Abstract
Pseudoxanthoma elasticum (PXE), a pleiotropic heritable disorder, is characterized by ectopic mineralization of the connective tissues. This disease is caused by mutations in the ABCC6 gene, which is expressed primarily in the baso-lateral surface of hepatocytes, and Abcc6−/− mice develop progressive mineralization mimicking human PXE. To investigate the hypothesis that PXE is a metabolic disorder, potentially caused by the absence of antimineralization factor(s) in circulation, we used parabiotic pairing, ie, surgical joining of two mice, to create a shared circulation between various Abcc6 genotypic mice. To prevent immune reaction between the parabiotic animals, all mice were bred to be Rag1−/−. Shared circulation between the parabiotic animals was confirmed by Evans blue dye injection and by quantitative PCR of blood cell genotypes. Pairing of Abcc6−/− mice with their wild-type counterparts halted the connective tissue mineralization in the knockout mice. Homogenetic wild-type and heterozygous pairings serving as controls were phenotypically unaffected by parabiosis. Consequently, the observations on the parabiotic mice support the notion that PXE is a metabolic disease, potentially due to absence of systemic antimineralization factor(s). These observations suggest that reintroduction of the critical antimineralization factors into circulation could provide a potential treatment for this, currently intractable, disease.
Pseudoxanthoma elasticum (PXE) is a multisystem heritable disorder characterized by ectopic mineralization of peripheral connective tissues, with cardinal clinical manifestations in the skin, the eyes, and the cardiovascular system.1,2 PXE is inherited in an autosomal recessive manner but is rarely present at birth, the average age of onset being in early teens. The complications of the disease include progressive loss of visual acuity, leading occasionally to central blindness, and cardiovascular manifestations presenting with intermittent claudication, bleeding from the gastric arteries, and occasionally, early myocardial infarcts. The clinical manifestations of PXE are a consequence of the slow, yet progressive, mineralization process affecting the elastic structures in the affected tissues. There is no effective or specific treatment for this disease at the present.1
PXE is caused by mutations in the ABCC6 gene, which encodes the ABCC6 protein, a putative transmembrane efflux transporter, and a member of the ATP binding cassette (ABC) family of proteins.3 ABCC6 is expressed at relatively high levels in the liver and the kidneys and to a lower extent, if at all, in tissues affected clinically by PXE.4,5,6 Several theories have been advanced to explain how mutations in a gene expressed primarily in the liver can result in the mineralization of peripheral connective tissues. One of them, the “metabolic hypothesis,” postulates that as a result of nonfunctional ABCC6 pump in the liver, factors physiologically required for prevention of the ectopic mineralization under normal calcium/phosphate homeostatic conditions become deficient in the circulation, allowing slow yet progressive mineralization of connective tissue in the peripheral tissues to ensue.7,8 In support of this hypothesis are recent identifications of a number of antimineralization factors that are required in appropriate balance with promineralization factors in the circulation under homeostatic conditions. Thus, an imbalance between the antimineralization/mineralization factors would allow the ectopic mineralization to proceed. Such antimineralization factors include fetuin A, matrix Gla protein, and osteopontin, and the antimineralization properties of these factors have been documented by development of the corresponding “knockout” (KO) mice, which demonstrate extensive mineralization of soft connective tissues.9,10,11 In addition to the “metabolic hypothesis,” the “PXE cell hypothesis” postulates that local absence of ABCC6 activity, even if normally at low levels, at resident cells, such as dermal fibroblasts or vascular smooth muscle cells, can result in changes in the morphology, migration, and/or by biosynthetic profiles of the cells, resulting in local mineralization.12,13 Finally, oxidative stress has been suggested to play a role in progression of the mineralization in PXE.14,15
To examine the “metabolic hypothesis” in the context of PXE, we have developed an innovative animal model system based on parabiotic pairing of Abcc6−/− mice with their wild-type Abcc6+/+ counterparts on immunodeficient (Rag1−/−) background. The immunodeficient Abcc6−/− mice demonstrate late onset (5 to 6 weeks) of progressive mineralization in a number of connective tissues, recapitulating the genetic, histopathological, and ultrastructural features of PXE in humans, similar to previously developed Abcc6−/− mice.16,17 The introduction of parabiosis, ie, experimental juncture of two animals, dates back to the 1860s18 and has been subsequently used to address a number of physiological and pharmacological questions. More recently, this approach has been used to assess the influence of the circulatory factors on muscle regeneration, transfer of bone phenotype from mice with generalized lymphoproliferative disorder to the wild-type mice, and the role of circulatory factors in the wound healing in diabetic mice.19,20,21 Thus, this model system is suitable to explore the effect of circulatory factors in pathological situations, such as development of ectopic mineralization in PXE.
