Abstract
Large cholangiocytes secrete bicarbonate in response to secretin and proliferate after bile duct ligation by activation of cyclic adenosine 3′, 5′-monophosphate signaling. The Ca2+-dependent adenylyl cyclase 8 (AC8, expressed by large cholangiocytes) regulates secretin-induced choleresis. Ca2+-dependent protein kinase C (PKC) regulates small cholangiocyte function. Because γ-aminobutyric acid (GABA) affects cell functions by activation of both Ca2+ signaling and inhibition of AC, we sought to develop an in vivo model characterized by large cholangiocyte damage and proliferation of small ducts. Bile duct ligation rats were treated with GABA for one week, and we evaluated: GABAA, GABAB, and GABAC receptor expression; intrahepatic bile duct mass (IBDM) and the percentage of apoptotic cholangiocytes; secretin-stimulated choleresis; and extracellular signal-regulated kinase1/2 (ERK1/2) phosphorylation and activation of Ca2+-dependent PKC isoforms and AC8 expression. We found that both small and large cholangiocytes expressed GABA receptors. GABA: (i) induced apoptosis of large cholangiocytes and reduced large IBDM; (ii) decreased secretin-stimulated choleresis; and (iii) reduced ERK1/2 phosphorylation and AC8 expression in large cholangiocytes. Small cholangiocytes: (i) proliferated leading to increased IBDM; (ii) displayed activation of PKCβII; and (iii) de novo expressed secretin receptor, cystic fibrosis transmembrane regulator, Cl−/HCO3− anion exchanger 2 and AC8, and responded to secretin. Therefore, in pathologies of large ducts, small ducts replenish the biliary epithelium by amplification of Ca2+-dependent signaling and acquisition of large cholangiocyte phenotypes.
Cholangiocytes line the intrahepatic biliary tree,1,2 a network of interconnecting ducts of different sizes and functions.1,2 A number of gastrointestinal hormones including secretin modify bile of canalicular origin before reaching the duodenum.3,4 In humans, cholangiocytes are the target cells in a number of chronic cholestatic liver diseases characterized by biliary proliferation/loss.5 Cholangiocytes proliferate or are damaged in animal models of cholestasis including bile duct ligation (BDL) or acute administration of carbon tetrachloride (CCl4).4,6,7,8 Secretin receptor (SR, expressed only by large cholangiocytes in rodent liver)1,2,8,9 is a unique pathophysiological tool for evaluating at the functional level the degree of biliary growth/loss.6,7,8,9 Whereas enhanced cholangiocyte growth is associated with increased SR expression and secretin-stimulated choleresis, biliary damage leads to decreased functional expression of SR.7,8
The human and rodent biliary epithelium is morphologically and functionally heterogeneous.1,2,10,11,12 In rat liver, purified small cholangiocytes (≈8 μm in size) derive from small ducts (<15 μm in diameter), whereas large cholangiocytes (≈15 μm in size) originate from large ducts (>15 μm in diameter).1,2 Whereas the secretory, apoptotic, and proliferative activities of large cholangiocytes are regulated by changes in cyclic adenosine 3′,5′-monophosphate (cAMP)-dependent signaling,1,6,8,9,11,13 the function of small cholangiocytes (normally mitotically dormant)6,8 is regulated by the D-myo-inositol 1,4,5-trisphosphate (IP3)/Ca2+/calmodulin-dependent protein kinase I signaling pathway.14,15 For example, large (but not small) rodent cholangiocytes express SR,1,2,10 cystic fibrosis transmembrane regulator (CFTR),1,2,10 and Cl−/HCO3− exchanger1,2,10 (recently identified as the Cl−/HCO3− anion exchanger 2 [AE2]),16 and secrete bile in response to secretin by activation of cAMP⇒protein kinase A (PKA)⇒CFTR⇒Cl−/HCO3− anion AE2.1,2,9 The Ca2+-dependent adenylyl cyclase 8 (AC8, expressed mainly by large cholangiocytes)17 regulates secretin-stimulated choleresis of large bile ducts.17 After BDL, large but not small cholangiocytes undergo mitosis (leading to enhanced large duct mass)6,8,18 by activation of cAMP signaling.6,8,18 A single dose of CCl4 to rats induces a functional loss of large cAMP-responsive cholangiocytes, whereas small cholangiocytes (resistant to CCl4-induced apoptosis) de novo proliferate to compensate for the loss of large biliary mass.8 Although some studies suggest that activation of Ca2+-dependent signaling may be important in the regulation of small cholangiocyte function,14,15 the mechanisms by which small cholangiocytes replenish the biliary tree in response to the damage of large bile ducts is unknown.
Gamma-aminobutyric acid (GABA) is the chief inhibitory neurotransmitter in the vertebrate central nervous system.19 In addition to the central nervous system, the liver represents the major site of synthesis and metabolism of GABA.20 GABA actions are mediated by three GABA receptor subtypes (GABAA, GABAB, and GABAC).21 Studies have shown that GABAergic activity inhibits hepatic regeneration after partial hepatectomy in rats.22 We have shown that GABA decreases both in vivo and in vitro cholangiocarcinoma growth.21 However, no data exist regarding the role of GABA in the regulation of cholangiocyte hyperplasia in cholestasis. Because GABA can affect cell functions by both activation of Ca2+ signaling and inhibition of AC activity,23 we tested the hypothesis that GABA regulates the proliferative, apoptotic, and secretory activities of small and large cholangiocytes by the differential activation/deactivation of Ca2+- and cAMP-dependent signaling pathways.
Materials and Methods
Materials
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise indicated. The RIA kits for the measurement of intracellular cAMP ([125I] Biotrak Assay System, RPA509) and IP3 (D-myo-inositol 1,4,5-trisphosphate (IP3) [3H] Biotrak Assay System, TRK1000) levels were purchased from GE Health care (Piscataway, NJ). The antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) unless otherwise indicated. The CFTR monoclonal (IgG1) antibody (M3A7, previously used by us in rodent cholangiocytes)10 was purchased from Thermo Fisher Scientific (Fremont, CA). The antibody (an affinity-purified rabbit anti-rat AE2 IgG) against Cl−/HCO3− AE216 was purchased from α Diagnostic International (San Antonio, TX). The RNeasy Mini Kit to purify total RNA from cholangiocytes was purchased from Qiagen Inc, Valencia, CA.
Animal Models
Male 344 Fischer rats (150 to 175 g) were purchased from Charles River (Wilmington, MA) and kept in a temperature-controlled environment (22°C) with a 12:12 hours light/dark cycle. Animals were fed ad libitum and had free access to drinking water. The studies were performed in normal rats, and in rats that immediately after BDL or bile duct incannulation (BDI, for bile collection)4 were treated with daily IP injections of NaCl or GABA (50 mg/kg body weight)24 for 1 week. Before each procedure, animals were anesthetized with sodium pentobarbital (50 mg/kg IP) according to the regulations of the panel on euthanasia of the American Veterinarian Medical Association and local authorities. In all animals, we measured wet liver weight, body weight, and wet liver weight to body weight ratio, an index of liver cell growth including cholangiocytes.4
Purification of Small and Large Cholangiocytes
Virtually pure (by γ-glutamyl transpeptidase histochemistry)25 and distinct subpopulations of small (mean diameter 8 μm) and large (mean diameter 14 μm) cholangiocytes2,9 were isolated by counterflow elutriation.2,6,9 Cell viability was approximately 98%.
