Abstract
Taste is a chemical sense that aids in the detection of nutrients and guides food choice. A limited number of primary qualities comprise taste. Accumulating evidence has raised a question about whether fat should be among them. Most evidence indicates triacylglycerol is not an effective taste stimulus, though it clearly contributes sensory properties to foods by carrying flavor compounds and altering texture. However, there is increasing anatomical, electrophysiological, animal behavior, imaging, metabolic, and psychophysical evidence that free fatty acids are detectable when non-taste cues are minimized. Free fatty acids varying in saturation and chain length are detectable, suggesting the presence of multiple transduction mechanisms and/or a nonspecific mechanism in the oral cavity. However, confirmation of “fatty” as a taste primary will require additional studies that verify these observations are taste specific. Oral exposure to free fatty acids likely serves as a warning signal to discourage intake and influences lipid metabolism.
Keywords: chemosensory, perception, lipid metabolism, taste transduction, cephalic phase, dietary
Introduction
The raison d'être of sensory systems is to collect information from the external and internal environments and to transduce it into a signal that may be used by an organism to make cognitive decisions or noncognitive responses that affect survival. All learning, purposeful behavior, and arguably, homeostasis, would be impossible without sensory input. Each organism possesses the requisite sensory systems for its particular needs. For example, homing pigeons monitor magnetic fields to aid long-distance migration (39); sharks sense electrical fields (67), and selected dolphins and whales echolocate (81) to obtain food in murky waters; while other species gain advantages by seeing in the infrared (76) or ultraviolet (131). Humans gather and process information about impending dangers and opportunities through five sensory systems that have their own classes of effective stimuli, detection mechanisms, pathways to the brain, and central locations for signal decoding. Thus, each is distinct (e.g., taste and smell are as independent as vision and audition). However, interactions are the rule when humans are exposed to complex stimuli such as food, which may concurrently activate thermoreceptors, mechanoreceptors, pain receptors, electromagnetic receptors, and chemoreceptors, as well as nonreceptor mediated transduction mechanisms. In the case of food, this combination of inputs results in the sensation termed flavor. Although taste is the colloquial descriptor for the sensations emanating from foods or beverages in the mouth, taste is actually a minor contributor. Many of the subtleties of foods and beverages stem from olfactory and tactile cues (26).
Taste is one of the chemical senses (olfaction and chemical irritation are the others), meaning that it is tuned to detect selected chemicals. Taste is currently regarded as a single sensory system, but there has been disagreement on this point. One view holds that each of the sensations subsumed under the term taste is a unique modality responding to nonoverlapping sets of stimuli and hence should be considered independent sensory systems. The alternate, and prevailing, view is that the array of sensations merely represents qualities within one sensory system. This issue highlights a fundamental question regarding how many qualities taste comprises. Some have argued that taste is a synthetic sense (28, 29, 128) wherein exposure to combinations of effective taste stimuli leads to a gradation of sensations. This is analogous to how wavelengths of electromagnetic energy blend to yield a continuous spectrum of colors. The alternative position is that a limited number of primary qualities comprise taste. Combinations of each may lead to complex sensations, but ultimately they can be decomposed to taste primaries (9, 73), much as a trained ear can identify each note in a chord played on a piano. This analytical interpretation currently predominates, but it begs the question of how many primaries exist. This question has been on the forefront of taste research for more than two millennia (5) and has yet to be resolved.
There is longstanding, widespread agreement for the existence of sweet, sour, salty, and bitter taste qualities. Umami, or more appropriately, “glutamate taste” (49), the sensation elicited by the prototypical stimulus monosodium glutamate (MSG), was proposed about a century ago (60). However, it was only widely accepted as a basic taste in the past decade, with the identification of a unique transduction system for glutamate (16) and other amino acids (102). Acceptance of glutamate taste as a primary was slow and is still not universal, in part because of the lack of a clear, identifiable quality label for the sensation, as is the case for sweet, sour, salty, and bitter. The latter point is relevant to the current purpose, as there is also a lack of a single identifiable sensory quality for dietary fats.
Fat has been identified as a basic quality sporadically over time, most notably by Aristotle (105) and later by Jean Fernell in his momentous text on physiology, De Naturali Parte Medicinae (32). Given fat's prominence in foods, its numerous physiological roles, and its potential threats to health, this is not surprising. Analogous to the teleological arguments for the existence of other taste qualities (i.e., sweet, salty, sour, bitter, and glutamate for the detection of carbohydrate, electrolytes, acids, toxins, and amino acids, respectively), a system to detect compounds with varied functions essential for survival, or a threat to it, would be expected. The principal challenges to acceptance of fat taste have been the recognition that dietary fats are predominantly in the form of triacylglycerol, so unlikely to bind to any expected receptor system in the oral cavity, as well as the absence of an identifiable universal sensation and descriptive label for fat stimuli. However, with increasing documentation that free fatty acids are important signaling compounds throughout the body, the idea that they may play such a role in the oral cavity has gained credibility. As was the case for glutamate taste, with the identification of plausible transduction mechanisms, the study of fat as a basic taste quality began in earnest.
Fat Detection
Unlike vision, audition, and olfaction, where the stimulus source may be distant from the body, taste is a contact sense. As a result, taste stimulation is typically coincident with activation of other sensory systems. Dietary fats, in particular, alter the appearance of foods (30, 142), their odors (99, 119), taste (47, 65), and physical properties (74, 94, 118). Thus, each of these cues may be, and probably is, used to detect and gauge the concentration of fats in foods and beverages. This hampers isolation and documentation of independent taste effects, but does not preclude an independent taste component, since none of the other cues fully account for findings on fat perception.
Appearance Cues from Fat
Common experience supports an important contribution of fat to a food's appearance. It is perhaps most obvious in fluid dairy products, but is also important to other fresh foods, such as meats, as well as processed items. Indeed, to obtain estimates of the impact of dietary fat on the other sensory properties of a food, it is often necessary to conduct sensory tests under conditions that minimize visual cues (e.g., testing participants blindfolded or under red light).
