Abstract
Vα14 invariant natural killer T (Vα14iNKT) cells are at the interface between the innate and adaptive immune responses and are thus critical for providing full engagement of host defense. We investigated the role of polyriboinosinic:polycytidylic acid (poly I:C), a replication-competent viral double-stranded RNA mimic and a specific agonist that recognizes the cellular sensor Toll-like receptor 3 (TLR3), in regulating Vα14iNKT cell activation. We established for the first time that hepatic Vα14iNKT cells up-regulate TLR3 extracellularly after poly I:C treatment. Notably, activation of TLR3-expressing hepatic Vα14iNKT cells by a TLR3 ligand was suppressed by TLR3 deficiency. Our studies also revealed that Vα14iNKT cell activation in response to poly I:C administration uniquely suppressed the accumulation and activation of intrahepatic γδT cells (but not natural killer cells) by inducing apoptosis. Furthermore, we established that activated hepatic Vα14iNKT cells (via cytokines and possibly reactive oxygen species) influenced the frequency and absolute number of intrahepatic γδT cells, as evidenced by increased hepatic γδT cell accumulation in Vα14iNKT cell-deficient mice after poly I:C treatment relative to wild-type mice. Thus, hepatic Vα14iNKT cells and intrahepatic γδT cells are functionally linked on application of TLR3 agonist. Overall, our results demonstrate a novel and previously unrecognized anti-inflammatory role for activated hepatic Vα14iNKT cells in negatively regulating intrahepatic γδT cell accumulation (probably through TLR3 signaling) and thereby preventing potentially harmful activation of intrahepatic γδT cells.
Vα14 invariant natural killer T (Vα14iNKT) cells are thymic-derived innate murine T lymphocytes with significant immunoregulatory effects in cardiovascular, infectious, and autoimmune diseases as well as in tumors.1,2,3,4,5,6,7,8,9,10 In contrast to conventional T cells, which recognize peptide antigens presented by major histocompatability class I and II molecules, Vα14iNKT cells respond to glycolipid antigen presented by CD1d expressed on antigen-presenting cells.11,12 In the last decade, several potential mechanisms underlying Vα14iNKT cell activation during immune responses have been revealed. Vα14iNKT cells are activated by lipids presented by CD1d.5,13 The established dogma is that the lipid tail of glycolipid antigen (including α-galactosylceramide or exogenous antigens from pathogens) is buried in CD1d, whereas the sugar head group of glycolipid antigen protrudes out of the CD1d to activate the T-cell receptor (TCR) α on the Vα14iNKT cell.2,13,14 After activation, Vα14iNKT cells exert multiple effects including the production of several cytokines (such as interferon [IFN]-γ, interleukin [IL]-4, and tumor necrosis factor [TNF]-α), chemokines (regulated on activation normal T cell expressed and secreted/CCL5, monocyte chemotactic protein-1/CCL2, and macrophage inflammatory protein-1α/CCL3)15,16,17,18 and cytotoxic proteins (such as tumor necrosis factor-related apoptosis-inducing ligand and Fas/Fas ligand).13,19 Through these mediators, activated Vα14iNKT cells can interact with and transactivate other immune cells.15,20,21 Thus, Vα14iNKT cells act as a “bridge” between the innate and adaptive immune systems.
Vα14iNKT cells are also activated by a TCR-independent mechanism involving Toll like receptors (TLRs). TLRs are pathogen recognition receptors that identify molecular patterns of components specific to microbes and play a critical role in initiating the innate immune response to microbes.22 To date, more than 10 TLRs have been reported in humans and mice, and each recognizes different microbial components.22 TLRs are located on the plasma membrane and in endosomal compartments of cells.22 Among the TLRs, TLR2, TLR4, and TLR5 recognize the bacterial signals peptidoglycan, lipopolysaccharide, and flagellin, respectively,22 whereas TLR3, TLR7, TLR8, and TLR9 play fundamental roles in detecting viral signals.23,24,25,26 An additional mechanism for activation of Vα14iNKT cells (in the absence of foreign antigen for their TCRs) is by IL-12 and/or IL-18 derived from antigen-presenting cells that have been activated via a TLR (4,7,8,9)-dependent pathway.13,14,27,28 The precise pathway of activation may depend on the pathogen. For example, TLR4 traditionally recognizes the bacterial signal lipopolysaccharide,22 whereas TLR7, TLR8, and TLR9 all sense viral signals.23,24,25,26 The potential contribution of the viral sensor TLR3 to Vα14iNKT cell activation has not yet been determined. Poly I:C is the specific TLR3 agonist and a replication-competent viral double-stranded RNA (dsRNA) mimic.23,29 dsRNA is a structure found in the genome of some viruses and is produced as a replication intermediate by viruses.23,29,30 Therefore, poly I:C is routinely used in experimental studies to assess the functional activity of TLR3 during immune responses.23,29,30,31
In the present study, we evaluated the potential role of TLR3 in promoting Vα14iNKT cell activation by examining the response of hepatic Vα14iNKT cells after treatment with the TLR3 ligand, poly I:C. In addition, we assessed the functional consequences of Vα14iNKT cell activation on the hepatic innate immune response after poly I:C treatment. We demonstrate that a functional consequence of hepatic Vα14iNKT cell activation in response to poly I:C administration is the subsequent induction of apoptotic death of hepatic γδT cells. Overall, our findings demonstrate a novel role for activated hepatic Vα14iNKT cells in negatively regulating the recruitment, activation, and potentially harmful effector function(s) of intrahepatic γδT cells on application of the TLR3 ligand, poly I:C.
Materials and Methods
Mice
Male C57BL/6 mice, B6129SF2/J mice, and TLR3−/− mice (on a B6129SF2/J background) were purchased from The Jackson Laboratory (Bar Harbor, ME). Breeding pairs of Jα18−/− mice (on a C57BL/6 background) were kindly provided by Dr. Mitch Kronenberg (La Jolla Institute of Allergy and Immunology) with permission from Dr. M. Taniguchi (RIKEN Research Center for Allergy and Immunology, Yokohoma, Japan).32 Jα18−/− mice were bred in a pathogen-free breeding facility at Louisiana State University Health Sciences Center–Shreveport.
