Abstract
Influenza viruses vary markedly in their efficiency of human-to-human transmission. This variation has been speculated to be determined in part by the tropism of influenza virus for the human upper respiratory tract. To study this tropism, we determined the pattern of virus attachment by virus histochemistry of three human and three avian influenza viruses in human nasal septum, conchae, nasopharynx, paranasal sinuses, and larynx. We found that the human influenza viruses—two seasonal influenza viruses and pandemic H1N1 virus—attached abundantly to ciliated epithelial cells and goblet cells throughout the upper respiratory tract. In contrast, the avian influenza viruses, including the highly pathogenic H5N1 virus, attached only rarely to epithelial cells or goblet cells. Both human and avian viruses attached occasionally to cells of the submucosal glands. The pattern of virus attachment was similar among the different sites of the human upper respiratory tract for each virus tested. We conclude that influenza viruses that are transmitted efficiently among humans attach abundantly to human upper respiratory tract, whereas inefficiently transmitted influenza viruses attach rarely. These results suggest that the ability of an influenza virus to attach to human upper respiratory tract is a critical factor for efficient transmission in the human population.
Influenza is an important cause of morbidity and mortality in humans during seasonal, pandemic, and zoonotic outbreaks. Seasonal influenza is estimated to cause 250,000 to 500,000 deaths per year worldwide. Pandemic influenza viruses of the previous century resulted in an estimated 1 to 4 million deaths for the 1957 H2N2 (Asian flu) and the 1968 H3N2 (Hong Kong flu) influenza pandemics, and 20 to 50 million deaths for the 1918 H1N1 (Spanish flu) influenza pandemic.1,2 The first influenza pandemic of the 21st century, the currently ongoing new H1N1 virus outbreak (Mexican flu), has caused at least 3486 deaths as of September 13, 2009 (http://www.who.int/csr/don/2009_09_18/en/index.html). The zoonotic highly pathogenic avian influenza virus (HPAIV) H5N1, which is causing an ongoing outbreak in poultry, only occasionally infects humans, but has a high mortality rate, with 262 deaths out of 400+ confirmed infections as of August 2009 (http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_08_11/en/index.html).
The pandemic potential of an influenza virus depends largely on its efficiency of human-to-human transmission. Human influenza viruses, including seasonal H1N1 and H3N2 viruses, and the pandemic H1N1 virus, are transmitted efficiently.3 In contrast, the zoonotic HPAIV H5N1 is only rarely transmitted from human to human.4 However, the factors determining efficient virus transmission among humans are poorly understood.
Tropism of influenza virus for the human upper respiratory tract (URT) has been speculated to be an important determinant for the efficiency of virus transmission, based both on receptor distribution and virus replication studies.5,6 Based on lectin histochemistry, the human URT has abundant receptors for human influenza viruses, which are efficiently transmitted.5,7 This fits with the ability for human influenza viruses to replicate in human URT tissues based on in vivo,8 ex vivo,7 and in vitro studies.9,10,11 In contrast, the human URT has only limited receptors for avian influenza viruses.5,7 This fits with the absence or rarity of HPAIV H5N1 transmission among humans.4 However, it is discordant with a study of Nicholls and others, who showed that HPAIV H5N1 can replicate in URT tissues. They explained this discordance by suggesting that HPAIV H5N1 attached to receptors not detected by the lectins used. Therefore, there is currently no consensus on the tropism of HPAIV H5N1 for the human URT. In addition, the studies to date have not studied the human URT systematically, and it is not known what the tropism of the new H1N1 virus is for the human URT.
