within the uppermost spinous and granular layers. The merged image shows that loricrin and pepK5 labeling clearly overlap (yellow) each other on the cell membrane of the granular layer keratinocytes (L). FITC-pepK5 labeling, green; anti-involucrin and anti-loricirn antibodies, red (TRITC); nuclear stain, blue (TOPRO). White dots, basement membrane zone. M–R: Detection of TGase1 activity in normal human skin sections using graded concentrations of pepK5 or cadaverine. Intense labeling is seen in the upper epidermis with 10 μmol/L (M) and 5 μmol/L (N) of FITC-pepK5. Only the granular layer keratinocytes are labeled with 1 μmol/L (O) of FITC-pepK5. Using 10 μmol/L (P) of FITC-cadaverine, all epidermal keratinocytes are labeled. With 5 μmol/L (Q) of FITC-cadaverine, entire epidermis is faintly labeled. No labeling is observed with 1 μmol/L (R) of FITC-cadaverine. M–O: FITC-pepK5 labeling, green; P–R: FITC-cadaverine labeling, green; nuclear stain, red (propidium iodide). Substrate concentrations, 10 μmol/L (M, P), 5 μmol/L (N, Q), 1 μmol/L (O, R). Scale bars = 50 μm.
The FITC-pepK5 labeling pattern corresponded well with the localization of TGase1 by immunostaining with anti-TGase1 antibody. Double labeling for in situ TGase1 activity assay using FITC-pepK5 and immunostaining for TGase1 molecule showed completely overlapping colocalization of these moieties at the cell periphery of both the upper spinous and granular layer cells (Figure 1, D–F).
Double Labeling for TGase1 Activity with pepK5 and CCE Precursor Proteins Demonstrated that pepK5 Labeling Precisely Localized to Sites of CCE Formation
Immunofluorescence labeling for involucrin, a major CCE precursor protein, was seen in the upper half of the epidermis (Figure 1H). Double labeling for in situ TGase1 activity assay using pepK5, and involucrin immunolabeling showed that, in the upper spinous and granular cell layers, pepK5 labeling and involucrin co-localized at the cell periphery (Figure 1, G–I). In addition, double labeling for the in situ TGase1 activity assay using pepK5, and immunolabeling for loricrin, another major CCE precursor protein, revealed almost complete colocalization of TGase1 activity and loricrin in the cell periphery of the upper spinous and granular layer cells (Figure 1, J–L).
PepK5 Detected in Situ TGase1 Activity Efficiently Compared with Cadaverine
We also compared the reactivity of FITC-pepK5 and FITC-cadaverine, which has been previously used for detection of in situ TGase activity in normal human skin at various concentrations, 10, 5, 1, and 0.1 μmol/L (Figure 1, M–R). At 10 μmol/L and 5 μmol/L concentrations, intense FITC-pepK5 labeling was observed mainly in the cell periphery of the upper spinous and granular layer keratinocytes in the normal human epidermis. At 1 μmol/L concentration, FITC-pepK5 labeled only the granular layer keratinocytes, and at 0.1 μmol/L concentration (data not shown) no FITC-pepK5 labeling was seen in the normal human epidermis. In contrast, using FITC-cadaverine at 10 μmol/L concentration, the entire epidermis was labeled, and at 5 μmol/L concentration only faint FITC-cadaverine labeling was seen in all of the layers of normal human epidermis. At 1 μmol/L or 0.1 μmol/L (data not shown) concentration, no FITC-cadaverine labeling was obtained in the epidermis. These results suggest that FITC-pepK5 detects endogenous TGase1 activity with greater sensitivity, at least more than ten times higher than FITC-cadaverine in human epidermis. In addition, considering the labeling patterns in the epidermis by the two substrates, specificity of pepK5 to TGase1 seemed to be much higher than that of cadaverine.
