Abstract
Alzheimer’s disease (AD) is characterized by neuronal death; thus, identifying neurotoxic proteins and their source is central to understanding and treating AD. The multifunctional protease thrombin is neurotoxic and found in AD senile plaques. The objective of this study was to determine whether brain endothelial cells can synthesize thrombin and thus be a source of this neurotoxin in AD brains. Microvessels were isolated from AD patient brains and from age-matched controls. Reverse transcription-PCR demonstrated that thrombin message was highly expressed in microvessels from AD brains but was not detectable in control vessels. Similarly, Western blot analysis of microvessels showed that the thrombin protein was highly expressed in AD- but not control-derived microvessels. In addition, high levels of thrombin were detected in cerebrospinal fluid obtained from AD but not control patients, and sections from AD brains showed reactivity to thrombin antibody in blood vessel walls but not in vessels from controls. Finally, we examined the ability of brain endothelial cells in culture to synthesize thrombin and showed that oxidative stress or cell signaling perturbations led to increased expression of thrombin mRNA in these cells. The results demonstrate, for the first time, that brain endothelial cells can synthesize thrombin, and suggest that novel therapeutics targeting vascular stabilization that prevent or decrease release of thrombin could prove useful in treating this neurodegenerative disease.
Although Alzheimer’s disease (AD) has traditionally been classified as a neurodegenerative dementia without cerebrovascular changes, current epidemiological, pathological, experimental, and imaging studies suggest that such classification is no longer tenable.1,2 Many vascular risk factors such as hypertension, hypercholesterolemia, obesity, homocysteinemia, apolipoprotein E4 genotype, and diabetes, have been shown to increase the risk of AD.3,4,5,6,7,8,9 Recent data from brain imaging studies in humans and animal models suggest that cerebrovascular dysfunction may precede cognitive decline and onset of neurodegenerative changes in AD and AD models.10 Phenotypic modulation of endothelium to a dysfunctional state is recognized to contribute to the pathogenesis of cardiovascular diseases such as atherosclerosis.11 Endothelial dysfunction is also increasingly implicated in the development of neurodegenerative diseases such as AD.12,13,14,15
Activated endothelial cells elaborate adhesion molecules, cytokines and chemokines, growth factors, vasoactive molecules, major histocompatibility complex molecules, procoagulant and anticoagulant moieties, and a variety of other gene products with biological activity.16 The activated endothelium exerts direct local effects by producing at least 20 paracrine factors that act on adjacent cells.17 Perturbations of the central nervous system microvascular endothelium are closely linked to the pathophysiology of several neuroinflammatory, neuroinfectious, and neurodegenerative disease states including multiple sclerosis, HIV-associated encephalopathy, and AD.18 Because endothelial cells are highly synthetic, producing a variety of soluble factors, an injured/altered brain endothelial cell could release factors that are injurious or toxic to neurons. This is especially relevant for diseases such as AD that are characterized by neuronal cell death. In this regard, work from our laboratory provides support for the idea that in AD the cerebral microvasculature is biochemically altered, functionally deranged, and a rich source of soluble factors that affect neurons and other cells in the brain.12 We have shown that in the AD brain microvessels release numerous inflammatory and/or neurotoxic proteins including tumor necrosis factor α, transforming growth factor-β, interleukin (IL) IL-1β, IL-6, IL-8, matrix metalloproteinases, and thrombin.19,20,21
Vascular release of the multifunctional protein thrombin is of particular relevance for AD as thrombin has been shown to be neurotoxic both in vitro and in vivo. Exposure of primary neuronal cultures or neuronal cell lines to thrombin results in significant apoptosis.22 Thrombin accumulation has been documented in the senile plaques of the AD.23 Also, traumatic brain injury, where neurons are exposed to high thrombin levels is associated with an increased incidence of AD.24,25 Some neurological diseases, such as AD and Parkinson’s disease are characterized by increased levels of both thrombin and the thrombin receptor protease activated receptor (PAR)-1.26,27 Activation or overexpression of the receptor PAR-1 has been shown to induce motor neuron degeneration.28 We have previously published that administration of thrombin directly into the rat brain results in neuronal cell death, glial scarring, and cognitive deficits.29
Whether brain endothelial cells can synthesize thrombin has not been reported. We have shown that brain microvessels in AD release thrombin,30 but this could reflect release of sequestered thrombin because endothelial cells can readily bind and internalize thrombin. Similarly, we have reported that cultured brain endothelial cells, under stress conditions, release thrombin31; but whether this involves synthesis or release is unknown.
The objective of this study is to determine whether brain endothelial cells can synthesis thrombin and whether the vasculature is a source of this neurotoxin in AD brains.
Materials and Methods
Culture and Treatment of Rat Brain Endothelial Cells
Rat brain endothelial cells were obtained from rat brain microvessels, as previously described.32 The endothelial identity of these cultures was confirmed using antibodies to endothelial cell surface antigen Factor VIII. Endothelial cells used in this study (passages 8 to 12) were maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, 1% glutamine, and 1% antibiotics in a humidified 5% CO2 incubator at 37°C. For experiments, rat endothelial cells were plated at a density of 300,000 cells per well onto 6-well plates. The cells were washed three times with Hanks’ balanced salt solution and the media changed to serum-free Dulbecco’s modified Eagle’s medium plus 0.1% bovine serum albumin. Endothelial cell cultures were treated with serum-free media, media containing 100 μmol/L H2O2, or media containing 1 μmol/L of the protein kinase C (PKC) inhibitor bisindolymalemide for 24 hours.