To address the question whether URT tropism of influenza viruses is linked to efficient transmission, we determined the pattern of attachment of selected influenza viruses in the human URT: human influenza viruses, including seasonal H1N1 and H3N2 viruses and pandemic H1N1 virus, which are transmitted efficiently, and avian influenza viruses, including a HPAIV H5N1, isolated from a fatal human case, which is not transmitted efficiently among humans. We measured the pattern of virus attachment by use of virus histochemistry instead of lectin histochemistry.6 Virus histochemistry measures the attachment of influenza virus to its host cell directly. Therefore, any receptors other than SA-α-2,3-Gal terminated saccharides and SA-α-2,6-Gal terminated saccharides also would be detected by virus histochemistry. We have used this technique previously to show that the pattern of attachment in the human lower respiratory tract is different for human and avian influenza viruses, which correlates with differences in primary disease.12
Materials and Methods
The pattern of attachment was determined by virus histochemistry of the seasonal H1N1 virus (A/Netherlands/35/05), seasonal H3N2 virus (A/Netherlands/213/03), HPAIV H5N1 (A/Vietnam/1194/04), low-pathogenic avian influenza virus (LPAIV) H5N9 (A/Mallard/Sweden/79/02), and LPAIV H7N7 (A/Mallard/Sweden/100/02). To study the pattern of attachment of the pandemic H1N1 virus, we used a reassortant virus rather than the pandemic H1N1 virus itself to obtain sufficiently high titers in cell culture for the virus histochemical assay. This reassortant virus consisted of six gene segments of A/PR/8/34 and the HA and NA of pandemic H1N1 virus (A/NL/602/09). Because the surface glycoproteins of this reassortant virus were those of the pandemic H1N1 virus, attachment of the reassortant virus was expected to be the same as that of the pandemic H1N1 virus and is referred to as such in the rest of the text. The pandemic H1N1 virus, the two seasonal human influenza viruses, and HPAIV H5N1 were grown on MDCK cells, and the two LPAIV were grown in the allantoic cavity of 11-day-old embryonated hens’ eggs. Viruses were purified, concentrated, inactivated, and labeled with FITC as described previously.6,12
Histologically normal, archival, formalin-fixed, paraffin-embedded, human URT tissues from the nasal septum (n = 2), nasal inferior concha (n = 5), medial concha (n = 3), nasopharynx (n = 5), paranasal sinuses (n = 2), and larynx (n = 5) were included. In total, tissues originated from 20 different individuals. Tissue sections were incubated with FITC-labeled influenza viruses, as described before.6,12 Briefly, binding of FITC-labeled influenza virus was detected with an peroxidase-labeled Rabbit-anti-FITC antibody (DAKO, Glostrup, Denmark). The signal was amplified with a tyramide signal amplification system (Perkin Elmer, Boston, MA). Peroxidase was revealed with 3-amino-9-ethyl-carbozole (Sigma, St Louis, MO) resulting as a granular to diffuse red precipitate. For each tissue tested, in each run, an omission control was included to check for nonspecific staining. Visualization by light microscopy provides a better overview of the tissues than is possible by fluorescence microscopy. For the precise localization of submucosal glands and goblet cells, serial sections were stained by HE or periodic acid Schiff stain. Analysis was performed on a Olympus BX51 microscope and photographs were made with a Colorview IIIu camera.
Results
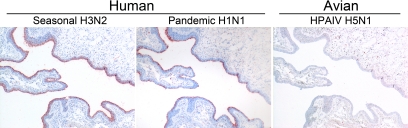
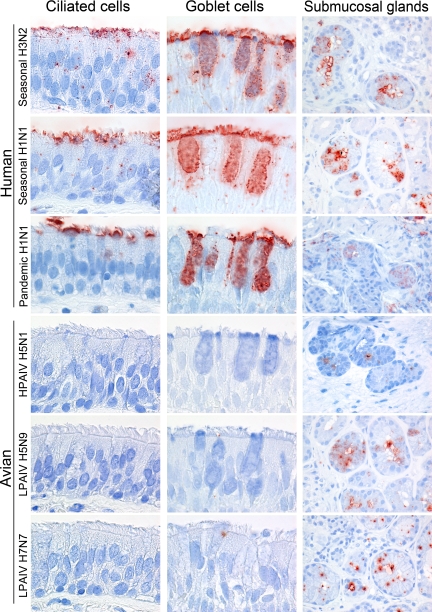
Attachment of the studied influenza viruses to the mucociliary epithelium showed two distinct patterns (Table 1; Figures 1 and 2). All human influenza viruses (seasonal H1N1 and H3N2 viruses, pandemic H1N1 virus) attached abundantly to the apical site of ciliated epithelial cells. Furthermore, all human influenza viruses attached to goblet cells, both to the apical site and intracellularly to the mucus. In contrast, the avian influenza viruses (HPAIV H5N1 and LPAIV H5N9 and H7N7) attach only rarely to ciliated epithelial cells and not to goblet cells.