Poly I:C Treatment
Poly I:C is the specific TLR3 agonist and a viral dsRNA mimic.23,29 dsRNA is a structure found in the genome of some viruses and produced as a replication intermediate by viruses.23,29,30 For this reason, poly I:C is widely used in experimental studies to assess the functional activity of TLR3 during immune responses.23,29,30 In our study, mice were treated with poly I:C (30 mg/kg i.p., Sigma-Aldrich, St. Louis, MO)29,33,34 and manipulated at indicated time points (0, 3, 16, and 24 hours) as described below. For cytokine blocking experiments, mice received murine IFN-γ antiserum (0.5 ml/mouse i.p.)17 or control serum 16 hours before poly I:C treatment. The murine IFN-γ antiserum was generously provided by Dr. Robert Strieter (University of Virginia, Charlottesville, VA). Murine TNF monoclonal antibody (mAb) (0.2 mg, clone 1B1, R&D Systems, Minneapolis, MN) or control IgG was administered intravenously 2 hours before poly I:C treatment. To investigate the role of endogenous reactive oxygen species (ROS) in modulating hepatic innate immune cell accumulation and/or activation, a single dose of N-acetylcysteine (NAC) (300 mg/kg i.p., Sigma-Aldrich)35 or vehicle PBS was administered simultaneously with poly I:C to C57BL/6 mice or Jα18 knockout (KO) mice, which were then sacrificed 16 hours later.
Isolation of Hepatic Lymphocytes and Flow Cytometry
Hepatic lymphocytes were isolated using our published protocols.16,17 For the specific identification of hepatic γδT cells, isolated hepatic lymphocytes were preincubated with anti-mouse CD16/32 mAb (clone 2.4G2, BD Pharmingen, San Diego, CA) to block Fcγ receptors and then incubated simultaneously with fluorochrome-labeled TCRγδ mAb (clone GL3, BD Pharmingen) and fluorochrome-labeled CD3ε mAb (clone 145-2C11, BD Pharmingen) as we described recently.16,17 Three-color staining was used to assess intracellular cytokine (IFN-γ and IL-17) or intracellular active caspase 3 expression by hepatic γδT cells. In brief, fluorochrome-labeled TCRγδ-CD3+ double-positive T cells were permeabilized with Cytofix/Cytoperm Plus (BD Pharmingen) and then stained intracellularly with either fluorochrome-labeled murine IFN-γ mAb (clone XMG1.2, BD Pharmingen),17 fluorochrome- labeled murine IL-17 mAb (clone TC11-18H10.1, BD Pharmingen),36 or fluorochrome-labeled active caspase 3 mAb (clone C92-605, BD Pharmingen).16,37
To determine cytokine (IFN-γ and TNF-α) or TLR3 expressions by hepatic Vα14iNKT cells, isolated hepatic lymphocytes were preincubated with anti-mouse CD16/32 mAb to block Fcγ receptors and then incubated simultaneously with fluorochrome-labeled TCRβ mAb (clone H57-597, BD Pharmingen) and fluorochrome-labeled Vα14iNKT cell tetramer (CD1d-PBS57, NIH Tetramer Core Facility, Atlanta, GA).17,38 PBS57 is an analog of α-galactosylceramide and the CD1d/PBS57 tetramer stain Vα14iNKT cells comparable to the CD1d/α-galactosylceramide tetramers.38 Next, a rat anti-mouse TLR3 mAb (R&D Systems)39 and phycoerythrin-labeled anti-rat IgG secondary antibody (eBioscience, San Diego, CA) were used to determine TLR3 expression on the surface of Vα14iNKT cell as well as within Vα14iNKT cells. Cytokine production (ie, intracellular IFN-γ and TNF) by hepatic Vα14iNKT cells was determined using fluorochrome-labeled murine IFN-γ mAb or fluorochrome-labeled murine TNF mAb (clone MP6-XT22, eBioscience) after cell permeabilization and then analysis by fluorescence-activated cell sorting (FACS). In all experiments, corresponding isotype antibodies/tetramers were used as controls. In addition, viable lymphocyte populations were gated using forward and side scatter characteristics, and data were analyzed using the FACSCalibur and FACScan Diva software (BD Biosciences). Notably, all intracellular cytokine stainings were performed without further reactivation in vitro.
Statistical Analysis
All data are shown as means ± SEM, For comparisons of means between two experimental groups, a Student’s unpaired t-test was used. Comparison among three or more experimental groups was performed using a one-way analysis of variance, followed by either Dunnett’s multiple comparison test or a Newman-Keuls post hoc test. P < 0.05 was considered significant.
Results
Vα14iNKT Cell-Deficient Mice Exhibit Increased Hepatic γδT Cell Accumulation after Poly I:C Treatment
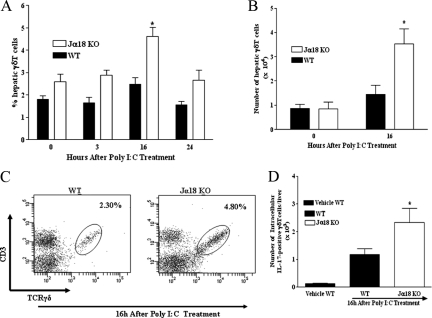
To evaluate the kinetics of γδT cell accumulation in the livers of C57BL/6 wild-type mice and Jα18 KO mice, flow cytometric-based techniques were used to determine the expression of cell surface markers for γδT cells (ie, TCRγδ/CD3 double-positive T cells) in the livers of untreated naive mice (ie, 0 hours) and in mice treated with poly I:C over several time points (ie, 3, 16, and 24 hours). We observed that γδT cells represent a minor population of innate immune T cells in the livers of untreated naive C57BL/6 wild-type mice (Figure 1, A and B). In addition, poly I:C treatment did not increase the frequency and absolute number of γδT cells accumulating in the livers of C57BL/6 wild-type mice at any of the time points examined relative to those in untreated naive C57BL/6 wild-type mice (Figure 1, A and B). In contrast, Vα14iNKT cell deficiency was associated with a significant increase in γδT cell accumulation in the livers of poly I:C-treated Jα18 KO mice. Specifically, the frequency (∼2-fold) and absolute number (>2-fold) of γδT cells in the livers of poly I:C-treated Jα18 KO mice were significantly increased at 16 hours (but not at 3 or 24 hours) compared with those in corresponding C57BL/6 wild-type mice and untreated naive C57BL/6 wild-type mice (Figure 1, A and B). A representative FACS dot plot is shown in Figure 1C. Based on these results, we selected 16 hours as the optimum time point for subsequent studies.
Figure 1.
Vα14iNKT cell deficiency promotes hepatic γδT cell accumulation and activation after poly I:C treatment. C57BL/6 wild-type (WT) and Jα18 KO mice were treated with poly I:C at the indicated time points. Next, hepatic lymphoid cells were isolated from mice. Hepatic γδT cells were identified by FACS after cell surface staining with TCRγδ and CD3 mAbs (as described in Materials and Methods) and then gating on TCRγδ/CD3 double-positive T cells. The percentage (A), absolute number (B), and representative FACS dot plot (C) of hepatic γδT cells (ie, TCRγδ/CD3 double-positive T cells) are depicted. Results are presented as means ± SEM with four to six mice per group from two independent experiments. *P ≤ 0.05 versus all other groups. D: Absolute number of intracellular IL-17 expression by hepatic γδT cells was determined by FACS (as described in Materials and Methods). Data are shown as means ± SEM with three to six mice per group from two separate experiments. *P ≤ 0.05 versus vehicle-treated wild-type mice.