Human Microvessel Isolation and Human Cerebrospinal Fluid
Brains were obtained from AD (69.6 ± 5.5 yrs) and age-matched control (69.8 ± 3.61 yrs) patients. Microvessels were isolated from the right cerebral hemispheres of control (postmortem times: 14.5, 14, 14, 20, 19; 15.4 ± 2.02 hours) and AD (9, 10, 21, 12, 15; 13.4 ± 2.16 hours) autopsy specimens and stored at −80°C until use. Left cerebral hemispheres were histologically processed for diagnostic and morphometric studies for the clinical diagnosis of primary degenerative AD dementia. Each case was examined for neuritic plaques and neurofibrillary tangles as recommended by National Institutes of Health Neuropathology Panel and each case fulfilled the rigorous morphometric criteria of AD.33,34 Control samples from age-matched patients without evidence of neuropathology and similar postmortem intervals were also collected. Microvessels were isolated from pooled temporal, parietal, and frontal cortices, as we have previously described.35 Briefly, brain microvessels were filtered through a 210 μm sieve and collected on a 53-μm sieve, resuspended in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and dimethyl sulfoxide and stored frozen in liquid nitrogen until use. This procedure yields approximately 6 to 10 mg microvessel protein from 15 g human cortex. A separate microvessel preparation was isolated from each human brain. The microvessel preparation has been previously characterized as largely capillary (>85%) and relatively free of nonvascular contaminants. The purity of the microvessel preparations was assessed by phase contrast microscopy. The morphology of the isolated vascular preparation was comparable between AD-and control-derived vessels.
Frozen cerebrospinal fluid (CSF) was quick-thawed, mixed with sample buffer (25 mmol/L Tris-HCl, pH 6.8,1% SDS, 10% glycerol, 2% β-mercaptoethanol, and 0.02% bromophenol blue) and processed as described in Western blot section.
Reverse Transcription-PCR
RNA from cultured rat brain endothelial cells and isolated human microvessels was prepared using the TRI Reagent RT (Molecular Research Center, Inc., Cincinnati, Ohio), according to manufacturer protocol. For the reverse transcription (RT)-PCR protocol (Roche Applied Science, Germany), 4 μg of total RNA, were reverse transcribed using random hexamer primer, 3 μl cDNA from RT-PCR reaction were used for PCR reaction. PCR conditions were: 95°C for 15 minutes, 38 cycles of 94°C for 30 seconds, 57°C for 30 seconds, and 72°C for 60 seconds, followed by last extension at 72°C for 10 minutes. β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as internal markers. PCR products were separated by electrophoresis in a 1.5% agarose gel and detected under the UV light. Human thrombin PCR products were sequenced by Texas Tech University Core Facility and confirmed by NCBI BLAST alignments. Specific gene primers for PCR are shown on Table 1.
Table 1.
Primers Used for RT-PCR
| Gene | RefSeq accession | Sequence | Amplicon |
|---|---|---|---|
| Human | |||
| Thrombin | NM_000506 | Fwd 5′-GAGTGCCAGCTATGGAGGAG-3′ | 388 bp |
| Rev 5′-GCTGCACAGCTGAGTTGAAG-3′ | |||
| GAPDH | NM_002046 | Fwd 5′-GAGTCAACGGATTTGGTCGT-3′ | 288 bp |
| Rev 5′-TTGATTTTGGAGGGATCTCG-3′ | |||
| Rat | |||
| Thrombin | NM_022924 | Fwd 5′-TGGGAGAGGAGAACCATGAC-3′ | 339 bp |
| Rev 5′-AGGGTGGGTACAGAATGCAG-3′ | |||
| β-actin | NM_031144 | Fwd 5′-TGTCACCAACTGGGACGATA-3′ | 391 bp |
| Rev 5′-TCTCAGCTGTGGTGGTGAAG-3′ | |||
Western Blot
Proteins were extracted from human microvessels using lysis buffer containing 150 mmol/L sodium chloride, 50 mmol/L Tris, 1% NP-40, and 2 mmol/L phenylmethyl sulfonylfluoride. CSF, devoid of macroscopic blood contamination, was also used. Protein samples were mixed with sample buffer (25 mmol/L Tris-HCl, pH 6.8, 1% SDS, 10% glycerol, 2% β-mercaptoethanol, and 0.02% bromophenol blue) boiled for 5 minutes, resolved in 10% SDS-polyacrylamide electrophoresis mini gel and transferred to polyvinylidene difluoride membrane. After transfer, the membranes were blocked in Tris-buffered saline containing 0.25% Tween-20 (TBST) and 4% nonfat dry milk at room temperature for 2 hours, and subsequently incubated with antibodies to: thrombin (1:200, Cat. No: HYB 109–04, Antibody Shop, Denmark), GAPDH (1:1000, Cat. No: MAB374, Millipore, Billerica, MA), or human serum albumin (1:2500, Cat. No: ab10241, Abcam, Cambridge, MA) diluted in TBST plus 2% nonfat dry milk overnight at 4°C. Bound antibody was detected using goat anti-mouse IgG conjugated to horseradish peroxidase (Cat. No: 170-6516, Bio-Rad Laboratories, Hercules, CA) and developed with chemiluminescence.