Tactile Cues from Fats
The prevailing view has been that fats are detected by the textural properties they impart to foods. In some instances, textural properties appear to be sufficient to quantify fat content. Comparable oiliness ratings of stimuli are reported when samples are rated in the oral cavity allowing olfactory and visual cues, in the oral cavity without these additional cues, and when samples are merely rubbed between the fingers (95). However, more often, subjective ratings by sensory panels reveal a predominant contribution of textural attributes, but also odor and taste dimensions (74, 112). There are also examples where textural cues hold no predictive power for fat content (96). This lack of consensus likely reflects the many roles fats play in foods and lack of a unitary sensory property. Unlike sweetness for carbohydrate and sourness for acids, common terms for fat-related properties include, but are not limited to, lubricity, viscosity, greasiness, oiliness, and creaminess. Each may be variously applicable in different foods, so study outcomes will be limited by the stimuli and dimensions assessed.
Electrophysiological studies in rats confirm the efficacy of free fatty acids as effective tactile stimuli (66). Several transduction mechanisms involving trigeminal stimulation have been proposed. First, free fatty acids could contribute to somatosensory sensations by chemosensory mechanisms. They are irritants, so could stimulate trigeminal nociceptors (151).
Second, there is recent evidence of delayed rectifying potassium channels (DRKs) and G protein–coupled receptors (GPCRs) on trigeminal fibers that bind fatty acids (33, 52). Third, the predominant view is that the textural properties of fats are conveyed through pressure-activated systems such as stimulation of free nerve endings and corpuscular receptors. It might be expected that oils or solid fats would be the primary stimuli in this case because free fatty acid concentrations are typically very low in foods. Electrophysiological studies in primates have identified neurons in the amygdala responsive to viscosity cues from oral exposure to nutritive or nonnutritive fats (66). Similar results are reported from recordings in the orbitofrontal cortex (123). Subsequent functional magnetic resonance imaging data also reveal human neural activation in the orbitofrontal cortex with oral fat exposure (25). The effective sensory property has not been fully characterized. Viscosity is a likely contributor since responses are observed with dietary fats as well as silicon oil and mineral oil (35), but tactile effects independent of viscosity are also apparent (25, 123). Several trials demonstrate that fats may also be detected by their contribution to oral lubricity (116, 143).
Olfactory Cues from Fat
Rancid fats are easily detected by odor cues, but sensitivity to nonoxidized fats is less well characterized. Exposure of rats to short- and medium-chain fatty acids (acetic, propionic, butyric, valeric, caproic, and caprylic) results in graded responses according to chain length and concentration (64). Differential neural spatial response patterns are noted and likely contribute to quality coding. Electrophysiological recordings in rhesus macaques are not fully consistent with these observations (122). Robust responses to hunger are observed for food odors, including high-fat foods such as cream. However, the contribution of fat odors is unclear because no increase of neural activity is noted with exposure to caprylic acid. Additionally, other work fails to note responses to the odors of lauric or linoleic acids (66). Behavioral studies indicate rats can discriminate between acetic and both caproic and propionic acids (8). However, they retain this ability after bilateral bulbar lesions that encompass most of the presumed fatty acid–responsive areas (8). The residual sensitivity may stem from remaining odor-responsive neurons (64) or tactile cues these stimuli may impart, although pungency thresholds for short-chain carboxylic acids are one, or more, orders of magnitude higher than olfactory thresholds (20, 21). Other behavioral studies in rats document a sensitivity to and preference for low concentrations (0.1%–1.0% w/w) of oil (115) and long-chain fatty acids (37, 141). After rendering rats anosmic by zinc sulfate irrigation (37) or olfactory bulbectomy (115), this preference, and presumably discriminatory ability, is lost. However, preferences are still exhibited, albeit attenuated, for more concentrated stimuli. Similar results are reported for mice (125). Again, this is consistent with a nonolfactory contribution to fatty acid detection.
Data on olfactory sensitivity to fatty acids in humans are also complex. Imaging studies demonstrate neural activation following exposure to short-chain fatty acids (i.e., caprylic and isovaleric acids) with modulation by their perceived hedonic properties based on prior learning (121). Oral exposure to vegetable oil, confirmed to be odorless in sensory tests, prompts a response that is independent of viscosity (25). These findings do not exclude a texture component since viscosity is only one dimension of fat's textural properties, but are consistent with an additional sensory (e.g., taste) contribution. Some psychophysical data indicate little or no contribution of olfactory cues since detection of fats is unaltered by testing with the nares open or closed (95, 127). Furthermore, the postprandial rise of serum triacylglycerols (TAGs) in humans following oral exposure to a high-fat food is not observed with orthonasal odor exposure alone (86, 87). A marked rise is observed with oral exposure when the nares are blocked, indicating either there was no discernable olfactory cue or it was not an effective elicitor of the TAG response.
Taste Cues from Fat
The case that fat is detected, in part, by a gustatory cue is not simply one by default. Animal electrophysiological and behavioral studies as well as psychophysical and clinical observations in humans support this property. Minimal criteria for establishing a taste component include (a) identification of an effective stimulus or class of stimuli, (b) a transduction mechanism to convert the chemical signal to an electrical one, (c) a pathway to carry the electrical signal to gustatory centers in the central nervous system, and (d) evidence of its decoding through a physiological response or some consistent sensation that is independent of other tastes.
