Introduction
It is widely assumed that the epidermis of early hominids lacked pigmentation, as does the skin of their primate ancestors (Jablonski and Chaplin, 2000; Westerhof, 2007). Several theories have been advanced to explain the subsequent latitude-dependent development and divergence of human pigmentation, including still widely held hypotheses that pigmentation evolved to protect against either genotoxic mutations that favor development of skin cancer (Goding, 2007; Robins, 1991), or against UV-induced nutrient photolysis (Jablonski, 1999; Jablonski and Chaplin, 2000). Although tyrosinase-positive, melanin-producing cells are widely distributed in exposed surfaces from fungi to primates (Epel et al., 1999), melanocytes are also present in some extracutaneous tissues with no exposure to the external environment. This wide distribution, plurifunctionality, and conservation throughout vertebrate evolution implies roles for melanin that extend beyond a need for defense against genotoxic or photolytic doses of ultraviolet light (UV) exposure (Blois, 1968; Deol, 1975; Mackintosh, 2001).
Other hypotheses regarding the evolution of pigmented skin
Skin cancer – pigment link
Melanin-producing cells are widely distributed from fungi to primates, and in extracutaneous tissues of humans, as well, implying that melanin has roles that extend beyond cutaneous UV-B defense (Blois, 1968). Yet, facultative pigmentation of epidermal melanocytes, such as the UV-B-induced increase in eumelanin production above constitutive levels (Quevedo et al., 1975), does not occur in extracutaneous (e.g., uveal tract) melanocytes (Li et al., 2006). Moreover, increased epidermal pigmentation could provide several potential adaptive advantages to hairless hominids, beyond protection against UV-B, including camouflage, sexual display, and as a free radical absorber (Jablonski and Chaplin, 2000; Parra, 2007; Robins, 1991; Schallreuter et al., 2008). More recent studies suggest additional neuroendocrine functions of epidermal melanocytes (Takeda et al., 2007), and an important role for melanocytes in cutaneous innate immunity (Mackintosh, 2001; Montefiori and Zhou, 1991).
But perhaps, the most widely held UV-B-based hypothesis proposes that pigmentation evolved from pale skin to protect against genotoxic mutations that favor the development of skin cancer (Table 1) (Goding, 2007; Robins, 1991). Indeed, lightly pigmented skin displays a much higher propensity to develop skin cancers than occurs in darker skin (Harrison, 1973), while conversely darkly pigmented skin transmits 10-fold less UV-B than does fair skin (Yamaguchi et al., 2007). Moreover, the supranuclear capping of melanin granules within keratinocytes represents a putative, photoprotective phenomena (Gibbs et al., 2000; Yamaguchi et al., 2006). Nonetheless, other studies suggest that melanin is a relatively ineffective UV filter (Hill, 1992) with a peak action spectrum in the low UV-B to UV-C range (<300 nm) (Hill and Hill, 2000). Moreover, experimental induction of pigmentation does not decrease UV-B-induced pyrimidine dimer formation (Niggli, 1990), an initial step in UV-B-induced mutagenesis. More importantly, while some skin cancers (e.g., malignant melanoma) can be lethal, they are relatively uncommon in comparison to the great majority of non-melanoma skin cancers which are slow-growing, only locally invasive, and non-lethal. Very lightly pigmented Europeans, living near the equator in northern Queensland in the presun-screen era did not develop skin cancers until late in the third decade, and even albinos living at equatorial latitudes developed skin cancers only in the third decade (Parra, 2007). Even taking into consideration ‘the grandmother effect’ (Diamond, 2005), these mostly non-lethal cancers develop relatively late in reproductive life, and therefore, they probably did not reduce reproductive success (Blum, 1961). Finally, there is no molecular genetic evidence to date for mutations in either anti-apoptotic or DNA repair mechanisms that support the genotoxic hypothesis. Together, these results suggest that skin cancer prevention was not the principal evolutionary ‘driver’ for the development of pigmentation.
Table 1.
Hypotheses commonly advanced to explain latitude-dependent increase in pigmentation
| Pigment evolved | Arguments for / against |
|---|---|
| To prevent | Against: ↑ photoisomerization to inactive isomers with ↑ UV-B; 1,25(OH2) Vit D-generation downregulated as serum Ca2+ increases |
| Vitamin D intoxication | Absence of molecular genetic correlates |
| Photodegradation of folic acid | Against: congenital neural tube defects too rare to influence reproduction rates |
| Skin cancers | For: melanin forms ‘caps’ over epidermal nuclei. Against: occurs too late to influence reproductive success |
| To improve | Against |
| Antioxidant defense | Melanin is a free radical absorber, but synthetic intermediates are themselves free radicals |
| Camouflage | No evidence for or against |
| Sexual display | No evidence for or against |
| Innate immunity | For Consistent with present hypothesis |
| Barrier function | Present hypothesis |
| Pigment de-evolved | Arguments for / against |
| To promote | Against |
| Cutaneous Vitamin D synthesis | No fossil evidence of rickets in early Homo (Industrial Age phenomenon) Clothing blocks more UV than pigment Sufficient vitamin D synthesis occurs in dark skin |
| Sexual selection | Against Reflects possible cultural bias |
| Metabolic cost of melanogenesis | For: increased polymorphisms in pigment-related genes in light-pigmented populations |
UV-nutrient photolysis – pigmentation link
Increased pigmentation has been proposed to protect against the photodegradation of serum folic acid (Branda and Eaton, 1978; Jablonski and Chaplin, 2000). Deficiency of this vitamin during pregnancy can result in both congenital neural tube anomalies (Jablonski, 1999) and reduced spermatogenesis (Mathur et al., 1977). Either or both would provide a strong evolutionary basis for the development of pigmentation. Whereas, there is indirect evidence for latitude-dependent photolysis of folic acid, and for reduced folic acid blood levels in lightly pigmented individuals living at equatorial latitudes (Jablonski and Chaplin, 2000), the overall incidence of congenital neural tube defects seems too low to exert evolutionary pressure, even in populations with a high incidence of folic acid-deficiency (Table 1). Thus, nutrient photolysis would not appear to have been the principal evolutionary ‘driver’ of cutaneous pigmentation.
Skin pigment hypotheses: dark skin into pale skin
Vitamin D – pigmentation link
Although there are several compelling reasons why pigmentation would have evolved in response to intense UVL (see next paragraph), two current hypotheses assume the opposite (i.e., that dark skin later devolved into pale skin). The first hypothesis proposes that H. sapiens lightened progressively as they radiated out of equatorial Africa, because of a critical requirement to generate vitamin D3 (Loomis, 1967; Murray, 1934; Reichrath, 2007), an intracutaneous process (Holick et al., 1980; Loomis, 1967; Murray, 1934). Fur-bearing mammals generate vitamin D from precursors in sebaceous secretions, but modern humans, a relatively hairless species, instead generate vitamin D3 by UV-B-induced photoconversion of 7-dehydrocholesterol into previtamin D3 in epidermis, which is followed by thermal conversion of previtamin D3 into vitamin D3 (cholecalciferol), and delivery of vitamin D3 into the circulation (Holick et al., 1980).
Though attractive in its simplicity, the vitamin D hypothesis is subject to criticism on several grounds (Aoki, 2002; Neer, 1975) (Table 1). As noted above, melanin pigments are distributed widely in the plant and animal kingdom, including several extracutaneous tissues in humans, suggesting that the capacity to synthesize melanin is highly conserved for reasons that predate the cutaneous production of vitamin D in humans (Blois, 1968). Moreover, sufficient vitamin D3 is formed in pigmented skin, even if sun exposure is restricted, depending upon latitude, time of day, and months of the year (Holick et al., 1981). In fact, substantial UV-B penetrates into the nucleated layers of the epidermis, regardless of pigment type (Hill, 1992; Holick et al., 1980; Loomis, 1967; Thomson, 1955). Instead, blockade of UV-B penetration into the epidermis has been attributed largely to structural proteins (Thomson, 1955) and to endogenous UV filters, such as trans-urocanic acid (Kripke, 1984), which absorb up to 70% of incident UV-B.
As humans moved from Africa to more temperate latitudes, animal pelt / clothing would have replaced hair to allow habituation to colder climates, perhaps restricting UV-B exposure as much as pigmentation had. Proponents of the vitamin D hypothesis point out that South Asians living in the United Kingdom display higher rates of rickets than do their non-Asian co-habitants (Reichrath, 2007). Yet, this difference might also reflect cultural practices like the sequestration of women and girls indoors, or the wearing of burkhas to cover the entire skin surface when outside. In fact, rickets is not common in other darkly pigmented groups (e.g., West Indians), even when living at the latitude of Scotland [cited in (Neer, 1975)]. Furthermore, there is no evidence of rickets in fossils of early H. sapiens living at European latitudes. Rickets and its adult variant, osteomalacia, only became common under the relatively recent, atmospheric pall of the Industrial Revolution (Aoki, 2002; Neer, 1975). Finally, none of the recently identified genes that underlie human pigment variations involve the vitamin D endocrine system [e.g., (Lao et al., 2007; Parra, 2007)]. Thus, the vitamin D hypothesis seems untenable as the principal basis for the latitude-dependent loss of pigment in modern humans (Table 1).
Sexual selection and pigmentation
While Darwin (1871) specifically proposed that humans could be preferentially attracted by individuals of different pigment-type, he did not suggest that such selection would be unidirectional, as recently proposed by Aoki (2002) and others. Based upon studies in divergent cultures, these workers propose that sexual selection would have favored lighter pigmentation (Frost, 1988; Van Den Berghe and Frost, 1986), a process that could have been amplified by parental selection (Harris, 2006). But the sexual selection hypothesis is inherently susceptible to considerable cultural bias. Indeed, recent studies on the period after the Nubian conquest of Egypt in the 8th century, B.C. have found no evidence of discrimination based upon pigmentation (Draper, 2008). Finally, there is little or no evidence that sexual selection necessarily translates into greater reproductive success when one compares developed (largely pale-skinned) and underdeveloped (largely dark-skinned) countries.
New hypothesis
Introduction
We are proposing here instead that pigmentation evolved both to protect against the devastating consequences of excess UV-B irradiation for cutaneous permeability barrier integrity, and because it endowed the skin with superior barrier function, which would have been highly advantageous in the extremely arid environment that prevailed in sub-Saharan Africa during the later stages of hominid evolution. In the reminder of this article, we will present evidence that links stress to the barrier to the development of epidermal pigmentation.