We first developed immunodeficient Abcc6−/−, Abcc6+/−, and Abcc6+/+ mice, and these mice were then paired by parabiotic surgery to develop a common circulatory system, allowing plasma and blood cells to move between the parabiotic partners. The immunodeficient background (Rag1−/−) of these mice prevents immune reaction between the parabiotic animals and allowed us to test the role of circulatory factors in the development of tissue mineralization up to several months of age of the animals. Our results demonstrated that heterogenetic pairing of Abcc6−/− mice together with their wild-type counterparts resulted in diminished progression of the ectopic mineralization. This model system, therefore, allows the study of the pathomechanism of PXE and provides a novel model system to explore treatment options for this, currently intractable, disease.
Materials and Methods
Mice and Study Design
We generated an immuodeficient PXE mouse by cross-breeding the traditional Abcc6−/− mouse17 with a well-established immunodeficient Rag1−/− mouse22 in C57/BL6 background (strain: 002216F; Jackson Labs, Bar Harbor, ME). As an initial step of generating Abcc6-deficient and Rag1-deficient double mutants, female Abcc6−/− mice (F0-PXE) were crossed with Rag1−/− male (F0-RAG1) mutants. Heterozygous Abcc6+/−/Rag1+/− male and female mice (F1-D HET) were then taken to generate the double mutants (Abcc6−/−/Rag1−/−-F2-D KO). The mice were maintained under pathogen-free conditions and were handled in accordance with the guidelines for animal experiments by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
The experiments were initiated when the mice were 4 weeks of age. Three groups of mice were formed, all on Rag1−/− background: KO mouse (Abcc6−/−) was joined in parabiosis with either the age-matched (a) wild-type (Abcc6+/+), group KO+wild-type; (b) HET (Abcc6+/−), group KO+HET; or (c) another KO mouse (Abcc6−/−), group KO+KO. Each group consisted of 4 to 10 pairs of surgically joined animals. In addition, control groups were established by joining two HET mice (group HET+HET) and a wild-type with another wild-type mouse (group wild-type+wild-type). The mice were sacrificed by CO2 asphyxiation 8 weeks after the surgery.
Parabiosis
Parabiotic surgery was performed in a laminar-flow hood by using sterile procedures. General anesthesia was accomplished with isoflurane delivered by a precision vaporizer. Mice were surgically joined following the previously published protocol.23 After shaving the corresponding lateral sides of each mouse, matching skin incisions were made from the elbow to the knee joint of each mouse. The elbow and knee joints with muscles were attached by a single 5-0 coated vicryl suture (Ethicon, Somerville, NJ), and then the dorsal and ventral skin was approximated by a discontinuous 5-0 vicryl suture.
To ensure the animals’ well-being, individual parabiotic pairs were placed in clean cages with food pellets provided on the floor to minimize the strain of reaching for food while adjusting to parabiotic existence. Also, the mice were provided with cotton squares construct nests to keep warm. After the suturing procedure, the mice were monitored daily for 3 days for signs of pain and distress according to Institutional Animal Care and Use Committee guidelines. If the animals showed any sign of pain or distress, such as shaking, lethargy, chewing of tail and/or legs, arched back, and/or unkept grooming, then they were administered an additional dose of 0.3 mg/ml of Buprenex at 0.4 mg to 0.6 mg per kilogram body weight to alleviate the pain as needed. After the first week postoperatively the animals were monitored weekly for signs of pain and distress.
Genotyping
The tail tissues were harvested before surgery, the blood samples were collected at different time points after surgery, and single-cell primary hepatocyte suspensions were obtained 8 weeks after surgery for genomic DNA isolation. Genomic PCR of different tissues or quantitative PCR of blood cell genotypes was performed with three primers designed for amplification of the KO (320-bp), wild-type (430-bp), or both alleles of Abcc6 in the same reaction, as described previously.17
Evans Blue Dye Assay
Established shared blood circulation was also determined by injecting Evans blue dye.24 Four hundred microliters of 0.5% Evans blue dye in Hanks’ Salt Solution was injected into one counterpart through the tail vein. Blood was collected by retro-orbital bleed at different time points after injection. Choice of eye to be used was alternated between consecutive bleeds. Parabiosis patency (functional cross-circulation) was determined by separating serum from blood cells by centrifugation, diluting the sera (1:50), and determining the absorbance at 620 nm.
Histopathology
The organs or biopsies of muzzle skin were fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned (5 μm), and stained with H&E, Alizarin Red, or von Kossa by using standard methods for histopathological analyses.
Computerized Morphometric Quantitation of Connective Tissue Mineralization by Histopathology
Computerized morphometric analysis of H&E-stained sections of muzzle skin was performed as described elsewhere.14,25 Briefly, two sections from each biopsy were examined with a Nikon model TE2000 microscope equipped with an AutoQuant Imaging system (Watervliet, New York, NY). The number of vibrissae, both those with evidence of mineralization and those without, is determined in 11 to 15 systemically selected fields, so that the images capture every visible vibrissae in each section. The degree of mineralization was expressed as pixels (arbitrary units) per section and calculated as the percentage of area of mineralization per total area of vibrissae.