Effective Stimuli
A variety of research approaches have been used to explore the possibility that free fatty acids are effective taste stimuli in animal models. The first is one- or two-bottle preference testing, where differences in responses to samples with and without fatty acids demonstrate the animal's ability to discriminate between the stimuli. In this paradigm, a heightened preference is observed for free fatty acids and esterified fatty acids in comparison with vehicle (141). However, free fatty acids are strongly preferred over their esterified forms (141). Furthermore, rats discriminate between the free fatty acids, showing the strongest preference for linolenic acid followed by linoleic and finally oleic. Support for an interpretation that these observations are based on taste requires a critical consideration of the testing paradigm to determine whether other sensory cues could account for the preference responses. Subtle sensory properties may be functional, as animals display clear preferences between extra light olive oil and extra virgin olive oil when the two have very similar fatty acid profiles (117). To minimize tactile cues, a low (1.0% w/w) fatty acid concentration was tested and a thickener, 0.3% xanthan gum, was added to the test solutions and vehicle to mask tactile cues contributed by the fatty acids. Such an approach is required because recent evidence indicates that free fatty acid concentrations as low as 3–100 uM elicit an intracellular rise of calcium, an integral component of the depolarization process, in lingual trigeminal neurons (152). Thus, low concentrations of free fatty acids may be detected via the somatosensory system. Whether xanthan gum adequately masks the lubricity properties of the fatty acids is not established and may itself contribute to preference responses in rats (116). Thus, this sensory attribute was not completely controlled. However, others have used different compounds to mask tactile cues [e.g., Tween-80 (100)] with comparable results. The low fatty acid concentration was also viewed as providing little olfactory stimulation, but again, this could not be excluded. However, comparable results have been reported with anosmic animals (37), thereby definitively demonstrating that olfactory cues are not necessary for discrimination in the preference-testing paradigm. To reduce the possibility that responses are based on postingestive cues (e.g., 24, 48, 130), testing was limited to five minutes. Lipid absorption may not begin for approximately 10 minutes (46, 140), but signaling systems that could affect responses may be activated more quickly. Evidence of free fatty acid detection in esophagostomized rats (54, 78) documents postingestive nutritive signaling is not necessary for free fatty acid responsiveness, although this was not used in a preference-testing paradigm. Finally, to more directly test the role of taste, trials have been reported with cluster of differentiation 36 (CD36; a putative fatty acid taste receptor discussed below) knock-out and wild-type mice (129). The knock-outs show no preference for dilute soybean oil, linoleic acid, or Sefa Soyate emulsions. However, it is possible that low concentrations of oxidation products are detectable and preferred by animals (114), so the true effective stimulus was not certain. Taken together, preference testing in rodents provides strong but not definitive evidence for fatty acid taste.
Another research approach entails conditioning preferred and aversive responses to the sensory properties of fats and subsequently determining their detection, specificity, and function. Conditioned place preference testing, where repeated exposure to a rewarding stimulus in one location leads to a preference for that location, is one paradigm used to document the efficacy of free fatty acids as oral cues (61). Provision of corn oil to six-week-old male ddY mice while they were in a light box, but only water when they were in a dark box, led to a preference for the former. This could have been due to the rewarding properties of the sensory stimulation or postingestive feedback due to the difference in energy administered. Thus, in another set of animals, the lipid loads were infused, bypassing oral exposure, and these animals failed to exhibit the same preference. This highlights the importance of the fat's sensory cues, but failure to control odor and texture precludes determination of an independent contribution of taste.
More definitive evidence is derived from conditioned taste aversions where the sensory properties of a stimulus such as fat are paired with malaise or illness (e.g., induced by LiCl injection after fat exposure) so that subsequent exposure to the fat or related compounds leads to an aversive or rejection response. Alternatively, the absence of a response to the sensory properties of a stimulus suggests its properties are not shared with the conditioned (fat) stimulus. Studies in rats reveal that aversions generalize between butter, margarine, and lard, but not to petroleum jelly, a substance with similar textural properties (77). Further evidence that tactile cues were not responsible for the differential response draws from trials where the ingested stimulus was a sugar-corn oil mixture, sugar-mineral oil mixture, or sham conditioning with saline administration (133). Based on two-bottle preference tests, the aversions were largely directed to the corn oil relative to mineral oil or sucrose. Anosmic rats showed responses similar to those of intact animals, indicating that odor was not the salient sensory cue. Limiting corn oil exposures to only 30 seconds did not alter the results, indicating that postingestive feedback was not a likely explanation. Evidence that free fatty acids may be involved stems from trials showing the aversive response generalized to linoleic acid. However, linoleic acid was not the primary target when conditioning involved exposure to sucrose/linoleic acid mixtures.
These findings were later extended with aversion conditioning to 44, 66, or 88 uM nonesterified oleic and linoleic fatty acids (93). Both fatty acids were readily targeted at the 66 and 88 uM concentrations and generalized between them. Texture was deemed an unlikely explanation for the findings because viscosity measurements of the acid solutions varied from water by only 1.5%. Other tactile properties were not evaluated. Olfaction was discounted because of the failure to note an aversion to the odor of ethanol, in which the free fatty acids were dissolved.
Accumulating human data indicate that free fatty acids varying in chain length and saturation may be effective taste stimuli. Human psychophysical studies have documented oral detection thresholds for an array of fatty acids in young adults. A masking procedure was used to isolate the taste contribution. Thresholds were measured with participants wearing nose clips to eliminate odor cues and under red light to minimize visual cues. The free fatty acids were presented in a vehicle containing 5% mineral oil and 5% gum acacia to mask effects the free fatty acids would have on lubricity and viscosity, respectively. To minimize the irritancy component, testing was conducted after participants had undergone capsaicin (the active irritant in red peppers) desensitization. This entails repeated presentation of 1% solutions of a capsaicin solution over short intervals followed by a pause. During this pause, desensitization occurs wherein responsiveness to the irritant is blunted. This technique minimizes, but does not eliminate, irritancy cues because not all trigeminal fibers are capsaicin sensitive. In addition, taste solutions were prepared daily, before testing, using procedures that minimized the formation of oxidation products. With these controls, low concentrations of 18 carbon polyunsaturated (linoleic), monounsaturated (oleic), and saturated (stearic) fatty acids were detected by nearly all study participants (14, 15). Using the same approach, oral detection thresholds were obtained in another group of participants for saturated fatty acids varying in chain length; caproic (C6:0), lauric (C12:0), and stearic (C18:0) (90). Although suggestive of a taste component, these findings are not definitive because all possible textural cues were not eliminated. The similarity of threshold values for the 18 carbon fatty acids suggests a common mechanism. It should be noted that the absolute threshold concentration is of limited importance. Detection thresholds are merely statistical concepts defined as the lowest concentration of a substance that can be detected at better-than-chance levels of performance under a given set of conditions. The probability of acceptable performance can vary at the researcher's discretion, depending on the concern for consequences of type I and type II errors. Furthermore, the ability to detect a signal among the noise (vehicle carrying the compound of interest) will depend greatly on the ratio of the two. It is generally easier to detect a stimulus in water than in a complex food because of the lower signal/noise ratio, so the absolute threshold concentration will be lower in the former case. The data from the human psychophysical trials indicate thresholds lie in the range of 1% w/w free fatty acid, but this is a context where noise was purposefully added to reduce the likelihood that nongustatory cues could be used for detection. Lower absolute concentrations (i.e., 10 uM) have been detected by subgroups of the population under different testing paradigms [i.e., adding conjugated linoleic acid (101) or the sodium salt of linoleic acid to ice cream (69)], but these trials were not designed to determine the detection threshold concentration of linoleic acid.