Primer on cutaneous barrier function and its importance
Despite the overarching importance of its numerous protective functions (Table 2), evolutionary biologists have not yet been considered the potential role of barrier requirements as a ‘driver’ of the evolution of pigmentation. The epidermal permeability barrier simultaneously prevents desiccation of the organism in a terrestrial environment, while excluding noxious chemicals, potential allergens, and microbial pathogens (Elias, 2005; Madison, 2003; Steinert, 2000). Notably, to protect from excessive evaporative water loss, organisms ranging from plants and insects to highly evolved mammals have utilized lipids (Tu et al., 2002), the universal waterproofing chemical entity. In mammals, this permeability barrier, like most of the skin’s other critical defensive functions, resides in the outermost, anucleate layers of the epidermis, the stratum corneum, a tissue organized into a two-compartment system of proteinaceous corneocytes embedded in a lipid-enriched extracellular matrix (analogous to the ‘bricks and mortar’ of a masonry wall) (Elias and Menon, 1991). These matrix lipids are enriched in three key lipids; i.e., a family of ceramides, both essential and non-essential free fatty acids, and free (unesterified) cholesterol (Schurer et al., 1991). Despite lacking phospholipids in mammals, these lipids self-organize into broad membrane multilayers that engorge the interstices, together accounting for ≈ 10% of the dry weight of the stratum corneum (Lampe et al., 1983).
Table 2.
Defensive functions of epidermis
| Function | Localization | Morphologic basis | Biochemical basis | How signalled |
|---|---|---|---|---|
| Permeability barrier + xenobiote penetration | Matrix | Lamellar bilayers | Cer:Chol:FFA (1:1:1 molar ratio) | Δ TEWL ? → TRPV4 |
| Antimicrobial defense | Matrix ? Cytosol | Lamellar bilayers ND |
LL-37, hBD2 RNase5, 7, psoriasin |
Δ TEWL ? |
| Cohesion / desquamation | Matrix | Corneodesmosomes | Protease / antiprotease; cholesterol sulfate | Local Δ in pH |
| Mechanical / rigidity | Corneocyte | Cornified envelope | Isopeptide (γ-glutamyl x-linking), Ca++ | TGase1 activation |
| Hydration | Corneocyte | CLE |
ω-OH-ceramides FLG → ‘NMF’ |
TRPV4 tonFAT → TAUT, GABA |
| Matrix | Sebaceous glands | Glycerol | AQP3 | |
| UV defense | Corneocyte cytosol | – | FLG → UCA | ↓ RH → TRPV4 ? |
| Antioxidant defense | Surface → Extracellular matrix |
Sebaceous glands | Vitamin E, CoQ | ? |
FFA, free fatty acid; TEWL, transcutaneous water loss; hBD2, human beta-defensin 2; CLE, Corneocyte lipid envelope.
The hierarchal importance of permeability barrier function is evidenced further by the complex series of metabolic responses, orchestrated by a variety of signaling mechanisms, that rapidly restore permeability barrier homeostasis after acute perturbations [rev. in (Feingold, 2007)]. A final clinical testament to the importance of barrier function comes from molecular genetics, which has identified several inherited abnormalities of either the lipid (‘mortar’) or protein (brick) constituents of the stratum corneum that result in both common [e.g., atopic dermatitis (eczemas)] and rare (e.g., the inherited ichthyoses) skin disorders (Elias et al., 2008a,b; Schmuth et al., 2008), whose clinical manifestations are ‘driven’ by the barrier abnormality (Elias et al., 2008a,b; Schmuth et al., 2007).
The consequences of a defective permeability barrier include a failure of antimicrobial defense, because these two critical functions that are both integrated and co-regulated (Aberg et al., 2008; Elias and Choi, 2005). The cohesive structure of normal stratum corneum, coupled with its low water content and its acidic pH, encourage the growth of the normal flora, while simultaneously providing a formidable outpost against pathogen colonization (Elias, 2007). Moreover, the free fatty acids (FFA) of the extracellular matrix of the stratum corneum are important both for the permeability barrier (Mao-Qiang et al., 1995), and for antimicrobial defense (Drake et al., 2008; Miller et al., 1988). The stratum corneum interstices also are laced with an array of antimicrobial peptides, at least two of which (i.e., the cathelicidin product, LL-37, and human β-defensin 2) (Aberg et al., 2008; Braff et al., 2005; Oren et al., 2003), provide a further, potent shield against invading pathogens (Elias, 2007). Conversely, structural defects in the extracellular matrix, resulting in a loss of corneocyte cohesion (Cork et al., 2006), or abnormalities in antimicrobial lipid / peptide expression (Ong et al., 2002) allow pathogens to penetrate into the skin [e.g., (Miller et al., 1988)]. Finally, the interdependence of permeability and antimicrobial functions is shown both by the stimulation of antimicrobial peptide expression that occurs in response to mechanical, chemical or UV-B-induced insults (Aberg et al., 2008; Hong et al., 2008), and perhaps most compellingly, in transgenic knock-out mice with a deletion of the cathelicidin homologue of LL-37; i.e., CAMP. These mice display not only increased susceptibility to cutaneous infections (Nizet et al., 2001), but also defective permeability barrier homeostasis (Aberg et al., 2008). Thus, the permeability barrier can also be considered a distal outpost of the cutaneous innate immune system (Elias, 2007).
Evidence in favor of the new hypothesis
Paleoclimatologic and ecologic events correlate with the development of pigmentation
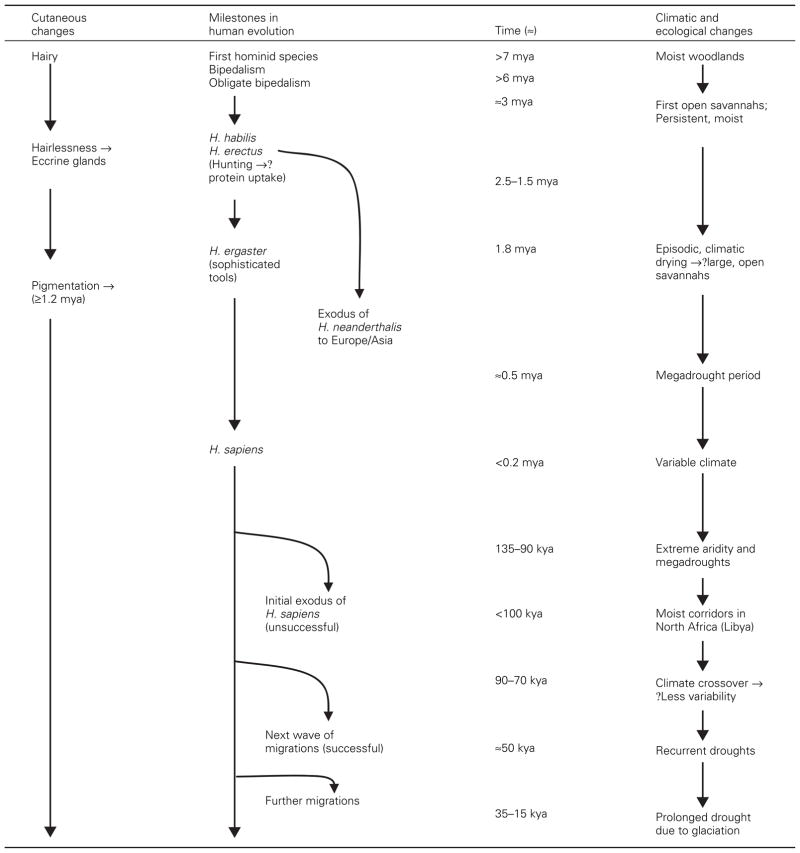
Hominids evolved in central Africa in the late Pliocene [over 7 million yr ago (mya)]. These early hominids rapidly became bipedal (Richmond and Jungers, 2008), eventually allowing them to hunt more efficiently (2.5–1.5 mya). The resultant ability to increase both the total intake and the variety of dietary protein likely fueled the development of a larger brain (Finch, 2007; Westerhof, 2007). Since early hominids were hairy, they likely still had pale, non-pigmented skin with melanocytes largely restricted to hair follicles, as is the case in modern chimpanzees and great apes (Jablonski and Chaplin, 2000). A long-term shift towards a drier climate, with the wide-spread replacement of tropical forest by sparsely forested savannah grasslands, may have both favored the emergence of Homo erectus and Homo neanderthalis (≈1.5 mya) (Bobe et al., 2002; Chaplin, 2004; Demenocal, 2004; Lahr and Foley, 1998), and widespread, often-severe arid episodes ultimately may have forced their exodus out of Africa into Europe and Western Asia around 800 000 yr ago (kya) (Table 3) (Demenocal, 2004).
Table 3.
Correlation of climatic changes with evolution of pigmentation
 |
Although hair provides a partial barrier against excessive transcutaneous water loss (TEWL), a hairy mantle would have severely impaired heat dissipation as Homo began to extend their hunting range into open savannahs (Jablonski, 2006). Hence, early hominids, who were both hairy and lightly pigmented, likely would have avoided excessive sun exposure, presumably restricting hunting activities to residual forested areas, while venturing into open savannahs only in the early morning or late afternoon. The subsequent loss of hair would have facilitated heat dissipation, but also resulted in a greater risk of dehydration in the exceedingly dry environment that prevailed between 1.5 and 0.8 mya. To facilitate heat dissipation, these highly active hominids must have developed eccrine sweat glands shortly after they became hairless, an adaptation that would have protected them from overheating (Jablonski, 2006)1.
Compared with mammals, the other major group of homeotherms, aves, could have dispensed with their epidermal appendages (feathers); they instead utilized an interconnected and continuous arrector muscle system which aids in raising feathers, thus facilitating increased evaporative cooling. Because the permeability barrier is much less competent in avians than in mammals (much higher TEWL values) (Menon et al., 1991, 1996), it also aids in evaporative cooling in the absence of sweat glands. However, adults of several large birds (storks, ibises, vultures) have either a complete loss or significantly reduced plumage on the head and / or neck regions (compared with juveniles with full feather cover), which facilitates heat dissipation, but they also can retract their necks into a ‘hunched position’ to reduce heat loss when necessary2.
