Abstract
Several studies have focused attention on cardio-respiratory function as an important indicator of development in infants. In the preterm infant, however, it remains unclear whether respiratory activity already affects heart beat variations at such an early development stage. In this work we investigate the presence of cardio-respiratory coupling in preterm infants by quantifying the interaction between heart rate variability and respiration using multivariate autoregressive analysis. We evaluated the frequency domain indices using standard methods. Results show a significantly higher coupling, as confirmed by surrogate data analysis, in the frequency range associated with regular breathing compared to other ranges. These observations indicate a mild, but present, respiratory sinus arrhythmia in preterm infants.
1. Introduction
Cardio-respiratory interaction has been considered an important indicator of development in infants; however it is unclear whether such interaction exists in preterm infants. There have been reports in the literature about the presence [1,2] as well as absence [3,4] of interaction between heart rate variability (HRV) and respiration (RESP) in preterm infants, known as respiratory sinus arrhythmia (RSA). It has also been reported that heart rate fluctuations may exist at the respiratory frequencies even in the absence of respiration [5]. It has been suggested that HRV is reduced in preterm infants [6] and the interaction between HRV and respiration may be weak. We here investigate the presence of RSA by means of standard frequency domain analysis using a multivariate autoregressive model. Furthermore we study the significance of such an interaction by performing surrogate data analysis.
2. Methods
Detection of R-wave peaks
Ten infants with post-conceptional age between 30 and 35 weeks have been used for the analysis. For each subject, we analyzed 1.5 hours of ECG recordings with relatively less artifacts for detecting the peaks. The R-wave peaks were detected using a derivative and threshold algorithm. The peak to peak (RR) series from each segment was visually inspected and corrected for any artifacts or erroneously detected peaks. To improve the detection, we applied a fourth order band-pass zero-phase Butterworth filter. The RR series, together with the corresponding respiratory signals from respiratory inductance plethysmography, were interpolated and resampled at 3 Hz. For each of the 10 subjects, we chose three epochs of approximately 500 seconds duration from the cleaned data sets.
Frequency domain analysis
Frequency domain analysis was performed on each of the epochs using a bivariate autoregressive model [7]. After preliminary optimal model order analysis, we considered a fixed autoregressive order of 32 for all epochs. We computed the bivariate autoregressive parameters and the noise variances, and also computed the RR and RESP power spectral densities, the RR-RESP coherence, and the RESP to RR (RSA) gain. As reported in the literature [2,4], the standard LF and HF frequency ranges classified for adult HRV analysis do not apply in the infant case. Generally, any frequency above 0.2 Hz has been classified as high frequency in the case of infant HRV. However, an agreement on a standard classification has not yet been reached. In this study, starting from the observation that our preterm infants have a predominant breathing frequency around 1Hz, we decided to introduce a more refined characterization of the respiratory range, up to the Nyquist frequency, by subdividing the HF range in three different regions, thereby classifying in total four different frequency ranges: the Low Frequency (LF: 0.01–0.15Hz), High Frequency (HF: 0.15–0.45 Hz), the Super High Frequency (sHF: 0.45–0.7Hz) and the Ultra High Frequency (uHF: 0.7–1.5Hz).
Surrogate data analysis
The significance of the coherence function is determined by setting a threshold using surrogate data analysis [8,9]. For each epoch we generated an ensemble of 50 pairs of Fourier Transform (FT) surrogate time series and calculated the coherence function. The FT surrogates were constructed by computing the Fourier Transform of the original time series and then randomizing the phases, while keeping the magnitude unchanged. We set the threshold level at the 95th percentile of the coherence sampling distribution. Any coherence value above threshold is significant.