Next, we investigated whether hepatic γδT cells displayed signs of activation. For this investigation, we determined (by FACS) intracellular expression of IFN-γ and IL-17 by hepatic γδT cells after poly I:C treatment. These cytokines (ie, IFN-γ and IL-17) are routinely secreted by activated γδT cells during inflammation.17,36,40 Figure 1D shows that Vα14iNKT cell deficiency induced hepatic γδT cell activation with poly I:C treatment because the absolute numbers of intracellular IL-17-expressing hepatic γδT cells were significantly increased in poly I:C-treated Jα18 KO mice (relative to vehicle-treated C57BL/6 wild-type mice). In contrast, hepatic γδT cells obtained from poly I:C-treated C57BL/6 wild-type mice did not induce a statistically significant increase in IL-17 production compared with vehicle-treated C57BL/6 wild-type mice (Figure 1D). A similar trend was seen when serum IL-17 was measured in these mice (data not shown). Notably, poly I:C treatment did not induce IFN-γ production by activated hepatic γδT cells derived from C57BL/6 wild-type mice or Jα18 KO mice (unpublished observation).
A unique feature of activated Vα14iNKT cells is that they promptly regulate the innate immune system by inducing the activation of natural killer (NK) cells in many model systems.20,41,42 Because poly I: C treatment promotes hepatic NK cell accumulation and activation,43,44 we also evaluated the effects of Vα14iNKT cell deficiency on hepatic NK cell accumulation and activation after poly I:C application. Vα14iNKT cell deficiency did not suppress hepatic NK cell accumulation and activation after poly I:C treatment compared with poly I:C-treated C57BL/6 wild-type mice; M. Ajuebor, unpublished observation.
Vα14iNKT Cell Deficiency Prevents Apoptosis of Intrahepatic γδT Cells in Response to Poly I:C Treatment
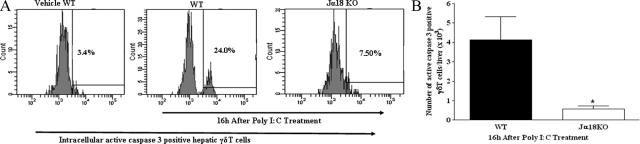
We showed in Figure 1, A and B, that Vα14iNKT cell deficiency was uniquely associated with increased hepatic γδT cell accumulation in response to poly I:C treatment. Next, we determined whether the lack of γδT cell accumulation in the livers of C57BL/6 wild-type mice after poly I:C application could be due to apoptotic death of hepatic γδT cells by Vα14iNKT cell-derived mediator(s). Moreover, viruses are capable of making immune cells more susceptible to apoptosis. We initially assessed whether γδT cells derived from the livers of poly I:C-treated C57BL/6 wild-type mice were dying by apoptosis. Active caspase 3 is a marker widely used to determine programmed cell death.16,37 Figure 2, A and B, depicts a significant increase in intracellular active caspase 3 expression by hepatic γδT cells obtained from poly I:C-treated C57BL/6 wild-type mice (relative to that seen in the livers of naive C57BL/6 wild-type mice), but this response was severely attenuated in Jα18 KO mice with poly I:C treatment. These results suggest that intrahepatic γδT cells are susceptible to apoptotic death in the absence of Vα14iNKT cells with poly I:C treatment.
Figure 2.
Activated hepatic Vα14iNKT cells promote apoptotic death of intrahepatic γδT cells after poly I:C treatment. Hepatic γδT cells were identified by FACS after cell surface staining with TCRγδ and CD3 mAbs. Hepatic γδT cells were fixed, permeabilized, and then stained intracellularly for active caspase 3. A representative FACS histogram (A) and absolute number (B) of intracellular active caspase 3-positive γδT cells are shown. Data are presented as means ± SEM with three to four mice per group from two independent experiments. *P ≤ 0.05 versus poly I:C-treated wild-type (WT) mice.
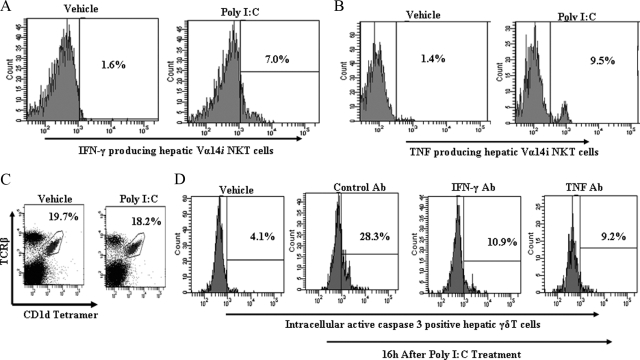
It is a well known fact that Vα14iNKT cells rapidly produce cytokines (including IFN-γ and TNF) after activation.16,18 Previous studies have documented elevated serum levels of IFN-γ and TNF in mice treated with poly I:C.23,33,44 Moreover, we observed that increased serum levels of IFN-γ and TNF in C57BL/6 wild-type mice after poly I:C treatment were significantly blunted by Vα14iNKT cell deficiency (data not shown). More importantly, we confirmed by intracellular staining and flow cytometry that hepatic Vα14iNKT cells produce IFN-γ and TNF at 16 hours after poly I:C treatment (Figure 3, A and B). Despite numerous reports16,37,45 that hepatic Vα14iNKT cells subsequently die by apoptosis after activation, no Vα14iNKT cell loss was observed in the liver on poly I:C application. Specifically, the frequency (Figure 3C) and absolute number (data not shown) of hepatic Vα14iNKT cells in poly I:C-treated C57BL/6 wild-type mice were comparable to that seen in the livers of vehicle-treated C57BL/6 wild-type mice. These cytokines can promote apoptosis of immune and nonimmune cells.46,47,48 Consequently, we verified whether blockade of endogenous IFN-γ and TNF secreted by activated hepatic Vα14iNKT cells on poly I:C treatment could suppress the apoptosis of hepatic γδT cells. As shown in Figure 3D, pretreatment of C57BL/6 wild-type mice with IFN-γ antiserum or TNF mAb before poly I:C treatment significantly suppressed apoptotic death of intrahepatic γδT cells as depicted by reduced intracellular active caspase 3 expression relative to that seen in poly I:C-treated C57BL/6 wild-type mice given control antibody.
Figure 3.