Chemical Quantification of Calcium Deposition
Muzzle skin biopsies were obtained with 6-mm biopsy punch and embedded in paraffin. Paraffin sections (10 μm) from the center of the biopsies were deparaffinized and decalcified with 0.6 N HCl for 2 weeks. The o-cresolphthalein complexone method was used to measure the calcium content in a colorimetric fashion (Calcium [CPC] Liquicolor; Stanbio Laboratory, Boerne, TX). The complete dissolving of calcium deposits was confirmed by H&E staining after decalcification.
Cell Cultures and the Effect of Parabiotic Abcc6−/− Serum on the Process of Calcification in Vitro
Human aortic smooth muscle cells (Cascade Biologics, Portland, OR) were routinely cultured in Dulbecco’s modified Eagle’s medium normal growth medium. At ∼80% confluence, the cells were switched to calcification-inducing medium (Dulbecco’s modified Eagle’s medium supplemented with 2 mmol/L Pi) in the presence of 10% mouse serum from different paired Abcc6−/− mice for up to 10 days. Meanwhile, three additional groups were set up as controls by using the serum from nonparabiotic Abcc6−/−, Abcc6+/−, or Abcc6+/+ mice. The medium was changed every 3 days. The calcium content of the cell layer was quantitated and normalized to cellular protein, as described previously.16
Statistical Analysis
Results are given as mean ± SE. The data were analyzed by using the Kruskal-Wallis test followed by the Wilcoxon Comparison test for the pairwise analyses.
Results
Development of Immunodeficient Abcc6−/− Mice
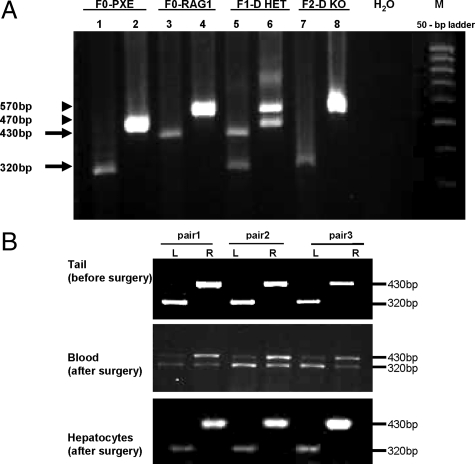
To develop a mouse model system for parabiotic experimentation, we used previously developed Abcc6−/− mice, which recapitulate the genetic, histopathological, and ultrastructural features of PXE.17 To allow pairing of these mice with HET (Abcc6+/−) or wild-type mice, we first developed immunodeficient knockout mice by crossing the Abcc6−/− mice with Rag1−/− mice.22 Crossing of Abcc6−/− mice with Rag1−/− mice resulted in Abcc6+/−/Rag1+/− double heterozygous mice (Figure 1A). Further crossing of the heterozygotes led to development of Abcc6−/−/Rag 1−/− (Figure 1A, lanes 7 and 8), as well as Abcc6+/− on Rag1−/− background. Thus, all mice used for parabiosis were immunodeficient on Rag1−/− background. The development of the double KO mice allowed the surgical pairing of these mice without immune reaction.
Figure 1.
A: Generation of an immunodeficient Abcc6−/− mouse is shown. Genotyping of Abcc6−/−/Rag1−/− mice: DNA was isolated from mouse tail. Each mouse was examined by two separate PCR reactions with two sets of primers designed for Abcc6 (lane 1, 3, 5, and 7) and Rag1 (lane 2, 4, 6, and 8), respectively. In Abcc6 amplification, the 430-bp band represents the wild-type allele, whereas the 320-bp PCR product is derived from amplification of the knockout (Abcc6−/−) allele (arrows). For the Rag1 gene, the knockout allele (570-bp), wild-type allele (470-bp), or both alleles were amplified in the PCR reaction (arrowheads). Note that F2 is null for both Abcc6 (bottom arrow) and Rag1 (top arrowhead) genes. H2O, blank control lane containing no DNA; M, molecular weight markers. B: Genotype of parabiotic mouse pairs is shown. Quantitative PCR for genotyping of the Abcc6 gene was performed with blood samples collected at 4 weeks postoperatively. The 430-bp and 320-bp bands represent the wild-type allele and knockout allele, respectively. Within each pair of parabiotic mice, the left (L) animal is always of KO genotype, whereas the right (R) animal is wild-type for Abcc6 before surgical pairing. Note the progressive mixing of blood cells at 4 weeks. Tail samples were harvested before surgery, and single-cell hepatocyte suspensions were obtained 8 weeks after surgery.
To ensure that the immune-deficient background of the Abcc6−/− mice did not interfere with the ectopic mineralization, we first examined the immunodeficient Abcc6−/− mice regarding the mineralization of the connective tissue capsules surrounding vibrissae, an early, progressive biomarker of the overall mineralization process in Abcc6−/− mice.16,17 Histopathological examination of muzzle skin containing the vibrissae revealed early foci of mineralization at about 1 month of age and the mineralization progressively increased up to 12 months of age (not shown). These observations are similar to those previously made on Abcc6−/− mice,17 indicating that the immune deficiency on Rag1−/− background does not interfere with the mineralization process, and suggesting that these mice can be used as a model of PXE.