Detection thresholds lie below the minimal concentration required to assign a quality label to the sensation (i.e., recognition threshold). Thus, these studies fail to provide information about how the study participants perceived the quality of the stimulus. This information is required to determine whether performance is based on a change of some established quality (e.g., sweet, sour) or one that is fat specific. A sour taste has been ascribed to selected short-chain fatty acids such as formic and proprionic acids (34), but there are no reports that any combination of currently recognized basic taste qualities results in a “fatty” sensation. To the contrary, studies in rats indicate that fat is a unique sensation. With aversion conditioning to taste mixtures, fat is specifically targeted for aversions with little generalization to sweet, salty, or alcohol components (93, 133). However, generalization does occur across free fatty acids such as linoleic and oleic acids, consistent with their holding a common sensory property (93). Evidence from humans indicates that correlations between detection thresholds for free fatty acid and sucrose are not significant (90).
There is a large literature on scaling (i.e., assigning intensity ratings to stimuli) of suprathreshold concentrations of fats in foods or model solutions, but only recently has this been reported where potential contributions from other sensory systems were controlled. Free fatty acids were presented in a congealed (linoleic, oleic) or viscous (stearic) medium to mask tactile cues. In addition, testing was conducted under red light to mask visual cues and with participants wearing nose clips to eliminate olfactory cues (2). For linoleic and oleic acids, the concentrations tested were 0.0, 0.4, 0.71, 1.26, 2.25, or 4.0% (w/w), whereas for stearic acid, the stimuli included 0.0, 0.5, 1.0, 2.0, 4.0, or 8.0% (fat w/w). The linoleic and oleic samples were served at room temperature, whereas stearic acid was served at approximately 70–75°C so that it was in fluid form. In addition to rating the intensity and palatability of the stimuli, participants also provided quality assessments. Although there was a graded increase in rated overall taste intensity with increasing fatty acid concentration, the slope was greater when ratings were focused on fat concentration. A wide variety of qualities were identified for the stimuli that were predominantly somesthetic, either tactile (e.g., fatty, oily, greasy, gummy, waxy, slimy, slippery, mouth-coating, heavy) or irritating (e.g., acidic, spicy). Other taste qualities were mentioned, but only by a small number of participants (e.g., sour, 1.5%–4%; bitter, 1.5%–11%; sweet, 1.5%–3%). There was also a high incidence of unpleasant reports—33% for oleic acid, 43% for linoleic acid, and 19% for stearic acid.
There are several reports of individual variability in fat taste. Individuals sensitive to the compound propylthiouracil are reportedly more sensitive to low concentrations of conjugated linoleic acid (101) and better discriminate between fat concentrations in high-fat foods (74, 138). However, the proposed mechanism is through enhanced trigeminal innervation due to the greater numbers of taste buds that are cradled in such fibers. If true, taste is not the likely mechanism. Other work suggests there are fatty acid tasters and nontasters independent of propylthiouracil-taster status (69), but lack of control over nongustatory cues precludes determination of the basis for the findings. Recent data from more controlled studies reveal fatty acid (taste?) thresholds range over several orders of magnitude, and the distributions are not normal (90). The question of whether there is a genetic basis to fat taste remains open.
Taken together, there are (a) biophysical data documenting the efficacy of free fatty acids in depolarizing taste receptor cells, (b) animal behavior data showing free fatty acids are salient targets for conditioning and that the quality does not generalize to other qualities but does generalize across fatty acids, (c) human psychophysical data that a range of free fatty acids can be detected in low concentrations when the contributions of other sensory properties are minimized, (d) human evidence of the ability to discriminate graded suprathreshold concentrations of free fatty acids when nontaste sensory systems are minimized, (e) human reports of fatty acid qualities that are vague and diverse, but rarely include other known “basic” taste qualities, and (f) no reports of combinations of other basic taste qualities that result in sensations described as fatty. Although not definitive, these data are consistent with a view that there is a gustatory detection system in the oral cavity for free fatty acids.
Independent of the evidence that free fatty acids may be detected in the oral cavity, the question as to whether they are present challenges the physiological relevance of such stimuli. Dietary fats, which are consumed as triacylglycerol, do not appear to be effective stimuli (e.g., 42). This is a non-issue in rats, which secrete ample lingual lipase and thus generate free fatty acids in the oral cavity (72). However, evidence does not support the secretion of this enzyme in the saliva of humans (127, 135). Thus, fat detection by gustatory cues would require the existence of free fatty acids in foods. Although there has been no systematic study of their concentrations across foods, numerous reports identify their presence and more may be liberated during mechanical disruption of the food matrix during preparation, cooking, and mastication (18, 87, 92, 98, 134, 145). Concentrations in edible oils generally range between 0.01% and 1% (17, 45, 80, 124) but can be markedly higher (7, 144). Furthermore, concentrations increase with hydrolysis during heating (104). So, concentrations are well within the effective range for human detection.
Gustatory Anatomy and Physiology
To facilitate discussion of fat taste transduction mechanisms, it is useful to briefly review the anatomy and physiology of the gustatory system. Taste receptor cells are located within taste buds, which are clusters of about 150 cells that are functional receptor cells, immature taste cells, or supporting cells. Taste buds are, in turn, primarily located within three types of papillae: in the apical region of fungiform papillae located on the dorsal anterior two-thirds of the tongue, in the folds of the foliate papillae on the lateral posterior tongue, and in the cleft of circumvallate papillae on the back of the tongue. However, taste buds are also present at the junction of the soft and hard palate on the roof of the mouth, pharynx, larynx, and upper esophagus. The taste cells in fungiform papillae are innervated by the chorda tympani nerve, a branch of the facial nerve, while taste cells in the foliate and circumvallate papillae synapse with axons from the glossopharyngeal nerve. The vagus nerve also conveys taste information from the back of the throat. Through various mechanisms, such as modification of membrane channels, binding to cell surface receptors or passage of compounds through channels or membranes, and activation of intracellular signaling systems, exposure to effective taste stimuli leads to an increased intracellular calcium concentration (via influx from the extracellular space and mitochondrial release), resulting in depolarization of the taste receptor cell. The consequence may be migration of vesicles containing neurotransmitters to the basal membrane and, through exocytosis, neurotransmitter release into the synapse with the gustatory nerve or, possibly, activation of a presynaptic taste cell followed by neurotransmitter release through the same sequence. In either case, this leads to the development of an action potential in the nerve that is propagated centrally to taste centers where the spatial-temporal signal is decoded.