Contribution of Vα14iNKT cell-derived cytokines to apoptosis of intrahepatic γδT cells after poly I:C treatment. A and B: C57BL/6 wild-type mice were treated with poly I:C or vehicle for 16 hours, and isolated hepatic Vα14iNKT cells were identified by FACS after cell surface staining with TCRβ mAb and PBS57-CD1d tetramer (described in Materials and Methods). The cells were fixed, permeabilized, and then stained intracellularly for IFN-γ or TNF. Representative FACS histograms demonstrating IFN-γ-positive hepatic Vα14iNKT cells and TNF-positive hepatic Vα14iNKT cells are shown in A and B, respectively, from two independent experiments of four mice per group. C: FACS dot plot (from two independent experiments of three mice per group) depicts hepatic Vα14iNKT cell profiles from poly I:C or vehicle-treated C57BL/6 wild-type mice at the 16-hour time point. D: C57BL/6 wild-type mice were pretreated with murine IFN-γ antiserum, murine TNF mAb, or control antibody before poly I:C treatment, and 16 hours later isolated hepatic γδT cells were fixed, permeabilized, and then stained intracellularly for active caspase 3. A representative FACS histogram highlighting active caspase 3-positive hepatic γδT cells from two independent experiments of four mice per group is shown.
Effects of Endogenous ROS on Hepatic Vα14iNKT Cell Activation during Poly I:C Treatment
Previous studies have reported that T-cell activation is associated with increased levels of ROS.49 Therefore, we evaluated whether endogenous ROS could regulate hepatic Vα14iNKT cell activation during poly I:C treatment. Treatment of C57BL/6 wild-type mice with the ROS scavenger, NAC, during poly I:C treatment significantly suppressed hepatic Vα14iNKT cell activation. Specifically, we found that the percentage of intracellular IFN-γ expressing Vα14iNKT cells in the livers of poly I:C-treated C57BL/6 wild-type mice was significantly attenuated by NAC treatment (Figure 4A). Next, we assessed the effect of impaired hepatic Vα14iNKT cell activation on γδT cell accumulation in the liver. Figure 4B shows that simultaneous treatment of C57BL/6 wild-type mice with NAC and poly I:C significantly increased (twofold) γδT cell accumulation in the liver compared with that in poly I:C-treated C57BL/6 wild-type mice given vehicle. Notably, the frequency of intrahepatic γδT cells in poly I:C/NAC-treated C57BL/6 wild-type mice was comparable to that seen in the livers of poly I:C-treated Vα14iNKT cell-deficient mice (ie, Jα18 KO mice) as shown in Figure 4B. Furthermore, concurrent treatment of Vα14iNKT cell-deficient mice with NAC and poly I:C augmented intrahepatic γδT cell accumulation relative to that of poly I:C-treated Vα14iNKT cell-deficient mice (Figure 4B). Taking these results together, we propose that blockade of endogenous ROS blunts hepatic Vα14iNKT cell activation in response to poly I:C application.
Figure 4.
Role of endogenous ROS in hepatic Vα14iNKT cell activation on poly I:C treatment. The ROS scavenger, NAC (300 mg/kg i.p.), was administered simultaneously with poly I:C in C57BL/6 wild-type (WT) mice or Jα18 KO mice. Control mice received vehicle PBS. A: Hepatic Vα14iNKT cells were identified by FACS using the CD1d tetramer and TCRβ mAb (see Materials and Methods) 16 hours after poly I:C treatment and then were stained for intracellular IFN-γ (as a marker for Vα14iNKT cell activation) after cell permeabilization. Results are presented as means ± SEM with four mice per group from two separate experiments. *P ≤ 0.05 versus vehicle. B: C57BL/6 wild-type mice and Jα18 KO mice were treated concurrently with NAC/poly I:C or PBS/poly I:C for intrahepatic γδT cell accumulation assessment by FACS 16 hours later. Data are presented as means ± SEM with four to six mice per group from two independent studies. ***P ≤ 0.05 versus all other groups; **P ≤ 0.05 versus vehicle-treated wild-type mice (no poly I:C) and poly I:C/PBS treated wild-type mice; *P ≤ 0.05 versus vehicle-treated wild-type mice (no poly I:C) and poly I:C/PBS-treated wild-type mice.
TLR3 Deficiency Inhibits Vα14iNKT Cell Activation in Response to Poly I:C Treatment
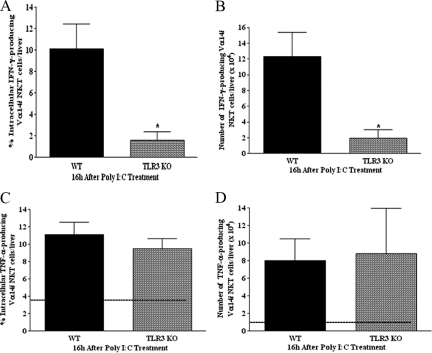
In this series of experiments, we established a critical role for TLR3 in promoting Vα14iNKT cell activation on poly I:C application. A representative FACS histogram in Figure 5 reveals that poly I:C treatment of B6129SF2/J wild-type mice was associated with increased (>3-fold) TLR3 expression on the surface of hepatic Vα14iNKT cells relative to that for vehicle-treated B6129SF2/J wild-type mice. Furthermore, we observed by FACS analysis/cell permeabilization that TLR3 was localized intracellularly in hepatic Vα14iNKT cells after vehicle or poly I:C treatment of B6129SF2/J wild-type mice (Figure 5). The specificity of TLR3 mAb was confirmed using peritoneal macrophages obtained from poly I:C-treated TLR3-deficient mice.50 To ascertain the potential role of the TLR3 ligand in regulating the activation of TLR3-expressing Vα14iNKT cells, we used TLR3-deficient mice. Figure 6, A and B, shows that TLR3 deficiency markedly reduced the frequency and absolute number of intracellular IFN-γ-expressing hepatic Vα14iNKT cells after poly I:C treatment compared with those in corresponding B6129SF2/J wild-type mice. In contrast, we found that TLR3 deficiency did not suppress the frequency and/or absolute number of TNF-producing hepatic Vα14iNKT cells with poly I:C treatment (Figure 6, C and D). Overall, our data provide the first demonstration of a crucial role for TLR3 in regulating the activation of TLR3-expressing Vα14iNKT cells after administration of the TLR3 ligand, poly I:C.
Figure 5.
Hepatic Vα14iNKT cell TLR3 expression before and after poly I:C treatment. B6129SF2/J wild-type mice were treated with poly I:C or vehicle for 16 hours and extracellular and intracellular TLR3 expression by hepatic Vα14iNKT cells was evaluated by flow cytometry as described in Materials and Methods. Representative FACS histograms are shown from two independent experiments.
Figure 6.