TGM1 Mutations and Clinical Features of LI Patients Involved in the Present Study
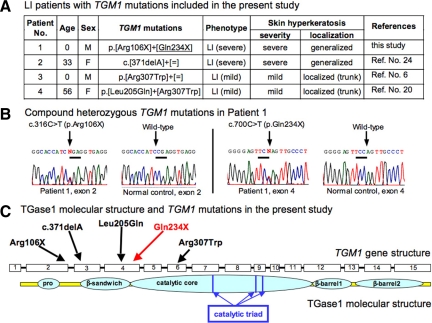
TGM1 mutations and clinical features of the patients included in the present study are summarized in Figure 2, A–C. Patients 1 and 2 showed a typical, classic LI phenotype. Patients 3 and 4 had a mild LI phenotype with mild hyperkeratosis mainly on the trunk. Patient 4 had a LI phenotype termed as “bathing suit ichthyosis”26 with restricted affected regions on the trunk.
Figure 2.
TGM1 mutations and clinical features of LI patients in the present study. A: Summary of the TGM1 mutations and phenotypes of the LI patients included in the present study. Note Patients 1 and 2 harbored truncation mutations in both alleles and exhibited a severe phenotype, and Patients 3 and 4 carried missense mutations in both alleles exhibiting a milder phenotype. An underlined mutation was a novel mutation. B: Direct sequence analysis of exons 2 and 4 of Patient 1 revealed heterozygous nonsense mutations, c.316C>T (p.Arg106X) and c.700C>T (p.Gln234X). C: Schematic sequential arrangement of the domain structure of the TGase1 polypeptide. Mutations in the present LI patients are marked by arrows. Red characters and arrows indicate novel mutations and black ones are previously reported mutations. Note that three truncation mutations are located upstream to the catalytic core domain. Two missense mutations are in the β-sandwich domain and the catalytic core domain, which are important for enzyme activity.
Patient 1 was a newly examined LI case. Patient 1 was compound heterozygous for the two TGM1 nonsense mutations, p.Arg106X and p.Gln234X (c.[316C>T]+ [700C>T]; p.[Arg106X]+[Gln234X]; Figure 2B) and showed a typical classic form of LI. One mutation p.Gln234X was a novel mutation and the other mutation p.Arg106X was previously reported.27 These mutations were not found in 100 normal control alleles (50 unrelated, healthy Japanese individuals) and were not thought to be polymorphisms. The three other patients included in the present study had been reported previously to have a total of three TGM1 mutations including p.Arg307Trp, a prevalent TGM1 mutation in the Japanese population.6,20,24
PepK5 Labeling Clearly Detected Defective TGase1 Activity in the Skin of LI Patients
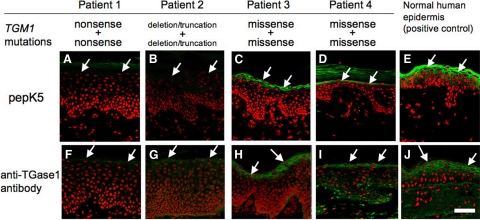
In Patients 1 and 2, membranous TGase 1 activity detected by FITC-pepK5 in the upper spinous and granular layers of the patients’ epidermis was completely lost (Figure 3, A and B). In Patient 3, membranous TGase 1 activity detected by FITC-pepK5 in the upper spinous and granular layers of the patient’s epidermis was observed, but remarkably weaker (Figure 3C) than that of normal control human epidermis (Figure 3E). In Patient 4, membranous TGase1 activity demonstrated by FITC-pepK5 in the upper spinous and granular layers of the patient’s epidermis was present, but restricted solely to the granular layer cells and cells just below the granular layer and was significantly weaker (Figure 3D) than that of normal control human epidermis (Figure 3E). In the epidermis of the two patients with ichthyosis caused by ABCA12 mutations, other than TGM1 mutations, intense membrane TGase1 activity was normally observed in the upper spinous and the granular layers by pepK5 labeling (data not shown).
Figure 3.