Immunolocalization in Brain Tissue Sections
Human brain tissue sections were processed for immunolocalization, as previously described.36 Brain tissue sections (5 μm) were fixed in ice-cold acetone for 30 minutes, and room temperature acetone for 10 minutes, then washed in TBST, and blocked in TBS with 10% donkey serum at room temperature for 2 hours. Sections were incubated at 4°C overnight with primary antibodies: affinity-purified goat polyclonal thrombin antibody (1:50, Cat. No: Sc-16972, Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit polyclonal to Von Willebrand Factor (1:500, Cat. No: ab6994, Abcam) in TBS containing 2.5% donkey serum overnight at 4°C. Sections were then washed in TBST, incubated with 0.3% hydrogen peroxidase blocking solution at room temperature for 15 minutes, washed in TBST and incubated with secondary antibodies (1:400, Alexa Fluor 488 donkey anti-goat IgG (H+L), Cat. No: A-11055; Alexa Flour 546 donkey anti-rabbit IgG, Cat. No. A-10040; Invitrogen, Carlsbad, CA) at room temperature for 1 hour and washed. To visualize nuclei, sections were incubated with IHC-Tek DAPI Solution (Cat. No: IW-1404, IHC World, Woodstock, MD) at room temperature for 25 minutes and viewed using an Olympus IX71 Meta Imaging series 7.1 microscope.
Results
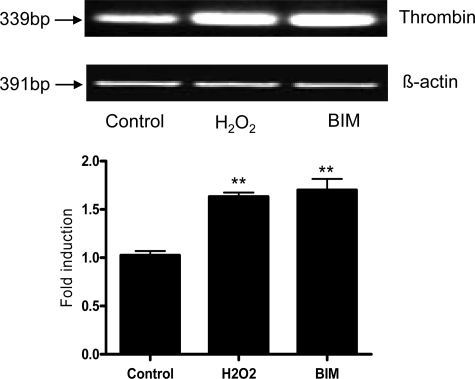
Rat brain endothelial cell cultures were treated with either serum-free media, H2O2 (100 μmol/L), or the protease inhibitor bisindolymalemide (1 μmol/L) for 24 hours. The cells were collected, mRNA isolated and RT-PCR performed using primers for rat thrombin (Table 1). The data showed that untreated endothelial cells express a basal level of thrombin in culture (Figure 1). Treatment with either H2O2 or PKC inhibitor caused a significant (P < 0.01) increase in the expression thrombin RNA (Figure 1).
Figure 1.
Cultured rat brain endothelial cells were exposed to either serum-free media containing 0.1% bovine serum albumin (control), media plus 100 μmol/L H2O2, or media plus 1 μmol/L PKC inhibitor bisindolymalemide (BIM) for 24 hours. RNA was extracted, reverse transcribed, and amplified using primers specific for rat thrombin and the housekeeping gene β-actin. The intensity of the bands is graphically shown below RT-PCR bands. The experiment was performed three times and a representative RT-PCR is shown. **P < 0.01 vs. control.
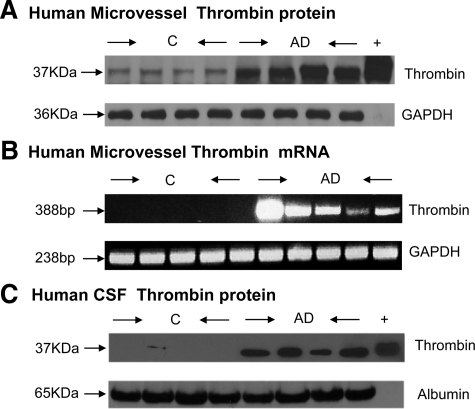
Microvessels isolated from AD brains and age-matched, nondemented controls were analyzed by Western blot and RT-PCR for the expression of thrombin protein and message, respectively. The Western blot from four control and four AD brains (Figure 2A) showed that the protein thrombin was very highly expressed in AD microvessels, while barely detectable in controls. Similarly, RT-PCR analysis showed robust expression of thrombin in AD microvessels, with no detectable expression in control vessels (Figure 2B). We also examined the presence of thrombin, by Western blot, in patient CSF and showed that CSF samples from AD patients express thrombin while in CSF samples from control brains thrombin was undetectable (Figure 2C). All samples were monitored for loading equivalency using GAPDH or albumin (Figure 2).
Figure 2.
A: Proteins from control- (C) and AD-derived microvessel lysates were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and immunoblotted for human thrombin (37 kDa). Loading equivalency was confirmed using GAPDH; n = 4. B: RNA from control (C) and AD-derived microvessels was extracted, reverse transcribed, and amplified using primers specific for human thrombin and the housekeeping gene GAPDH; n = 4. C: Proteins from control- (C) and AD-derived CSF were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and immunoblotted for human thrombin (37 kDa). Loading equivalency was confirmed using albumin; n = 4.