Heterogenetic Pairing of Abcc6−/− Mice
The Abcc6−/− mice were surgically joined either with another Abcc6−/− mouse, with heterozygous Abcc6+/− mice, or with their corresponding wild-type littermate (Abcc6+/+) to form KO+KO, KO+HET, and KO+wild-type pairs, respectively. In addition, two Abcc6+/− mice were joined to form HET+HET pairs, and two wild-type mice, also on Rag1−/− background, were joined to form wild-type+wild-type pairs, to serve as controls to ensure that the surgical procedures will not cause ectopic mineralization. The mice were observed periodically as described in Materials and Methods. No obvious signs of stress were noted during observations after 3 weeks after surgery.
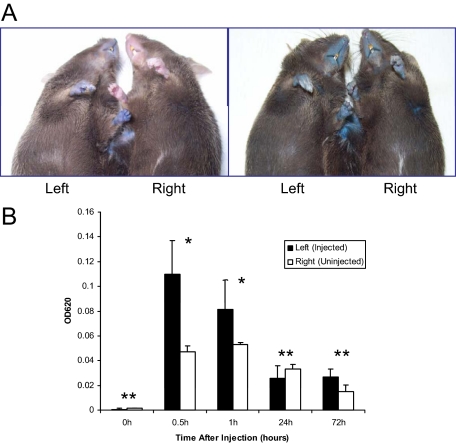
The formation of shared blood circulation between the parabiotic animals was tested by injection of Evans blue dye to the tail vein of one of the mice in parabiosis at 7 weeks postsurgically.26 The mice were first visually observed for appearance of blue color on the glabrous skin (Figure 2A). The injected mice turned immediately blue, whereas the uninjected counterpart remained normal pink colored at the 5-minute time point. However, examination of mice 24 hours after injection of Evans blue revealed equal bluish color of both mice (Figure 2A, right panel). The concentrations of Evans blue in circulation of both mice in each pair were then spectrophotometrically determined at 0, 0.5, 1, 24, and 72 hours after injection (Figure 2B). Although the injected mouse (left) in all cases had higher concentration of the Evans blue at 0.5 and 1 hour after injection, the relative concentration was approximately equal in both mice at 24 and 72 hours (Figure 2B). It should be noted that the total amount of Evans blue in the mice was reduced from the peak at 0.5 hours, probably due to urinary and fecal excretion.
Figure 2.
Documentation of shared circulation between the parabiotic animals by using Evans blue. A: Photographs of the mice were taken 5 minutes (A, left) and 24 hours (A, right) after the injection of Evans blue into the tail vein of the parabiotic mouse on the left side at 7 weeks after surgery. Photographs illustrate that Evans blue stained both mice at 24 hours after injection. B: The serum concentrations of Evans blue in both mice in each pair were measured at 620 nm by using spectrophotometry at 0, 0.5, 1, 24, and 72 hours after injection. Evans blue dye appeared in the contralateral mouse at 0.5 hours, and the concentrations in serum were equalized between the injected and noninjected mice at 24 and 72 hours after injection, even though the total amount of Evans blue in the circulation was reduced at that time point as compared with earlier time points. The values are mean ± SE, n = 4. Statistical difference between the injected and noninjected mice was noted at the time points of 0.5 and 1 hour (*P < 0.01, Wilcoxon test), but not at the 0, 24, and 72 hours (**P > 0.01, Wilcoxon test).
The shared circulation was also demonstrated by examination of the genomic DNA of blood cells in paired mice at 1, 3, and 4 weeks after parabiotic surgery. Three pairs of mice (Figure 1B, middle panel) revealed heterogenetic chimerism. Specifically, the left mouse (KO) in each pair at 1 week showed primarily the Abcc6−/− genotype, whereas the right pair showed predominantly wild-type genotype (not shown). At 4 weeks of parabiosis, the right mice (originally wild-type) showed the presence of the mutant allele in their circulations (Figure 1B). The genotype in these three pairs of mice was further examined in detail at 2 months after surgery. Genomic PCR of DNA isolated from the tails of these mice before surgery confirmed that the left mice of each pair were KO mice, whereas the right ones were wild-type (Figure 1B, upper panel). Also, hepatocytes isolated from the liver of these mice, and examined in single cell suspension without contamination of blood cells, revealed the original genotypes (Figure 1B, bottom panel), suggesting that there was no transfer of blood-derived stem cells to the liver from the contralateral side that might have differentiated into hepatocytes. Collectively, these observations revealed that parabiotic surgery of Abcc6−/− mice with their wild-type counterparts results in shared circulation, with transfer of plasma components (as reflected by Evans blue) and blood cells (as identified by DNA phenotyping).