TGase1 deficiency detected by pepK5 labeling in the LI patients. A and F: Patient 1, a compound heterozygote for two TGM1 nonsense mutations: FITC-pepK5 labeling (green) shows complete absence of TGase1 activity in the upper epidermis (arrows; A), and TGase1 immunostaining (green) is also negative in the upper epidermis (arrows; F). B and G: Patient 2, a homozygote for a TGM1 deletion mutation causing truncation of the peptide: FITC-pepK5 labeling (green) reveals completely abolished TGase1 activity in the upper epidermis (arrows; B) and no TGase1 immunolabeling (in green) is seen in the upper epidermis (arrows; G). C and H: Patient 3, a homozygote for a TGM1 missense mutation: detectable, but reduced membranous TGase1 activity is seen in the upper epidermis (arrows) by FITC-pepK5 labeling (green; C). TGase1 immunostaining (green) in the upper epidermis (arrows) confirms expression of TGase1 molecule (H). D and I: Patient 4, a compound heterozygote for two TGM1 missense mutations: FITC-pepK5 labeling (green) shows faint TGase1 activity restricted to the granular layers (arrows; D). Immunofluorescence labeling for TGase1 (green) reveals a positive staining in the granular layer (arrows) in the patient’s epidermis (I). E and J: In a normal human skin without any TGM1 mutations, intense TGase1 activity is seen in the upper epidermis (arrows) using FITC-pepK5 labeling (green; E). TGase1 immunolabeling (green) is also positive in the upper epidermis (arrows; J). A–E: FITC-pepK5 labeling, green; F–J: rabbit polyclonal anti-TGase1 antibody staining, green (FITC); A–J: nuclear stain, red (propidium iodide). Scale bar = 50 μm.
Immunofluorescent labeling using rabbit polyclonal anti-TGase1 antibody revealed that TGase1 immunostaining was not seen in the epidermis of Patients 1 and 2 (Figure 3, F and G). In the epidermis of Patients 3 and 4, positive immunostaining for TGase1 molecule was observed mainly in the granular layer (Figure 3, H, I, and J). From the results of pepK5 labeling and immunostaining for the TGase1 molecule, in Patients 1 and 2, it was thought that immunoreactive, intact TGase1 molecule was absent from the epidermis, resulting in the absence of FITC-pepK5 labeling. In Patients 3 and 4, although immunoreactivity for TGase1 was detected in the epidermis, FITC-pepK5 labeling was remarkably weak, suggesting reduced enzyme activity of TGase1 molecules expressed in the epidermis of these patients.
In the epidermis of any LI patient, no significant difference in pepK5 labeling pattern and intensity was seen under various experimental conditions, pH 7.4, 8.0, and 8.4, temperature 25°C, 33°C, and 37°C (data not shown).
Using FITC-conjugated pepT26 (FITC-pepT26), a preferable substrate for TGase2, only faint labeling was obtained around the granular layer cells in all of the skin samples from the patients (data not shown).
Discussion
In the first half of the present study, we examined the ability of pepK5 to detect endogenous TGase1 activity in normal human skin sections. Ca2+-dependent incorporation of FITC-pepK5 into glutamine acceptor substrates was clearly seen in human epidermal keratinocytes, mainly in the upper spinous and granular layers. To date, detection of cross-linked TGase products using tissue sections has used an FITC-labeled primary amine (FITC-cadaverine) or FITC-labeled substrate peptides.28,29 The pattern of TGase activity that we observed was consistent with that seen in the skin using FITC-cadaverine.29 In addition, the staining sensitivity of pepK5 was remarkably higher than that of cadaverine in normal human epidermis.