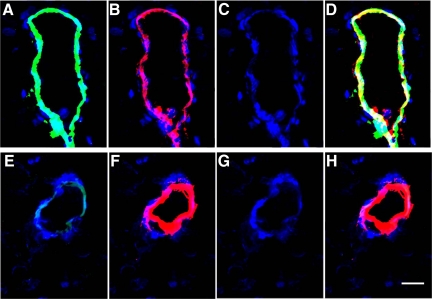
Sections from AD and control brains brain sections were examined by immunofluorescence for the presence of the endothelial cell marker Von Willebrand factor and for thrombin (Figure 3). Vessels showed staining for Von Willebrand factor in both AD (Figure 3B) and control (Figure 3F) tissues. Also, 4,6-diamidino-2-phenylindole staining for nuclei was comparable in both AD (Figure 3C) and control (Figure 3G) sections. In contrast, the presence of green immunofluorescence denoting reactivity to the thrombin antibody along the vessel wall was heavily expressed in AD (Figure 3A) but barely detectable in control (Figure 3E) sections. Similarly, the yellow/white staining of vessels, which reflects a merged image of Von Willebrand and thrombin staining, was strongly present in AD sections (Figure 3D) but not discernible in controls (Figure 3H).
Figure 3.
Alzheimer’s disease (A–D) and control (E–H) brain sections were examined by immunofluorescence for the presence of the endothelial cell marker Von Willebrand factor and for thrombin (×20). 4,6-Diamidino-2-phenylindole staining for nuclei was comparable in both AD (C) and control (G) sections. Staining for Von Willebrand factor was detectable in both AD (B) and control (F) tissues. In contrast, reactivity to the thrombin antibody (green immunofluorescence) was clearly present in AD (A) but barely detectable in control (E) vessels. Similarly, yellow/white immunofluorescence, which reflects a merged image of Von Willebrand and thrombin staining, was strong in AD sections (D) but not discernible in controls (H). Scale bar = 15 μm.
Discussion
Alzheimer’s disease is characterized by neuronal death; thus identifying neurotoxic proteins and their source is central to understanding and treating this disease. The multifunctional protease thrombin appears involved in neurodegenerative processes following traumatic brain injury and stroke.26 Although a protease, the nonproteolytic functions of thrombin have been well described.28,37,38 Thrombin regulates cell signaling by cleaving and activating G protein-coupled protease activated receptors (PARs 1 to 4) to unmask a tethered receptor-triggering ligand. Activation of PARs by thrombin can mediate cell death or survival in the brain depending on amplitude and duration of agonist; high thrombin levels trigger apoptosis.22,37 In the current study we show that thrombin is elevated in the CSF and cerebromicrovasculature in AD brains but not in those of age-matched control brains. Our data show that brain endothelial cells can synthesize thrombin and thus could be a heretofore unrecognized source for this neurotoxic protein. Our results are consistent with the observation that immunoreactivity for the major brain thrombin inhibitor, protease nexin-1 is significantly decreased around blood vessels in AD brains, suggesting vascular release of thrombin.39
Alzheimer’s disease is characterized by increased levels of both active thrombin and PAR-1, suggesting that thrombin and its receptor are involved in ongoing neurodegenerative processes.26,27 Thrombin is a neurotoxin of relevance for AD because it can affect multiple mechanisms that contribute to neuronal cell death. Thrombin can directly injure neurons by activating cell cycle pathways that initiate neuronal cell apoptosis.40 Thrombin can also induce neurotoxicity by nicotinamide adenine dinucleotide phosphate oxidase-mediated oxidative stress.41 Thrombin can indirectly contribute to neuronal cell death by activating glial cells that in turn increase oxidative stress and neuroinflammation.42,43,44 Thrombin can increase apolipoprotein E levels in the brain and potent neurotoxicity is evoked by a 22-kDa N-terminal thrombin-cleavage fragment of apolipoprotein E,45 thus thrombin can also cause neuronal cell injury and death through its actions on apolipoprotein E proteins.
Thrombin may also play a role in AD pathogenesis via interactions with Aβ. In platelets release of Aβ is regulated by thrombin.46 Thrombin in vitro can stimulate production of the amyloid precursor protein (APP) and cleavage of APP into fragments that are found in amyloid plaques of AD brains.47,48 In endothelial cells, thrombin induces surface expression and intracellular secretion of APP via a PKC-dependent mechanism.48 Treatment of cultured rat hippocampal neurons with thrombin causes a dose-dependent increase in Aβ and redistribution of APP.49 Also in these cultures toxicity of Aβ is significantly enhanced by co-incubation with thrombin and attenuated by incubation with the thrombin inhibitor protease nexin-1. Aβ induces an increase in intracellular calcium and peroxides that is augmented by thrombin and diminished protease nexin-1.49 Finally, because Aβ causes expression of inflammatory genes and proteins in endothelial cells50 and inflammatory proteins increase thrombin expression in endothelial cells,31 the vasculature could be an important nexus for the interaction of these two neurotoxic proteins.
Thrombin is also important for the proteolytic processing of the microtubule-associate protein tau, a primary component of the neurofibrillary tangle.51 Nanomolar concentrations of thrombin induce rapid tau hyperphosphorylation and aggregation in murine hippocampal neurons via PARs, which is followed by delayed synaptophysin reduction and apoptotic neuronal death.52 Persistent thrombin signaling via protease-activated receptor 4 and prolonged downstream p44/42 mitogen activated protein kinase activation appear to mediate this effect. Thrombin could be a contributor to processes that favor formation of hyperphosphorylated, insoluble tau in the AD brain.