Expression of GABAA, GABAB, and GABAC Receptors
The expression of GABA receptors was evaluated by: (i) immunohistochemistry in paraffin-embedded liver sections (4 to 5 μm thick) from the aforementioned groups of animals; and (ii) immunofluorescence in cell smears of purified small and large BDL cholangiocytes. For immunohistochemistry,26 endogenous peroxidase activity was blocked by a 30-minute incubation in methanolic hydrogen peroxide (2.5%). The endogenous biotin was blocked by Biotin Blocking System (Dako, Copenhagen, Denmark) according to the instructions supplied by the vendor. Sections were hydrated in graded alcohol and rinsed in PBS (pH 7.4), then the primary antibodies GABAA (Santa Cruz, #21336; 1: 50 dilution), GABAB (Santa Cruz, #14006; 1: 50 dilution), GABAc (Santa Cruz, #23362; 1: 50 dilution) were applied and incubated overnight at 4°C. Samples were rinsed with PBS for 5 minutes, incubated for 10 minutes at room temperature with secondary biotinylated antibody (Dako LSAB Plus System, Milan, Italy), then with Dako ABC (Dako LSAB Plus System, Milan, Italy) and finally developed with 3-3′ diaminobenzidine. To demonstrate the specificity of the immunoreactions, negative controls (the primary antibody was replaced with the same dilution—with normal serum from the same species) were performed for all immunoreactions. Sections were analyzed in a coded manner using a BX-51 light microscope (Olympus, Tokyo, Japan) with a video cam (Spot Insight; Diagnostic Instrument, Inc., Sterling Heights, MI) and processed with an Image Analysis System (IAS: Delta Sistemi, Rome, Italy). Immunofluorescence for GABA receptors was performed as described.27 After staining, images were visualized using an Olympus IX-71 confocal microscope. For all immunoreactions, negative controls were included.
To evaluate the message expression of GABAA, GABAB, and GABAC in purified small and large cholangiocytes, we used the RT2 Real-Time assay from SABiosciences (Frederick, MD).14 A ΔΔCT (delta delta of the threshold cycle) analysis was performed using brain tissue as the control sample.14 The primers (purchased from SABiosciences) for GABAA, GABAB, and GABAC were designed according to the NCBI GenBank Accession number, NM 017289 for GABAA28; NM 031028 for GABAB29; and NM 017291 for GABAC.30 Data were expressed as relative mRNA levels ± SEM of GABA receptors to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ratio.
In Vivo Effect of GABA on Liver Morphology, Cholangiocyte Apoptosis, and Proliferation
We evaluated lobular morphology, necrosis, and portal inflammation by hematoxylin & eosin staining of paraffin-embedded liver sections (4 to 5 μm thick). Liver sections were examined in a coded fashion by BX-51 light microscopy (Olympus, Tokyo, Japan) equipped with a camera. Six slides were analyzed for each group and six nonoverlapping fields (magnification ×20) for each slide were evaluated for each parameter.
Apoptosis of small and large cholangiocytes was measured by quantitative terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) kit (Apoptag; Chemicon International, Inc) in liver sections (4 to 5 μm thick) from BDL rats treated with NaCl or GABA. Six slides were analyzed for each group using a BX-51 light microscopy (Olympus, Tokyo, Japan). Positive cholangiocytes were counted in six nonoverlapping fields (magnification ×20) for each slide, and the data are expressed as percentage of positive cells.
Immunoblots for BCL2-associated X protein (Bax, a pro-apoptotic protein)31 expression was performed in protein (10 μg) from whole cell lysates from small and large cholangiocytes. Blots were normalized by β-actin.14 The intensity of the bands was determined by scanning video densitometry using the phospho-imager, Storm 860, (GE Health care, Piscataway, NJ) and the ImageQuant TL software version 2003.02 (GE Health care, Little Chalfont, Buckinghamshire, England).
Proliferation of small (<15 μm diameter)1 and large (>15 μm diameter)1 bile ducts was measured by evaluating intrahepatic bile duct mass (IBDM) in liver sections. IBDM was measured as area occupied by cytokeratin-19 positive-bile duct/total area × 100. Proliferation was evaluated by immunoblots for proliferating cellular nuclear antigen (PCNA) in protein (10 μg) from whole cell lysate from purified small and large cholangiocytes. Blots were normalized to β-actin14 and visualized as described above.
Membrane Translocation and Phosphorylation of Ca2+-Dependent PKC Isoforms (Expression of cAMP-Dependent Signaling)
We next determined whether small BDL cholangiocytes proliferate and secrete (to compensate for GABA-induced damage of large cAMP-dependent BDL cholangiocytes) by both: (i) the activation of Ca2+-dependent signaling evaluated by the enhanced translocation and phosphorylation of Ca2+-dependent protein kinase C (PKC) isoforms (ie, αI, βI, βII and γ), which are important in the regulation of cholangiocyte function7,32,33; and (ii) the de novo acquisition of cAMP-dependent phenotypes (ie, expression of SR, CFTR, Cl−/HCO3− AE2 and AC8, and cAMP and Cl− efflux in response to secretin), which are key in the modulation of large cholangiocyte functions.1,2,6,8,9,13,34
The activation of Ca2+-dependent PKCα, βI, βII, or γ was evaluated by: (i) immunofluorescence (membrane translocation) in cell smears; and (ii) immunoblots (phosphorylation)7,14 in protein (10 μg) from whole cell lysate from small and large cholangiocytes. In purified small and large cholangiocytes, we also evaluated the phosphorylation of ERK1/2 (expressed by a ratio to the corresponding total protein),18 a protein linked to cAMP-dependent signaling pathway.18
Evaluation of Secretory Activity of Small and Large Cholangiocytes
We performed experiments to demonstrate that following chronic GABA administration to BDL rats: (i) small cholangiocytes acquire functional phenotypes of large cholangiocytes; and (ii) there is down-regulation of cAMP-dependent secretory activity1,2,8,9 in large cholangiocytes. To achieve this, we measured the protein expression for SR, CFTR, and Cl−/HCO3− AE2 by immunohistochemistry in liver sections (4 to 5 μm thick), and immunofluorescence14,27 in small and large cholangiocytes; (ii) AC8 protein expression in liver sections (4 to 5 μm) by immunohistochemistry,14 and AC8 gene expression by real-time PCR in total RNA (1 μg) from small and large cholangiocytes; and (iii) basal and secretin-stimulated cAMP levels by RIA6,7,13,34 and 36Cl− efflux (a functional index of CFTR activity).9,35,36 The primers (purchased from SABiosciences, Frederick, MD) for AC8 were designed according to the NCBI GenBank Accession number NM 017142.37
The secretory activity of small and large cholangiocytes was also assessed by measuring basal and secretin-stimulated bile and bicarbonate secretion in bile fistula BDI rats.4 After anesthesia, rats were surgically prepared for bile collection as described.4 When steady-state bile flow was reached (60 to 70 minutes from the intravenous infusion of Krebs-Ringer-Henseleit solution, KRH), rats were infused with secretin (100 nmol/L) for 30 minutes followed by IV infusion of KRH for 30 minutes. Bicarbonate concentration in bile was determined by an ABL 520 Blood Gas System (Radiometer Medical A/S, Copenhagen, Denmark).