Localization
Evidence that animals and humans can detect free fatty acids is de facto confirmation that there are transduction mechanisms. However, their nature is a current area of active research. A first requirement to connect free fatty acid detection to taste is to demonstrate transduction mechanisms are present in taste receptor cells. There is now documentation for a number of candidate mechanisms on such cells or surrounding epithelia. Delayed rectifying potassium channels sensitive to selected free fatty acids have been identified in isolated taste receptor cells from rat fungiform papillae (42). The fatty acid transporter CD36 has been localized to the apical region of cells in the taste buds of foliate and circumvallate papillae of rats (36, 78). A number of GPCRs known to bind free fatty acids are also present on taste cells. GPCR120 is present in the apical region of rat taste bud cells from circumvallate, foliate, and fungiform papillae (23, 83) and is also expressed in human taste cells (23). It has not been detected in rat nongustatory oral epithelium. There is inconsistent evidence for the presence of GPCR40 (23, 83), but where it was identified, it was located in rat circumvallate papillae. GPCR41 and GPCR43 are expressed in taste cells from rat foliate and circumvallate papillae (52). In addition to these presumptive receptors, diffusion is a plausible mechanism that would hold for all taste cells.
Stimulus Specificity
As noted above, humans are able to detect free fatty acids varying in saturation and chain length. This then calls for mechanisms capable of transducing this array of stimuli. Aside from diffusion, each of the current putative mechanisms has unique ligand specificity (Figure 1). Only cis-long-chain, unsaturated fatty acids block delayed rectifying potassium channels. Short- and medium-chain fatty acids, unsaturated fatty acids in the trans configuration, and saturated fatty acids are not effective as measured by the reduction of outward potassium currents (42). Long-chain fatty acids varying in saturation bind with CD36 with a binding affinity in the nanomolar range (4, 58), and binding is reversible (4). Short- and medium-chain fatty acids are not bound to this receptor. The range of effective stimuli extends to medium- and long-chain fatty acids with the addition of GPCRs. Saturated fatty acids with chain lengths between C14 and C18, as well as unsaturated fatty acids with chain lengths of C16 to C22, are ligands for GPCR120 (55). However, this receptor does not bind esterified fatty acids or free fatty acids with chain lengths less than C12 (23, 55). GPCR40 binds saturated free fatty acids with an affinity directly related to chain length in the range of C8 to C18 (11), whereas only longer-chain unsaturated fatty acids, C18 to C22, are bound by this receptor (97). GPCR40 does not bind short-chain free fatty acids (11, 62). The latter may be accomplished by GPCR41 and GPCR43. These receptors bind free fatty acids in the range of C1 to C6 but not medium- or long-chain free fatty acids (12, 79, 150). Generally, binding affinities increase with chain length for GPCR43 and decrease with GPCR41 (12).
Figure 1.
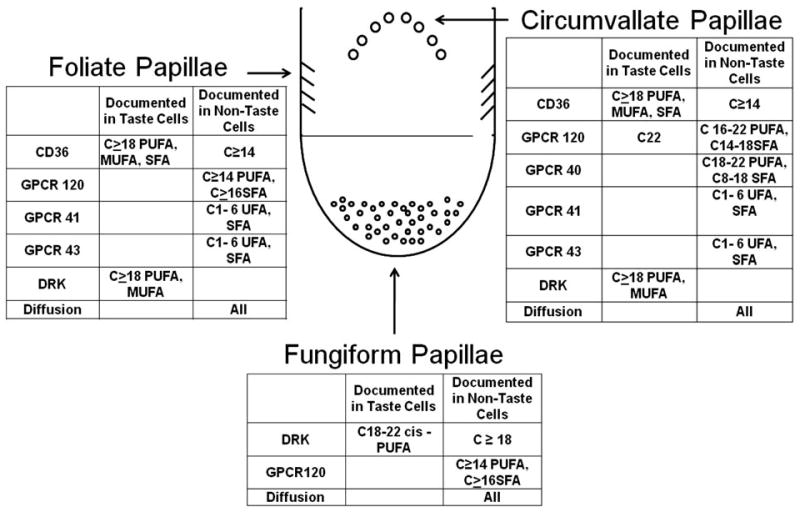
Putative taste transduction mechanisms: location and effective stimuli. DRK, delayed rectifying potassium channel; GPCR, G protein–coupled receptor; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
Thus, the combination of presumptive fatty acid receptors covers the range of stimuli detected by humans in psychophysical studies (14, 90), but no one receptor is sufficiently broadly tuned. This suggests either the presence of multiple systems, a currently unidentified broadly tuned receptor system, or a nonreceptor-mediated mechanism for fatty acid detection in humans. Although not negating the function of receptor systems, diffusion may also contribute to detection of the array of dietary fatty acids tested because they are capable of crossing cell membranes by diffusion.
Transduction Mechanisms
The mechanisms by which free fatty acids induce taste cell depolarization vary. As their name implies, delayed rectifying potassium channels (DRKs) allow positively charged potassium ions to exit the cell and thereby aid in maintaining the intracellular electronegativity relative to the extracellular space. Selected free fatty acids block these channels so may elicit depolarization or hold the cell in an excited state and reduce the stimulus threshold for depolarization. Fatty acid concentrations as low as 10 uM are effective blockers, and depolarization may occur in milliseconds (55). Taste cells may be broadly tuned, meaning they respond to stimuli eliciting different taste qualities. Thus, the possibility that fatty acid blockage of DRKs will nonspecifically hold the cell in a heightened state of activation raises the possibility that fatty acids are not primary stimuli, but rather increase responsiveness of taste cells to other taste qualities. Support for this mechanism was provided by an early report showing heightened preference responses to a subthreshold 0.5 mM saccharin solution containing 5 or 20 uM linoleic acid in S5B rats relative to the same concentrations of saccharin or linoleic acid alone (43). Consistent with the electrophysiological data, no enhancement was noted with the addition of lauric acid to saccharin. However, further support for this phenomenon is weak. The authors did not observe similar effects with Osborne-Mendel rats. A later study reported that 88 uM linoleic or oleic acids, or a combination of the two, led to increased lick rates to 20 second exposures to several concentrations of sucrose and glucose (109). Reductions of licking were observed with sodium chloride, citric acid, and quinine solutions. A reduction of responses to the generally aversive sour and bitter stimuli may reflect heightened sensitivity, but this cannot explain the lower response rate for salt, which is generally preferred. More recent work has failed to replicate the findings of free fatty acid modulation of other taste qualities in rats (93). This issue has also been examined in humans. The addition of 1% linoleic acid to solutions of sucrose, sodium chloride, citric acid, and caffeine elevated rather than decreased taste thresholds (i.e., lowered sensitivity) and either did not change or lowered intensity ratings for suprathreshold concentrations of the taste compounds (89). The higher concentration of linoleic acid used in the human trial ensured it was present at an adequate concentration but may have also led to a masking effect, accounting for the discrepant findings. Other work has revealed that the addition of oil enriched with docosahexaenoic acid does not alter the intensity ratings of sweet, salty, and sour taste compounds, although the bitterness of quinine sulfate was reduced whereas a mixture of inosine monophosphate (IMP) and MSG was enhanced (75). Thus, the preponderance of evidence indicates that free fatty acids are not detected by their modulation of sensitivity of taste receptor cells to other taste compounds.