Vascular-derived thrombin may be key regulator of events in the AD brain because of its ability to regulate the expression of other inflammatory and bioactive proteins. In this regard, thrombin causes endothelial activation and enhanced expression and/or release of many pro-inflammatory proteins including monocyte chemoattractant protein-1, intercellular adhesion molecule-1, IL-1, IL-6, and IL-853,54,55,56,57; these inflammatory proteins are all up-regulated in the cerebromicrovasculature in AD.19,20,21 Also, both thrombin and matrix metalloproteinase-9 are elevated in the AD brain vasculature. Synthesis and release of multiple factors from the vasculature suggests that these proteins may synergize and produce a locally intense neurotoxic insult. This is supported by results showing that the in vitro neurotoxicity as well as in vivo cell death in intracerebral hemorrhage evoked by either thrombin or matrix metalloproteinase-9 are significantly greater when both proteases are present.58 Our demonstration that endothelial cells can produce thrombin and data showing that brain endothelial cells have functionally active thrombin (PAR-1 and PAR-3) receptors59 suggest that thrombin may act as an autocrine factor, stimulating a noxious feed-forward cycle. Furthermore, the paracrine effects of thrombin released from endothelial cells are also important because of the ability of thrombin to activate other central nervous system cells, such as microglia and astrocytes.
Microglia, astrocytes, and microvascular endothelial cells co-exist in intimate proximity in nervous tissues, and their homeostatic interactions in health, as well as coordinated response to injury, have led to the concept that they form the basic elements of a functional “neurovascular unit.”60 Therefore, secretory products from one cell type profoundly influence the behavior of neighboring cells. Pro-inflammatory effects of thrombin on both microglia and astrocytes have been demonstrated. Intranigral injection of thrombin injures the dopaminergic neurons in the substantia nigra via thrombin-induced microglial activation and release of nitric oxide.42 Thrombin has been shown to stimulate the JAK2-STAT3 signaling pathway and increase transcription of inflammation-associated genes tumor necrosis factor α and inducible nitric oxide synthase in microglia.43 In astrocytes, activation of PAR-1 by thrombin leads to increased matrix metalloproteinase-9 expression through regulation of Erk1/2.44 Thus, vascular-derived thrombin may directly injure neurons or affect neuronal viability indirectly via activation of microglia and astrocytes.
What are the pathophysiologic signals that cause cerebral endothelial cells to produce thrombin? Our data show that in culture induction of stress by either exposure to the oxidant stressor H2O2 or by inhibition of PKC causes endothelial cells to produce thrombin. Both oxidative stress and perturbation of vascular signaling cascades are relevant for AD.
Considerable evidence points to oxidative stress as an important trigger in the complex chain of events leading to AD.61,62 A long list of surrogate markers for oxidant stress including lipid, DNA, and protein oxidation are elevated in AD.63,64 Brain microvessels in AD release high levels of the reactive oxygen species, nitric oxide, and express elevated levels of oxidized proteins.65 Hypoxia and oxidative stress and are interrelated processes and both contribute to AD pathogenesis.66 When injured by hypoxia, cerebral microvessels release reactive oxygen species.67 We have shown that brain microvessels from AD patients express higher levels of transcription factor hypoxia-inducible factor 1-α.30 Interestingly, treatment of cultured brain endothelial cells with thrombin causes an increase in hypoxia-inducible factor 1-α expression.30
Regarding PKC, we have previously documented abnormalities in signaling pathways in the cerebromicrovasculature including a pronounced inhibition of PKC activity in AD.68 Current data suggest that both generalized cellular stress caused by reactive oxygen species or perturbation of specific signaling mechanisms can lead to pathological activation of endothelial cells and the production of thrombin. Thrombin in the brain could be acutely deleterious in traumatic brain injury, where large amounts enter the brain via a disrupted blood-brain barrier. Alternatively, in neurodegenerative diseases such as AD thrombin, and the cascade of mediators it evokes, could be chronically deleterious resulting in neuronal injury in death over a number of years. Identification of brain endothelial cells as a source of neurotoxin thrombin suggests that novel therapeutics targeting vascular stabilization that prevent or decrease release of thrombin could prove useful in treating this neurodegenerative disease.
Acknowledgments
We gratefully acknowledge the secretarial assistance of Terri Stahl and the technical assistance of Janet Dertien.
Footnotes
Address reprint requests to Paula Grammas, Ph.D., Garrison Institute on Aging, Texas Tech University Health Sciences Center, 3601 4th Street Stop 9424, Lubbock, Texas 79430. E-mail: paula.grammas@ttuhsc.edu.
Supported in part by grants from the National Institutes of Health (AG15964, AG020569, and AG028367). Human Tissue was provided by the Garrison Institute on Aging Brain Bank at Texas Tech University Health Sciences Center, Indiana University Brain Bank and the New York Brain Bank at Columbia University. CSF was provided by the New York Brain Bank at Columbia University. Dr. Grammas is the recipient of the Shirley and Mildred Garrison Chair in Aging.