As observed in immunostaining analysis, TGase1 protein localizes to the peripheral regions of the keratinocytes in the granular and upper spinous layers, consistent with previous reports.30,31 Double fluorescence staining clearly indicated that TGase1 activity labeled with pepK5 precisely colocalized with TGase1 immunolabeling at these sites. In addition, TGase1 activity demonstrated with pepK5 overlapped with the major CCE precursor proteins, loricrin and involucrin. These findings confirm that pepK5 labeling specifically demonstrates TGase1 activity at sites of CCE formation. In the in vitro assay with TGase2, pepK5 reacted to a small extent at high peptide concentration.21 Thus, in the present study, it was necessary to check endogenous TGase2 activity in the skin samples and we confirmed that there was no significant TGase2 activity in the skin sections by FITC-labeled pepT26 labeling. From these results, we conclude that pepK5 can act as a highly sensitive and specific probe to detect in situ endogenous TGase1 activity in the human epidermis.
In the last half of the present study, to assess the efficacy and usefulness of pepK5 as a preferred substrate for TGase1 in evaluating TGase1 activity in LI patients, we performed in situ TGase1 activity assays using pepK5 as a substrate in four LI patients with TGM1 mutations.
From the nature and sites of TGM1 mutations in each patient and their effect on TGase1 activity, according to the protein modeling of TGase1 based on the structure of the human factor XIIIa subunit,32 a level of remnant TGase1 activity was theoretically speculated in each case as follows.
Patient 1 is a compound heterozygote for TGM1 nonsense mutations (Figure 2). Both nonsense mutations led to truncation of the catalytic core domain and are expected to result in a complete loss of function of TGase1 activity. Patient 2 is a homozygote for a TGM1 deletion mutation resulting in a frameshift and premature termination in an upstream of the catalytic core domain (Figure 2). Thus, TGase1 activity is also expected to be completely abolished in the epidermis of Patient 2. In addition, all of the three truncation mutations in Patients 1 and 2 led to early termination codons. This would probably lead to complete lack of the polypeptide in the present Patients 1 and 2. Furthermore, genomic premature termination codon mutations are subject to nonsense-mediated mRNA decay resulting in mRNA degradation in some instances, depending on the mutation site.33,34
Patient 3 is a homozygote of a missense mutation in the center of catalytic core domain of TGase1 peptide (Figure 2). Homozygosity of this mutation is expected to result in a significant, but not complete loss of TGase1 function. Patient 4 is a compound heterozygote harboring a missense mutation in the β-sandwich domain, and the missense mutation in the center of catalytic core domain, identical to the mutation harbored by Patient 3 (Figure 2). As described above, the latter mutation in the catalytic core domain is expected to lead to a significant but only partial loss of activity of TGase1. The former mutation p.Leu204Gln in the β-sandwich domain is considered to alter protein folding, which in turn affects the protein stability of TGase 1, as suggested in other missense mutations in the β-sandwich domain.12 This instability may result in rapid degradation of the TGase1 polypeptide and reduce TGase1 activity in the patient’s epidermis, although the reduction in activity might not be as serious compared with truncation mutations in Patients 1 and 2. In addition to this simplistic view based on the position of missense mutations in the primary structure, it has been demonstrated that TGM1 mutations in specific residues have their specific effects on the TGase1 activity, leading to specific phenotypes. For example, the distinct phenotype of self-healing collodion baby can be caused by compound heterozygous TGM1 mutations p.Gly278Arg and p.Asp490Gly.35 Molecular modeling and biochemical assays suggested that the high hydrostatic pressure in utero significantly inhibit the mutant TGase1 activity. After birth, the mutant TGase1 molecules become partially active under ordinary hydrostatic pressure, resulting in the dramatic improvement of skin symptoms in a self-healing collodion baby.35 In addition, several TGM1 missense mutations in specific residues were reported to cause another specific phenotype, bathing suit ichthyosis, characterized by pronounced scaling restricted to the bathing suit areas.26,36 The affected sites are warmer body areas, and bathing suit ichthyosis is thought to be a temperature-sensitive phenotype.26 A marked decrease of in situ TGase1 activity was revealed at high temperature (37°C) in the patients with bathing suit ichthyosis.26 Recent findings have shown that wild-type TGase1 activity is clearly reduced at 25°C compared with 37°C by in vivo activity analysis with cadaverine as a substrate. On the other hand, in case of reconstituted mutant TGase1 molecules with the specific mutations in bathing suit ichthyosis, such as p.Arg307Gly, the TGase1 activity is increased at 33°C (and even higher at 31°C) compared with 37°C.37 In the present study, under various temperature incubation conditions, 25°C, 33°C, and 37°C, no significant difference in the pepK5 labeling intensity was observed in normal human epidermis or in the epidermis of any LI patient, although Patient 4 had a missense mutation in Arg307 (p.Arg307Trp) in which another mutation p.Arg307Gly causing bathing suit ichthyosis phenotype was previously reported.26 We think these discrepancies on temperature sensitivity between previous reports26,37 and our present results may be attributable to the fact that fluorescence labeling is not completely a quantitative method. In addition, we incubated tissue sections with a substrate solution for 90 minutes in our in situ TGase1 activity assay. Thus, we cannot exclude the possibility that the long-time incubation might make the enzymatic reaction almost saturated and make it difficult to detect fine difference in TGase1 activity.