Fatty acid–stimulated Ca2+ uptake is lost in the taste cells of CD36 knock-out mice (40), but the mechanism remains to be determined. A transporter analogous to human CD36 was identified in rats more than a decade ago (36), but evidence of fatty acid transport is lacking. Indeed, long-chain free fatty acid uptake by adipocytes from CD36 knock-out and wild-type mice is similar (31), and short- and medium-chain free fatty acid uptake in cells is independent of CD36 (59). Alternatively, CD36 may serve as a binding site that facilitates blocking of DRK (41). However, the ability of CD36 to bind saturated free fatty acids that fail to alter DRK, but are detected by humans, raises questions about this role. Fatty acid peroxidation products may also bind to CD36 (4). These oxidation products have a marked odor that could serve as the effective stimulus, but elimination of odor cues in human trials has not altered detection thresholds. In addition, sensitivity to oxidized fatty acids was similar to nonoxidized samples of the same acid (14).
Nevertheless, the functionality of CD36 is well supported. CD36 knock-out animals do not express the preference for fatty acids exhibited by wild-type animals (38, 78), nor do they prefer 5% or 10% linoleic acid solutions over paraffin oil in one-hour preference tests, as do wild-type mice (137). Knock-out animals show a clear preference for sucrose and avoidance of bitterness, indicating that the deletion led to lipid-specific changes of sensory responsiveness (78).
A number of previously orphaned GPCRs have now been identified as receptors for sweet, bitter, and umami taste compounds in the gut (35) and oral cavity (56, 102, 103). Evidence is accumulating that they may also mediate fatty acid detection. For example, cell culture studies indicate transfection of Chinese hamster ovary cells with human GPR40 cDNA increases their responsiveness to fatty acids as measured by rises of intracellular calcium (62). Furthermore, GPCR knock-out mice have a lower preference for 30% corn oil and 0.3% oleic acid (23), as well as a lower intake of 30% corn oil, 3% lauric acid, and 1% intralipid, compared with wild-type mice. These differences are relative rather than absolute, indicating that the knock-outs are capable of responding and, again, that there are likely multiple fatty acid–detection mechanisms present.
Passive diffusion is an alternative or, more likely, complementary mechanism for fatty acid detection. The efficacy of this mechanism for detection of lipophilic sweet and bitter compounds is documented (27, 107, 154). Free fatty acids are amphipathic and can cross cell membranes via diffusion and then interact with intracellular signaling systems. Such a mechanism has been largely discounted because of skepticism that the time course would coincide with common taste experience. However, studies with lipid bilayers and model systems indicate that translocation of free fatty acids may occur in milliseconds to a few seconds (50, 51, 68). The rate would be dependent on diffusion coefficients (4), which vary inversely with chain length. Recent work demonstrates that Ca2+ influx in mouse taste bud cells, an index of responsiveness, occurs with exposure to palmitic, linoleic, and docosahexaenoic acid, in that order (40). Furthermore, intracellular free fatty acids can rapidly bind to intracellular proteins or be trapped through metabolic processes (82). Receptor-mediated processes are saturable [e.g., (4)], but where free fatty acid availability is not rate limiting, diffusion is the predominant route of passage across membranes (59). Thus, it has been hypothesized that receptor-mediated processes dominate when free fatty acid concentrations are low, and at higher concentrations, they are supplanted by diffusion processes (4). Unlike conditions in the circulation, where unbound free fatty acids are present in nanomolar concentrations (3), concentrations in the oral cavity while eating may be six to eight orders of magnitude higher.
Neural Conduction
Another defining feature of a sensory system is that information captured by its detection systems is conveyed centrally through unique neural processes. Data are limited but are consistent with central transmission of signals generated by free fatty acids. In one trial, bilateral chorda tympani transection led to a doubling of linoleic acid taste thresholds in rats (136). This procedure also eliminated avoidance of an 88 uM solution of linoleic acid to which an aversion had been conditioned, whereas preference for corn oil was not affected (110). Retention of sensitivity would be expected with chorda tympani cuts since the other cranial nerves conveying taste information were not altered, and they ramify with taste cells on the posterior tongue that may harbor the greatest abundance of fatty acid receptors. Glossopharyngeal nerve cuts also diminish, but do not eliminate, the preference for 2% linoleic acid in mice in 30-minute, two-bottle choice tests (40). However, sectioning both the glossopharyngeal and chorda tympani nerves eliminates differential responses to linoleic acid (40). It also eliminates the preference for 4% sucrose and avoidance of 0.1 mM denatonium. In addition, pancreatic exocrine secretions elicited by oral exposure to linoleic acid are reduced after transection of the glossopharyngeal alone or in combination with the chorda tympani (40). These data are consistent with fatty acids being effective taste stimuli. The observation that fat preferences were disrupted with sectioning of the taste nerves, but leaving the trigeminal nerve intact, suggests somesthetic cues from fats are not sufficient to account for fatty acid responses.