3. Results
Frequency domain analysis
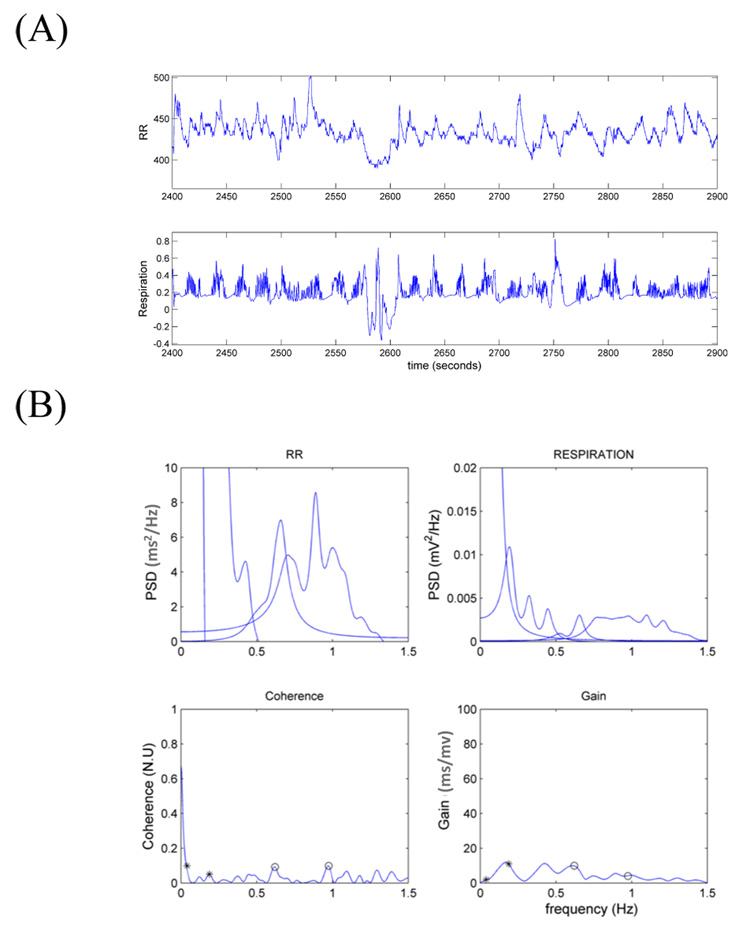
Figure 1 shows results from a single 500s epoch. Note the irregular breathing patterns in the time series including clear apnea episodes (i.e., pauses in breathing). These irregularities are reflected in the absence of a clear peak in the RESP spectral density distribution, with lower powers in the sHF and uHF ranges, as well as very low values of coherence in all frequency ranges.
Figure 1.
(A) RR and respiration time series from one of the 500s epochs used for the analysis and (B) the correspondent frequency domain measures. The small circles indicate the maximum coherence in each frequency range. Gains are computed at maximum coherence.
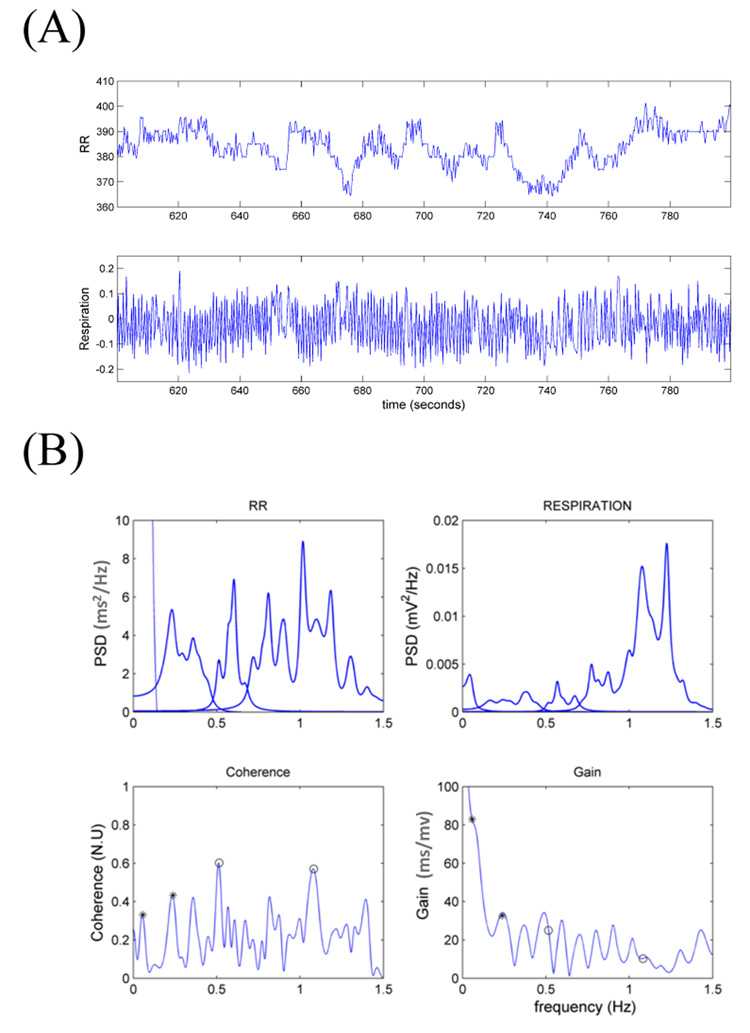
To further stress the importance of breathing patterns in cardio-respiratory coupling, Figure 2 shows results from a 200s sub-segment in which the infant is breathing regularly. In this case, the RESP power spectral density (PSD) shows clear high peaks in the infant’s natural breathing frequency range, between 1Hz and 1.3 Hz. Importantly, the RR spectrum also reveals substantial oscillations in the sHF and uHF ranges.