The threat to eccrine glands from excess UV-B (Jablonski and Chaplin, 2000) could have been a stimulus to the development of pigmentation (Jablonski and Chaplin, 2000; Westerhof, 2007). But even more importantly, erythemogenic UV-B also would have compromised permeability barrier function in still lightly pigmented, hairless hominids (see next paragraph) (Haratake et al., 1997). Thus, stress to the barrier from extensive and repeated UV-B exposure (next section), coupled with xeric stress changes, from concurrent climatic changes, could have been the initial stimulus to the development of epidermal pigmentation in hominids. Pigmentation then would have developed where both xeric conditions and intense UV-B prevailed; i.e., open savannahs and deserts; while it would have been delayed in heavily forested areas of Africa’s ‘humid tropics’. Natural selection would therefore have favored mutations that enhance and protect permeability barrier function from xeric and UV-B-induced stress. We will discuss below evidence that pigmentation of interfollicular epidermis would have represented such an effective adaptation, simultaneously providing defense against damage from excessive UV-B, as well as providing a superior permeability barrier in a desiccating external environment. Population genetic studies show that the melanocortin 1 receptor (MC1R) gene stabilized around 1.2 ± 500 mya (Harding et al., 2000; Rana et al., 1999), which correlates with both a period of megadroughts and residence by ancestral Homo in open savannahs (Table 3). This date provides an approximate chronology for the development of epidermal pigmentation in hominids, which conflicts with current views that pigmentation developed much later; i.e., after the appearance of modern humans (Jablonski and Chaplin, 2000; Westerhof, 2007).
Both genetic and archeological studies suggest that Homo sapiens, likely already pigmented, as noted above, first appeared in Eastern Africa about 200 kya (Wade, 2006; Westerhof, 2007). Mitochondrial DNA analyses reveal that about 100 kya later, the original matrilineal line, which corresponds to today’s Bushmen, split off a second lineage, the progeny of all other humans living today (Wade, 2006). An explosion of new matrilineages appeared soon thereafter, which coincided with prolonged drought conditions between 115 and 95 kya (Cohen et al., 2007; Demenocal, 2004), perhaps propelling the initial (unsuccessful) radiation of modern humans out of Africa about 90 kya (Osborne et al., 2008) (Table 3). A successful exodus by the small numbers of remaining modern humans began from Ethiopia ≈60–50 kya, accounted for the rapid, subsequent colonization of South Asia, Southeast Asia, and Australia (Wade, 2006). This later migration was also likely driven by severe droughts, triggered by the most recent ice age (Peltier and Fairbanks, 2006), which reduced sea levels, thereby facilitating these and subsequent migrations into Europe (35 kya) (Ke et al., 2001) and North America (<20 kya) (Goebel et al., 2008).
Erythemogenic UV-B damages the barrier, but stimulates pigmentation
Evolutionary biologists have not yet considered the importance of barrier function for the survival of terrestrial animals; or conversely, the negative consequences of excess UV-B for the barrier3. Erythemogenic doses of UV-B (i.e., 290–320 nm), the most energetic form of electromagnetic energy to breach the atmosphere, produce dose-dependent defects in the permeability barrier (Haratake et al., 1997)4. Development of the barrier defect after a single exposure to erythemogenic UV-B is, however, delayed for 2–4 days, an interval that reflects the time required for a burst of T-cell-driven DNA synthesis (Haratake et al., 1997), to lift a band of metabolically incompetent, apoptotic cells outward until they reach the stratum granulosum-stratum corneum interface (Holleran et al., 1997). The transient nature of the defect reflects the time required for the arrival of nascent, competent keratinocytes that regenerate a functional barrier. Pertinently, UV-B stimulates pigmentation of interfollicular epidermis (Quevedo et al., 1965; Staricco and Miller-Milinska, 1962), a response that is most evident in neonatal, hairless mice (Walker et al., 2009).
If pale-skinned hominids, living primarily in open savannahs, were repeatedly assaulted with erythemogenic UV-B, such sustained exposure would have had disastrous consequences for permeability barrier homeostasis. Development of cutaneous pigmentation, and the concurrent tanning response, would have provided an important protective mechanism against excess UV-B. Importantly, development of epidermal pigmentation, and the concurrent tanning response, would have shifted most episodes of UV-B exposure from erythemogenic to suberythemogenic doses. In contrast to erythemogenic UV-B, suberythemogenic UV-B enhances both permeability barrier homeostasis and antimicrobial peptide expression (Hong et al., 2008). The net result of epidermal pigmentation then provides ‘feed-forward’ advantages that extend beyond protection from UV-B-induced barrier disruption alone.
Xeric stress as a co-stimulant for the evolution of pigmentation
In light of the acknowledged importance of optimal permeability barrier function for life in a dry terrestrial environment, we are proposing here that pigmentation also could have evolved in response to paleoclimatologic events that produced a steep decline in ambient humidity in Africa. This conjecture is based, in part, upon our prior observation that reductions in external humidity, if prolonged, upregulate metabolic processes in the epidermis that enhance permeability barrier function (Denda et al., 1998a), a useful adaptation to a dry environment. The known metabolic processes that account for superior function in skin exposed to xeric stress include both enhanced epidermal DNA synthesis (Denda et al., 1998b) and accelerated lipid synthesis / secretion (Denda et al., 1998a).
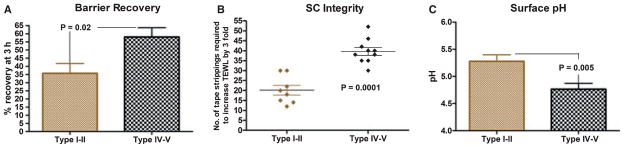
Could development of epidermal pigmentation reflect another such metabolic response to xeric stress, along with concurrent UV-B exposure? Our recent studies show that permeability barrier function is superior in darkly pigmented in comparison to lightly pigmented humans (Figure 1A). Darkly pigmented skin, independent of ethnicity, displays much more rapid barrier recovery after acute external perturbations than does lightly pigmented skin (Gunathilake et al., 2009; Reed et al., 1995). Using the Fitzpatrick I through VI-point pigment scale, in which the darkest skin type is type VI, the advantages of added pigment extend up to individuals with both types IV and V skin. Conversely, pigment-type I subjects (redheads, who freckle and sunburn readily) and type II subjects (blond-haired, blue-eyed individuals, who burn easily) fare poorly. Notably, darkly pigmented Filipinos and Sinhalese (Sri Lankans) exhibit the same pigment-type-endowed functional advantages as found in African-American skin (Gunathilake et al., 2009; Reed et al., 1995). Thus, development of pigmentation would have provided hominids with enhanced permeability barrier function in an arid environment (see ‘Basis for pigmentation-induced changes… ’ section for the mechanistic basis for such an improvement in function).
Figure 1.
Functional differences among divergent pigment groups are independent of geographic location and occupation: barrier recovery, epidermal integrity and forearm surface pH were assessed in a cohort of subjects with type I–II and IV–V skin, living in the same geographic location (San Francisco, California). None of the subjects were involved in nursing or related occupations. SC integrity was assessed as the number of D-squame tape strippings required to increase TEWL by threefold. Transcutaneous water loss was assessed immediately and 3 h after barrier disruption and percentage recovery was calculated as previously described. The baseline TEWL for the two pigment groups was ≤10mg / cm2/h. Surface pH of the volar forearm was measured using a flat glass electrode (modified from Gunathilake et al., 2009).
Increased epidermal pigment also endows humans with significant improvements in at least two related functions, stratum corneum integrity (the resistance of the stratum corneum to repeated sheer forces, such as tape stripping) (Figure 1B), and stratum corneum cohesion [the strength of the linkage between adjacent corneocytes, expressed as amount of protein removed per tape stripping (Gunathilake et al., 2009; Reed et al., 1995)]. The superior integrity and cohesion of darkly pigmented skin likely also provided a substantial adaptive advantage over the more fragile stratum corneum of lightly pigmented skin. These experimental results, supported by strong, correlative paleoclimatologic data (Table 3), provide the basis for our hypothesis that pigmentation evolved in response to a combination of stress to the barrier from UV-B and low humidity.
Role of the melanocyte in cutaneous antimicrobial defense
We have detailed above the intimate link between permeability barrier homeostasis and cutaneous antimicrobial defense (Aberg et al., 2008; Elias, 2005), as well as those structural and biochemical characteristics of a competent permeability barrier that contribute to the antimicrobial barrier (Elias, 2007). The tropics abound with potential pathogenic microorganisms, as well as suitable temperatures for optimal growth of pathogens. Prior, largely anecdotal studies noted that darkly pigmented humans living in the tropics experience fewer skin infections than do their lightly pigmented counterparts [e.g., (Wassermann, 1965)]. But, why would pigmented skin exhibit superior antimicrobial defense? As noted above, the physical characteristics of a superior permeability barrier alone would endow darkly pigmented individuals with a superior antimicrobial barrier (Elias, 2007). In addition, it is the ability of darkly pigmented melanocytes to further acidify the outer epidermis that likely further contributes to the enhanced cutaneous antimicrobial defense of darkly pigmented skin (see next section). Finally, melanocytes and their principle product, eumelanin, could impact antimicrobial defense directly in numerous other ways, including (Mackintosh, 2001): (i) Melanosomes are specialized phagolysosomes that can destroy encapsulated organisms, such as P. vivax, through generation of melanin intermediates with antimicrobial activity, perhaps also accounting for the observation that pigmented rabbits are less susceptible to botulism than are albino rabbits (Mackintosh, 2001; Schallreuter et al., 2008). (ii) Moreover, the melanin intermediates, L-dopa and L-tyrosine, are antiviral (Montefiori and Zhou, 1991), and melanin itself can neutralize fungal and bacterial exotoxins (Kuo and Alexander, 1967). (iii) Melanocytes also elaborate at least one important toll-like receptor (TLR4), indicating that this cell type itself is a participant in cutaneous innate immunity (Ahn et al., 2008). (iv) Melanocytes, like their more distally positioned neighbors in the epidermis, Langerhans cells, are dendritic cells with a large surface area, located in a site (the dermo-epidermal interface), where they could, in theory, serve both in antigen-processing and as microbial scavengers (Mackintosh, 2001). Together, these results suggest that superior antimicrobial defense, which is in large part a characteristic of a superior permeability barrier, likely confers a substantial, additional evolutionary advantage to darkly pigmented subjects.