Functions of Fat Detection
For the present purpose, a fourth element in establishing the existence of fat taste is evidence of its functionality. Teleological arguments may be made that sensitivity to fats provided a selective evolutionary advantage by facilitating detection of important sources of energy and essential nutrients, but the validity of such a claim cannot be established. Indeed, the case would be hard to make for fats because the sensory properties of the presumptive taste signal, free fatty acids, is aversive. Like bitterness, fat taste may have served to reduce exposure to potential toxins, in this case, rancid foods.
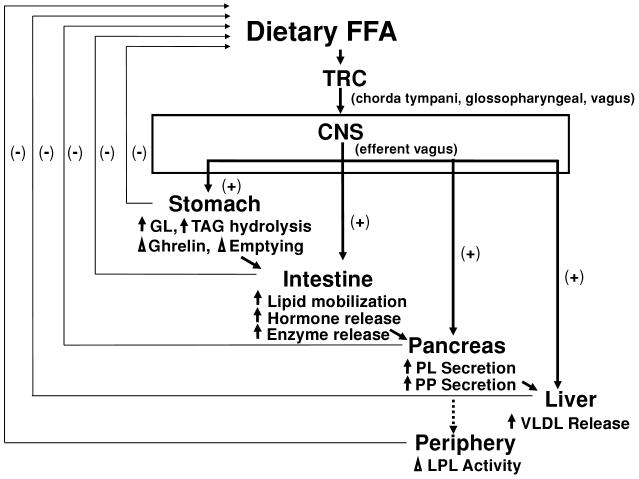
There is growing evidence of the physiological importance of fatty acid signaling (1, 19, 57, 59, 150). This may be especially true in the oral cavity, where dietary fats elicit an array of cephalic phase responses. These are acute, neurally mediated, physiological responses to sensory stimulation. Typically, tests of the phenomenon involve sham or modified sham feeding. The former allows normal ingestion of foods to maximize sensory exposure, but a gastric fistula drains the load to prevent feedback effects due to nutrient absorption. Modified sham feeding is more common in human studies and entails allowing participants to masticate food to derive sensory exposure; the food is then expectorated. Although cephalic phase responses are often rapid, transient, and of small magnitude, they are believed to serve as signals that guide homeostatic responses (111). Figure 2 is a useful guide to consider the likely influences of oral exposure to dietary fats on lipid metabolism.
Figure 2.
Physiological responses to oral exposure to dietary fatty acids. Free fatty acids are detected by various mechanisms on taste receptor cells, resulting in cell depolarization. The signal is conveyed to the central nervous system by the chorda tympani and glossopharyngeal and/or vagus nerves. This leads to generation of an efferent signal carried by the vagus nerve to multiple (e.g., stomach, intestine, pancreas, liver) sites, leading to a cascade of physiological responses related to lipid metabolism. Many of these responses provide feedback to modify feeding. FFA, free fatty acid; CNS, central nervous system; GL, gastric lipase; LPL, lipoprotein lipase; PL, pancreatic lipase; PP, pancreatic polypeptide; TAG, triacylglycerol; TRC, taste receptor cell; VLDL, very-low-density lipoprotein.
As proposed above, free fatty acids derived from food are detected by various sensory properties and, in the case of taste, lead to depolarization of taste receptor cells. The resulting signal is conveyed to taste centers (initially the nucleus of the solitary tract) in the central nervous system by cranial nerves VII, IX, and X. One outcome of this is activation of descending fibers to the dorsal motor nucleus of the vagus, which is conveyed through vagal efferents to essentially all sites involved with nutrient processing. Modified sham feeding stimulates pancreatic polypeptide secretion, a marker of neural activation (6, 44).
Stomach
At the level of the stomach, cephalic phase stimulation prompts enzyme and hormonal secretions, as well as effects on emptying. Modified sham feeding of a meal containing fat by humans leads to increased gastric lipase secretion (147). The effect is blocked by administration of atropine, a cholinergic antagonist, supporting a role for neural mediation. The contribution of fat to the response is not known, as no challenge with fat alone was administered, and a response is also elicited by merely chewing gum (148). Gastric lipase is estimated to account for up to 17% of triacylglycerol hydrolysis because it is active in the acidic environment of the stomach and retains 50% of its activity at a pH of 6.5, as would occur in the duodenum (13). Modified sham feeding of a high-fat meal with or without a liquid oral fat load (50 g of long-chain triacylglycerol) leads to a significantly greater initial ghrelin secretion followed by a more rapid decline during the postprandial period (53). A stimulatory effect of modified sham feeding on gastric emptying is also documented (53). Once again, the independent role of fat cannot be ascertained because responses to a fat-free stimulus have not been tested. Other work with rats suggests that oral fat plays a signaling role that modulates the rate of gastric emptying. Although gastric emptying is altered by gastric lipid infusion duration and rate, this is not the case with intraoral infusions (70). This constellation of augmented responses to modified sham feeding has been related to enhanced satiety as measured by questionnaire (53). This may then provide feedback that inhibits further feeding. However, these effects also feed forward to activate processes in the intestine.
Intestine
Oral fat exposure, perhaps particularly activation of the gustatory system, modifies the intestinal digestion, uptake, storage, and secretion of lipids. In rats, oral stimulation with oleic, linoleic, and linolenic fatty acids as well as corn oil stimulates pancreatic enzyme secretion (54). To ensure responses were attributable to sensory exposure, stimulation was provided by oral administration of only 0.2 ml of test solution to animals that were esophagostomized, and bile-pancreatic secretions were measured via fistula. No augmentation of secretion was observed with oral exposure to caprylic acid, esters of the long-chain fatty acids, trilinolein, or saline. Atropine blocked secretory responses to the fatty acids. Although details of stimulus administration were insufficient to exclude nongustatory cues as the basis for these effects, other workers have confirmed and added greater specificity to the findings (78). Wild-type mice with esophageal ligation show increased pancreatobiliary secretion with exposure to oleic, linoleic, and linolenic fatty acids, but not to caprylic or stearic acids. Furthermore, application of linoleic acid to the tongue of wild-type mice leads to an increased protein-enriched secretion, whereas comparable stimulation of CD36 knock-out mice does not (78). Application of linoleic to nongustatory oral tissue (i.e., soft palate) is also ineffective.
Dietary fats are important signals for secretion of gut peptides, such as cholecystokinin and glucagon-like peptide-1, with reported satiety effects (149). Evidence that this occurs with sensory stimulation is mixed. The most widely studied gut hormone is cholecystokinin. Some authors fail to detect an effect of sham feeding on its release (63, 71, 148), whereas others note a rise (146) that is blocked by atropine administration (126). The latter study also noted a rise of pancreatic polypeptide.