Parabiosis of Abcc6−/− Mice with Wild-Type Counterparts Halts the Ectopic Mineralization
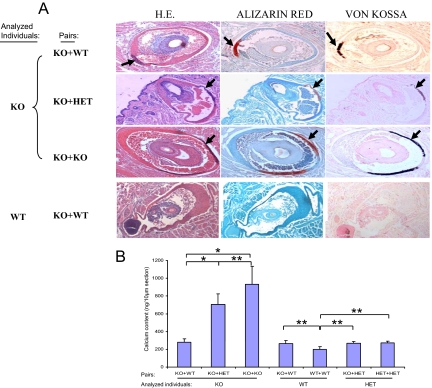
We subsequently examined the degree of mineralization in KO mice paired with another Abcc6−/− mouse (KO+KO), with a HET mouse (KO+HET), or with a wild-type mouse (KO+wild-type) by biopsying the muzzle skin and other soft tissues. First, histopathological examination of the vibrissae in KO mice in KO+KO pair demonstrated extensive mineralization, as visualized by H&E, Alizarin Red, and von Kossa stains (Figure 3A). These observations were similar to those in KO mice without parabiosis at the comparable age. Examination of the KO mice in KO+HET and KO+wild-type pairings revealed noticeably less mineralization (Figure 3A, two upper panels). At the same time, wild-type mice either in KO+wild-type or wild-type+wild-type pairs showed no evidence of mineralization (Figure 3A, bottom panel). These histological observations were quantitated by computerized morphometric analysis of 4 to 10 mice in each group (Table 1). Statistical analyses (Kruskal-Wallis test) confirmed that KO mice in KO+wild-type pairings had significantly less mineralization in their vibrissae than KO mice in KO+KO pairing (Table 1). Secondly, the degree of mineralization was also determined by quantitative assay of the calcium content in biopsies of muzzle skin from the same mice (Figure 3B). These assays demonstrated that the pairing of KO mice with HET or wild-type mice (KO+HET and KO+wild-type) significantly reduced the mineral deposition. HET mice in KO+HET or in HET+HET pairings showed similar levels of calcium as the wild-type mice in the wild-type+wild-type pairs (Figure 3B). Collectively, these observations indicate that pairing of KO mice with wild-type mice, the surgery being performed at 1 month of age, markedly reduces the subsequent deposition of calcium in the vibrissae of these mice. In addition, examination of other soft connective tissues revealed the presence of calcification in the eyes of KO mice in KO+KO pairs, but such foci were not found in KO mice either in KO+wild-type or KO+HET pairs (not shown).
Figure 3.
The effect of parabiosis on aberrant mineralization of the connective tissue capsule of vibrissae. A: Two months after parabiotic surgery, the muzzle skin was biopsied and the mineralization was examined by H&E (left), Alizarin red (middle), and von Kossa (right) stains. The connective tissue capsule of vibrissae from the muzzle skin of KO mice paired with the wild-type (WT) mice developed less mineralization (the top panel, arrows) when compared with the KO mice paired either with KO (the third panel, arrows). Note that no mineralization was observed in the vibrissae of wild-type parabiotic mice in KO+wild-type pairing (bottom panel of A). B: Content of calcium in the vibrissae of KO mice in the different parabiotic pairs was determined by chemical assay. The calcium content was determined colorimetrically by using o-cresolphthalein complexone method, and the values are presented as mean ± SE. The total exact P value for the Kruskal-Wallis Test is 0.001; the exact P values for statistical comparison between KO mice in different groups are as follows: KO+wild-type versus KO+KO, 0.011; KO+wild-type versus KO+HET, 0.014; and KO+HET versus KO+KO, 0.242. Wild-type or HET in the pairs did not differ from the wild-type in wild-type+wild-type pair (*P < 0.05; **P > 0.05).
Table 1.
Quantification of Mineralization of Vibrissae 2 Months After Parabiotic Surgery
| Experimental group*
|
Total vibrissae per mouse† (n) | Mineralized vibrissae per mouse† (n) | Percent mineralized | Area of mineralization per total area‡ (%) | Fold§ | P | |
|---|---|---|---|---|---|---|---|
| Mouse analyzed | Parabiotic pairs | ||||||
| KO (n = 5) | KO+WT | 14.6 | 4.8 | 34.3 | 0.71 ± 0.232 (0.58; 0.20–1.40) | 1.0 | |
| KO (n = 10) | KO+KO | 11.0 | 7.0 | 66.6 | 5.26 ± 1.572 (4.55; 1.19–11.25) | 7.4 | 0.007¶ |
| KO (n = 4) | KO+HET | 12.5 | 6.8 | 48.8 | 1.89 ± 0.658 (1.74; 0.69–3.38) | 2.7 | 0.07∥; 0.06** |
| WT (n = 5) | KO+WT | 14.8 | 0 | 0 | 0 | NA | |
| HET (n = 4) | KO+HET | 13.4 | 0 | 0 | 0 | NA | |
WT, wild-type; NA, not applicable.
Skin sections from KO mice at the age of 3 months, ie, 8 weeks after parabiotic surgery, were stained with H&E and examined for mineralization by light microscopy, followed by quantitative computerized morphometric analysis.
n, number of vibrissae examined.
Values presented as mean ± SE, as well as median and range in parentheses.
Calculated by using KO mice in KO+WT group as 1.0.
P value for KO comparison between KO+KO and KO+WT groups by using the Kruskal-Wallis test.
P value for KO comparison between KO+HET and KO+WT groups by using the Kruskal-Wallis test.