Figure 2.
(A) RR and respiration time series from an infant during regular breathing (200s interval) and (B) the correspondent frequency domain measures. The small circles indicate the maximum coherence in each frequency range. Gains are computed at maximum coherence.
Furthermore, when compared to the previous example of irregular breathing pattern, significantly higher values of coherence in the uHF and sHF ranges are accompanied by higher values of RSA gain in the presence of a regular breathing pattern. Of note, of all 500s segments considered, none presented continuous regular pattern. In fact, most of the breathing patterns are irregular with wide range of periodicities and frequent occurrence of apnea episodes.
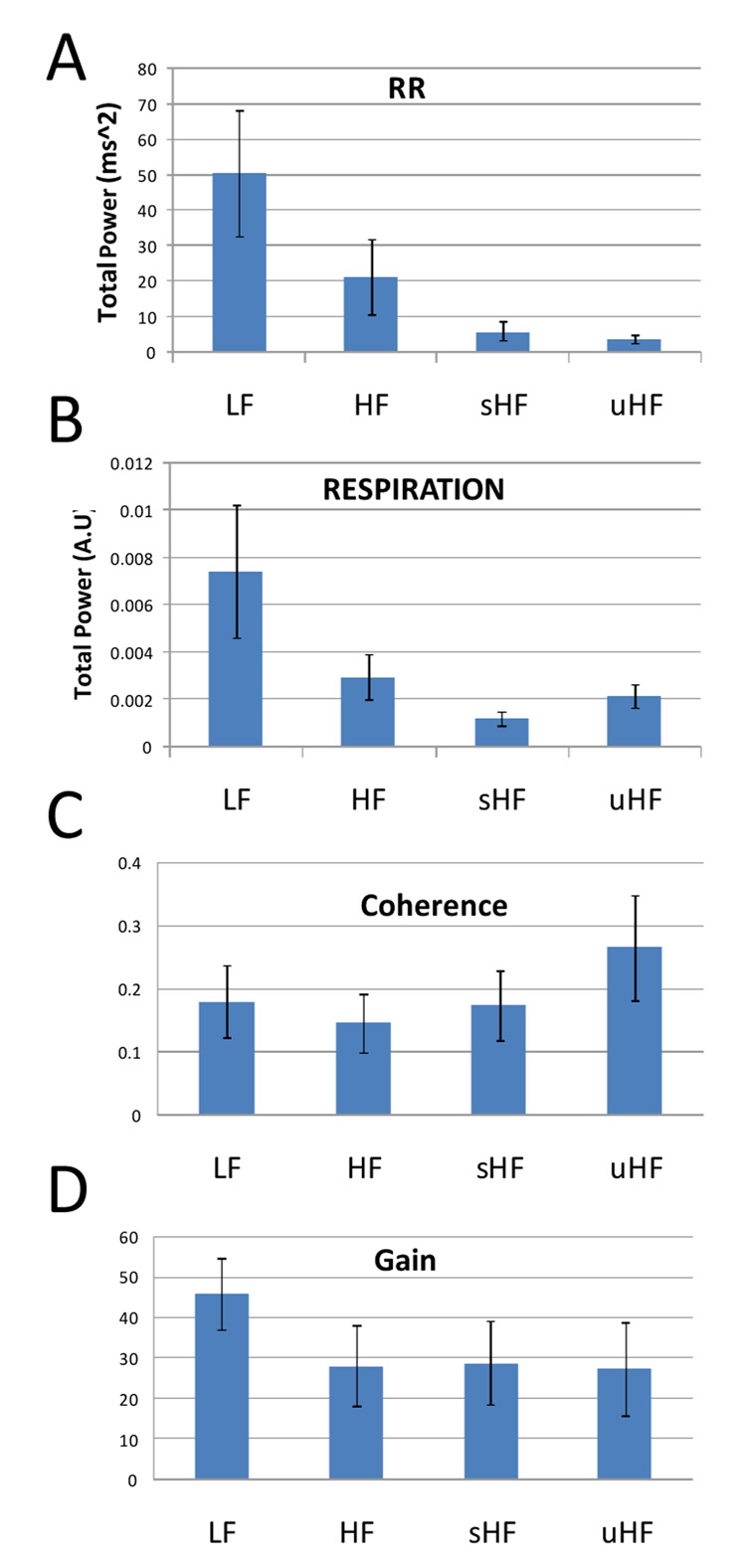
Results were averaged along all epochs and all subjects for each frequency range and are summarized in Figure 3. The RR distribution of power (A) decreases exponentially as frequencies get higher, indicating a generally very irregular modulation of heart beat variations. In contrast, higher respiratory uHF power than sHF power point at the presence of regular breathing patterns (B). It is in this very range that the coherence value is larger than the other frequency ranges (C). The uHF coherence is significantly higher compared to HF with a p-value of 0.01 and marginally higher than LF and sHF, with a p-value of 0.076 and 0.074 respectively. Coherence values are the lowest in HF, whereas gain values are the highest in the LF range (not significant).
Figure 3.
Total power in different frequency ranges for (A) RR and (B) respiration. (C) Coherence and (D) gain between RR and respiration. Indices are averaged for all epochs and all subjects. Bars indicate standard errors.
Surrogate data analysis
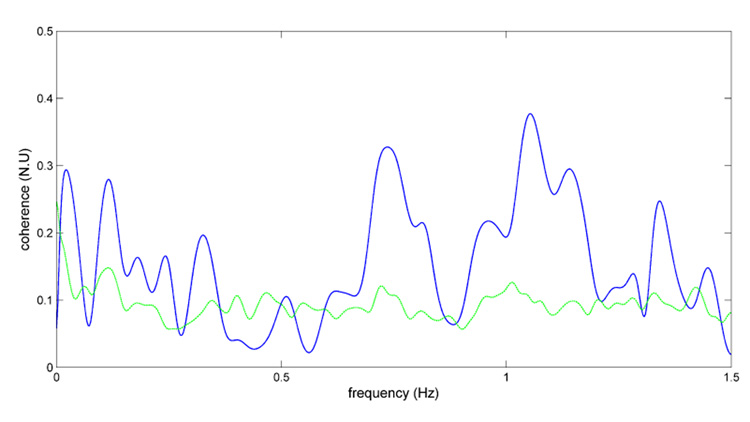