Effects of TLR3 deficiency on hepatic Vα14iNKT cell cytokine production in response to poly I:C application. TLR3-deficient mice and B6129SF2/J wild-type (WT) mice were treated with poly I:C for 16 hours for the isolation and identification of hepatic Vα14iNKT cells by FACS. Intracellular staining was performed on hepatic Vα14iNKT cells (after cell permeabilization) using fluorochrome-labeled cytokine antibodies (see Materials and Methods) to determine the percentage and absolute numbers of both IFN-γ expressing Vα14iNKT cells (A and B) as well as TNF-producing Vα14iNKT cells (C and D). The dotted line in C and D represents the value in wild-type mice treated with vehicle. All results are presented as means ± SEM with three to four mice per group from two independent experiments. *P ≤ 0.05 versus wild-type mice.
Discussion
Vα14iNKT cells provide an important link between the innate and adaptive immune systems by being able to rapidly produce both T helper (Th) 1 and Th2 cytokines, most notably IFN-γ and IL-4, after activation.15,16,17,18 Activated Vα14i NKT cells subsequently transactivate innate and adaptive immune cells, including neutrophils, NK cells, dendritic cells, macrophages, and T and B cells.15,20,21 Two major mechanisms for Vα14iNKT cell activation have been proposed. In the first model, the presentation of glycolipid antigens (such as α-galactosylceramide or exogenous antigens from pathogens) by CD1d-expressing antigen-presenting cells promptly induces activation of Vα14iNKT cells.2,13,14 The second model proposes that Vα14iNKT cells are indirectly activated (in the absence of foreign antigen for the TCR) by IL-12 and/or IL-18 secreted by TLR (4,7,8,9)-stimulated antigen-presenting cells.13,27,28,51,52 Thus, TLRs serve a critical role in the initiation of innate immunity in microbes.13,14,27,28 Although TLR7, TLR8, and TLR9 are vital for the recognition of viruses23,24,25,26 and indirectly induce Vα14iNKT cell activation,27,51,52 the potential role of TLR3, an innate immune receptor that is also typically involved in sensing viral danger signals in mediation of Vα14iNKT cell activation remains undefined. The results presented in this study demonstrate an important role for the TLR3 ligand, poly I:C, in promoting hepatic Vα14iNKT cell activation (without prior activation by exogenous viral administration). Furthermore, we identified a novel and previously unrecognized anti-inflammatory role for activated Vα14iNKT cells in negatively regulating intrahepatic γδT cell accumulation and thereby preventing potentially harmful activation of intrahepatic γδT cells on application of poly I:C.
There is general consensus that TLR3 recognizes dsRNA (such as poly I:C), a structure found in the genome of some viruses and produced as a replication intermediate by viruses.23,29,30 Therefore, we assessed TLR3 expression by Vα14iNKT cells in response to poly I:C treatment. Using flow cytometric-based techniques, we observed a significant increase in TLR3 expression on the surface of Vα14iNKT cells with poly I:C treatment relative to the control. In addition, TLR3 was localized intracellularly in hepatic Vα14iNKT cells before and after poly I:C treatment. Our data are consistent with previous reports that TLR3 is expressed intracellularly and/or extracellularly by immune and nonimmune cells (including NK cells, dendritic cells, smooth muscle cells, epithelial cells, γδT cells, and mesangial cells).31,53,54,55,56,57 To our knowledge this is the first demonstration of TLR3 expression by Vα14iNKT cells. These preliminary findings of surface and intracellular TLR3 expression by Vα14iNKT cells support our pursuit of TLR3 as a potential mediator of Vα14iNKT cell activation.
Poly I:C is routinely used to trigger the TLR3 signaling pathway.23,29,56 After binding a dsRNA such as poly I:C, TLR3 signals through the adapter protein TRIF to activate transcription factors including nuclear factor-κB and interferon regulatory factor 3.23 These transcription factors induce multiple inflammatory cytokines (such as TNF-α, IFN-γ, and IL-12)22,23,56,58 and type I IFNs (IFN-α and IFN-β).23 Many of the molecules regulated by TLR3 may subsequently regulate the immune response. To ascertain the functional activity of TLR3-expressing Vα14iNKT cells on poly I:C treatment, we used TLR3-deficient mice. Poly I:C treatment was associated with increased hepatic Vα14iNKT cell activation in wild-type mice as shown, by significant increases in intracellular hepatic Vα14iNKT cell IFN-γ and TNF production. These two cytokines are routinely produced by activated Vα14iNKT cells.1,16,59 More importantly, TLR3 deficiency markedly diminished Vα14iNKT cell activation because intracellular hepatic Vα14iNKT cell IFN-γ (but not TNF) production was almost completely suppressed in TLR3-deficient mice with poly I:C treatment compared with wild-type mice. This finding that Vα14iNKT cell intracellular TNF production was not suppressed by TLR3 deficiency with poly I:C treatment suggests that other receptors for poly I:C (such as melanoma differentiation associated gene-5 and retinoic acid inducible gene-I) could potentially contribute to Vα14iNKT cell activation on poly I:C application. It is conceivable that TNF derived from other cells (such as macrophages) may also contribute to the apoptotic death of intrahepatic γδT cells. Nonetheless, these novel studies strongly suggest a crucial role for the TLR3 ligand, poly I:C, in promoting the activation of TLR3-expressing Vα14iNKT cells (without prior Vα14iNKT cell activation by exogenously administered virus).
Next, we investigated the functional consequences of Vα14iNKT cell activation on the hepatic innate immune response in response to poly I:C administration. A unique feature of activated Vα14iNKT cells is that they alter innate immune responses by selectively regulating the activation of NK cells in many model systems.15,20,21,41,42,60 Poly I:C treatment is associated with increased hepatic NK cell accumulation and activation.43,61 For this reason, we evaluated the effects of Vα14iNKT cell deficiency on hepatic NK accumulation and activation on poly I:C application. Vα14iNKT cell deficiency did not suppress hepatic NK cell accumulation and/or activation after poly I:C application. Interestingly, Vα14iNKT cell deficiency caused increased accumulation of intrahepatic γδT cells in Jα18 KO mice after poly I:C treatment compared with that in corresponding wild-type mice (discussed below).