P value for KO comparison between KO+HET and KO+KO groups by using the Kruskal-Wallis test.
In Vitro Evidence of Lack of Antimineralization Factors in Serum of PXE Mice
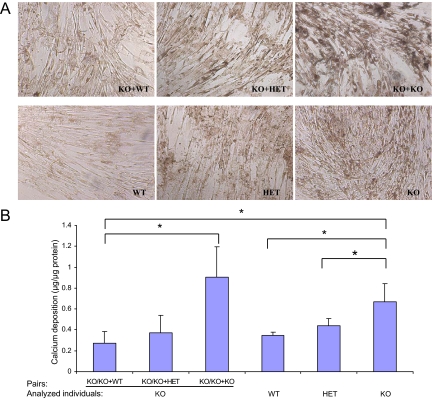
To examine the direct effects of circulatory factors on the mineralization process in the KO mice in parabiotic pairings, we used an in vitro mineralization assay based on monolayer cultures of human aortic smooth muscle cells.16,27 In this culture system, the cells are allowed to reach approximately 80% confluence, and the medium is then supplemented with 2 mmol/L Pi in the presence of 10% mouse serum, which results in precipitation of calcium phosphate on the cell layer. Assay of the cell-layer associated calcium then serves as an indicator of the extent of the mineralization process. As reported previously,16 addition of 10% serum from Abcc6−/− mice enhances the calcium deposition process over wild-type mouse serum, and intermediate values can be observed with serum from HET mice (Figure 4, A and B). Incubation of cells in the presence of 10% serum from KO mice that had been kept in KO+KO pairing for 10 days revealed high level of calcium precipitates, whereas the values obtained with serum from KO mice in KO+wild-type or KO+HET pairings were similar to those noted with wild-type and HET mouse sera. These experiments suggest that KO mice lack circulatory factors that in wild-type mice prevent precipitation of calcium phosphate in this in vitro mineralization assay.
Figure 4.
Effect of parabiotic Abcc6 −/− serum on calcium deposition in human aortic smooth muscle cell culture. A: Cells were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, and after having reached ∼80% confluence, the cells were incubated in medium supplemented with Pi (2 mmol/L) in the presence of 10% mouse serum from Abcc6−/− mice paired with either Abcc6+/+ (KO+wild-type), Abcc6+/− (KO+HET), or Abcc6−/− (KO+KO) mice, for up to 10 days. Three additional groups were set up as controls by using the serum from nonparabiotic Abcc6+/+, Abcc6+/−, or Abcc6−/− mice. The medium was changed every 3 days. At 10 days after addition of mouse serum, mineral deposition was assessed by phase-contrast light microscopy. Note that more mineralization was apparent in the cultures with the serum of Abcc6−/− mice paired with Abcc6−/− mice (KO+KO), in comparison with KO+wild-type and KO+HET pairs. B: The calcium content was quantitated by o-cresolphthalein complexone method, normalized by cellular protein content, and presented as mean ± SE (n = 3 or 4 cultures per group). The general P value for the Kruskal-Wallis test is 0.044. Multiple comparisons demonstrated significant differences in the cultures between KO+wild-type and KO+KO (P = 0.05), in the cultures between KO+wild-type and KO alone (P = 0.034), in the cultures between wild-type (WT) and KO (P = 0.021), and in the cultures between KO and HET (P = 0.043), as shown in the graph (*P = 0.05).
Discussion
The ABCC6 gene, which harbors mutations in PXE,1,3 a multisystem ectopic mineralization disorder, is expressed primarily in the liver and kidneys, and at a very low level, if at all, in cells in the clinically affected tissues, such as dermal fibroblasts and arterial smooth muscle cells.4,5,6 This situation has raised the question as to how, pathomechanistically, mutations in the gene expressed in the liver and the kidneys can result in ectopic mineralization of the peripheral soft connective tissues. The “metabolic hypothesis” postulates that absent ABCC6−/− activity in the liver results in a deficiency or absence of critical circulatory factors that are physiologically required for prevention of aberrant mineralization under normal homeostatic conditions.1,7,8 In this study, we have combined two technical advances to create a model system to address the pathomechanisms of PXE. First, we developed a double mutant mouse, Abcc6−/−/Rag1−/−, which allows pairing of immunodeficient PXE mice in parabiosis. The Rag1−/− mice lack mature T- and B-cells, while granulocytes and monocyte macrophages are intact, resulting in “non-leaky” severe combined immune deficiency.22 Next, we performed surgical parabiotic pairing of these mice with homozygous, heterozygous, or wild-type Abcc6 mice, also on Rag1−/− background (KO+KO, KO+HET, and KO+wild-type, respectively). No evidence of immune reaction between the parabiotic mice was noted up to 2 months after surgery, and importantly, the immune deficient background did not interfere with the ectopic mineralization in Abcc6−/− mice, as detected by the mineralization of the vibrissae in the muzzle skin as an early, progressive biomarker of the mineralization process.16 The latter observations suggest that immunological factors may not play a significant role in the development of mineralization in PXE, consistent with histopathology of the mineralized lesions in patients with PXE, which demonstrate little, if any, evidence of inflammation.2