Basis for pigmentation-induced changes in epidermal barrier function
Our studies have begun to delineate the reasons for the pigment type-determined enhancement of epidermal function. Contrary to common belief (Gambichler et al., 2005), the stratum corneum of darkly pigmented individuals is not thicker than that of pale-skinned subjects (Gunathilake et al., 2009). In contrast, an important difference in darkly pigmented subjects is their more acidic stratum corneum (pH 4.5–5.0 versus 5.5–6.0) (Figure 1C), and it is this highly acidic pH that activates or deactivates key stratum corneum enzymes that regulate both permeability barrier homeostasis and stratum corneum integrity / cohesion, respectively (Gunathilake et al., 2009). At a lower pH, the activities of two key enzymes, β-glucocerebrosidase and acidic sphingomyelinase, that hydrolyze secreted lipid precursors, glucosylceramides and sphingomyelin, respectively (Hachem et al., 2003, 2005), into a key family of barrier lipids (i.e., ceramides), which comprise 50% of stratum corneum lipids (5% of total dry weight). In parallel, the activities of serine proteases (kallikreins) in the stratum corneum decline at the lower pH of darkly pigmented skin, with the net result of a more cohesive, stratum corneum (Gunathilake et al., 2009). Conversely, these proteases become more active at the higher pH of lightly pigmented stratum corneum (op. cit.), with a host of negative, downstream consequences, including deactivation / degradation of the two ceramide-generating enzymes, and premature dissolution of corneodesmosomes (Hachem et al., 2005). Loss of these specialized intercellular junctions, which are unique to the stratum corneum, compromises stratum corneum integrity and cohesion in lightly pigmented subjects (Gunathilake et al., 2009). Furthermore, the lower pH of darkly pigmented stratum corneum favors the growth of the normal cutaneous microflora, while conversely, the higher pH of lightly pigmented skin favors colonization by pathogenic microorganisms (Korting et al., 1990). Growth of common microbial pathogens, such as Staphylococcus aureus and Group A Streptococcus pyogenes, which replicate preferentially at a neutral pH (Korting et al., 1990), slows as the pH of stratum corneum declines. Finally, the highly acidic pH of darkly pigmented stratum corneum activates acid-pH-dependent proteases, such as the aspartic protease, cathepsin D, accounting for the timely desquamation, and equivalent thickness of stratum corneum (Gunathilake et al., 2009). Thus, by increasing the acidity of the outer epidermis, increased pigmentation could have provided a substantial adaptive advantage for three key functions: (i) permeability barrier function; (ii) stratum corneum integrity / cohesion; and (iii) cutaneous antimicrobial defense.
Cellular basis for epidermal acidification by melanosomes
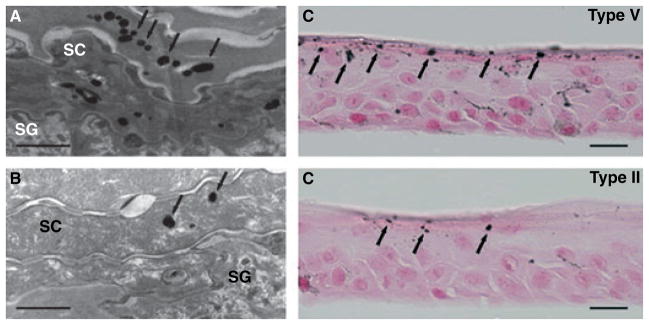
Melanocytes, through their multiple dendritic processes, are positioned to distribute melanosomes to ≈30–40 overlying keratinocytes (Figure 2). Melanosomes, like other proton-pump-containing secretory vesicles formed along the endocytic pathway (Yamaguchi et al., 2007), initially are highly acidic, as required for the early steps of melanin synthesis (Schallreuter et al., 2008). The classic studies of Szabo, et al. (1969) showed that multiple, sparsely melanized melanosomes of lightly pigmented individuals are collected together in large phagocytic vacuoles in keratinocytes. In contrast, the more heavily melanized melanosomes of darker subjects are taken up by keratinocytes as single melanosomes (Jimbow et al., 1976; Thong et al., 2003). While the smaller melanin granules in the melanosome complexes of lightly pigmented individuals are degraded early in epidermal differentiation, the single melanosome complexes of darker subjects persist into the outer epidermis, and even into the stratum corneum, before they dissipate (Jimbow et al., 1976) (Figure 2).
Figure 2.
Melanosomes persist into the stratum corneum in organotypic keratinocytes co-cultured with type V melanocytes: human second-passage keratinocytes from type II subjects were co-cultured at an air-medium interface with either type II or type V human melanocytes. (A, B) Electron microscopy demonstrates persistence of larger numbers of melanin particles (arrows) into the stratum corneum in cultures with type V, then with type II melanocytes (Bar = 1 μm). (C, D) Co-cultures with type V melanocytes demonstrate more melanin particles (arrows) in the stratum corneum, Fontana–Masson stain (Bars = 20 μm). (Modified from Gunathilake et al., 2009).
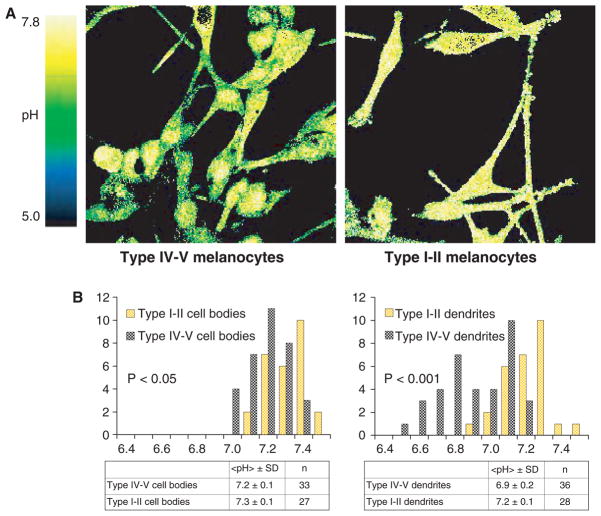
The delayed dissolution of multiple, singly packaged melanosomes in the outer epidermis likely further acidifies an already-acidic milieu (Behne et al., 2002). Using a pH-sensitive fluorophore (SNARF), applied to freshly obtained melanocytes from darkly and lightly pigmented individuals, we could visualize the acidity of melanocytes and their dendrites by dual-channel confocal microscopy. Cell bodies of melanocytes from darkly pigmented individuals are significantly more acidic than their counterparts from lightly pigmented skin (Gunathilake et al., 2009), and their pigment-transferring dendrites are even more acidic (Figure 3). Pertinently, with high-resolution confocal microscopy, this acidity appears to localize further to vesicles with the characteristics of melanosomes (Gunathilake et al., 2009). As these dendrites are positioned to transfer melanosomes that are both more persistent and more acidic, to adjacent keratinocytes, darkly pigmented subjects are endowed with an enhanced capacity to acidify the outer epidermis.
Figure 3.
Melanocytic dendrites are significantly more acidic in the darkly pigmented subjects: Two channel confocal imaging of human melanocytes stained with the pH sensitive probe SNARF-5F shows that dendrites, as well as the cell bodies, of darkly pigmented melanocytes (type IV–V skin) are significantly more acidic, in comparison to those of lightly pigmented melanocytes (type I–II skin). The color bar on the left of the image indicates the pH range corresponding to the R color coding used in the figures, with green indicating a more acidic pH, while green denotes a more neutral pH (modified from Gunathilake et al., 2009).
Population-based, molecular genetic studies show that genes that contribute to the acidification of melanosomes [e.g., OCA2 (p protein), MATP, SLC24A5, SLC45A2] are highly conserved in African populations (Cook et al., 2009; Graf et al., 2005; Lamason et al., 2005; Lao et al., 2007; Parra, 2007; Soejima and Koda, 2007; Stokowski et al., 2007). In fact, these genes, more than any other category, develop single nucleotide polymorphisms that are thought to result in reduced pigmentation in European and East Asian populations (Anno et al., 2008; Parra, 2007). Thus, generation of highly acidic melanosomes, and the transfer of these contents to adjacent keratinocytes, together could account for several of the evolutionary advantages of pigmented skin.
Discussion and predictions
Melanocyte–keratinocyte interactions that could have stimulated the development of pigmentation
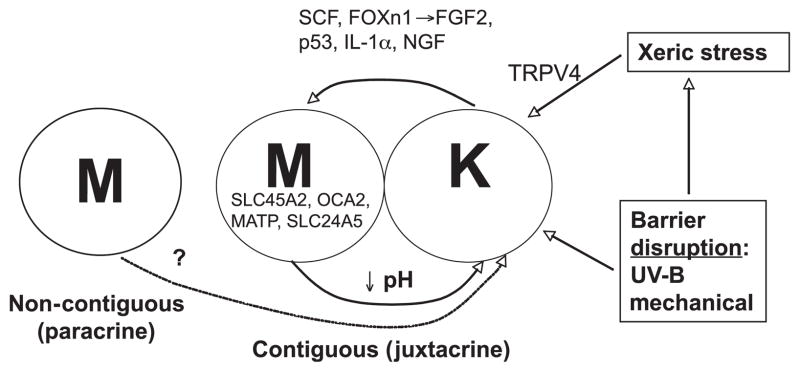
Although keratinocytes regulate melanocyte function by a variety of signaling mechanisms (Yamaguchi et al., 2007), less is known about how melanocytes influence epidermal function. In addition to providing coloration and a UV shield, melanosome transfer of acidity provides an additional mechanism whereby melanocytes regulate function in adjunct keratinocytes (Figure 4). The density of melanocytes in epidermis, which remains constant in humans of all pigment-types (Fitzpatrick, 1988), appears to be regulated by the keratinocyte (Weiner et al., 2007). In addition, in vitro mixtures of melanocytes and keratinocytes have shown that keratinocytes potently regulate both melanocyte growth and pigment phenotype (Minwalla et al., 2001; Scott and Haake, 1991).
Figure 4.
Potential cross-talk between keratinocytes and melanocytes – potential influence of stress to barrier (see text for abbreviations).