Figure 4 represents the coherence function (blue) along with the 95% threshold function (green) computed by the surrogate data analysis procedure for one 500s epoch. In this example the maximum coherence in the sHF and uHF ranges is far greater than the threshold function, and generally over the threshold in the LF range. On the other hand, the coherence function lies mostly below the threshold in the HF range.
Figure 4.
Example of surrogate data analysis of coherence function (blue line) with 95% percentile threshold (green line).
In Table I, coherence values are averaged for each subject in each frequency range whenever at least one epoch showed significance according to surrogate data analysis. We found significant coherence levels in all frequency ranges in five infants, with generally higher values in the uHF range. One additional subject (Subject 4) showed a high and significant level of coherence in this range as well.
Table I.
Surrogate data analysis summary. Only significant coherences are reported.
| Subject | LF | HF | sHF | uHF |
|---|---|---|---|---|
| 1 | - | - | - | - |
| 2 | 0.29 | 0.14 | 0.25 | 0.35 |
| 3 | 0.17 | 0.31 | 0.18 | 0.2 |
| 4 | - | - | - | 0.43 |
| 5 | - | - | - | - |
| 6 | - | - | - | - |
| 7 | 0.24 | 0.16 | 0.15 | 0.17 |
| 8 | 0.15 | 0.2 | 0.28 | 0.36 |
| 9 | - | - | - | - |
| 10 | 0.2 | 0.13 | 0.23 | 0.29 |
4. Discussion and Conclusions
Our results point at a clear presence of RSA in 60% of the subjects. This outcome is important, also considering that it was achieved without limiting the analysis to shorter segments including only regular breathing patterns. However, significance levels were not observed in four of the ten infants. Although there is interaction between heart rate and respiration, these interactions are not continuous as in adults, and/or may develop later in some infants.