Statistical Analysis
All data are expressed as mean ± SEM. Differences between groups were analyzed by Student unpaired t test when two groups were analyzed and analysis of variance when more than two groups were analyzed, followed by an appropriate post hoc test.
Results
Cholangiocytes Express GABA Receptors
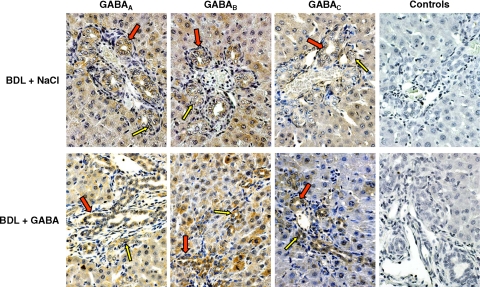
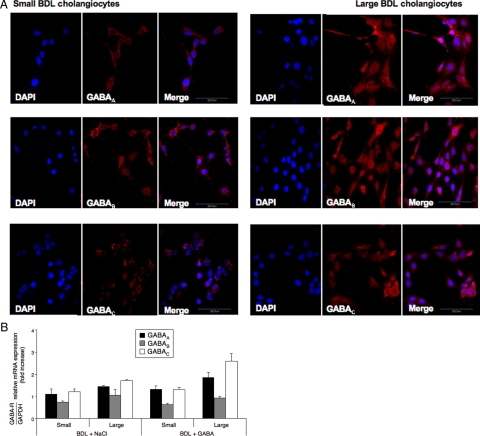
Immunohistochemistry in liver sections from normal (not shown) and BDL (Figure 1) rats treated with NaCl or GABA shows that both small (yellow arrows) and large (red arrows) bile ducts express GABAA, GABAB, and GABAC receptors. By immunofluorescence, there was positive immunoreactivity for the three GABA receptors in small and large BDL cholangiocytes (Figure 2A). Negative controls are shown in Figure 2A. By real-time PCR, both small and large cholangiocytes from BDL rats treated with NaCl or GABA express the message for the three GABA receptors (Figure 2B).
Figure 1.
Representative immunohistochemistry for GABAA, GABAB, and GABAC in liver sections from BDL rats treated with NaCl or GABA for 1 week. Both small (yellow arrows) and large (red arrows) bile ducts express the three subtypes of GABA receptors. No staining was visible when primary antibodies were replaced with nonimmune serum from the same species. Original magnification, ×40.
Figure 2.
A: By immunofluorescence, both small and large cholangiocytes from BDL rats express the three GABA receptor subtypes. Specific immunoreactivity of representative fields is shown in red; cell nuclei were stained with DAPI (blue). Scale bar = 200 μm. B: Real-time PCR shows that purified small and large cholangiocytes from BDL rats treated with NaCl or GABA express the message for all three GABA receptors. Data are mean ± SEM of 3 experiments.
Effect of GABA on Liver Histology, Liver and Body Weight, Apoptosis, and Proliferation of Small and Large Bile Ducts
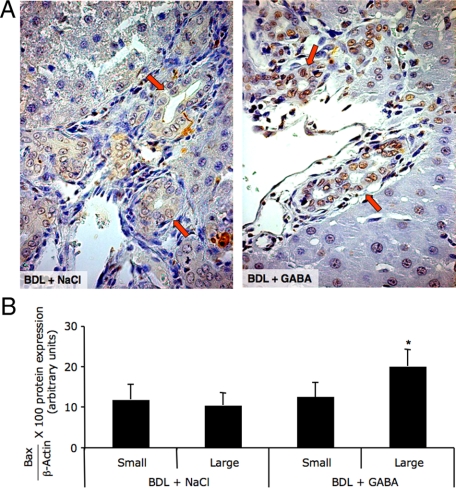
No significant differences in wet liver weight, body weight, liver to body weight ratio, degree of portal inflammation, necrosis, and lobular damage were observed in BDL rats treated with NaCl or GABA for 1 week (not shown). Chronic in vivo administration of GABA to normal rats did not alter cholangiocyte apoptosis or proliferation (not shown). Administration of GABA to BDL rats induced apoptosis of large bile ducts (red arrows, by TUNEL in liver sections) (Figure 3A and Table 1) and purified large cholangiocytes (by Bax immunoblots; Figure 3B). No changes in apoptosis were seen in small ducts (Figure 3A) and purified small cholangiocytes (Figure 3B) from BDL rats treated with NaCl or GABA.
Figure 3.
Evaluation of cholangiocyte apoptosis by TUNEL analysis in liver sections (A), and Bax immunoblots in small and large cholangiocytes (B) from BDL rats treated with NaCl or GABA for 1 week. GABA induced apoptosis of large ducts (A, red arrows, for quantitative data see Table 1) and large cholangiocytes (B) from BDL rats treated with GABA compared with BDL rats treated with NaCl. A and B: No changes in apoptosis were seen in small ducts and small cholangiocytes from BDL rats treated with NaCl or GABA. A: Original magnification, ×40. B: Data are mean ± SEM of 8 blots. *P < 0.05 versus large cholangiocytes from BDL rats treated with NaCl for 1 week.
Table 1.
Evaluation of % of Tunel-Positive Cholangiocytes in Small and Large Bile Ducts and Measurement of Bile Duct Mass by Cytokeratin 19 Staining
| Groups | Bile ducts | Apoptosis | IBDM |
|---|---|---|---|
| Normal + NaCl | Small | ND | 0.05 ± 0.01 |
| Large | ND | 0.21 ± 0.03 | |
| Normal + GABA | Small | ND | 0.07 ± 0.01 |
| Large | ND | 0.19 ± 0.02 | |
| BDL + NaCl | Small | 12.04 ± 2.77 | 0.90 ± 0.08 |
| Large | 15.14 ± 2.46 | 4.65 ± 0.34 | |
| BDL + GABA | Small | 10.62 ± 1.85 | 1.70 ± 0.15† |
| Large | 40.77 ± 2.68* | 2.61 ± 0.31‡ |
Small bile ducts = <15 μm diameter; large bile ducts = >15 μm diameter. Apoptosis of small and large bile ducts was measured by TUNEL analysis in liver sections. Proliferation of small and large bile ducts was measured by evaluating intrahepatic bile duct mass (IBDM) in liver sections. IBDM was measured as area occupied by CK19-positive bile duct/total area × 100. ND indicates not detected. Data are expressed as mean ± SEM.