As the results of the present study, in situ TGase1 activity assays using pepK5 demonstrated a remarkably reduced or a complete lack of membrane-associated labeling in the epidermis in all patients with TGM1 mutations compared with normal human epidermis and ichthyosis patients with TGM1-unrelated genetic defects. The present results indicate that pepK5 labeling can distinguish LI patients with TGM1 mutations from normal healthy individuals and from ichthyosis patients with other causative gene mutations. In this context, specific and sensitive detection of TGase1 activity using pepK5 is thought to be a powerful tool for screening TGase1 deficiency in LI patients. Furthermore, in the present LI patients, we demonstrated that the TGase1 molecule was missing in a compound heterozygote and a homozygote for TGM1 nonsense/truncation mutations and was present in a compound heterozygote and a homozygote for missense mutations. Accordingly, pepK5 labeling was missing in the patients with nonsense/truncation mutations, although there were weaker pepK5 signals in the patients with missense mutations. In this context, it might be possible to differentiate LI patients with nonsense/truncation mutations and those with missense mutations, and to predict patients’ clinical severity and courses from pepK5 labeling results. However, pepK5 fluorescence labeling is not a completely quantitative method and further accumulation of the pepK5 labeling data in LI cases with TGM1 mutations is needed for its diagnostic application, especially for the prediction of clinical severity in patients.
Acknowledgments
We thank Akari Nagasaki, M.S. for her technical assistance and Associate Professor James R. McMillan for proofreading this manuscript.
Footnotes
Address reprint requests to Masashi Akiyama, M.D., Ph.D., Department of Dermatology, Hokkaido University Graduate School of Medicine, North 15 West 7, Kita-ku, Sapporo 060-8638, Japan. E-mail: akiyama@med.hokudai.ac.jp.
Supported in part by a grant from the Ministry of Education, Science, and Culture of Japan to M.A. (Kiban B 20390304) and by a grant from Ministry of Health, Labor, and Welfare of Japan (Health and Labor Sciences Research grants; Research on intractable diseases; H21-047) to M.A.