Parabiotic pairing of mice was performed at 4 weeks of age, at the time of weaning, and just before histologically detectable mineralization in the vibrissae of the KO mice takes place, ie, at or around 5 to 6 weeks of age.16,17 Two different approaches were used to confirm that blood circulation between the parabiotic mice indeed had been established. First, genotyping of blood cells from the individual mice in pairs of KO and wild-type mice provided evidence of mixing of the circulation as early as 1 week after the surgical procedure. At 3 and 4 weeks, there was progressive appearance of the mutated allele of Abcc6 in the circulation of the wild-type mice and vice versa. Shared circulation was also confirmed by Evans blue injection into the tail vein of one of the mice, followed by measurement of the dye concentrations in the serum of the individual mice at approximately 7 weeks postoperatively. Evans blue appeared in the circulation of the contralateral mouse as early as 30 minutes after injection, and the Evans blue concentrations were equal in the circulation of both mice at 24 hours. Evans blue is an azo compound dye with a molecular mass of 960.81g Mol−1 and has a very high affinity for serum albumin.24 Thus, collectively, measurement of cellular genotypes and Evans blue indicate that cells, such as monocyte macrophages (10 to 30 μm in diameter), as well as albumin (molecular mass of 68 kDa) readily cross to the contralateral side of the paired mice. Thus, if deficiency of antimineralization factors in Abcc6−/− mice would be the cause of ectopic mineralization, as postulated by the “metabolic hypothesis,” parabiotic pairing of KO+wild-type mice would halt the mineralization in KO mice. Conversely, it would be expected that no mineralization in the wild-type or HET mice paired with KO mice occurs.
Histopathological observations of the mineralization process confirmed that KO mice, when paired with wild-type mice, developed much less mineralization than the KO mice in KO+KO pairs. In the latter mice, the mineralization was progressive, similar to Abcc6−/− mice without parabiosis. The lack of such antimineralization factors has also been demonstrated in an in vitro mineralization system by using human smooth muscle cells in culture.16 In this system, the cells, after having reached ∼80% confluence, are transferred to a mineralization-inducing medium containing 2 mmol/L Pi, and mineralization of the cell layer can be quantitated by chemical assay of calcium.27 In the presence of 10% serum from wild-type mice, significant mineralization occurs, but if 10% KO mouse serum is used instead, the mineralization is enhanced. Similarly, testing of serum from KO mice that had been in parabiosis in KO+KO pair demonstrated significantly enhanced mineralization, as compared with serum from KO mice that were in parabiosis with wild-type mice (KO+wild-type). These observations, indeed, support the notion that antimineralization factors traveled in parabiosis from wild-type mice to KO mice. Collectively, these observations provide new understanding of the pathomechanisms leading to ectopic mineralization in PXE.
The observations on PXE by using the parabiotic model system may have relevance to understanding of the pathomechanisms of more common mineralization disorders, such as arteriosclerosis, and similar mechanisms may be operative in these systemic disorders as well. Although the molecules being transported by ABCC6 from the liver to the circulation in vivo are currently unknown, small molecular weight anionic molecules and glutathione conjugates, such as vitamin K derivatives, may serve as substrates for ABCC6.1,28,29 Once the physiological substrates for ABCC6 have been identified, our current results indicating the circulatory nature of the antimineralization factors in PXE suggest that direct introduction of such molecules into circulation may provide treatment and perhaps a cure for this, currently intractable, disease.
Acknowledgments
We thank GianPaolo Guercio for assistance in article preparation. We also thank Adekemi Akingboye for assistance.
Footnotes
Address reprint requests to Jouni Uitto, M.D., Ph.D., Department of Dermatology and Cutaneous Biology, Jefferson Medical College, 233 S. 10th St, Suite 450 BLSB, Philadelphia, PA 19107. E-mail: Jouni.uitto@jefferson.edu.
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01 AR28450 and R01 AR55225. Q.J. was the recipient of the Research Career Development Award from the Dermatology Foundation.
A guest editor acted as editor-in-chief for this article. No person at Thomas Jefferson University was involved in the peer review process or final disposition for this article.