Table 1.
Attachment of Human and Avian Influenza Viruses to Different Parts of the Human Upper Respiratory Tract
| n | Human
|
Avian
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seasonal H3N2
|
Seasonal H1N1
|
Pandemic H1N1
|
HPAIV H5N1
|
LPAIV H5N9
|
LPAIV H7N7
|
||||||||
| Cilia | Goblet | Cilia | Goblet | Cilia | Goblet | Cilia | Goblet | Cilia | Goblet | Cilia | Goblet | ||
| Nasal septum | 2 | ++ | + | ++ | + | ++ | + | − | − | +/− | − | +/− | − |
| Concha inferior | 5 | ++ | + | ++ | + | ++ | + | − | − | − | − | +/− | − |
| Concha media | 3 | ++ | + | ++ | + | + | + | − | − | +/− | − | +/− | − |
| Nasopharynx | 5 | ++ | + | ++ | + | ++ | + | +/− | − | + | − | +/− | − |
| Paranasal sinuses | 2 | ++ | + | ++ | + | ++ | + | +/− | − | +/− | − | +/− | − |
| Larynx | 5 | ++ | + | ++ | + | +/− | + | − | − | − | − | − | − |
Scores are median scores from individual tissues. Attachment of influenza viruses to the apical site of ciliated epithelial cells (− indicates no attachment; +/−, < 10% cells positive; +, < 50% cells positive; ++, ≥ 50% cells positive) and to the cytoplasm of goblet cells (− indicates no attachment; +, intracellular attachment) was scored. All viruses attached occasionally to epithelial cells of the submucosal glands.
Figure 1.
Overview of attachment of seasonal H3N2, pandemic H1N1, and HPAIV H5N1 to the inferior concha.
Figure 2.
Attachment of human and avian viruses to ciliated epithelial cells, goblet cells, and submucosal glands in the human URT.
Attachment to the submucosal gland epithelium was similar for all viruses tested (Figure 2). All human and avian influenza viruses attached occasionally to the apical side and cytoplasm of submucosal gland epithelial cells.
In general, the pattern of virus attachment was similar among the different sites of the human URT for each virus tested (Table 1). Exceptions were less attachment of pandemic H1N1 virus to ciliated epithelial cells of the larynx and medial concha. Furthermore, attachment of avian influenza viruses to different sites of the human URT varied slightly.
Discussion
We here show that seasonal H1N1 and H3N2 viruses and pandemic H1N1 virus, which are transmitted efficiently among humans, attach abundantly to ciliated epithelial cells in the human upper respiratory tract. In contrast HPAIV H5N1, which is inefficiently transmitted among humans, attach rarely. These results indicate that the ability of an influenza virus to bind to human URT epithelium is a critical factor for efficient transmission in the human population.
Although there is no proof for the causal link between attachment and infection, our virus histochemistry data on human influenza viruses correspond with data from lectin histochemistry and from in vitro, ex vivo, and in vivo infections.5,7,8,9,10,11 They also correspond with clinical data on human influenza virus infections, which commonly cause URT disease, specifically rhinitis, paranasal sinusitis, pharyngitis, and laryngitis (or croup).13,14,15,16,17,18,19,20,21,22 The similarity that we found between pandemic H1N1 virus and seasonal influenza viruses for the attachment to the URT corresponds to the similarity between these viruses for attachment to the trachea.23
Our virus histochemistry data on HPAIV H5N1 and other avian influenza viruses correspond with some previous studies, but not with others. They correspond with lectin histochemistry studies,5,7 which showed poor binding of MAA2, which is considered to specifically recognize SA-α-2,3-Gal—the preferred receptor of avian influenza viruses—to URT epithelium. They also correspond with clinical data on H5N1 virus infection, where URT symptoms are only present in a minority of hospitalized patients.24 However, it contrasts with productive replication of HPAIV H5N1 in ex vivo cultures of human URT epithelium.7 Although attachment does not necessarily lead to infection or attachment could be below detection limit, our virus histochemistry results suggests that infection of epithelial cells in the URT by H5N1 virus is possible, but likely not very widespread.