γδT cells are also considered to be part of the innate immune response. γδT cells, a unique T-cell lineage that possess a distinct TCR, are composed of two glycoprotein chains called γ and δ.17,36,40 Although γδT cells are less widespread in tissues than in conventional αβT cells, they are found in a number of different anatomic sites, with the highest frequency in the mucosa.36,40 An important feature of γδT cells is to regulate the innate and adaptive immune systems by producing Th1-and/or Th2-type cytokines and more recently, IL-17.17,36,40 In addition, γδT cells exert cytotoxic-mediated killing of a variety of target cells via Fas/Fas ligand.17,36,40 Numerous studies have used γδT cell-deficient mice and/or γδT cell-depleting antibody to confirm a regulatory role for γδT cells in the pathophysiology of numerous infectious and autoimmune diseases.17,36,40 However, just one study62 has attempted to investigate whether γδT cells and Vα14iNKT cells are functionally linked in vivo. Specifically, Jin et al62 reported that either γδT cells or Vα14iNKT cells alone were ineffective in mediating allergic airway hyperresponsiveness and the synergy of both cells was required to mediate lung inflammation and injury associated with airway hyperresponsiveness. As discussed above, we observed that Vα14iNKT cell deficiency was associated with increased accumulation of intrahepatic γδT cells on poly I:C treatment. Therefore, we specifically assessed how Vα14iNKT cell deficiency may lead to increased accumulation of activated hepatic γδT cells after poly I:C treatment.
We provide the following evidence to validate the fact that γδT cells and Vα14iNKT cells are functionally linked on application of the TLR3 ligand, poly I:C. First, poly I:C treatment was associated with hepatic Vα14iNKT cell activation as shown by elevated hepatic intracellular IFN-γ and TNF production. In addition, Vα14iNKT cell deficiency was associated with significant reductions in serum levels of IFN-γ and TNF. Our approach of using intracellular IFN-γ and/or TNF production by Vα14iNKT cells to denote activation is supported by multiple studies.1,16,20,42,59,63 These cytokines subsequently induced apoptosis of hepatic γδT cells because blockade of endogenous hepatic IFN-γ and TNF with specific antibodies suppressed intrahepatic γδT-cell active caspase 3 expression in wild-type mice after poly I:C treatment. Moreover, these cytokines are known to exert pro-apoptotic effects.46,47,48 Second, intrahepatic γδT cells derived from Vα14iNKT cell-deficient mice (ie, Jα18 KO mice) were resistant to apoptotic death on poly I:C application compared with corresponding wild-type mice. These results suggest that activated Vα14iNKT cells are capable of influencing the frequency (and absolute number) of intrahepatic γδT cells, as evidenced by increased γδT cell numbers in Jα18-deficient mice relative to wild-type mice in response to poly I:C application.
Last, blockade of endogenous ROS with the antioxidant, NAC, suppressed hepatic Vα14iNKT cell activation in wild-type mice after poly I:C administration. Interestingly, dampening of hepatic Vα14iNKT cell activation by blockade of ROS generation was also associated with significant increases in intrahepatic γδT cell accumulation in wild-type mice after poly I:C treatment. Notably, the frequency of intrahepatic γδT cells in NAC/poly I:C-treated wild-type mice was comparable to that seen in the livers of poly I:C-treated Vα14iNKT cell-deficient mice (ie, Jα18 KO mice). Thus, inhibiting Vα14iNKT cell activation by blockade of endogenous ROS generation subsequently promotes intrahepatic γδT cell accumulation on poly I:C application. However, our finding that concurrent treatment of Vα14iNKT cell-deficient mice with NAC and poly I:C augmented intrahepatic γδT cell accumulation relative to that of poly I:C-treated Vα14iNKT cell-deficient mice, suggests that ROS generation and Vα14iNKT cell activation are acting in parallel, rather than as part of a single pathway. Although previous studies have reported that activation of conventional T cells is associated with increased levels of ROS,49 further studies are required to specifically determine whether activated Vα14iNKT cells are capable of directly releasing ROS after poly I:C treatment. In addition, we cannot completely exclude the potential contribution of ROS generated from other cells such as macrophages in promoting intrahepatic γδT cell accumulation in Jα18 KO mice after poly I:C treatment. Moreover, it is well recognized that macrophages are capable of generating ROS.64,65 Collectively, we firmly believe that we have established an important anti-inflammatory role for activated hepatic Vα14iNKT cells in negatively regulating intrahepatic γδT cell accumulation on poly I:C administration via the induction of apoptosis in a response largely dependent on endogenous cytokines and possibly ROS.
In summary, a novel concept that has been demonstrated by data derived from our study relates to the previously undefined/unrecognized biological role for activated Vα14iNKT cells in negatively regulating γδT cell accumulation and activation during the hepatic innate immune response. γδT cells are frequently activated by a variety of pathogens to induce cytokines (IL-17 and IFN-γ), which mediate inflammation and autoimmunity and help fight infection by pathogens.17,36,66,67 γδT cells displayed signs of activation (as shown by a preferential elevation in intracellular IL-17 but not IFN-γ expression) during Vα14iNKT cell deficiency after poly I:C application. Thus, activated γδT cells could play a key role in early defense against viral and bacterial infections and also regulate the pathology of autoimmune diseases. On the other hand, γδT cells may sustain hepatic NK cell accumulation during Vα14iNKT cell deficiency so that NK cells subsequently regulate the hepatic inflammatory response through cytokine production and death receptors as described in many model systems.42,68,69 In conclusion, our studies have revealed that Vα14iNKT cell activation in response to the TLR3 ligand, poly I:C, induced a complex cascade of cellular activation that uniquely involved γδT cells (but not NK cells). Specifically, we have provided the first direct evidence that Vα14iNKT cells and γδT cells are functionally linked during liver inflammation in response to the TLR3 ligand, poly I:C. Furthermore, we have highlighted a novel and previously unrecognized anti-inflammatory role for activated hepatic Vα14iNKT cells in negatively regulating γδT cell accumulation in the liver possibly via TLR3 signaling and thereby preventing potentially harmful activation of intrahepatic γδT cells (schematized in Figure 7). Our finding has important implications for the role that Vα14iNKT cell-γδT cell cross-talk may play in controlling inflammatory responses to an infection. Our study highlights a novel concept with a new avenue through which activated Vα14iNKT cells can regulate the innate immune response, and this should be explored in infectious disease models.
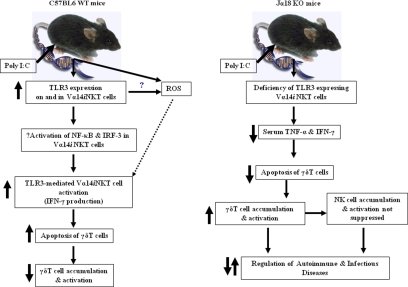
Figure 7.