References
- Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995;70:17702–17711. doi: 10.1074/jbc.270.30.17702. [DOI] [PubMed] [Google Scholar]
- Akiyama M. Harlequin ichthyosis and other autosomal recessive congenital ichthyoses: the underlying genetic defects and pathomechanisms. J Dermatol Sci. 2006;42:83–89. doi: 10.1016/j.jdermsci.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Huber M, Rettler I, Bernasconi K, Frenk E, Lavrijsen SP, Ponec M, Bon A, Lautenschlager S, Schorderet DF, Hohl D. Mutations of keratinocyte transglutaminase in lamellar ichthyosis. Science. 1995;267:525–528. doi: 10.1126/science.7824952. [DOI] [PubMed] [Google Scholar]
- Russell LJ, DiGiovanna JJ, Rogers GR, Steinert PM, Hashem N, Compton JG, Bale SJ. Mutations in the gene for transglutaminase 1 in autosomal recessive lamellar ichthyosis. Nat Genet. 1995;9:279–283. doi: 10.1038/ng0395-279. [DOI] [PubMed] [Google Scholar]
- Herman ML, Farasat S, Steinbach PJ, Wei MH, Toure O, Fleckman P, Blake P, Bale SJ, Toro JR. Transglutaminase-1 gene mutations in autosomal recessive congenital ichthyosis: summary of mutations (including 23 novel) and modeling of TGase-1. Hum Mutat. 2009;30:537–547. doi: 10.1002/humu.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Akiyama M, Yanagi T, McMillan JR, Suzuki T, Tsukamoto K, Sugiyama H, Hatano Y, Hayashitani M, Takamori K, Nakashima Keiko, Shimizu H. ABCA12 is a major causative gene for non-bullous congenital ichthyosiform erythroderma. J Invest Dermatol. 2009;129:2306–2309. doi: 10.1038/jid.2009.23. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Shimizu H. An update on molecular aspects of the non-syndromic ichthyoses. Exp Dermatol. 2008;17:373–382. doi: 10.1111/j.1600-0625.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- Jobard F, Lefévre C, Karaduman A, Blanchet-Bardon C, Emre S, Weissenbach J, Ozgüc M, Lathrop M, Prud'homme JF, Fischer J. Lipoxygenase-3 (ALOXE3) and 12(R)-lipoxygenase (ALOX12B) are mutated in non-bullous congenital ichthyosiform erythroderma (NCIE) linked to chromosome 17p13.1. Hum Mol Genet. 2002;1:107–113. doi: 10.1093/hmg/11.1.107. [DOI] [PubMed] [Google Scholar]
- Lefévre C, Audebert S, Jobard F, Bouadjar B, Lakhdar H, Boughdene-Stambouli O, Blanchet-Bardon C, Heilig R, Foglio M, Weissenbach J, Lathrop M, Prud'homme JF, Fischer J. Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum Mol Genet. 2003;12:2369–2378. doi: 10.1093/hmg/ddg235. [DOI] [PubMed] [Google Scholar]
- Lefévre C, Bouadjar B, Karaduman A, Jobard F, Saker S, Ozgüc M, Lathrop M, Prud'homme JF, Fischer J. Mutations in ichthyin a new gene on chromosome 5q33 in a new form of autosomal recessive congenital ichthyosis. Hum Mol Genet. 2004;13:2473–2482. doi: 10.1093/hmg/ddh263. [DOI] [PubMed] [Google Scholar]
- Lefévre C, Bouadjar B, Ferrand V, Tadini G, Mégarbané A, Lathrop M, Prud'homme JF, Fischer J. Mutations in a new cytochrome P450 gene in lamellar ichthyosis type 3. Hum Mol Genet. 2006;15:767–776. doi: 10.1093/hmg/ddi491. [DOI] [PubMed] [Google Scholar]
- Laiho E, Ignatius J, Mikkola H, Yee VC, Teller DC, Niemi KM, Saarialho-Kere U, Kere J, Palotie A. Transglutaminase 1 mutations in autosomal recessive congenital ichthyosis: private and recurrent mutations in an isolated population. Am J Hum Genet. 1997;61:529–538. doi: 10.1086/515498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennies HC, Küster W, Wiebe V, Krebsová A, Reis A. Genotype/phenotype correlation in autosomal recessive lamellar ichthyosis. Am J Hum Genet. 1998;62:1052–1061. doi: 10.1086/301818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho E, Niemi K-M, Ignatius J, Kere J, Palotie A, Saarialho-Kere U. Clinical and morphological correlations for transglutaminase 1 gene mutations in autosomal recessive congenital ichthyosis. Eur J Hum Genet. 1999;7:625–632. doi: 10.1038/sj.ejhg.5200353. [DOI] [PubMed] [Google Scholar]