P < 0.05 versus the number of large bile ducts (positive by TUNEL) from BDL rats treated with NaCl.
P < 0.05 versus the number of small bile ducts from BDL NaCl-treated rats.
P < 0.05 versus the number of large bile ducts from BDL NaCl-treated rats.
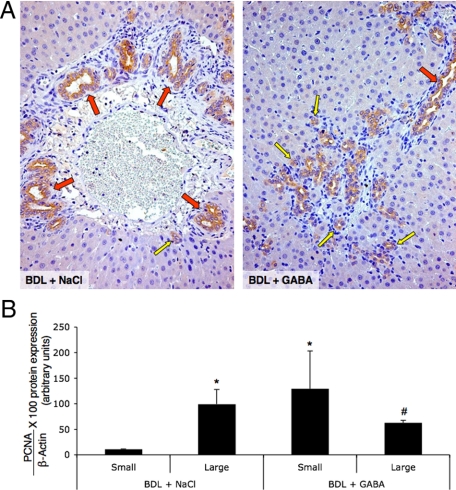
In agreement with previous studies,6 after BDL only large cholangiocytes proliferated leading to increased large IBDM (Figure 4A and Table 1). Concomitant with GABA-induced apoptosis of large cholangiocytes (Figure 3, A and B), there was a significant decrease in large (red arrows) IBDM (Figure 4A and Table 1) and the de novo proliferation of small ducts (yellow arrows) leading to an increase in small IBDM (Figure 4A and Table 1). The overall IBDM was similar between the BDL rats treated with NaCl or GABA (Table 1). After BDL, large cholangiocytes displayed higher proliferative activity compared with small BDL cholangiocytes (Figure 4B).6,8 Furthermore, there was decreased proliferation in large cholangiocytes and increased PCNA protein expression in purified small cholangiocytes from BDL rats treated with GABA compared with small and large cholangiocytes from BDL rats treated with NaCl (Figure 4B).
Figure 4.
Measurement of IBDM of small and large bile ducts by immunohistochemistry for cytokeratin-19 in liver sections (A), and PCNA protein expression in protein (10 μg) from whole cell lysate from small and large cholangiocytes (B) from BDL rats treated with NaCl or GABA for 1 week. A: After BDL, large (red arrows) bile ducts proliferate leading to an increase in large IBDM; there were no changes in the number of small (yellow arrow) bile ducts. After GABA administration, there were decreased large IBDM and the de novo proliferation of small bile ducts (yellow arrows) leading to an increase in small IBDM (for quantitative data see Table 1). Original magnification, ×20. Data are mean ± SEM of 36 cumulative values obtained from the six slides evaluated per each group of animals. B: After BDL, only large cholangiocytes displayed higher proliferative activity. There was decreased proliferation in large cholangiocytes and increased PCNA protein expression in purified small cholangiocytes from BDL rats treated with GABA compared with BDL rats treated with NaCl. Data are mean ± SEM of 8 blots. *P < 0.05 versus small cholangiocytes from BDL rats treated with NaCl for 1 week. #P < 0.05 versus large cholangiocytes from BDL rats treated with NaCl for 1 week.
Membrane Translocation and Phosphorylation of Ca2+-Dependent PKC Isoforms and Phosphorylation of ERK1/2
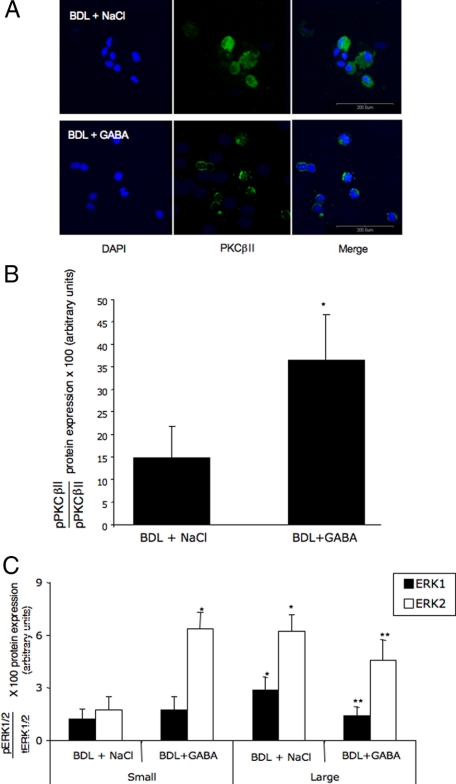
We observed membrane translocation (by immunofluorescence, Figure 5A) and enhanced phosphorylation of PKCβII (by immunoblots, Figure 5B) in small cholangiocytes from BDL GABA-treated rats compared with small cholangiocytes from BDL rats treated with NaCl. No membrane translocation of PKCα, PKCβI, and PKCγ was observed in small cholangiocytes from BDL rats treated with GABA compared with small cholangiocytes from BDL rats treated with NaCl (not shown). After GABA administration to BDL rats, there was: (i) decreased phosphorylation of ERK1/2 in large cholangiocytes; and (ii) increased ERK2 (but not ERK1) phosphorylation in small cholangiocytes compared with small and large cholangiocytes from BDL rats treated with NaCl (Figure 5C).
Figure 5.
Evaluation of the membrane translocation (A) and phosphorylation (B) of PKCβII in small cholangiocytes from BDL rats treated with NaCl or GABA for 1 week. We observed enhanced membrane translocation (by immunofluorescence [A]) and phosphorylation (by immunoblots [B]) of PKCβII in small cholangiocytes from BDL GABA-treated rats compared with small cholangiocytes from BDL rats treated with NaCl. A: Specific immunoreactivity of representative fields is shown in green; cell nuclei were stained with DAPI (blue). Bar = 200 μm. C: Evaluation of ERK1/2 phosphorylation in small and large cholangiocytes from BDL rats treated with NaCl or GABA for 1 week. After GABA administration to BDL rats, there was: (i) decreased phosphorylation of ERK1/2 in large cholangiocytes; and (ii) increased ERK2 (but not ERK1) phosphorylation in small cholangiocytes compared with small and large cholangiocytes from BDL rats treated with NaCl. Data are mean ± SEM of 8 blots. *P < 0.05 versus small cholangiocytes from BDL rats treated with NaCl for 1 week. **P < 0.05 versus large cholangiocytes from BDL rats treated with NaCl for 1 week.