A schematic model to illustrate the mechanisms of TLR3 ligand-dependent hepatic Vα14iNKT cell activation and the subsequent induction of apoptotic death of intrahepatic γδT cells on poly I:C application. Left panel: Vα14iNKT cells up-regulate TLR3 extracellularly on C57BL/6 wild-type (WT) mice on poly I:C treatment and activation of TLR3-expressing Vα14iNKT cells by poly I:C-induced Vα14iNKT cell cytokine production is through a signaling pathway, which could be dependent on the transcription factors nuclear factor-κB (NF-κB) and interferon regulatory factor-3 (IRF-3). Notably, these responses were suppressed by TLR3 deficiency. In addition, our study also revealed that the functional consequences of hepatic Vα14iNKT cell activation by the TLR3 ligand, poly I:C, is the negative regulation of γδT cells as shown by decreased accumulation of activated intrahepatic γδT cells due to apoptosis by Vα14iNKT cell-derived mediators (ie, cytokines and possibly ROS). Right panel: In contrast with that of C57BL/6 wild-type mice, poly I:C treatment of Jα18 KO mice was associated with lower serum levels of prototypical Vα14iNKT cell-derived cytokines (ie, TNF and IFN-γ), most likely due to a deficiency of TLR3-expressing activated hepatic Vα14iNKT cells. The functional consequence of lower cytokine levels is the suppression of apoptotic death of intrahepatic γδT cells (and thus increased survival of intrahepatic γδT cells) as shown by increased accumulation of γδT cells in the livers of poly I:C-treated Jα18 KO mice. Notably, the lack of suppression of hepatic NK cell accumulation and activation in Jα18 KO mice after poly I:C treatment may be due in part to the increased accumulation of γδT cells in the livers of these mice because we recently demonstrated that γδT cells promote NK cell accumulation in the liver after poly I:C treatment.43 Our study provides the first direct evidence that intrahepatic Vα14iNKT cells and γδT cells are functionally linked via TLR3 signaling on application of poly I:C because activated intrahepatic Vα14iNKT cells negatively regulate the recruitment, activation, and potentially harmful effector function(s) of intrahepatic γδT cells on application of the TLR3 ligand, poly I:C.
Acknowledgments
We are grateful to the Flow Cytometry Core Laboratory and Catherine Hickman (Breeding Colony) at Louisiana State University Health Sciences Center–Shreveport for technical assistance. The CD1d tetramers were generously provided by the National Institutes of Health Tetramer Core Facility (Emory University, Atlanta, GA).
Footnotes
Address reprint requests to Dr. Maureen N. Ajuebor, Department of Molecular & Cellular Physiology, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130-3932. E-mail: majueb@lsuhsc.edu.
Supported by the Louisiana State University Health Sciences Center–Shreveport seed package.
References
- Van Kaer L. α-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Wilson MT, Singh AK, Van Kaer L. Immunotherapy with ligands of natural killer T cells. Trends Mol Med. 2002;8:225–231. doi: 10.1016/s1471-4914(02)02325-0. [DOI] [PubMed] [Google Scholar]
- Wilson MT, Van Kaer L. Natural killer T cells as targets for therapeutic intervention in autoimmune diseases. Curr Pharm Des. 2003;9:201–220. doi: 10.2174/1381612033392080. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Rogers L, Burchat S, Gage J, Hasu M, Thabet M, Wilcox L, Ramsamy TA, Whitman SC. Deficiency of invariant Vα14 natural killer T cells decreases atherosclerosis in LDL receptor null mice. Cardiovasc Res. 2008;78:167–174. doi: 10.1093/cvr/cvn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderLaan PA, Reardon CA, Sagiv Y, Blachowicz L, Lukens J, Nissenbaum M, Wang CR, Getz GS. Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am J Pathol. 2007;170:1100–1107. doi: 10.2353/ajpath.2007.060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name?. Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- Seino K, Taniguchi M. Functional roles of NKT cell in the immune system. Front Biosci. 2004;9:2577–2587. doi: 10.2741/1418. [DOI] [PubMed] [Google Scholar]
- Tessmer MS, Fatima A, Paget C, Trottein F, Brossay L. NKT cell immune responses to viral infection. Expert Opin Ther Targets. 2009;13:153–162. doi: 10.1517/14712590802653601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdin N, Kronenberg M. CD1-mediated immune responses to glycolipids. Curr Opin Immunol. 1999;11:326–331. doi: 10.1016/s0952-7915(99)80052-1. [DOI] [PubMed] [Google Scholar]
- Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Seino K, Nakayama T. The NKT cell system: bridging innate and acquired immunity. Nat Immunol. 2003;4:1164–1165. doi: 10.1038/ni1203-1164. [DOI] [PubMed] [Google Scholar]
- Ajuebor MN, Aspinall AI, Zhou F, Le T, Yang Y, Urbanski SJ, Sidobre S, Kronenberg M, Hogaboam CM, Swain MG. Lack of chemokine receptor CCR5 promotes murine fulminant liver failure by preventing the apoptosis of activated CD1d-restricted NKT cells. J Immunol. 2005;174:8027–8037. doi: 10.4049/jimmunol.174.12.8027. [DOI] [PubMed] [Google Scholar]
- Ajuebor MN, Jin Y, Gremillion GL, Strieter RM, Chen Q, Adegboyega PA. γδT cells initiate acute inflammation and injury in adenovirus-infected liver via cytokine-chemokine cross talk. J Virol. 2008;82:9564–9576. doi: 10.1128/JVI.00927-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, Locksley RM, Kronenberg M. Mouse Vα14i natural killer T cells are resistant to cytokine polarization in vivo, Proc Natl Acad Sci USA. 2003;100:8395–8400. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: nKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- Ajuebor MN, Wondimu Z, Hogaboam CM, Le T, Proudfoot AE, Swain MG. CCR5 deficiency drives enhanced natural killer cell trafficking to and activation within the liver in murine T cell-mediated hepatitis. Am J Pathol. 2007;170:1975–1988. doi: 10.2353/ajpath.2007.060690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886–1900. doi: 10.1053/j.gastro.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition, Proc Natl Acad Sci USA. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: The mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- Londhe VA, Belperio JA, Keane MP, Burdick MD, Xue YY, Strieter RM. CXCR2 is critical for dsRNA-induced lung injury: relevance to viral lung infection. J Inflamm (Lond) 2005;2:4. doi: 10.1186/1476-9255-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun R, Wei H, Dong Z, Tian Z. Pre-activation of T lymphocytes by low dose of concanavalin A aggravates Toll-like receptor-3 ligand-induced NK cell-mediated liver injury. Int Immunopharmacol. 2006;6:800–807. doi: 10.1016/j.intimp.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS ONE. 2008;3:e1861. doi: 10.1371/journal.pone.0001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen WF, Jr, Voellmy R, Roberts SM. Effect of N-acetylcysteine on heat shock protein induction by acetaminophen in mouse liver. J Pharmacol Exp Ther. 1998;286:519–524. [PubMed] [Google Scholar]