Several keratinocyte-derived signaling mechanisms are known to regulate pigmentation either under basal conditions (Schauer et al., 1994; Wintzen and Gilchrest, 1996), or in response to UV-B exposure (Chakraborty et al., 1996; Slominski et al., 1993). These signals regulate melanocyte localization, proliferation, dendricity, and eumelanin synthesis. Some keratinocyte signals operate at the level of the melanocytes’ G-protein-coupled, MC1R that regulates eumelanin synthesis (Lin and Fisher, 2007; Yamaguchi et al., 2007). But keratinocytes also can increase pigmentation by stimulating their own production of pro-opiomelanocortin (POMC)-derived peptides, such as α-melanocyte-stimulating hormone (αMSH), βMSH, and adrenocorticotropic hormone (ACTH), and / or endothelin (Schallreuter et al., 2008; Slominski et al., 1993; Yamaguchi et al., 2007).
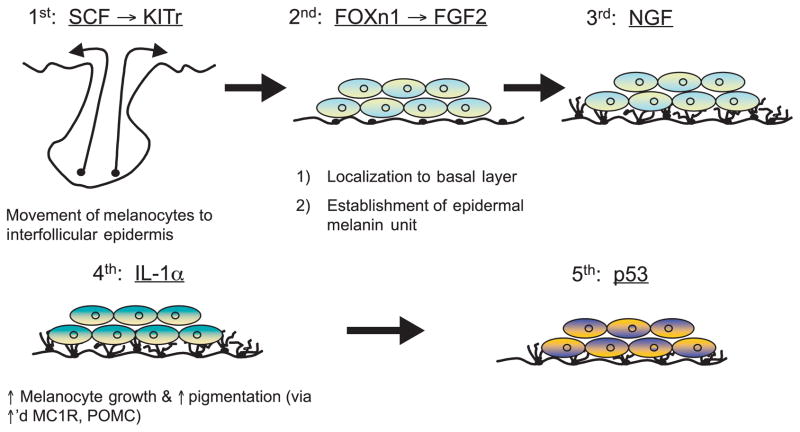
Which keratinocyte-derived signaling mechanisms could have stimulated the sequential development of epidermal pigmentation in response to stress to the barrier in hominids? As early hominids were initially pale-skinned, with pigment localized to hair follicles (Jablonski and Chaplin, 2000; Westerhof, 2007), a necessary first step in the pigmentary response to stress to the barrier would be for melanocytes to translocate from hair follicles to the interfollicular epidermis (Figure 5). A potential initiating signal for this sequence could be epidermal stem cell factor (SCF), the ligand for melanocyte KIT tyrosine kinase (Longley et al., 1993). The KIT gene is highly conserved in dark-skinned populations (Miller et al., 2007), consistent with our hypothesis that this mechanism could have been a key step in the development of pigmentation. KIT activation allows melanocytes to migrate to, and persist in, interfollicular epidermis (Kunisada et al., 1998). Transgenic mice that express scf postnatally on basal cell membranes display interfollicular melanocytes (D’orazio et al., 2006). Accordingly, the absence of interfollicular melanocytes in early hominids could have reflected a lack of postnatal SCF (Yoshida et al., 1996). Conversely, transgenic mice engineered to express various levels of scf postnatally display a corresponding increase in the density of interfollicular melanocytes (Carter et al., 2008). Together, these results suggest that increased epidermal SCF expression could have provided a key, initial signal leading to the development of pigmentation during human development.
Figure 5.
Proposed sequential keratinocyte-derived signals of pigmentation in response to stress to the barrier.
The next signaling mechanism that could have been involved in the development of human epidermal pigmentation could have been FOXn1 (Whn, Hfn 11), a transcription factor-like protein that is required for the development of numerous epithelial tissues (Brissette et al., 1996). Recent studies have shown that keratinocytes use FOXn1 to recruit melanocytes to the basal layer of the epidermis (Weiner et al., 2007) (Figure 5). Upregulation of FOXn1 increases expression of epidermal FGF2 (Hirobe, 1992, 1994), which in turn, promotes melanocyte multiplication, migration, and survival (Halaban et al., 1988; Wu et al., 2006), as well as MC1R expression (Scott et al., 2002). As FOXn1-bearing transcripts rapidly induce FGF2 mRNA expression, while antibody-neutralization of FGF2 decreases pigmentation, FGF2 is the likely effector of FOXN1 in the control of pigmentation (Weiner et al., 2007). Weiner et al. (2007) have proposed further that this mechanism could regulate the ratio of melanocytes within epidermis (the ‘epidermal-melanin unit’).
Two additional signals, IL-1α and nerve growth factor (NGF), are already known to be upregulated by either mechanical-, solvent-, or UV-B-induced stress to the barrier (Liou et al., 1997; Wood et al., 1992). Pertinently, both IL-1α and NGF are known to potently regulate pigmentation, but they do so by divergent mechanisms (Figure 5; see also below). Not only are both IL-1α and NGF rapidly upregulated by changes in permeability barrier status, but also in the case of NGF (but not IL-1α), upregulation is prevented when barrier function is artificially restored by application of a vapor-impermeable membrane over freshly abrogated skin sites (Liou et al., 1997). Thus, the NGF signal is specifically linked to barrier requirements, while IL-1α may be important not only for re-establishing barrier homeostasis (Barland et al., 2004; Ye et al., 2002), but also as a pro-inflammatory signal. Nerve growth factor inhibits UV-B-induced apoptosis of melanocytes by increasing bcl-2 levels (Stefanato et al., 2003), and it signals melanocyte proliferation, migration, dendricity, and eumelanin synthesis via activation of both low-(p75)NGFr and high-affinity (trk) receptors on melanocytes (Hirobe, 2005; Marconi et al., 2003; Truzzi et al., 2008; Yaar et al., 1994). In contrast, IL-1α, whose levels also increase markedly after UV-B, stimulates the MC1R, melanocyte differentiation (i.e., eumelanin synthesis) (Funasaka et al., 1998; Hirobe and Ootaka, 2007), αMSH / ACTH generation from POMC (Chakraborty et al., 1996), and hepatocyte growth factor production by fibroblasts (Mildner et al., 2007), which serves as a further growth factor for melanocytes.
Finally, recent studies strongly suggest that a fifth regulatory signal could be p53 (Figure 5), a transcription factor that increases expression of the keratinocyte POMC gene, leading to increased secretion of αMSH and stimulation of MC1R function in neighboring melanocytes (Cui et al., 2007). Notably, p53 expression increases in response to doses of UV-B (Cui et al., 2007) that can also abrogate the permeability barrier (Table 3) (Haratake et al., 1997).
Could xeric stress have signaled a pigmentary response via TRPV4?
As noted above, xeric stress upregulates both DNA and lipid biosynthetic pathways that enhance permeability barrier function (Denda et al., 1998a,b). A member of the transient vanilloid receptor family, TRPV4, has emerged as the most-likely sensor of changes in external humidity (Denda et al., 2007). Very recent, as yet unpublished, study from our group show further that TRPV4 k.o. mice cannot restore permeability barrier function after acute abrogations, and even minor insults, such as removal of hair, provoke disproportionate abnormalities in barrier function. Conversely, a topically applied TRPV4 agonist, 4-α-phorbol 12, 13 didecanone, accelerates barrier recovery in normal skin after acute perturbations (Denda et al., 2007). Although TRPV4 is not yet known to influence pigmentation, its dominant role as a sensor of external humidity makes it a possible candidate to have regulated incremental improvements in barrier function during human evolution, perhaps including stimulation of epidermal pigmentation.
Conclusions and further predictions
Loss of hair in a UV-B-enriched, extremely arid environment likely stimulated migration of melanocytes, with an eumelanin-generating system, from hair follicles into the interfollicular epidermis, and an increasingly active, eumelanin-synthetic system, which enhanced barrier function by acidifying the outer epidermis. If modern humans evolved with a eumelanin-generating capacity in their interfollicular epidermis, their hairless skin likely could quickly adapt to the high levels of UV-B in equatorial Africa. Because of other advantages endowed by pigmentation, this adaptation would simultaneously allow early humans to survive in the dry environment that prevailed at that time, and to combat environmental pathogens more efficiently. Hence, the net result would have been not only superior UV protection, but perhaps even more importantly, a parallel, incremental improvement in epidermal permeability barrier and cutaneous antimicrobial defense. Could this set of functions provide a sufficient evolutionary advantage to allow emergence of cutaneous pigmentation in early humans? Clearly, a superior permeability / innate immune barrier in a potentially desiccating environment would have provided a large evolutionary advantage. Hominids became bipedal and hairless, and along with the development of eccrine glands, these changes together would have provided the distinct evolutionary advantage of heat dissipation. They would, however, have lost an outer insulating layer that provides a partial permeability barrier, and development of interfollicular pigmentation would have restored a level of barrier competence. The hypothesis developed here is consistent with several predictions: (i) climatic conditions during the time that humans developed pigmentation were very dry, and even today the Rift Valley and Old-uvai Gorge display strikingly low ambient humidities; (ii) loss of hair likely preceded the dissemination of pigmentation from hair structures into the epidermis; and (iii) epidermal pigmentation should be inducible by sustained exposure to a low humidity, with or without additional sub-erythemal doses of UV irradiation, an eminently testable proposition.
Acknowledgments
This work was supported by NIH grants AR19098, AI059311, and the Medical Research Service, Department of Veterans Affairs, San Francisco, CA. Andrea Lucky, Ph.D., and Mary L. Williams, M.D. made many useful suggestions. Ms. Joan Wakefield provided superb editorial assistance. There are no conflicts of interests for any of the authors.
Footnotes
TEWL itself also contributes to heat dissipation, independent of eccrine glands (Moskovitz, et al., 2004).
A relation between the loss of epidermal appendages and skin pigmentation is also apparent in the birds that lose feathers in the head / neck regions mentioned above. The ‘neo-apteria’ (secondarily featherless regions) acquire either dark (ibises) or bright (painted storks) pigmentation. The latter have been shown to accumulate carotenoids in the stratum basale, providing a barrier to UV damage (Menon & Menon, 2000), analogous to the melanin capping of muclei of the stratum basale in pigmented human skin.
To be sure, there are numerous statements that pigmentation protects against the debilitating effects of UV-induced sunburn (e.g., Skin: a Natural History, Jablonski, 2006; and Before the Dawn: Recovering the Lost History of Our Ancestors, Wade, 2006).