- Shevchenko YO, Compton JG, Toro JR, DiGiovanna JJ, Bale SJ. Splice-site mutation in TGM1 in congenital recessive ichthyosis in American families: molecular, genetic, genealogic, and clinical studies. Hum Genet. 2000;106:492–499. doi: 10.1007/s004390000284. [DOI] [PubMed] [Google Scholar]
- Aeschlimann D, Wetterwald A, Fleisch H, Paulsson M. Expression of tissue transglutaminase in skeletal tissues correlates with events of terminal differentiation of chondrocytes. J Cell Biol. 1993;120:1461–1470. doi: 10.1083/jcb.120.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunath M, Hennies HC, Velten F, Wiebe V, Steinert PM, Reis A, Traupe H. A novel in situ method for the detection of deficient transglutaminase activity in the skin. Arch Dermatol Res. 1998;290:621–627. doi: 10.1007/s004030050362. [DOI] [PubMed] [Google Scholar]
- Hohl D, Aeschlimann D, Huber M. In vitro and rapid in situ transglutaminase assays for congenital ichthyoses—a comparative study. J Invest Dermatol. 1998;110:268–271. doi: 10.1046/j.1523-1747.1998.00132.x. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Takizawa Y, Kokaji T, Shimizu H. Novel mutations of TGM1 in a child with congenital ichthyosiform erythroderma. Br J Dermatol. 2001;144:401–407. doi: 10.1046/j.1365-2133.2001.04037.x. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Takizawa Y, Suzuki Y, Ishiko A, Matsuo I, Shimizu H. Compound heterozygous TGM1 mutations including a novel missense mutation L204Q in a mild form of lamellar ichthyosis. J Invest Dermatol. 2001;116:992–995. doi: 10.1046/j.0022-202x.2001.01367.x. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Hosono M, Kitamura M, Tsuda T, Yamanishi K, Maki M, Hitomi K. Identification of preferred substrate sequences for transglutaminase 1 – development of a novel peptide that can efficiently detect cross-linking enzyme activity in the skin. FEBS J. 2008;275:5667–5677. doi: 10.1111/j.1742-4658.2008.06692.x. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Hosono M, Wada F, Yoshimura T, Maki M, Hitomi K. Screening for the preferred substrate sequence of transglutaminase using a phage-displayed peptide library. Identification of peptide substrates for TGase2 and factor XIIIa. J Biol Chem. 2006;281:17699–17706. doi: 10.1074/jbc.M513538200. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Smith LT, Shimizu H. Expression of transglutaminase activity in developing human epidermis. Br J Dermatol. 2000;142:223–225. doi: 10.1046/j.1365-2133.2000.03288.x. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Takizawa Y, Suzuki Y, Shimizu H. A novel homozygous mutation 371delA in TGM1 leads to a classic lamellar ichthyosis phenotype. Br J Dermatol. 2003;148:149–153. doi: 10.1046/j.1365-2133.2003.05041.x. [DOI] [PubMed] [Google Scholar]
- Natsuga K, Akiyama M, Kato N, Sakai K, Sugiyama-Nakagiri Y, Nishimura M, Hata H, Abe M, Arita K, Tsuji-Abe Y, Onozuka T, Aoyagi S, Kodama K, Ujiie H, Tomita Y, Shimizu H. Novel ABCA12 mutations identified in two cases of non-bullous congenital ichthyosiform erythroderma associated with multiple skin malignant neoplasia. J Invest Dermatol. 2007;127:2669–2673. doi: 10.1038/sj.jid.5700885. [DOI] [PubMed] [Google Scholar]