This preliminary analysis does not provide a final answer to the conflicting reports in the literature about the presence of RSA in preterm infants, but it is an encouraging starting point for our investigation. One limitation is that we employed a bivariate model that is suitable for stationary data set, thus assuming that each of the 500s segments is stationary; however there are nonstationary events in these segments, particularly associated with apnea. In an immature system, both the cardiovascular and respiratory mechanisms may exhibit transient synchronization, but due to system vulnerability, the interactions are fast-changing and impersistent. To understand such changes we need a model which estimates the parameters in a time varying manner.
A second limitation is that we derived a continuous representation of RR using an interpolation method. The low HRV in these preterm infants requires a fine time resolution, and a simple interpolation may not accurately represent the real dynamic process associated with RR variations. A point process model along with respiration as one of the covariates [10] may provide a more accurate determination of cardio-respiratory coupling, and this work is in progress.
Acknowledgements
This work was supported by CIMIT under U.S. Army Medical Research Acquisition Activity Cooperative Agreement W81XWH-07-2-0011 (R.B.), by National Institutes of Health (NIH) Grants R01-HL084502 (R.B), R01-DA015644 (E.N.B), DP1-OD003646 (E.N.B), R01-HL49848 (D.P.), R01-HL071884 (D.P.), by American Heart Association Scientist Development Grant (E.B.S.), and the Clinical and Translational Science Pilot Project Program of the University of Massachusetts Medical School (D.P.). The information contained herein does not necessarily reflect the position or policy of the Government, and no official endorsement should be inferred.
References
- 1.Rassi D, Mishin A, Zhuravlev YE, Matthes J. Time domain correlation analysis of heart rate variability in preterm neonates. Early Hum Dev. 2005;81:341–350. doi: 10.1016/j.earlhumdev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Patzak A, Lipke K, Orlow W, Stauss H, Windt E, Persson PB, Schubert E. Development of heart rate power spectra reveals neonatal peculiarities of cardiorespiratory control. Am J Physiol. 1996;271:1025–1032. doi: 10.1152/ajpregu.1996.271.4.R1025. [DOI] [PubMed] [Google Scholar]
- 3.Longin E, Gerstner T, Schaible T, Lenz T, Konig S. Maturation of the autonomic nervous system: differences in heart rate variability in pre-mature vs. term infants. J Pernat Med. 2006;34:303–308. doi: 10.1515/JPM.2006.058. [DOI] [PubMed] [Google Scholar]
- 4.Giddens DP, Kitney RI. Neonatal heart rate variability and its relation to respiration. J Theor Biol. 1985;113:759–780. doi: 10.1016/s0022-5193(85)80192-2. [DOI] [PubMed] [Google Scholar]
- 5.Berntson GG, Cacioppo JT, Qugley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms and psychophysiological implications. Psychophysiology. 1993;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstock EG, Cassuto Y, Zmora E. Heart rate variability in the neonate and infant: analytical methods, physiological and clinical observations. Acta Paediatr. 1999;88:477–482. doi: 10.1080/08035259950169422. [DOI] [PubMed] [Google Scholar]
- 7.Barbieri R, Waldmann RA, Di Virgilio V, Triedman JK, Bianchi AM, Cerutti S, Saul JP. Continuous quantification of baroreflex and respiratory control of heart rate by use of bivariate autoregressive techniques. Ann Noninvasive Electrocardiol. 1996;1:264–277. [Google Scholar]
- 8.Theiler J, Eubank S, Longtin A, Galdrikian B, Farmer JD. Testing for nonlinearity in time series: The method of surrogate data. Physica D. 1992;58:77–94. [Google Scholar]
- 9.Faes L, Pinna GD, Porta A, Maestri R, Nollo G. Surrogate data analysis of the coherence function. IEEE Trans Biomed. Eng. 2004;51:1156–1166. doi: 10.1109/TBME.2004.827271. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Brown EN, Barbieri R. A study of probabilistic models for characterizing human heart beat dynamics in autonomic blockade control. Proceedings of ICASSP’08; March 30–April 4; Las Vegas, USA. 2008. pp. 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]