Evaluation of Biliary Markers and Secretory Activity of Small and Large Cholangiocytes
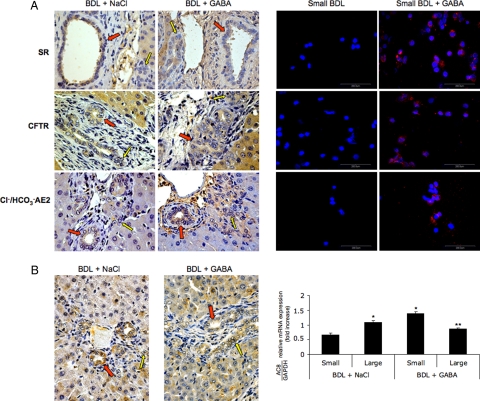
The protein for SR, CFTR, and Cl−/HCO3− AE2 was expressed only by large bile ducts (red arrows, Figure 6A) and large cholangiocytes (by immunofluorescence, not shown)1,2 from BDL rats. By immunofluorescence, small BDL cholangiocytes do not express SR, CFTR, and Cl−/HCO3− AE2 (Figure 6A). In BDL rats treated with GABA, small ducts (yellow arrows, Figure 6A) and small cholangiocytes (Figure 6A) express de novo SR, CFTR, and Cl−/HCO3− AE2. Parallel to our previous studies,17 immunohistochemistry in liver sections shows that AC8 was mostly expressed by large cholangiocytes from BDL rats treated with NaCl (Figure 6B). In liver sections from BDL rats treated with GABA, the expression of AC8 in liver sections seemed lower in large ducts and present de novo in small bile ducts (Figure 6B). Similarly, by real-time PCR we demonstrated that: (i) AC8 was present in large and at lower levels in small BDL cholangiocytes17; and (ii) AC8 mRNA expression decreased in large cholangiocytes and significantly increased in small cholangiocytes from BDL rats treated with GABA (Figure 6B).
Figure 6.
A: Representative immunohistochemistry for SR, CFTR, Cl−/HCO3− AE 2 and immunofluorescence in liver sections (left panel) and immunofluorescence in freshly isolated small cholangiocytes (right panel) from BDL rats treated with NaCl or GABA for 1 week. In liver sections, the protein for SR, CFTR, and Cl−/HCO3− AE2 was expressed only by large (red arrows) bile ducts from BDL rats; no expression for SR, CFTR, and Cl−/HCO3− AE2 was seen in small (yellow arrows) bile ducts. In BDL rats treated with GABA, small bile ducts (yellow arrows) express de novo SR, CFTR, and Cl−/HCO3− AE2. Original magnification, ×40. By immunofluorescence, small BDL cholangiocytes do not express SR, CFTR, and Cl−/HCO3− AE2. After administration of GABA to BDL rats, small cholangiocytes express de novo SR, CFTR, and Cl−/HCO3− AE2. Scale bar = 200 μm. B: Expression of AC8 was evaluated by immunohistochemistry in liver sections and real-time PCR in freshly isolated cholangiocytes from BDL rats treated with NaCl or GABA for 1 week. In liver sections from BDL rats treated with GABA, the expression of AC8 in liver sections seemed lower in large ducts (red arrows) and present de novo in small bile ducts (yellow arrows, left panel). By real-time PCR we demonstrated that: (i) AC8 was present in large and at lower levels in small BDL cholangiocytes; and (ii) AC8 mRNA expression decreased in large cholangiocytes and significantly increased in small cholangiocytes from BDL rats treated with GABA (right panel). Data are mean ± SEM of 3 experiments. *P < 0.05 versus small cholangiocytes from BDL rats treated with NaCl for 1 week. **P < 0.05 versus large cholangiocytes from BDL rats treated with NaCl for 1 week.
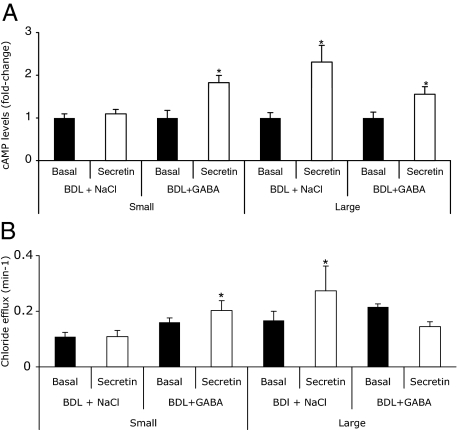
Parallel to other studies,6,9,32,38 secretin increased cAMP levels (Figure 7A) and Cl− efflux (Figure 7B) of large (but not small) BDL cholangiocytes compared with their corresponding basal values. In large cholangiocytes from BDL GABA-treated rats, there was a decrease in secretin-stimulated cAMP levels (Figure 7A) and Cl− efflux (Figure 7B) compared with their corresponding values of large BDL cholangiocytes. Small cholangiocytes from BDL GABA-treated rats de novo respond to secretin with increased cAMP levels (Figure 7A) and Cl− efflux (Figure 7B).
Figure 7.
Measurement of basal and secretin-stimulated (A) cAMP levels and (B) Cl− efflux in small and large cholangiocytes from BDL rats treated with NaCl or GABA for 1 week. A: Secretin increased cAMP levels of large (but not small) BDL cholangiocytes compared with their corresponding basal values. In BDL rats treated with GABA, secretin did not increase cAMP levels in large cholangiocytes, but significantly increased cAMP levels of purified small cholangiocytes compared with small cholangiocytes from BDL rats treated with NaCl. Data are mean ± SEM of six experiments. *P < 0.05 versus the corresponding basal value. B: Secretin increased Cl− efflux of large (but not small) BDL cholangiocytes. In large cholangiocytes from BDL rats treated with GABA, secretin did not increase Cl− efflux. In small cholangiocytes from BDL GABA-treated rats, secretin induced a de novo increase in Cl− efflux. Data are mean ± SEM of 6 experiments. *P < 0.05 versus the corresponding basal value.
Intravenous infusion of secretin increased bile flow and bicarbonate secretion of BDI rats (Table 2).4,32,38,39 In BDL rats treated with GABA, secretin-stimulated bicarbonate rich choleresis was lower (although significant) compared with its corresponding values of BDI rats treated with NaCl (Table 2). The smaller but significant increase in secretin-stimulated choleresis (observed in BDL GABA-treated rats) is likely attributable to small proliferating cholangiocytes, which de novo respond to secretin in this model of large cholangiocyte damage.
Table 2.
Measurement of Basal and Secretin-Stimulated Bile Flow and Bicarbonate Secretion
| Treatment | Bile flow (μl/min/kg BW) (basal) | Bile flow (μl/min/kg BW) (secretin) | Bicarbonate secretion (μEq/min/kg BW) (basal) | Bicarbonate secretion (μEq/min/kg BW) (secretin) |
|---|---|---|---|---|
| BDI+ NaCl | 120.3 ± 13.0 | 186.3 ± 17.3* | 5.2 ± 0.6 | 11.3 ± 1.5† |
| BDI+ GABA | 105.7 ± 8.3 | 139.5 ± 11.1*‡ | 4.1 ± 0.5 | 6.7 ± 0.5†§ |
BDI indicates bile duct ligation. Data are mean ± SEM of 5 experiments.
P < 0.05 versus its corresponding value of basal bile flow.
P < 0.05 versus its corresponding value of basal bicarbonate secretion.
P < 0.05 versus secretin-stimulated bile flow in BDI rats.
P < 0.05 versus secretin-stimulated bicarbonate secretion in BDI rats.