Conversely, repeated suberythemogenic doses of UV-B benefit both permeability barrier function and cutaneous antimicrobial defense (Hong et al., 2008).
References
- Aberg KM, Man MQ, Gallo RL, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JH, Park TJ, Jin SH, Kang HY. Human melanocytes express functional Toll-like receptor 4. Exp Dermatol. 2008;17:412–417. doi: 10.1111/j.1600-0625.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- Anno S, Abe T, Yamamoto T. Interactions between SNP alleles at multiple loci contribute to skin color differences between caucasoid and mongoloid subjects. Int J Biol Sci. 2008;4:81–86. doi: 10.7150/ijbs.4.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K. Sexual selection as a cause of human skin colour variation: Darwin’s hypothesis revisited. Ann Hum Biol. 2002;29:589–608. doi: 10.1080/0301446021000019144. [DOI] [PubMed] [Google Scholar]
- Barland CO, Zettersten E, Brown BS, Ye J, Elias PM, Ghadially R. Imiquimod-induced interleukin-1 alpha stimulation improves barrier homeostasis in aged murine epidermis. J Invest Dermatol. 2004;122:330–336. doi: 10.1046/j.0022-202X.2004.22203.x. [DOI] [PubMed] [Google Scholar]
- Behne MJ, Meyer JW, Hanson KM, et al. NHE1 regulates the stratum corneum permeability barrier homeostasis. Microenvironment acidification assessed with fluorescence life-time imaging. J Biol Chem. 2002;277:47399–47406. doi: 10.1074/jbc.M204759200. [DOI] [PubMed] [Google Scholar]
- Blois MS. Vitamin D, sunlight, and natural selection. Science. 1968;159:652. [PubMed] [Google Scholar]
- Blum HF. Does the melanin pigment of human skin have adaptive value? An essay in human skin have adaptive value? An essay in human ecology and the evolution of race Q. Rev Biol. 1961;36:50–63. doi: 10.1086/403275. [DOI] [PubMed] [Google Scholar]
- Bobe R, Behrensmeyer AK, Chapman RE. Faunal change, environmental variability and late Pliocene hominin evolution. J Hum Evol. 2002;42:475–497. doi: 10.1006/jhev.2001.0535. [DOI] [PubMed] [Google Scholar]
- Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- Branda RF, Eaton JW. Skin color and nutrient photolysis: an evolutionary hypothesis. Science. 1978;201:625–626. doi: 10.1126/science.675247. [DOI] [PubMed] [Google Scholar]
- Brissette JL, Li J, Kamimura J, Lee D, Dotto GP. The product of the mouse nude locus, Whn, regulates the balance between epithelial cell growth and differentiation. Genes Dev. 1996;10:2212–2221. doi: 10.1101/gad.10.17.2212. [DOI] [PubMed] [Google Scholar]
- Carter EL, O’herrin S, Woolery C, Jack Longley B. Epidermal stem cell factor augments the inflammatory response in irritant and allergic contact dermatitis. J Invest Dermatol. 2008;128:1861–1863. doi: 10.1038/sj.jid.5701247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim Biophys Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- Chaplin G. Geographic distribution of environmental factors influencing human skin coloration. Am J Phys Anthropol. 2004;125:292–302. doi: 10.1002/ajpa.10263. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Stone JR, Beuning KR, et al. Ecological consequences of early Late Pleistocene megadroughts in tropical Africa. Proc Natl Acad Sci U S A. 2007;104:16422–16427. doi: 10.1073/pnas.0703873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AL, Chen W, Thurber AE, et al. Analysis of cultured human melanocytes based on polymorphisms within the SLC45A2 / MATP, SLC24A5 / NCKX5, and OCA2 / P loci. J Invest Dermatol. 2009;129:392–405. doi: 10.1038/jid.2008.211. [DOI] [PubMed] [Google Scholar]
- Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, Macgowan A, Duff GW, Ward SJ, Tazi-Ahnini R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21. doi: 10.1016/j.jaci.2006.04.042. quiz 22-3. [DOI] [PubMed] [Google Scholar]
- Cui R, Widlund HR, Feige E, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- D’orazio JA, Nobuhisa T, Cui R, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- Demenocal PB. African climate change and faunal evolution during the Pliocene-Pleistocene. Earth Planet Sci Lett. 2004;220:3–24. [Google Scholar]
- Denda M, Sato J, Masuda Y, Tsuchiya T, Koyama J, Kuramoto M, Elias PM, Feingold KR. Exposure to a dry environment enhances epidermal permeability barrier function. J Invest Dermatol. 1998a;111:858–863. doi: 10.1046/j.1523-1747.1998.00333.x. [DOI] [PubMed] [Google Scholar]
- Denda M, Sato J, Tsuchiya T, Elias PM, Feingold KR. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: implication for seasonal exacerbations of inflammatory dermatoses. J Invest Dermatol. 1998b;111:873–878. doi: 10.1046/j.1523-1747.1998.00364.x. [DOI] [PubMed] [Google Scholar]
- Denda M, Sokabe T, Fukumi-Tominaga T, Tominaga M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J Invest Dermatol. 2007;127:654–659. doi: 10.1038/sj.jid.5700590. [DOI] [PubMed] [Google Scholar]
- Deol MS. Racial differences in pigmentation and natural selection. Ann Hum Genet. 1975;38:501–503. doi: 10.1111/j.1469-1809.1975.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Diamond J. Evolutionary biology: geography and skin colour. Nature. 2005;435:283–284. doi: 10.1038/435283a. [DOI] [PubMed] [Google Scholar]
- Drake DR, Brogden KA, Dawson DV, Wertz PW. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- Draper R. Black Pharaohs. Nat Geosci. 2008;213:34–59. [Google Scholar]
- Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29:3–14. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- Elias PM, Choi EH. Interactions among stratum corneum defensive functions. Exp Dermatol. 2005;14:719–726. doi: 10.1111/j.1600-0625.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008a;121:1337–1343. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Williams ML, Holleran WM, Jiang YJ, Schmuth M. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J Lipid Res. 2008b;49:697–714. doi: 10.1194/jlr.R800002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel D, Hemela K, Shick M, Patton C. Development in the floating world: defences of eggs and embryos against damage from UV radiation. Am Zool. 1999;39:271–278. [Google Scholar]
- Feingold KR. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res. 2007;48:2531–2546. doi: 10.1194/jlr.R700013-JLR200. [DOI] [PubMed] [Google Scholar]
- Finch C. The Biology of Human Longevity: Inflammation, Nutrition and Aging in the Evolution of Lifespans. Chapter 6.2. Burlington, MA: Academic Press; 2007. The human life span: present, past, and future. [Google Scholar]
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- Frost P. Human skin color: a possible relationship between its sexual dimorphism and its social perception. Perspect Biol Med. 1988;32:38–58. doi: 10.1353/pbm.1988.0010. [DOI] [PubMed] [Google Scholar]
- Funasaka Y, Chakraborty AK, Hayashi Y, Komoto M, Ohashi A, Nagahama M, Inoue Y, Pawelek J, Ichihashi M. Modulation of melanocyte-stimulating hormone receptor expression on normal human melanocytes: evidence for a regulatory role of ultraviolet B, interleukin-1alpha, interleukin-1beta, endothelin-1 and tumour necrosis factor-alpha. Br J Dermatol. 1998;139:216–224. doi: 10.1046/j.1365-2133.1998.02357.x. [DOI] [PubMed] [Google Scholar]
- Gambichler T, Boms S, Stucker M, Moussa G, Kreuter A, Sand M, Sand D, Altmeyer P, Hoffmann K. Acute skin alterations following ultraviolet radiation investigated by optical coherence tomography and histology. Arch Dermatol Res. 2005;297:218–225. doi: 10.1007/s00403-005-0604-6. [DOI] [PubMed] [Google Scholar]
- Gibbs S, Murli S, De Boer G, Mulder A, Mommaas AM, Ponec M. Melanosome capping of keratinocytes in pigmented reconstructed epidermis–effect of ultraviolet radiation and 3-isobutyl-1-methyl-xanthine on melanogenesis. Pigment Cell Res. 2000;13:458–466. doi: 10.1034/j.1600-0749.2000.130608.x. [DOI] [PubMed] [Google Scholar]
- Goding CR. Melanocytes: the new Black. Int J Biochem Cell Biol. 2007;39:275–279. doi: 10.1016/j.biocel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Goebel T, Waters MR, O’rourke DH. The late Pleistocene dispersal of modern humans in the Americas. Science. 2008;319:1497–1502. doi: 10.1126/science.1153569. [DOI] [PubMed] [Google Scholar]
- Graf J, Hodgson R, Van Daal A. Single nucleotide polymorphisms in the MATP gene are associated with normal human pigmentation variation. Hum Mutat. 2005;25:278–284. doi: 10.1002/humu.20143. [DOI] [PubMed] [Google Scholar]
- Gunathilake R, Schurer NY, Shoo BA, Celli A, Hachem JP, Crumrine D, Sirimanna G, Feingold KR, Mauro TM, Elias PM. H-regulated mechanisms account for pigment-type differences in epidermal barrier function. J Invest Dermatol. 2009;129:1719–1729. doi: 10.1038/jid.2008.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity / cohesion. J Invest Dermatol. 2003;121:345–353. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, Roseeuw D, Feingold KR, Elias PM. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005;125:510–520. doi: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- Halaban R, Langdon R, Birchall N, Cuono C, Baird A, Scott G, Moellmann G, Mcguire J. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J Cell Biol. 1988;107:1611–1619. doi: 10.1083/jcb.107.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haratake A, Uchida Y, Schmuth M, Tanno O, Yasuda R, Epstein JH, Elias PM, Holleran WM. UVB-induced alterations in permeability barrier function: roles for epidermal hyperproliferation and thymocyte-mediated response. J Invest Dermatol. 1997;108:769–775. doi: 10.1111/1523-1747.ep12292163. [DOI] [PubMed] [Google Scholar]