- Oji V, Hautier JM, Ahvazi B, Hausser I, Aufenvenne K, Walker T, Seller N, Steijlen PM, Küster W, Hovnanian A, Hennies HC, Traupe H. Bathing suit ichthyosis is caused by transglutaminase-1 deficiency: evidence for a temperature-sensitive phenotype. Hum Mol Genet. 2006;15:3082–3097. doi: 10.1093/hmg/ddl249. [DOI] [PubMed] [Google Scholar]
- Esposito G, Tadini G, Paparo F, Viola A, Ieno L, Pennacchia W, Messina F, Giordano L, Piccirillo A, Auricchio L. Transglutaminase 1 deficiency and corneocyte collapse: an indication for targeted molecular screening in autosomal recessive congenital ichthyosis. Br J Dermatol. 2007;157:808–810. doi: 10.1111/j.1365-2133.2007.08070.x. [DOI] [PubMed] [Google Scholar]
- Furutani Y, Kato A, Notoya M, Ghoneim MA, Hirose S. A simple assay and histochemical localization of transglutaminase activity using a derivative of green fluorescent protein as substrate. J Histochem Cytochem. 2001;49:247–258. doi: 10.1177/002215540104900212. [DOI] [PubMed] [Google Scholar]
- Oji V, Oji ME, Adamini N, Walker T, Aufenvenne K, Raghunath M, Traupe H. Plasminogen activator inhibitor-2 is expressed in different types of congenital ichthyosis: in vivo evidence for its cross-linking into the cornified cell envelope by transglutaminase-1. Br J Dermatol. 2006;154:860–867. doi: 10.1111/j.1365-2133.2005.07109.x. [DOI] [PubMed] [Google Scholar]
- Hiiragi T, Sasaki H, Nagafuchi A, Sabe H, Shen SC, Matsuki M, Yamanishi K, Tsukita S. Transglutaminase type 1 and its cross-linking activity are concentrated at adherens junctions in simple epithelial cells. J Biol Chem. 1999;274:34148–34154. doi: 10.1074/jbc.274.48.34148. [DOI] [PubMed] [Google Scholar]
- Iizuka R, Chiba K, Ohmi-Imajoh S. A novel approach for the detection of proteolytically activated transglutaminase 1 in epidermis using cleavage site-directed antibodies. J Invest Dermatol. 2003;121:457–464. doi: 10.1046/j.1523-1747.2003.12403.x. [DOI] [PubMed] [Google Scholar]
- Yee VC, Pedersen LC, Le Trong I, Bishop PD, Stenkamp RE, Teller DC. Three-dimensional structure of a transglutaminase: human blood coagulation factor XIII. Proc Natl Acad Sci USA. 1994;91:7296–7300. doi: 10.1073/pnas.91.15.7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat LE. Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat Rev Mol Cell Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- Lejeune F, Maquat LE. Mechanistic links between nonsense-mediated mRNA decay and pre-mRNA splicing in mammalian cells. Curr Opin Cell Biol. 2005;17:309–315. doi: 10.1016/j.ceb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Raghunath M, Hennies HC, Ahvazi B, Vogel M, Reis A, Steinert PM, Traupe H. Self-healing collodion baby: a dynamic phenotype explained by a particular transglutaminase-1 mutation. J Invest Dermatol. 2003;120:224–228. doi: 10.1046/j.1523-1747.2003.12032.x. [DOI] [PubMed] [Google Scholar]
- Arita K, Jacyk WK, Wessagowit V, van Rensburg EJ, Chaplin T, Mein CA, Akiyama M, Shimizu H, Happle R, McGrath JA. The South African “bathing suit ichthyosis” is a form of lamellar ichthyosis caused by a homozygous missense mutation, p.R315L, in transglutaminase 1. J Invest Dermatol. 2007;127:490–493. doi: 10.1038/sj.jid.5700550. [DOI] [PubMed] [Google Scholar]
- Aufenvenne K, Oji V, Walker T, Becker-Pauly C, Hennies HC, Stöcker W, Traupe H. Transglutaminase-1 and bathing suit ichthyosis: molecular analysis of gene/environment interactions. J Invest Dermatol. 2009;129:2068–2071. doi: 10.1038/jid.2009.18. [DOI] [PubMed] [Google Scholar]