Discussion
The findings of this study relate to the heterogeneous effects of GABA on the apoptotic, proliferative, and secretory functions of small and large cholangiocytes in cholestatic BDL rats. Chronic administration of GABA to BDL rats: (i) induced apoptosis of large cholangiocytes; (ii) reduced large cholangiocyte proliferation and IBDM by down-regulation of cAMP signaling; and (iii) decreased AC8 expression and reduced secretin-stimulated choleresis in large cholangiocytes. After GABA administration, small cholangiocytes: (i) were resistant to GABA-induced biliary apoptosis and de novo proliferate leading to an increased number of small ducts; (ii) displayed membrane translocation and phosphorylation of Ca2+-dependent PKCβII; and (iii) de novo express SR, CFTR, Cl−/HCO3− AE2 and AC8, and secrete water and electrolytes in response to secretin. During damage of large cholangiocytes, small ducts replenish the intrahepatic biliary tree by amplification of both Ca2+-dependent signaling and the acquisition of large cholangiocyte phenotypes.
We first demonstrated that both small and large cholangiocytes express GABAA, GABAB, and GABAC receptors at similar levels; the expression of the GABA receptors in small and large cholangiocytes did not change with GABA administration. These findings suggest that the heterogeneous effects of GABA on small and large cholangiocyte function are not attributable to the differential expression of these receptors in the two cell types. In our in vivo model it is difficult to pinpoint the specific receptors involved in GABA modulation of small and large cholangiocyte functions. Likely, these actions are mediated by all three GABA receptor subtypes. Our concept is supported by our previous in vitro studies21 in human cholangiocarcinoma cells showing that blocking of GABAA, GABAB, and GABAC receptors (by specific receptor antagonist) prevents GABA inhibition of cholangiocarcinoma proliferation.
Importantly, GABA administration induces large cholangiocyte damage only in rats with extrahepatic cholestasis, which can be explained based on the fact that: (i) GABA damages large ducts due to sensitization from obstructive cholestasis and consequent biliary/seric accumulation40; (ii) GABA damages only proliferating large cholangiocytes, and this is consistent with our previous findings in cholangiocarcinoma cells21; and (iii) GABA metabolism is dysregulated during liver damage induced by cholestasis.41
A number of speculations and studies support the view that small cholangiocytes are more resistant than large cholangiocytes to hepatic injury/toxins.8,42 For example, the anti-apoptotic protein bcl-2 is expressed at higher levels by ductules and small bile ducts in normal human liver and human liver with cirrhosis and focal nodular hyperplasia.43 The higher resistance of small cholangiocytes to injury/toxins may be attributable to their primordial undifferentiated nature, whereas large cAMP-dependent cholangiocytes (more differentiated) are more susceptible to injury. Indeed, the presence of a larger nucleus and a smaller cytoplasm in small cholangiocytes44 suggests the undifferentiated primitive nature of small bile ducts.42,44 On the other hand, large cholangiocytes (displaying a larger cytoplasmic area)42,44 are more differentiated/senescent cells and more likely more susceptible to damage. However, these points are speculative, and further studies are necessary to support this view.42,44
The PKC signaling pathway modulates cell resistance to apoptosis in a number of systems.45,46 For example, the expression of PKCβI confers resistance to tumor necrosis factor-α and paclitaxel-induced apoptosis in HT-29 colon carcinoma cells.45 In this regard, the activation of GABA receptors stimulates PKC activity in hippocampal and cultured spinal neurons.47,48 Although our data do not demonstrate that the activation of IP3/Ca2+-dependent PKC signaling is the key factor for the higher resistance of small cholangiocytes to GABA, they do suggest that activation of this signaling pathway is important for the activation of a “small bile duct compartment” to replenish the biliary epithelium during the damage of large bile ducts.14,15 The IP3/Ca2+-dependent signaling pathway and its cross talk with cAMP signaling are important regulators of small cholangiocyte functions.14 We hypothesize that the trigger for the de novo activation of Ca2+/PKC-dependent proliferation of small cholangiocytes is attributable to the down-regulation of cAMP-dependent signaling in large cholangiocytes damaged by GABA treatment resulting in the absence of required functional capacity, which must be replenished by the activation of the small cholangiocyte compartment. In support of our hypothesis, studies have shown that GABA activity mediating cytosolic Ca2+ increases in developing neurons is triggered by changes (activation or inhibition) in cAMP-dependent signal transduction.49
The de novo acquisition of proliferative and secretory phenotypes of large cholangiocytes by small cholangiocytes is likely attributable to the IP3/Ca2+-dependent activation of AC8, a Ca2+-dependent AC isoform that plays a key role in the proliferative and secretory functions of large bile ducts.17 Nine AC isoforms exist in mammalian cells.50 All mammalian ACs are activated by GTP-bound stimulatory G protein α subunit (Gαs).50 Whereas activation of Gαs increases AC-mediated cAMP synthesis, inhibitory G protein (Gαi) inhibits all AC isoforms except AC2 and AC4.50 The effects of [Ca2+]i are very diverse depending on the AC isoform.50 The activities of AC1, AC3, and AC8 are positively regulated by [Ca2+]i and calmodulin, whereas AC5 and AC6 are negatively modulated by [Ca2+]I.50 Several studies demonstrate the role of AC in the regulation of cholangiocyte functions.13,17,51 Changes in the activity of Gαs and Gαi protein subunits are associated with alteration of cholangiocyte growth of BDL rats.51 Increased cholangiocyte cAMP levels prevent bile duct damage by total vagotomy.13 Also, cross talk between the IP3/Ca2+/PKC pathway and AC plays a key role in the regulation of cholangiocyte functions. Gastrin inhibits cholangiocyte proliferation through activation of Ca2+-dependent PKCα.7 The D2 dopaminergic agonist, quinelorane, inhibits secretin-stimulated ductal secretion of BDL rats by activation of the Ca2+-dependent PKCγ, which leads to decreased PKA activity.32 The α−1 adrenergic receptor, phenylephrine, increases secretin-stimulated ductal secretion by activation of PKCα and PKCβII.33
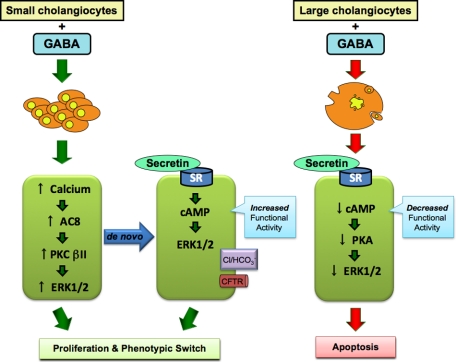
In summary, we have developed a novel in vivo model characterized by impaired function of large cAMP-dependent cholangiocytes (Figure 8). In this model, small cholangiocyte function is regulated by both the activation of IP3/Ca2+-dependent PKC signaling and the acquisition of large cholangiocyte functional markers to compensate for the damage of large ducts (Figure 8). In pathological conditions in which large cAMP-dependent bile ducts are damaged (eg, such as cystic fibrosis),5 the de novo activation of the IP3/Ca2+-/PKC-dependent small cholangiocyte compartment may be important compensatory mechanism for the replenishment of the biliary epithelium.
Figure 8.