The significance of influenza virus attachment to submucosal glands and goblet cells is not clear. Our attachment results correspond with in vivo data showing infection of human submucosal glands by human seasonal influenza viruses.25 Infection of submucosal glands and goblet cells could not only lead to the production of progeny virus but also decrease their production of mucus, which is known to inhibit virus infection.26
In conjunction with our previous study6,12 we now have an overview of the pattern of attachment of influenza viruses at different levels of both upper and lower respiratory tract. However, attachment is only the first step in the replication cycle of influenza virus in its host cell. Therefore, the next challenge will be to systematically investigate how influenza viruses replicate in tissues at different levels of the human respiratory tract. Together, these studies will help us to understand how respiratory tract tropism of influenza viruses affect both their pathogenicity and their transmission in the human host.
Acknowledgments
We thank Wilina Lim for providing the H5N1 virus isolate and Frank van der Panne and Peter van Run for technical assistance.
Footnotes
Address reprint requests to Thijs Kuiken, D.V.M., Ph.D., DACVP, Erasmus Medical Center, Department of Virology, P.O. Box 2040, 3000 CA Rotterdam, The Netherlands. E-mail: t.kuiken@erasmusmc.nl.
See related Commentary on page 1584
Supported by the VIRGO consortium (BSIK 03012), CRISP (HHSN266200700010C), and EMPIRIE (HEALTH-F3-2009-223498).
References
- Oxford JS. Influenza A pandemics of the 20th century with special reference to 1918: virology, pathology and epidemiology. Rev Med Virol. 2000;10:119–133. doi: 10.1002/(sici)1099-1654(200003/04)10:2<119::aid-rmv272>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Cox NJ, Tamblyn SE, Tam T. Influenza pandemic planning. Vaccine. 2003;21:1801–1803. doi: 10.1016/s0264-410x(03)00076-8. [DOI] [PubMed] [Google Scholar]
- Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, Jombart T, Hinsley WR, Grassly NC, Balloux F, Ghani AC, Ferguson NM, Rambaut A, Pybus OG, Lopez-Gatell H, Alpuche-Aranda CM, Chapela IB, Zavala EP, Guevara DM, Checchi F, Garcia E, Hugonnet S, Roth C. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;19;324:1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MD. H5N1 transmission and disease: observations from the frontlines. Pediatr Infect Dis J. 2008;27:S54–S56. doi: 10.1097/INF.0b013e3181684d2d. [DOI] [PubMed] [Google Scholar]
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. H5N1 virus attachment to lower respiratory tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- Nicholls JM, Chan MC, Chan WY, Wong HK, Cheung CY, Kwong DL, Wong MP, Chui WH, Poon LL, Tsao SW, Guan Y, Peiris JS. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat Med. 2007;13:147–149. doi: 10.1038/nm1529. [DOI] [PubMed] [Google Scholar]
- Tateno I, Kitamoto O, Kawamura A., Jr Diverse immunocytologic findings of nasal smears in influenza. N Engl J Med. 1966;274:237–242. doi: 10.1056/NEJM196602032740502. [DOI] [PubMed] [Google Scholar]