- O'Brien RL, Roark CL, Born WK. IL-17-producing γδT cells. Eur J Immunol. 2009;39:662–666. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs JA, Cho S, Roberts TJ, Sriram V, Zhang J, Xu M, Brutkiewicz RR. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J Virol. 2001;75:10746–10754. doi: 10.1128/JVI.75.22.10746-10754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage PB, Teyton L, Bendelac A. Glycolipids for natural killer T cells. Chem Soc Rev. 2006;35:771–779. doi: 10.1039/b510638a. [DOI] [PubMed] [Google Scholar]
- Cho WG, Albuquerque RJ, Kleinman ME, Tarallo V, Greco A, Nozaki M, Green MG, Baffi JZ, Ambati BK, De Falco M, Alexander JS, Brunetti A, De Falco S, Ambati J. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth, Proc Natl Acad Sci USA. 2009;106:7137–7142. doi: 10.1073/pnas.0812317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, Wands JM, Johnston M, Born WK. γδT-cell receptors: functional correlations. Immunol Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-γ by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of α-galactosylceramide. Blood. 2002;99:1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK cell-like behavior of Vα14i NK T cells during MCMV infection. PLoS Pathog. 2008;4:e1000106. doi: 10.1371/journal.ppat.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner T, Chen Q, Jin Y, Ajuebor MN. Characterization of the role of TCR γδ in NK cell accumulation during viral liver inflammation. Exp Mol Pathol. 2009;86:32–35. doi: 10.1016/j.yexmp.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun R, Wei H, Dong Z, Gao B, Tian Z. Poly I:C prevents T cell-mediated hepatitis via an NK-dependent mechanism. J Hepatol. 2006;44:446–454. doi: 10.1016/j.jhep.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Chiba A, Dascher CC, Besra GS, Brenner MB. Rapid NKT cell responses are self-terminating during the course of microbial infection. J Immunol. 2008;181:2292–2302. doi: 10.4049/jimmunol.181.4.2292. [DOI] [PubMed] [Google Scholar]
- Li X, McKinstry KK, Swain SL, Dalton DK. IFN-γ acts directly on activated CD4+ T cells during mycobacterial infection to promote apoptosis by inducing components of the intracellular apoptosis machinery and by inducing extracellular proapoptotic signals. J Immunol. 2007;179:939–949. doi: 10.4049/jimmunol.179.2.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-α-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model, J Hepatol. 2009;51:168–175. doi: 10.1016/j.jhep.2009.02.032. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Gollapudi S, Gupta S. Increased TNF-α-induced apoptosis in lymphocytes from aged humans: changes in TNF-α receptor expression and activation of caspases. J Immunol. 1999;162:2154–2161. [PubMed] [Google Scholar]
- Hildeman DA, Mitchell T, Kappler J, Marrack P. T cell apoptosis and reactive oxygen species. J Clin Invest. 2003;111:575–581. doi: 10.1172/JCI18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, Platt F, Trottein F. Activation of invariant NKT cells by Toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, Besra GS, Platt FM, Cerundolo V. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation, Proc Natl Acad Sci USA. 2007;104:20490–20495. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KN, Leung B, Kwong M, Zarember KA, Satyal S, Navas TA, Wang F, Godowski PJ. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesch D, Beetz S, Oberg HH, Marget M, Krengel K, Kabelitz D. Direct costimulatory effect of TLR3 ligand poly(I:C) on human γδT lymphocytes. J Immunol. 2006;176:1348–1354. doi: 10.4049/jimmunol.176.3.1348. [DOI] [PubMed] [Google Scholar]
- Patole PS, Grone HJ, Segerer S, Ciubar R, Belemezova E, Henger A, Kretzler M, Schlondorff D, Anders HJ. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J Am Soc Nephrol. 2005;16:1326–1338. doi: 10.1681/ASN.2004100820. [DOI] [PubMed] [Google Scholar]
- Niimi K, Asano K, Shiraishi Y, Nakajima T, Wakaki M, Kagyo J, Takihara T, Suzuki Y, Fukunaga K, Shiomi T, Oguma T, Sayama K, Yamaguchi K, Natori Y, Matsumoto M, Seya T, Yamaya M, Ishizaka A. TLR3-mediated synthesis and release of eotaxin-1/CCL11 from human bronchial smooth muscle cells stimulated with double-stranded RNA. J Immunol. 2007;178:489–495. doi: 10.4049/jimmunol.178.1.489. [DOI] [PubMed] [Google Scholar]
- Negishi H, Osawa T, Ogami K, Ouyang X, Sakaguchi S, Koshiba R, Yanai H, Seko Y, Shitara H, Bishop K, Yonekawa H, Tamura T, Kaisho T, Taya C, Taniguchi T, Honda K. A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity, Proc Natl Acad Sci USA. 2008;105:20446–20451. doi: 10.1073/pnas.0810372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biburger M, Tiegs G. α-Galactosylceramide-induced liver injury in mice is mediated by TNF-α but independent of Kupffer cells. J Immunol. 2005;175:1540–1550. doi: 10.4049/jimmunol.175.3.1540. [DOI] [PubMed] [Google Scholar]
- Wintermeyer P, Cheng CW, Gehring S, Hoffman BL, Holub M, Brossay L, Gregory SH. Invariant natural killer T cells suppress the neutrophil inflammatory response in a mouse model of cholestatic liver damage. Gastroenterology. 2009;136:1048–1059. doi: 10.1053/j.gastro.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Wei H, Sun R, Hu Z, Gao B, Tian Z. Involvement of natural killer cells in poly I:C-induced liver injury. J Hepatol. 2004;41:966–973. doi: 10.1016/j.jhep.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Jin N, Miyahara N, Roark CL, French JD, Aydintug MK, Matsuda JL, Gapin L, O'Brien RL, Gelfand EW, Born WK. Airway hyperresponsiveness through synergy of γδT cells and NKT cells. J Immunol. 2007;179:2961–2968. doi: 10.4049/jimmunol.179.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniai H, Hines IN, Bharwani S, Maloney RE, Nimura Y, Gao B, Flores SC, McCord JM, Grisham MB, Aw TY. Susceptibility of murine periportal hepatocytes to hypoxia-reoxygenation: role for NO and Kupffer cell-derived oxidants. Hepatology. 2004;39:1544–1552. doi: 10.1002/hep.20217. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277–284. doi: 10.3109/10715769109105223. [DOI] [PubMed] [Google Scholar]
- Peck A, Mellins ED. Breaking old paradigms: Th17 cells in autoimmune arthritis. Clin Immunol. 2009;132:295–304. doi: 10.1016/j.clim.2009.03.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- Johansson S, Berg L, Hall H, Hoglund P. NK cells: elusive players in autoimmunity. Trends Immunol. 2005;26:613–618. doi: 10.1016/j.it.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, Yagita H, Okumura K. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon γ-dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193:661–670. doi: 10.1084/jem.193.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]