Working model of GABA-induced changes in small and large biliary proliferation/damage. Right: GABA stimulates a down-regulation of large cholangiocyte proliferation that results in the down-regulation of the functional capacity of cholangiocytes (ie, SR->cAMP->PKA->RK1/2 signaling mechanisms) and subsequently activates apoptosis. Left: GABA stimulates the proliferation of small cholangiocytes via the activation of calcium->AC8->PKCβII->ERK1/2-dependent signaling. In addition, GABA stimulates a phenotypic switch in small cholangiocytes resulting in the de novo expression of SR and the downstream cAMP->ERK1/2 signaling mechanisms. Also, GABA stimulates the de novo expression of CFTR and Cl−/HCO3− exchanger, which a partners in cholangiocyte functional activity. AC indicates adenylyl cyclase; cAMP, cyclic adenosine 3′,5′-monophosphate; ERK1/2, extracellular signal-regulated kinase1/2; GABA, γ-aminobutyric acid; PKA, protein kinase A; PKC, protein kinase C; SR, secretin receptor.
Footnotes
Address reprint requests to Gianfranco Alpini, Ph.D., Texas A & M Health Science Center, Medical Research Building, 702 SW H.K. Dodgen Loop, Temple, TX, 76504. E-mail: galpini@tamu.edu or galpini@medicine.tamhsc.edu.
Supported in part by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, the VA Research Scholar Award, a VA Merit Award, and National Institutes of Health grants DK58411, DK062975, and DK76898 (to G.A.), a National Institutes of Health K01 grant award (DK078532; to S.D.), and by University funds (to P.O.) and PRIN 2007 and Federate Athenaeum funds from University of Rome “La Sapienza” (to E.G.).
R.M. and A.F. contributed equally to this work.
References
- Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage G. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1064–G1074. doi: 10.1152/ajpgi.1997.272.5.G1064. [DOI] [PubMed] [Google Scholar]
- Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, LaRusso NF. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- Glaser SS, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med. 2009;11:e7. doi: 10.1017/S1462399409000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules, J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, Schachter D, Shafritz DA, editors. Philadelphia, PA: Lippincott Williams & Wilkins,; The Liver; Biology & Pathobiology, 4E. 2001:pp. 421–435. [Google Scholar]
- Alpini G, Glaser S, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274:G767–G775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- Glaser S, Benedetti A, Marucci L, Alvaro D, Baiocchi L, Kanno N, Caligiuri A, Phinizy JL, Chowdhury U, Papa E, LeSage G, Alpini G. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-. Ca(2+)-, and protein kinase C alpha-dependent mechanisms, Hepatology. 2000;32:17–25. doi: 10.1053/jhep.2000.8265. [DOI] [PubMed] [Google Scholar]
- LeSage G, Glaser S, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, Papa E, Tretjak Z, Jezequel AM, Holcomb LA, Alpini G. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1289–1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- Alpini G, Ulrich C, Roberts S, Phillips JO, Ueno Y, Podila PV, Colegio O, LeSage G, Miller LJ, LaRusso NF. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1997;272:G289–G297. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- Glaser SS, Gaudio E, Rao A, Pierce LM, Onori P, Franchitto A, Francis HL, Dostal DE, Venter JK, DeMorrow S, Mancinelli R, Carpino G, Alvaro D, Kopriva SE, Savage JM, Alpini GD. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno N, LeSage G, Glaser S, Alvaro D, Alpini G. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology. 2000;31:555–561. doi: 10.1002/hep.510310302. [DOI] [PubMed] [Google Scholar]
- Martinez-Anso E, Castillo JE, Diez J, Medina JF, Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400–1406. [PubMed] [Google Scholar]
- LeSage G, Alvaro D, Benedetti A, Glaser S, Marucci L, Baiocchi L, Eisel W, Caligiuri A, Phinizy JL, Rodgers R, Francis H, Alpini G. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191–199. doi: 10.1016/s0016-5085(99)70567-6. [DOI] [PubMed] [Google Scholar]
- Francis H, Glaser S, Demorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, Onori P, Franchitto A, Marzioni M, Vaculin S, Vaculin B, Katki K, Stutes M, Savage J, Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–C513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpini G, Ueno Y, Glaser SS, Marzioni M, Phinizy JL, Francis H, Lesage G. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology. 2001;34:868–876. doi: 10.1053/jhep.2001.28884. [DOI] [PubMed] [Google Scholar]
- Banales JM, Arenas F, Rodriguez-Ortigosa CM, Saez E, Uriarte I, Doctor RB, Prieto J, Medina JF. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43:266–275. doi: 10.1002/hep.21042. [DOI] [PubMed] [Google Scholar]
- Strazzabosco M, Fiorotto R, Melero S, Glaser S, Francis H, Spirli C, Alpini G. Differentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation. Hepatology. 2009;50:244–252. doi: 10.1002/hep.22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis H, Franchitto A, Ueno Y, Glaser S, DeMorrow S, Venter J, Gaudio E, Alvaro D, Fava G, Marzioni M, Vaculin B, Alpini G. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest. 2007;87:473–487. doi: 10.1038/labinvest.3700533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- Erlitzki R, Gong Y, Zhang M, Minuk G. Identification of gamma-aminobutyric acid receptor subunit types in human and rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G733–G739. doi: 10.1152/ajpgi.2000.279.4.G733. [DOI] [PubMed] [Google Scholar]
- Fava G, Marucci L, Glaser S, Francis H, De Morrow S, Benedetti A, Alvaro D, Venter J, Meininger C, Patel T, Taffetani S, Marzioni M, Summers R, Reichenbach R, Alpini G. gamma-Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437–11446. doi: 10.1158/0008-5472.CAN-05-1470. [DOI] [PubMed] [Google Scholar]
- Minuk GY. Gamma-aminobutyric acid and the liver. Dig Dis. 1993;11:45–54. doi: 10.1159/000171400. [DOI] [PubMed] [Google Scholar]
- Martin C, Jacobi JS, Nava G, Jeziorski MC, Clapp C, Martinez de la Escalera G. GABA inhibition of cyclic AMP production in immortalized GnRH neurons is mediated by calcineurin-dependent dephosphorylation of adenylyl cyclase 9. Neuroendocrinology. 2007;85:257–266. doi: 10.1159/000103557. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gong YW, Minuk GY. The effects of ethanol and gamma aminobutyric acid alone and in combination on hepatic regenerative activity in the rat. J Hepatol. 1998;29:638–641. doi: 10.1016/s0168-8278(98)80160-8. [DOI] [PubMed] [Google Scholar]