References
- Castellani RJ. Vascular dementia and Alzheimer’s disease: a waning dichotomy. J Alzheimers Dis. 2007;12:343–344. doi: 10.3233/jad-2007-12407. [DOI] [PubMed] [Google Scholar]
- Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, Dekosky ST, Kuller LH. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E affects the rate of Alzheimer disease expression: beta amyloid burden in a secondary consequence on APOE genotype and duration of disease. J Neuropathol Exp Neurol. 1994;53:429–437. doi: 10.1097/00005072-199409000-00002. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Coronary artery disease, hypertension, ApoE, and cholesterol: a link to Alzheimer’s disease. Ann NY Acad Sci. 1997;826:128–146. doi: 10.1111/j.1749-6632.1997.tb48466.x. [DOI] [PubMed] [Google Scholar]
- Tariska P, Klein V, Panczel G, Vitrai J, Knolmayer J, Meszaros A, Urbanics K, Kiss E. Vascular disease risk factors and findings in patients with Alzheimer’s disease. Arch Gerontol Geriatr. 1997;25:237–243. doi: 10.1016/s0167-4943(97)00015-0. [DOI] [PubMed] [Google Scholar]
- Marx J. Alzheimer’s disease. Bad for the heart, bad for the mind? Science. 2001;294:508–509. doi: 10.1126/science.294.5542.508. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. Vascular factors in Alzheimer’s disease. Int Psychogeriatr. 2003;15:47–52. doi: 10.1017/S1041610203008950. [DOI] [PubMed] [Google Scholar]
- Sadowski M, Pankiewicz J, Scholtzova H, Li YS, Quartermain D, Duff K, Wisniewski T. Links between the pathology of Alzheimer’s disease and vascular dementia. Neurochem Res. 2004;29:1257–1266. doi: 10.1023/b:nere.0000023612.66691.e6. [DOI] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardeña G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann NY Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- Grammas P. A damaged microcirculation contributes to neuronal cell death in Alzheimer’s disease. Neurobiol Aging. 2000;21:199–205. doi: 10.1016/s0197-4580(00)00102-0. [DOI] [PubMed] [Google Scholar]
- Grammas P, Yamada M, Zlokovic B. The cerebromicrovasculature: a key player in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis. 2002;4:217–223. doi: 10.3233/jad-2002-4311. [DOI] [PubMed] [Google Scholar]
- Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubio A, Sagare A, Liu D, Li F, Armstrong D, Gasiewicz T, Zidovetzki R, Song X, Hofman F, Zlokovic BV. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- Schultheiss C, Blechert B, Gaertner FC, Drecoll E, Mueller J, Weber GF, Drzezga A, Essier M. In vivo characterization of endothelial cell activation in a transgenic mouse model of Alzheimer’s disease. Angiogenesis. 2006;9:59–65. doi: 10.1007/s10456-006-9030-4. [DOI] [PubMed] [Google Scholar]
- Milkiewicz M, Ispanovic E, Doyle JL, Haas TL. Regulators of angiogenesis and strategies for their therapeutic manipulation. Int J Biochem Cell Biol. 2006;38:333–357. doi: 10.1016/j.biocel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Felmeden DC, Blann AD, Lip GYH. Angiogenesis: basic pathophysiology and implications for disease. Eur Heart J. 2003;24:585–603. doi: 10.1016/s0195-668x(02)00635-8. [DOI] [PubMed] [Google Scholar]
- Andjelkovic AV, Pachter JS. Central nervous system endothelium in neuroinflammatory, neuroinfectious, and neurodegenerative disease. J Neurosci Res. 1998;51:423–430. doi: 10.1002/(SICI)1097-4547(19980215)51:4<423::AID-JNR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging. 2001;22:837–842. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Cerebrovascular TGF-β contributes to inflammation in the Alzheimer’s brain. Am J Pathol. 2002;160:583–1587. doi: 10.1016/s0002-9440(10)61105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumangakudi L, Ghatreh-Samany P, Owoso A, Wiskar B, Grammas P. Angiogenic proteins are expressed by brain blood vessels in Alzheimer’s disease. J Alzheimers Dis. 2006;10:111–118. doi: 10.3233/jad-2006-10114. [DOI] [PubMed] [Google Scholar]
- Reimann-Philipp U, Ovase R, Weigel PH, Grammas P. Mechanisms of cell death in primary cortical neurons and PC12 cells. J Neurosci Res. 2001;64:654–660. doi: 10.1002/jnr.1119. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Ikeda K, Kondo H, McGeer PL. Thrombin accumulation in patients with Alzheimer’s disease. Neurosci Lett. 1992;146:152–154. doi: 10.1016/0304-3940(92)90065-f. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A. Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20:S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, Kurland LT. Traumatic brain injury and time to onset of Alzheimer’s disease: a population based study. Am J Epidemiol. 1999;149:32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- Nishino A, Suzuki M, Ohtani H, Motohashi O, Umezawa K, Nagura H, Yoshimoto T. Thrombin may contribute to the pathophysiology of central nervous system injury. J Neurotraum. 1993;10:167–179. doi: 10.1089/neu.1993.10.167. [DOI] [PubMed] [Google Scholar]