References
- Li Q, Jiang Q, Pfendner E, Váradi A, Uitto J. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics, and putative pathomechanisms. Exp Dermatol. 2009;18:1–11. doi: 10.1111/j.1600-0625.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neldner KH, Struk B. Pseudoxanthoma elasticum. Royce PM, Steinmann B, editors. New York: Wiley-Liss, Inc,; Connective tissue and its heritable disordersmolecular, genetic, and medical aspects. 2002:pp 561–583. [Google Scholar]
- Pfendner EG, Vanakker O, Terry SF, Vourthis S, McAndrew PE, McClain MR, Fratta S, Marais AS, Hariri S, Coucke PJ, Ramsay M, Viljoen D, Terry PF, De Paepe A, Uitto J, Bercovitch LG. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44:621–628. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky MG, Kruh GD. MOAT-E (ARA) is a full length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer. 1999;80:1342–1349. doi: 10.1038/sj.bjc.6690527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y, Nakano A, Jiang Q, Pukkinen L, Uitto J. Tissue-specific expression of the ABCC6 gene. J Invest Dermatol. 2005;125:900–905. doi: 10.1111/j.0022-202X.2005.23897.x. [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82:515–518. doi: 10.1038/labinvest.3780444. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Uitto J. Pseudoxanthoma elasticum: a metabolic disease? J Invest Dermatol. 2006;126:1440–1441. doi: 10.1038/sj.jid.5700267. [DOI] [PubMed] [Google Scholar]
- Uitto J, Pulkkinen L, Ringpfeil F. Molecular genetics of pseudoxanthoma elasticum: a metabolic disorder at the environment-genome interface? Trends Mol Med. 2001;7:13–17. doi: 10.1016/s1471-4914(00)01869-4. [DOI] [PubMed] [Google Scholar]
- Jahnen-Dechent W, Schinke T, Trindl A, Müller-Esterl W, Sablitzky F, Kaiser S, Blessing M. Cloning and targeted deletion of the mouse fetuin gene. J Biol Chem. 1997;272:31496–31503. doi: 10.1074/jbc.272.50.31496. [DOI] [PubMed] [Google Scholar]
- Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1.). J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- Passi A, Albertini R, Baccarani Contri M, de Luca G, de Paepe A, Pallavicini G, Pasquali Ronchetti I, Tiozzo R. Proteoglycan alterations in skin fibroblast cultures from patients affected with pseudoxanthoma elasticum. Cell Biochem Funct. 1996;14:111–120. doi: 10.1002/cbf.653. [DOI] [PubMed] [Google Scholar]
- Quaglino D, Boraldi F, Barbieri D, Croce A, Tiozzo R, Pasquali-Ronchetti I. Abnormal phenotype of in vitro dermal fibroblasts from patients with pseudoxanthoma elasticum (PXE). Biochim Biophys Acta. 2000;1501:51–62. doi: 10.1016/s0925-4439(00)00007-7. [DOI] [PubMed] [Google Scholar]
- Li Q, Jiang Q, Uitto J. Pseudoxanthoma elasticum: oxidative stress and antioxidant diet in a mouse model (Abcc6−/−). J Invest Dermatol. 2008;128:1160–1164. doi: 10.1038/sj.jid.5701145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali-Ronchetti I, Garcia-Fernandez MI, Boraldi F, Quaglino D, Gheduzzi D, De Vincenzi Paolinelli C, Tiozzo R, Bergamini S, Ceccarelli D, Muscatello U. Oxidative stress in fibroblasts from patients with pseudoxanthoma elasticum: possible role in the pathogenesis of clinical manifestations. J Pathol. 2006;208:54–61. doi: 10.1002/path.1867. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li Q, Uitto J. Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: systemic and local regulatory factors. J Invest Dermatol. 2007;127:1392–1402. doi: 10.1038/sj.jid.5700729. [DOI] [PubMed] [Google Scholar]
- Klement JF, Matsuzaki Y, Jiang Q, Terlizzi J, Choi HY, Fujimoto N, Li K, Pulkkinen L, Birk DE, Sundberg JP, Uitto J. Targeted ablation of the ABCC6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–8310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bert P. Experiences et considerations sur la greffe animale. J de la Anat et de la Physiol. 1864;1:69–87. [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Welssman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Lukic IK, Kovacic N, Katavic V, Grcevic D, Ivcevic S, Marusic A. Shared circulation in parabiosis leads to the transfer of bone phenotype from gld to the wild-type mice. Cellular Immunol. 2005;233:133–139. doi: 10.1016/j.cellimm.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Pietramaggiori G, Scherer SS, Alperovich M, Chen B, Orgill DP, Wagers AJ. Improved cutaneous healing in diabetic mice exposed to healthy peripheral circulation. J Invest Dermatol. 2009;129:2265–2274. doi: 10.1038/jid.2009.60. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- Mackie WS. Plasma volume measurements in sheep using Evans’ blue and continuous blood sampling. Res Vet Sci. 1976;21:108–109. [PubMed] [Google Scholar]
- LaRusso J, Jiang Q, Li Q, Uitto J. Ectopic mineralization of connective tissue in Abcc6−/− mice: effects of dietary modifications and a phosphate binder: a preliminary study. Exp Dermatol. 2008;17:203–207. doi: 10.1111/j.1600-0625.2007.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed H, Goodall SR, Hainsworth R. Re-evaluation of Evans blue dye dilution method of plasma volume measurement. Clin Lab Haematol. 1995;17:189–194. [PubMed] [Google Scholar]
- Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:10–17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- Borst P, van de Wetering K, Schlingemann R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with Pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle. 2008;7:1575–1579. doi: 10.4161/cc.7.11.6005. [DOI] [PubMed] [Google Scholar]
- Iliás A, Urbán Z, Seidl TL, Le Saux O, Sinkó E, Boyd CD, Sarkadi B, Váradi A. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6). J Biol Chem. 2002;277:16860–16867. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]