- Rutenburg AM, Kim H, Fischbein JW, Hanker JS, Wasserkrug HL, Seligman AM. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969;17:517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- DeMorrow S, Francis H, Gaudio E, Ueno Y, Venter J, Onori P, Franchitto A, Vaculin B, Vaculin S, Alpini G. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G506–G519. doi: 10.1152/ajpgi.00304.2007. [DOI] [PubMed] [Google Scholar]
- DeMorrow S, Glaser S, Francis H, Venter J, Vaculin B, Vaculin S, Alpini G. Opposing actions of endocannabinoids on cholangiocarcinoma growth: recruitment of Fas and Fas ligand to lipid rafts. J Biol Chem. 2007;282:13098–13113. doi: 10.1074/jbc.M608238200. [DOI] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Mehta AK, Ticku MK. Effect of chronic administration of ethanol on the regulation of the delta-subunit of GABA(A) receptors in the rat brain. Brain Res. 2007;1174:47–52. doi: 10.1016/j.brainres.2007.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grampp T, Notz V, Broll I, Fischer N, Benke D. Constitutive, agonist-accelerated, recycling and lysosomal degradation of GABA(B) receptors in cortical neurons. Mol Cell Neurosci. 2008;39:628–637. doi: 10.1016/j.mcn.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Yang L, Nakayama Y, Hattori N, Liu B, Inagaki C. GABAC-receptor stimulation activates cAMP-dependent protein kinase via A-kinase anchoring protein 220. J Pharmacol Sci. 2008;106:578–584. doi: 10.1254/jphs.fp0071362. [DOI] [PubMed] [Google Scholar]
- Sheng G, Guo J, Warner BW. Epidermal Growth Factor Receptor Signaling Modulates Apoptosis via p38{alpha} MAPK-Dependent Activation of Bax in Intestinal Epithelial Cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G599–G606. doi: 10.1152/ajpgi.00182.2007. [DOI] [PubMed] [Google Scholar]
- Glaser S, Alvaro D, Roskams T, Phinizy JL, Stoica G, Francis H, Ueno Y, Barbaro B, Marzioni M, Mauldin J, Rashid S, Mancino MG, LeSage G, Alpini G. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am J Physiol Gastrointest Liver Physiol. 2003;284:G683–G694. doi: 10.1152/ajpgi.00302.2002. [DOI] [PubMed] [Google Scholar]
- LeSage G, Alvaro D, Glaser S, Francis H, Marucci L, Roskams T, Phinizy JL, Marzioni M, Benedetti A, Taffetani S, Barbaro B, Fava G, Ueno Y, Alpini G. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology. 2004;40:1116–1127. doi: 10.1002/hep.20424. [DOI] [PubMed] [Google Scholar]
- Francis H, Glaser S, Ueno Y, LeSage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Francis H, LeSage G, DeMorrow S, Alvaro D, Ueno Y, Venter J, Glaser S, Mancino MG, Marucci L, Benedetti A, Alpini G. The alpha2-adrenergic receptor agonist UK 14,304 inhibits secretin-stimulated ductal secretion by downregulation of the cAMP system in bile duct-ligated rats. Am J Physiol Cell Physiol. 2007;293:C1252–C1262. doi: 10.1152/ajpcell.00031.2007. [DOI] [PubMed] [Google Scholar]
- Fitz JG, Basavappa S, McGill J, Melhus O, Cohn JA. Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest. 1993;91:319–328. doi: 10.1172/JCI116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masada N, Ciruela A, Macdougall DA, Cooper DM. Distinct mechanisms of regulation by Ca2+/calmodulin of type 1 and 8 adenylyl cyclases support their different physiological roles. J Biol Chem. 2009;284:4451–4463. doi: 10.1074/jbc.M807359200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage G, Marucci L, Alvaro D, Glaser S, Benedetti A, Marzioni M, Patel T, Francis H, Phinizy JL, Alpini G. Insulin inhibits secretin-induced ductal secretion by activation of PKC alpha and inhibition of PKA activity. Hepatology. 2002;36:641–651. doi: 10.1053/jhep.2002.35537. [DOI] [PubMed] [Google Scholar]
- Tietz PS, Alpini G, Pham LD, LaRusso NF. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1995;269:G110–G118. doi: 10.1152/ajpgi.1995.269.1.G110. [DOI] [PubMed] [Google Scholar]
- Nathwani RA, Kaplowitz N. Drug hepatotoxicity. Clin Liver Dis. 2006;10:207–217, vii. doi: 10.1016/j.cld.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Levy LJ, Losowsky MS. Plasma gamma aminobutyric acid concentrations provide evidence of different mechanisms in the pathogenesis of hepatic encephalopathy in acute and chronic liver disease. Hepatogastroenterology. 1989;36:494–498. [PubMed] [Google Scholar]
- Marzioni M, Glaser SS, Francis H, Phinizy JL, LeSage G, Alpini G. Functional heterogeneity of cholangiocytes. Semin Liver Dis. 2002;22:227–240. doi: 10.1055/s-2002-34501. [DOI] [PubMed] [Google Scholar]
- Charlotte F, L'Hermine A, Martin N, Geleyn Y, Nollet M, Gaulard P, Zafrani ES. Immunohistochemical detection of bcl-2 protein in normal and pathological human liver. Am J Pathol. 1994;144:460–465. [PMC free article] [PubMed] [Google Scholar]
- Benedetti A, Bassotti C, Rapino K, Marucci L, Jezequel AM. A morphometric study of the epithelium lining the rat intrahepatic biliary tree. J Hepatol. 1996;24:335–342. doi: 10.1016/s0168-8278(96)80014-6. [DOI] [PubMed] [Google Scholar]
- Cesaro P, Raiteri E, Demoz M, Castino R, Baccino FM, Bonelli G, Isidoro C. Expression of protein kinase C beta1 confers resistance to TNFalpha- and paclitaxel-induced apoptosis in HT-29 colon carcinoma cells. Int J Cancer. 2001;93:179–184. doi: 10.1002/ijc.1314. [DOI] [PubMed] [Google Scholar]
- Shankar E, Sivaprasad U, Basu A. Protein kinase C epsilon confers resistance of MCF-7 cells to TRAIL by Akt-dependent activation of Hdm2 and downregulation of p53. Oncogene. 2008;27:3957–3966. doi: 10.1038/onc.2008.39. [DOI] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988;1:585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Taniyama K, Niwa M, Kataoka Y, Yamashita K. Activation of protein kinase C suppresses the gamma-aminobutyric acidB receptor-mediated inhibition of the vesicular release of noradrenaline and acetylcholine. J Neurochem. 1992;58:1239–1245. doi: 10.1111/j.1471-4159.1992.tb11334.x. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. GABA activity mediating cytosolic Ca2+ rises in developing neurons is modulated by cAMP-dependent signal transduction. J Neurosci. 1997;17:4785–4799. doi: 10.1523/JNEUROSCI.17-12-04785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa G, Kumagai S, Yano M, Wang YG, Kobayashi Y, Saito Y. 12(S)-Hydroxyeicosatetraenoic acid induces cAMP production via increasing intracellular calcium concentration. FEBS Lett. 2003;554:127–132. doi: 10.1016/s0014-5793(03)01128-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Henche N, Guijarro LG, Couvineau A, Carrero I, Arilla E, Laburthe M, Prieto JC. G proteins in rat liver proliferation during cholestasis. Hepatology. 1994;20:1041–1047. doi: 10.1002/hep.1840200437. [DOI] [PubMed] [Google Scholar]