- Sokolova E, Reiser G. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: localization, expression and participation in neurodegenerative diseases. Thromb Haemost. 2008;100:576–581. [PubMed] [Google Scholar]
- Luo W, Wang Y, Reiser G. Protease-activated receptors in the brain: receptor expression, activation, and functions in neurodegeneration. Brain Res Rev. 2007;56:331–345. doi: 10.1016/j.brainresrev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Mhatre M, Nguyen A, Kashani S, Pham T, Adesina A, Grammas P. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol Aging. 2004;25:783–793. doi: 10.1016/j.neurobiolaging.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Grammas P, Samany PG, Thirumangalakudi L. Thrombin and inflammatory proteins are elevated in Alzheimer’s disease microvessels: implications for disease pathogenesis. J Alzheimers Dis. 2006;9:51–58. doi: 10.3233/jad-2006-9105. [DOI] [PubMed] [Google Scholar]
- Grammas P, Ottman T, Reimann-Philipp U, Larabee J, Weigel PH. Injured brain endothelial cells release neurotoxic thrombin. J Alzheimers Dis. 2004;6:275–281. doi: 10.3233/jad-2004-6308. [DOI] [PubMed] [Google Scholar]
- Diglio CA, Liu W, Grammas P, Giacomelli F, Wiener J. Isolation and characterization of cerebral resistance vessel endothelium in culture. Tissue Cell. 1993;25:833–846. doi: 10.1016/0040-8166(93)90032-g. [DOI] [PubMed] [Google Scholar]
- Ball MJ, Griffin-brooks S, MacGregor JA, Ojalvo-Rose E, Fewster PH. Neuropathological definition of Alzheimer disease: multivariate analyses in the morphometric distinction between Alzheimer dementia and normal aging. Alzheimer Dis Assoc Disord. 1988;2:29–37. doi: 10.1097/00002093-198802010-00004. [DOI] [PubMed] [Google Scholar]
- Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Grammas P, Moore P, Weigel PH. Microvessels from Alzheimer’s disease brains kill neurons in vitro. Am J Pathol. 1999;154:337–342. doi: 10.1016/S0002-9440(10)65280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira HA, Kumar P, Grammas P. Expression of CAP37, a novel inflammatory mediator, in Alzheimer’s disease. Neurobiol Aging. 1996;17:753–759. [PubMed] [Google Scholar]
- Rohatgi T, Sedehizade F, Reymann KG, Reiser G. Protease-activated receptors in neuronal development, neurodegeneration, and neuroprotection: thrombin as signaling molecule in brain. Neuroscientist. 2004;10:501–512. doi: 10.1177/1073858404269955. [DOI] [PubMed] [Google Scholar]
- Hansen nKK, Oikonomopoulou K, Baruch A, Ramachandran R, Beck P, Diamandis EP, Hollenberg MD. Proteinases as hormones: targets and mechanisms for proteolytic signaling. Biol Chem. 2008;389:971–982. doi: 10.1515/BC.2008.120. [DOI] [PubMed] [Google Scholar]
- Vaughan PL, Su J, Cotman CW, Cunningham D. Protease nexin-1, a potent thrombin inhibitor, is reduced around cerebral blood vessels in Alzheimer’s disease. Neuroreport. 1994;5:2529–2533. doi: 10.1016/0006-8993(94)90521-5. [DOI] [PubMed] [Google Scholar]
- Rao HV, Thirumangalakudi L, Desmond P, Grammas P. Cyclin D1, cdk4, and Bim are involved in thrombin-induced apoptosis in cultured cortical neurons. J Neurochem. 2007;101:498–505. doi: 10.1111/j.1471-4159.2006.04389.x. [DOI] [PubMed] [Google Scholar]
- Park KW, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons: role of neuronal NADPH oxidase. J Neurosci Res. 86:1053–1063. doi: 10.1002/jnr.21571. [DOI] [PubMed] [Google Scholar]
- Huang CF, Li G, Ma R, Sun SG, Chen JG. Thrombin-induced microglial activation contributes to the degeneration of nigral dopaminergic neurons in vivo. Neurosci Bull. 2008;24:66–72. doi: 10.1007/s12264-008-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ma R, Sun S, Wei G, Fang Y, Liu R, Li G. JAK2-STAT3 signaling pathway mediates thrombin-induced proinflammatory actions of microglia in vitro. J Neuroimmunol. 2008;204:118–125. doi: 10.1016/j.jneuroim.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Choi MS, Kim YE, Lee WJ, Choi JW, Park GH, Kim SD, Jeon SJ, Go HS, Shin SM, Kim WK, Shin Cy, Ko KH. Activation of protease-activated receptor1 mediates induction of matrix metalloproteinase-9 by thrombin in rat primary astrocytes. Brain Res Bull. 2008;76:368–375. doi: 10.1016/j.brainresbull.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Tolar M, Marques MA, Harmony JA, Crutcher KA. Neurotoxicity of the 22 kDa thrombin-cleavage fragment of apolipoprotein E and related synthetic peptides is receptor-mediated. J Neurosci. 1997;17:5678–5686. doi: 10.1523/JNEUROSCI.17-15-05678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov A, Trupp A, Henkel AW, Bloch E, Reulbach U, Lewczuk P, Riggert J, Kornhuber J, Wiltfang J. Differential processing and secretion of Abeta peptides and sAPPalpha in human platelets is regulated by thrombin and prostaglandin E2. Neurobiol Aging. 2008;30:1552–1562. doi: 10.1016/j.neurobiolaging.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Murai H, Asaka J. Proteolytic processing of amyloid beta protein precursor (APP) by thrombin. Biochem Biophys Res. 1992;185:1000–1004. doi: 10.1016/0006-291x(92)91726-7. [DOI] [PubMed] [Google Scholar]