- Thompson CI, Barclay WS, Zambon MC, Pickles RJ. Infection of human airway epithelium by human and avian strains of influenza a virus. J Virol. 2006;80:8060–8068. doi: 10.1128/JVI.00384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibricevic A, Pekosz A, Walter MJ, Newby C, Battaile JT, Brown EG, Holtzman MJ, Brody SL. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J Virol. 2006;80:7469–7480. doi: 10.1128/JVI.02677-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure T, Suzuki T, Takahashi T, Miyamoto D, Hidari KI, Guo CT, Ito T, Kawaoka Y, Suzuki Y. Human trachea primary epithelial cells express both sialyl(alpha2-3)Gal receptor for human parainfluenza virus type 1 and avian influenza viruses, and sialyl(alpha2-6)Gal receptor for human influenza viruses. Glycoconj J. 2006;23:101–106. doi: 10.1007/s10719-006-5442-z. [DOI] [PubMed] [Google Scholar]
- van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol. 2007;171:1215–1223. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle WJ, Gentile DA, Skoner DP. Viral and bacterial rhinitis. Clin Allergy Immunol. 2007;19:177–195.:177–195. [PubMed] [Google Scholar]
- Winther B, Gwaltney JM, Jr, Mygind N, Hendley JO. Viral-induced rhinitis. Am J Rhinol. 1998;12:17–20. doi: 10.2500/105065898782102954. [DOI] [PubMed] [Google Scholar]
- Gwaltney JM, Jr, Scheld WM, Sande MA, Sydnor A. The microbial etiology and antimicrobial therapy of adults with acute community-acquired sinusitis: a fifteen-year experience at the University of Virginia and review of other selected studies. J Allergy Clin Immunol. 1992;90:457–461. doi: 10.1016/0091-6749(92)90169-3. [DOI] [PubMed] [Google Scholar]
- Silvennoinen H, Peltola V, Lehtinen P, Vainionpaa R, Heikkinen T. Clinical presentation of influenza in unselected children treated as outpatients. Pediatr Infect Dis J. 2009;28:372–375. doi: 10.1097/INF.0b013e318191eef7. [DOI] [PubMed] [Google Scholar]
- Louie JK, Hacker JK, Gonzales R, Mark J, Maselli JH, Yagi S, Drew WL. Characterization of viral agents causing acute respiratory infection in a San Francisco University Medical Center Clinic during the influenza season. Clin Infect Dis. 2005;41:822–828. doi: 10.1086/432800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MJ, Attia MW. Clinical predictors of influenza in children. Arch Pediatr Adolesc Med. 2004;158:391–394. doi: 10.1001/archpedi.158.4.391. [DOI] [PubMed] [Google Scholar]
- Gentile DA, Doyle WJ, Fireman P, Skoner DP. Effect of experimental influenza A infection on systemic immune and inflammatory parameters in allergic and nonallergic adult subjects. Ann Allergy Asthma Immunol. 2001;87:496–500. doi: 10.1016/S1081-1206(10)62263-6. [DOI] [PubMed] [Google Scholar]
- Chiu TF, Huang LM, Chen JC, Lee CY, Lee PI. Croup syndrome in children: five-year experience. Acta Paediatr Taiwan. 1999;40:258–261. [PubMed] [Google Scholar]
- Paisley JW, Bruhn FW, Lauer BA, McIntosh K. Type A2 influenza viral infections in children. Am J Dis Child. 1978;132:34–36. doi: 10.1001/archpedi.1978.02120260036007. [DOI] [PubMed] [Google Scholar]
- Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science. 2009;325:484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- Guarner J, Paddock CD, Shieh WJ, Packard MM, Patel M, Montague JL, Uyeki TM, Bhat N, Balish A, Lindstrom S, Klimov A, Zaki SR. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003–2004 season. Clin Infect Dis. 2006;43:132–140. doi: 10.1086/505122. [DOI] [PubMed] [Google Scholar]
- Gentry SE, Culp DJ, Roberts NJ, Jr, Marin MG, Simons RL, Latchney LR. Influenza virus infection of tracheal gland cells in culture. J Virol. 1988;62:1524–1529. doi: 10.1128/jvi.62.5.1524-1529.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]