- Harding RM, Healy E, Ray AJ, et al. Evidence for variable selective pressures at MC1R. Am J Hum Genet. 2000;66:1351–1361. doi: 10.1086/302863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JR. Parental selection: a third selection process in the evolution of human hairlessness and skin color. Med Hypotheses. 2006;66:1053–1059. doi: 10.1016/j.mehy.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Harrison GA. Differences in human pigmentation: measurement, geographic variation, and causes. J Invest Dermatol. 1973;60:418–426. doi: 10.1111/1523-1747.ep12702616. [DOI] [PubMed] [Google Scholar]
- Hill HZ. The function of melanin or six blind people examine an elephant. BioEssays. 1992;14:49–56. doi: 10.1002/bies.950140111. [DOI] [PubMed] [Google Scholar]
- Hill HZ, Hill GJ. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 2000;13(Suppl 8):140–144. doi: 10.1034/j.1600-0749.13.s8.25.x. [DOI] [PubMed] [Google Scholar]
- Hirobe T. Control of melanocyte proliferation and differentiation in the mouse epidermis. Pigment Cell Res. 1992;5:1–11. doi: 10.1111/j.1600-0749.1992.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Hirobe T. Keratinocytes are involved in regulating the developmental changes in the proliferative activity of mouse epidermal melanoblasts in serum-free culture. Dev Biol. 1994;161:59–69. doi: 10.1006/dbio.1994.1007. [DOI] [PubMed] [Google Scholar]
- Hirobe T. Role of keratinocyte-derived factors involved in regulating the proliferation and differentiation of mammalian epidermal melanocytes. Pigment Cell Res. 2005;18:2–12. doi: 10.1111/j.1600-0749.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- Hirobe T, Ootaka H. Interleukin-1alpha stimulates the differentiation of melanocytes but inhibits the proliferation of melanoblasts from neonatal mouse epidermis. Zool Sci. 2007;24:959–970. doi: 10.2108/zsj.24.959. [DOI] [PubMed] [Google Scholar]
- Holick MF, Maclaughlin JA, Clark MB, Holick SA, Potts JT, Jr, Anderson RR, Blank IH, Parrish JA, Elias P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- Holick MF, Maclaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- Holleran WM, Uchida Y, Halkier-Sorensen L, Haratake A, Hara M, Epstein JH, Elias PM. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatol Photoimmunol Photomed. 1997;13:117–128. doi: 10.1111/j.1600-0781.1997.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Hong SP, Kim MJ, Jung MY, Jeon H, Goo J, Ahn SK, Lee SH, Elias PM, Choi EH. Biopositive effects of low-dose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J Invest Dermatol. 2008;128:2880–2887. doi: 10.1038/jid.2008.169. [DOI] [PubMed] [Google Scholar]
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581–582. doi: 10.1054/mehy.1997.0697. [DOI] [PubMed] [Google Scholar]
- Jablonski NG. Skin: A Natural History. Berkeley: University of California Press; 2006. [Google Scholar]
- Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Quevedo WC, Jr, Fitzpatrick TB, Szabo G. Some aspects of melanin biology: 1950–1975. J Invest Dermatol. 1976;67:72–89. doi: 10.1111/1523-1747.ep12512500. [DOI] [PubMed] [Google Scholar]
- Ke Y, Su B, Song X, et al. African origin of modern humans in East Asia: a tale of 12,000 Y chromosomes. Science. 2001;292:1151–1153. doi: 10.1126/science.1060011. [DOI] [PubMed] [Google Scholar]
- Korting HC, Hubner K, Greiner K, Hamm G, Braun-Falco O. Differences in the skin surface pH and bacterial microflora due to the long-term application of synthetic detergent preparations of pH 5.5 and pH 7.0. Results of a crossover trial in healthy volunteers. Acta Derm Venereol. 1990;70:429–431. [PubMed] [Google Scholar]
- Kripke ML. Skin cancer, photoimmunology, and urocanic acid. Photodermatology. 1984;1:161–163. [PubMed] [Google Scholar]
- Kunisada T, Lu SZ, Yoshida H, et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998;187:1565–1573. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MJ, Alexander M. Inhibition of the lysis of fungi by melanins. J Bacteriol. 1967;94:624–629. doi: 10.1128/jb.94.3.624-629.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahr MM, Foley RA. Towards a theory of modern human origins: geography, demography, and diversity in recent human evolution. Am J Phys Anthropol Suppl. 1998;27:137–176. doi: 10.1002/(sici)1096-8644(1998)107:27+<137::aid-ajpa6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lamason RL, Mohideen MA, Mest JR, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- Lampe MA, Burlingame AL, Whitney J, Williams ML, Brown BE, Roitman E, Elias PM. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983;24:120–130. [PubMed] [Google Scholar]
- Lao O, De Gruijter JM, Van Duijn K, Navarro A, Kayser M. Signatures of positive selection in genes associated with human skin pigmentation as revealed from analyses of single nucleotide polymorphisms. Ann Hum Genet. 2007;71:354–369. doi: 10.1111/j.1469-1809.2006.00341.x. [DOI] [PubMed] [Google Scholar]
- Li L, Hu DN, Zhao H, Mccormick SA, Nordlund JJ, Boissy RE. Uveal melanocytes do not respond to or express receptors for alpha-melanocyte-stimulating hormone. Invest Ophthalmol Vis Sci. 2006;47:4507–4512. doi: 10.1167/iovs.06-0391. [DOI] [PubMed] [Google Scholar]
- Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- Liou A, Elias PM, Grunfeld C, Feingold KR, Wood LC. Amphiregulin and nerve growth factor expression are regulated by barrier status in murine epidermis. J Invest Dermatol. 1997;108:73–77. doi: 10.1111/1523-1747.ep12285638. [DOI] [PubMed] [Google Scholar]
- Longley BJ, Jr, Morganroth GS, Tyrrell L, Ding TG, Anderson DM, Williams DE, Halaban R. Altered metabolism of mast-cell growth factor (c-kit ligand) in cutaneous mastocytosis. N Engl J Med. 1993;328:1302–1307. doi: 10.1056/NEJM199305063281803. [DOI] [PubMed] [Google Scholar]
- Loomis WF. Skin-pigment regulation of vitamin-D biosynthesis in man. Science. 1967;157:501–506. doi: 10.1126/science.157.3788.501. [DOI] [PubMed] [Google Scholar]
- Mackintosh JA. The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J Theor Biol. 2001;211:101–113. doi: 10.1006/jtbi.2001.2331. [DOI] [PubMed] [Google Scholar]
- Madison KC. Barrier function of the skin: ‘la raison d’etre’ of the epidermis. J Invest Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- Mao-Qiang M, Feingold KR, Jain M, Elias PM. Extracellular processing of phospholipids is required for permeability barrier homeostasis. J Lipid Res. 1995;36:1925–1935. [PubMed] [Google Scholar]
- Marconi A, Terracina M, Fila C, Franchi J, Bonte F, Romagnoli G, Maurelli R, Failla CM, Dumas M, Pincelli C. Expression and function of neurotrophins and their receptors in cultured human keratinocytes. J Invest Dermatol. 2003;121:1515–1521. doi: 10.1111/j.1523-1747.2003.12624.x. [DOI] [PubMed] [Google Scholar]
- Mathur U, Datta SL, Mathur BB. The effect of aminopterin- induced folic acid deficiency on spermatogenesis. Fertil Steril. 1977;28:1356–1360. doi: 10.1016/s0015-0282(16)42984-5. [DOI] [PubMed] [Google Scholar]
- Menon GK, Menon JG. Avian epidermal lipids: functional considerations and relationship to feathering. Amer Zoologist. 2000;40:540–552. [Google Scholar]
- Menon GK, Hou SY, Elias PM. Avian permeability barrier function reflects mode of sequestration and organization of stratum corneum lipids: reevaluation utilizing ruthenium tetroxide staining and lipase cytochemistry. Tissue Cell. 1991;23:445–456. doi: 10.1016/0040-8166(91)90003-c. [DOI] [PubMed] [Google Scholar]
- Menon GK, Maderson PF, Drewes RC, Baptista LF, Price LF, Elias PM. Ultrastructural organization of avian stratum corneum lipids as the basis for facultative cutaneous waterproofing. J Morphol. 1996;227:1–13. doi: 10.1002/(SICI)1097-4687(199601)227:1<1::AID-JMOR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Mildner M, Mlitz V, Gruber F, Wojta J, Tschachler E. Hepatocyte growth factor establishes autocrine and paracrine feedback loops for the protection of skin cells after UV irradiation. J Invest Dermatol. 2007;127:2637–2644. doi: 10.1038/sj.jid.5700938. [DOI] [PubMed] [Google Scholar]
- Miller SJ, Aly R, Shinefeld HR, Elias PM. In vitro and in vivo antistaphylococcal activity of human stratum corneum lipids. Arch Dermatol. 1988;124:209–215. [PubMed] [Google Scholar]
- Miller CT, Beleza S, Pollen AA, Schluter D, Kittles RA, Shriver MD, Kingsley DM. cis-Regulatory changes in Kit ligand expression and parallel evolution of pigmentation in sticklebacks and humans. Cell. 2007;131:1179–1189. doi: 10.1016/j.cell.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minwalla L, Zhao Y, Le Poole IC, Wickett RR, Boissy RE. Keratinocytes play a role in regulating distribution patterns of recipient melanosomes in vitro. J Invest Dermatol. 2001;117:341–347. doi: 10.1046/j.0022-202x.2001.01411.x. [DOI] [PubMed] [Google Scholar]
- Montefiori DC, Zhou JY. Selective antiviral activity of synthetic soluble L-tyrosine and L-dopa melanins against human immunodeficiency virus in vitro. Antiviral Res. 1991;15:11–25. doi: 10.1016/0166-3542(91)90037-r. [DOI] [PubMed] [Google Scholar]
- Moskowitz DG, Fowler AJ, Heyman MB, Cohen SP, Crumrine D, Elias PM, Williams ML. Pathophysiologic basis for growth failure in children with ichthyosis: an evaluation of cutaneous ultrastructure, epidermal permeability barrier function, and energy expenditure. J Pediatr. 2004;145:82–92. doi: 10.1016/j.jpeds.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Murray F. Pigmentation, sunlight, and nutritional disease. Am Anthropol. 1934;36:438–445. [Google Scholar]
- Neer RM. The evolutionary significance of vitamin D, skin pigment, and ultraviolet light. Am J Phys Anthropol. 1975;43:409–416. doi: 10.1002/ajpa.1330430322. [DOI] [PubMed] [Google Scholar]