- Ciallela JR, Figueiredo H, Smith-Swintosky V, McGillis JP. Thrombin induces surface and intracellular secretion of amyloid precursor protein from human endothelial cells. Thromb Haemost. 1999;81:630–637. [PubMed] [Google Scholar]
- Smith-Swintosky VL, Zimmer S, Fenton JW, 2nd, Mattson MP. Opposing actions of thrombin and protease nexin-1 on amyloid beta-peptide toxicity and on accumulation of peroxides and calcium in hippocampal neurons. J Neurochem. 65:1415–1418. doi: 10.1046/j.1471-4159.1995.65031415.x. [DOI] [PubMed] [Google Scholar]
- Vukic V, Callaghan D, Walker D, Lue LF, Liu QY, Couraud PO, Romero IA, Weksler B, Stanimirovic DB, Zhang W. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer’s brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis. 34:95–106. doi: 10.1016/j.nbd.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Guo JP, McGeer PL. Proteolysis of non-phosphorylated and phosphorylated tau by thrombin. J Biol Chem. 2005;280:5145–5153. doi: 10.1074/jbc.M409234200. [DOI] [PubMed] [Google Scholar]
- Suo Z, Wu M, Citron BA, Palazzo RE, Festoff BW. Rapid tau aggregation and delayed hippocampal neuronal death induced by persistent thrombin signaling. J Biol Chem. 2003;278:37681–37689. doi: 10.1074/jbc.M301406200. [DOI] [PubMed] [Google Scholar]
- Colotta F, Sciacca FL, Sironi S, Luini W, Rabiet MJ, Mantovani A. Expression of monocyte chemoattractant protein-1 by monocytes and endothelial cells exposed to thrombin. Am J Pathol. 1994;144:975–985. [PMC free article] [PubMed] [Google Scholar]
- Marin V, Montero-Julian FA, Gres S, Boulay V, Bongrand P, Farnarier C, Kapanski G. The IL-6-soluble IL-6Ralpha autocrine loop of endothelial activation as an intermediate between acute and chronic inflammation: an experimental model involving thrombin. J Immunol. 2001;167:3435–3442. doi: 10.4049/jimmunol.167.6.3435. [DOI] [PubMed] [Google Scholar]
- Miho N, Ishida T, Kuwaba N, Ishida M, Shimote-Abe K, Tabuchi K, Oshima T, Yoshizumi M, Chayama K. Role of the JNK pathway in thrombin-induced ICAM-1 expression in endothelial cells. Cardiovasc Res. 2005;68:289–298. doi: 10.1016/j.cardiores.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Okada M, Suzuki K, Takada K, Nakashima M, Nakanishi T, Shinohara T. Detection of up-regulated genes in thrombin-stimulated human umbilical vein endothelial cells. Thromb Res. 2006;118:715–721. doi: 10.1016/j.thromres.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Smadja DM, Biéche I, Susen S, Mauge L, Laurendeau I, d'Audigier C, Grelac F, Emmerich J, Aiach M, Gaussem P. Interleukin 8 is differentially expressed and modulated by PAR-1 activation in early and late endothelial progenitor cells. J Cell Mol Med. 2008;13:2534–2546. doi: 10.1111/j.1582-4934.2008.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Hollenberg MD, Yong VW. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J Neurosci. 2006;26:10281–10291. doi: 10.1523/JNEUROSCI.2806-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha K, Dömötör E, Lanza F, Adam-Vizi V, Machovick R. Identification of thrombin receptors in rat brain capillary endothelial cells. J Cereb Blood Flow. 2000;20:175–182. doi: 10.1097/00004647-200001000-00022. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Cui JG, Lukiw WJ. Natural secretory products of human neural and microvessel endothelial cells: implications in pathogenic “spreading” and Alzheimer’s disease. Mol Neurobiol. 2006;34:181–192. doi: 10.1385/MN:34:3:181. [DOI] [PubMed] [Google Scholar]
- Butterfield DA. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- Gella A, Durany N: Oxidative stress in Alzheimer’s disease. Cell Adh Migr 2009, 3:[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenova MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer’s disease. Neuroscience. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Ratio of 8-hydroxyguanine in intact DNA to free 8-hydroxyguanine is increased in Alzheimer’s disease ventricular cerebrospinal fluid. Arch Neurol. 2001;58:392–396. doi: 10.1001/archneur.58.3.392. [DOI] [PubMed] [Google Scholar]
- Dorheim MA, Tracey WR, Pollock JS, Grammas P. Nitric oxide is elevated in Alzheimer’s brain microvessels. Biochem Biophys Res Comm. 1994;205:659–665. doi: 10.1006/bbrc.1994.2716. [DOI] [PubMed] [Google Scholar]
- Guglielmotto M, Tamagno E, Danni O. Oxidative stress and hypoxia: contribute to Alzheimer’s disease pathogenesis: two sides of the same coin. Scient World J. 2009;9:781–791. doi: 10.1100/tsw.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P, Liu GJ, Wood K, Floyd RA. Anoxia/reoxygenation induces hydroxyl free radical formation in brain microvessels. Free Radic Biol Med. 1993;14:553–557. doi: 10.1016/0891-5849(93)90113-9. [DOI] [PubMed] [Google Scholar]
- Grammas P, Moore P, Botchlet T, Hanson-Painton O, Cooper DR, Ball MJ, Roher A. cerebral microvessels in Alzheimer’s have reduced protein kinase C activity. Neurobiol Aging. 1995;16:563–569. doi: 10.1016/0197-4580(95)00048-j. [DOI] [PubMed] [Google Scholar]