- Niggli HJ. Comparative studies on the correlation between pyrimidine dimer formation and tyrosinase activity in cloudman S91 melanoma cells after ultraviolet-irradiation. Photochem Photobiol. 1990;52:519–524. doi: 10.1111/j.1751-1097.1990.tb01794.x. [DOI] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Oren A, Ganz T, Liu L, Meerloo T. In human epidermis, beta-defensin 2 is packaged in lamellar bodies. Exp Mol Pathol. 2003;74:180–182. doi: 10.1016/s0014-4800(02)00023-0. [DOI] [PubMed] [Google Scholar]
- Osborne AH, Vance D, Rohling EJ, Barton N, Rogerson M, Fello N. A humid corridor across the Sahara for the migration of early modern humans out of Africa 120,000 years ago. Proc Natl Acad Sci U S A. 2008;105:16444–16447. doi: 10.1073/pnas.0804472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra EJ. Human pigmentation variation: evolution, genetic basis, and implications for public health. Am J Phys Anthropol Suppl. 2007;45:85–105. doi: 10.1002/ajpa.20727. [DOI] [PubMed] [Google Scholar]
- Peltier W, Fairbanks R. Global glacial ice volume and last glacial maximum duration from an extended barbados sea level record. Quat Sci Rev. 2006;25:3322–3337. [Google Scholar]
- Quevedo WC, Jr, Szabo G, Virks J, Sinesi SJ. Melanocyte populations in UV-irradiated human skin. J Invest Dermatol. 1965;45:295–298. doi: 10.1038/jid.1965.131. [DOI] [PubMed] [Google Scholar]
- Quevedo WC, Fitzpatrick TB, Pathak MA, Jimbow K. Role of light in human skin color variation. Am J Phys Anthropol. 1975;43:393–408. doi: 10.1002/ajpa.1330430321. [DOI] [PubMed] [Google Scholar]
- Rana BK, Hewett-Emmett D, Jin L, et al. High polymorphism at the human melanocortin 1 receptor locus. Genetics. 1999;151:1547–1557. doi: 10.1093/genetics/151.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JT, Ghadially R, Elias PM. Skin type, but neither race nor gender, influence epidermal permeability barrier function. Arch Dermatol. 1995;131:1134–1138. [PubMed] [Google Scholar]
- Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618–625. doi: 10.1111/j.1600-0625.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- Richmond BG, Jungers WL. Orrorin tugenensis femoral morphology and the evolution of hominin bipedalism. Science. 2008;319:1662–1665. doi: 10.1126/science.1154197. [DOI] [PubMed] [Google Scholar]
- Robins A. Biological perspectives on human pigmentation. 1991. [Google Scholar]
- Schallreuter KU, Kothari S, Chavan B, Spencer JD. Regulation of melanogenesis–controversies and new concepts. Exp Dermatol. 2008;17:395–404. doi: 10.1111/j.1600-0625.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- Schauer E, Trautinger F, Kock A, Schwarz A, Bhardwaj R, Simon M, Ansel JC, Schwarz T, Luger TA. Pro-opiomelanocortin- derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93:2258–2262. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuth M, Gruber R, Pm E, Williams M. Ichthyosis update: towards a function-driven model of pathogenesis of the disorders of cornification and the role of corneocyte proteins in these disorders. Adv Dermatol. 2007;23:231–256. doi: 10.1016/j.yadr.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic review series: skin lipids. Peroxisome proliferator- activated receptors and liver X receptors in epidermal biology. J Lipid Res. 2008;49:499–509. doi: 10.1194/jlr.R800001-JLR200. [DOI] [PubMed] [Google Scholar]
- Schurer NY, Plewig G, Elias PM. Stratum corneum lipid function. Dermatologica. 1991;183:77–94. doi: 10.1159/000247644. [DOI] [PubMed] [Google Scholar]
- Scott GA, Haake AR. Keratinocytes regulate melanocyte number in human fetal and neonatal skin equivalents. J Invest Dermatol. 1991;97:776–781. doi: 10.1111/1523-1747.ep12486726. [DOI] [PubMed] [Google Scholar]
- Scott MC, Suzuki I, Abdel-Malek ZA. Regulation of the human melanocortin 1 receptor expression in epidermal melanocytes by paracrine and endocrine factors and by ultraviolet radiation. Pigment Cell Res. 2002;15:433–439. doi: 10.1034/j.1600-0749.2002.02051.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Wortsman J. On the potential role of proopiomelanocortin in skin physiology and pathology. Mol Cell Endocrinol. 1993;93:C1–C6. doi: 10.1016/0303-7207(93)90131-3. [DOI] [PubMed] [Google Scholar]
- Soejima M, Koda Y. Population differences of two coding SNPs in pigmentation-related genes SLC24A5 and SLC45A2. Int J Legal Med. 2007;121:36–39. doi: 10.1007/s00414-006-0112-z. [DOI] [PubMed] [Google Scholar]
- Staricco RG, Miller-Milinska A. Activation of the amelanotic melanocytes in the outer root sheath of the hair follicle following ultra violet rays exposure. J Invest Dermatol. 1962;39:163–164. doi: 10.1038/jid.1962.97. [DOI] [PubMed] [Google Scholar]
- Stefanato CM, Yaar M, Bhawan J, Phillips TJ, Kosmadaki MG, Botchkarev V, Gilchrest BA. Modulations of nerve growth factor and Bcl-2 in ultraviolet-irradiated human epidermis. J Cutan Pathol. 2003;30:351–357. doi: 10.1034/j.1600-0560.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- Steinert PM. The complexity and redundancy of epithelial barrier function. J Cell Biol. 2000;151:F5–F8. doi: 10.1083/jcb.151.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokowski RP, Pant PV, Dadd T, et al. A genomewide association study of skin pigmentation in a South Asian population. Am J Hum Genet. 2007;81:1119–1132. doi: 10.1086/522235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Gerald AB, Pathak MA, Fitzpatrick TB. Racial differences in the fate of melanosomes in human epidermis. Nature. 1969;222:1081–1082. doi: 10.1038/2221081a0. [DOI] [PubMed] [Google Scholar]
- Takeda K, Takahashi NH, Shibahara S. Neuroendocrine functions of melanocytes: beyond the skin-deep melanin maker. Tohoku J Exp Med. 2007;211:201–221. doi: 10.1620/tjem.211.201. [DOI] [PubMed] [Google Scholar]
- Thomson ML. Relative efficiency of pigment and horny layer thickness in protecting the skin of Europeans and Africans against solar ultraviolet radiation. J Physiol. 1955;127:236–246. doi: 10.1113/jphysiol.1955.sp005252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong HY, Jee SH, Sun CC, Boissy RE. The patterns of melanosome distribution in keratinocytes of human skin as one determining factor of skin colour. Br J Dermatol. 2003;149:498–505. doi: 10.1046/j.1365-2133.2003.05473.x. [DOI] [PubMed] [Google Scholar]
- Truzzi F, Marconi A, Lotti R, Dallaglio K, French LE, Hempstead BL, Pincelli C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J Invest Dermatol. 2008;128:2031–2040. doi: 10.1038/jid.2008.21. [DOI] [PubMed] [Google Scholar]
- Tu MC, Lillywhite HB, Menon JG, Menon GK. Postnatal ecdysis establishes the permeability barrier in snake skin: new insights into barrier lipid structures. J Exp Biol. 2002;205:3019–3030. doi: 10.1242/jeb.205.19.3019. [DOI] [PubMed] [Google Scholar]
- Van Den Berghe P, Frost P. Skin color preference, sexual dimorphism and sexual selection: a case of gene culture co-evolution? Ethn racial stud. 1986;9:87–113. [Google Scholar]
- Wade N. Before the Dawn: Recovering the Lost History of our Ancestors. New York: Penguin Books; 2006. [Google Scholar]
- Walker GJ, Kimlin MG, Hacker E, Ravishankar S, Muller HK, Beermann F, Hayward NK. Murine neonatal melanocytes exhibit a heightened proliferative response to ultraviolet radiation and migrate to the epidermal basal layer. J Invest Dermatol. 2009;129:184–193. doi: 10.1038/jid.2008.210. [DOI] [PubMed] [Google Scholar]
- Wassermann HP. Human pigmentation and environmental adaptation. Arch Environ Health. 1965;11:691–694. doi: 10.1080/00039896.1965.10664280. [DOI] [PubMed] [Google Scholar]
- Weiner L, Han R, Scicchitano BM, Li J, Hasegawa K, Grossi M, Lee D, Brissette JL. Dedicated epithelial recipient cells determine pigmentation patterns. Cell. 2007;130:932–942. doi: 10.1016/j.cell.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Westerhof W. Evolutionary, biologic, and social aspects of skin color. Dermatol Clin. 2007;25:293–302. vii. doi: 10.1016/j.det.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Wintzen M, Gilchrest BA. Proopiomelanocortin, its derived peptides, and the skin. J Invest Dermatol. 1996;106:3–10. doi: 10.1111/1523-1747.ep12326950. [DOI] [PubMed] [Google Scholar]
- Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CS, Lan CC, Chiou MH, Yu HS. Basic fibroblast growth factor promotes melanocyte migration via increased expression of p125(FAK) on melanocytes. Acta Derm Venereol. 2006;86:498–502. doi: 10.2340/00015555-0161. [DOI] [PubMed] [Google Scholar]
- Yaar M, Eller MS, Dibenedetto P, Reenstra WR, Zhai S, Mcquaid T, Archambault M, Gilchrest BA. The trk family of receptors mediates nerve growth factor and neurotrophin-3 effects in melanocytes. J Clin Invest. 1994;94:1550–1562. doi: 10.1172/JCI117496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, Berens W, Beer JZ, Hearing VJ. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282:27557–27561. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- Ye J, Garg A, Calhoun C, Feingold KR, Elias PM, Ghadially R. Alterations in cytokine regulation in aged epidermis: implications for permeability barrier homeostasis and inflammation. I. IL-1 gene family. Exp Dermatol. 2002;11:209–216. doi: 10.1034/j.1600-0625.2002.110303.x. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Shultz LD, Yamamura K, Nishikawa S, Nishikawa S, Kunisada T. Neural and skin cell-specific expression pattern conferred by steel factor regulatory sequence in transgenic mice. Dev Dyn. 1996;207:222–232. doi: 10.1002/(SICI)1097-0177(199610)207:2<222::AID-AJA10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]