Evidence of a cephalic phase component to lipid absorption was noted more than 50 years ago in studies of vitamin A metabolism. Following a serendipitous observation that eating acutely augmented plasma vitamin A concentrations, controlled modified sham-feeding trials with a mixed meal revealed oral stimulation alone was also effective (97). This is consistent with an effect on lipid absorption, as vitamin A can serve as a marker for lipid absorption. Blockage by atropine administration supported the neural basis of the response. This was followed by studies in rats, where oral exposure specifically to lipid (0.3 ml of 2% oil in water emulsion of corn oil) led to a marked and sustained elevation of serum triacylglycerol concentration (113). Oral exposures to water and a saccharin solution were less effective. This has now been confirmed and extended in human studies, where participants ingest lipid in capsules to load the gut while avoiding oral fat exposure. Participants then receive various forms of oral stimulation while serum triacylglycerol concentrations are monitored (84, 86–88, 91, 106). This work has revealed that oral stimulation with full-fat cream cheese is more effective at eliciting a triacylglycerol rise than is comparable stimulation with a nonfat version of the cream cheese (84). In addition, butter was more effective than fat-based, protein-based, or carbohydrate-based fat replacers or vehicle, indicating the importance of the availability of fat (87). Whether the various fat-replacers adequately mimicked the tactile properties of the butter is open to question, but the findings suggest the response was not based on this property. Expectorated samples contained approximately 1 mM oleic acid and 0.35 mM linoleic acid, which indicates there was sufficient stimulus present to be detected. To address the possibility that the response was based on an olfactory cue, another trial tested responses to stimulation by odor alone, taste (nares were blocked) alone, or a combination of the two (86). The triacylglycerol rise was greater for the taste-only condition compared to odor, and the response to taste was nearly identical to that of oral exposure, indicating that taste was the likely effective cue. Another trial suggested unsaturated fats were more effective oral stimuli than were saturated fats, but this trial used a more highly saturated fat as the preload, complicating comparison of the effects with previous work (139). Modified sham feeding of meals with linoleic acid or olive oil, rich in oleic acid, led to greater triacylglycerol concentration rises than did oral stimulation with water, but only the former led to a greater increase of nonesterified fatty acids (132). Differential responses to these classes of fatty acids have also been noted in animal studies (110, 152).
More recent work has sought to better characterize salient stimulation characteristics. Earlier studies used a two-hour stimulation period, but fat exposures of only ten seconds (91), or three minutes (22) are adequate for most individuals. The most recent trial (91) observed a response in 80% of 34 participants to exposures of 20 minutes or less, indicating the response is ecologically relevant (i.e., may occur within a customary meal period).
There have also been recent advances in identifying the origin of the increased serum triacylglycerol after oral stimulation. In one trial, all 25 participants exhibited an early (i.e., within about 30 minutes) peak (88). Analyses of serum fatty acid profiles revealed that a portion of the lipid was derived from the meal consumed hours prior to testing (33, 88). This has now been confirmed through studies with stable isotopes that indicate almost half of the lipid in the initial triacylglycerol peak derives from the last meal consumed prior to testing, even after an overnight interval (106). Where this lipid is stored is not established, but it may be in jejunal enterocytes (120).
Pancreas, Liver, and Peripheral Tissues
Products of the intestinal phase of digestion as well as direct vagal activation stimulate pancreatic exocrine and endocrine secretions (85, 153). Pancreatic polypeptide release is neurally mediated (6, 44), whereas the mix of nutrients in the gastrointestinal tract stimulates the release of digestive enzymes, including pancreatic lipase. One function of pancreatic polypeptide is to stimulate hepatic very-low-density lipoprotein synthesis and secretion, and the plasma very-low-density lipoprotein concentration is elevated by modified sham feeding (120). Clearance of lipoprotein particles is also modified by sensory stimulation, probably through cephalic phase insulin release. Modified sham feeding initially enhances lipoprotein lipase activity in myocytes and suppresses it in adipocytes (108).
Thus, sensory stimulation has the potential to modify lipid metabolism at multiple levels, from decisions about fat ingestion to its digestion, absorption from the gastrointestinal tract, de novo hepatic synthesis, and clearance. However, a quantitative estimate of the independent effect of the cephalic component is not yet possible. Nevertheless, these actions further support an orosensory detection system for dietary fats that, in some instances (e.g., mobilization of lipid stored in the gastrointestinal tract), specifically implicates taste.
Summary
Dietary fat is a multimodal stimulus. In the oral cavity, there is no question that triacylglycerol may be detected by the tactile properties it contributes to foods and that free fatty acids and oxidation products are detectable by olfactory cues. Whether free fatty acids are also effective taste stimuli, and whether “fatty” should be considered another basic taste quality, is under active investigation. To date, strong data indicate there are transduction mechanisms on taste cells that, upon exposure to free fatty acids, generate signals conveyed by neurons containing taste fibers projecting to central sites that result in an array of physiological responses as well as sensations not characterized by current basic taste qualities. Although the data are strong, they cannot be considered definitive. Among the limitations is the fact that there are marked species differences in taste responsiveness (10) and little evidence for the existence and functionality of fatty acid transduction systems on human taste receptor cells. In addition, it is still uncertain whether somesthetic cues, which are highly confounded with taste, underlie fatty acid detection. Resolution of this issue is warranted, as this proposed component of the taste system is tuned to a class of nutrients essential for health as well as potential toxins, and thus hold a wide array of health implications.
Summary Points
Taste is an analytical sense composed of a limited number of primary qualities. Only sweet, salty, sour, bitter, and umami are currently widely recognized as taste primaries. Whether fatty constitutes a sixth primary is under study.
Dietary fats are detected by the tactile and olfactory properties they impart to foods, but they may also have a taste component. Tactile and olfactory properties do not account for the full range of sensations and responses elicited by oral fat exposure.
Taste function has multiple facets. Sensitivity is defined as the lowest stimulus concentration that may be detected or recognized under a given set of conditions at a defined level of probability. Scaling refers to the ability to judge gradations of physical concentration. Hedonics includes measures of liking and preference (i.e., relative liking). Measurements of each dimension provide support, albeit not definitive, for fat detection.
Current evidence indicates that esterified fatty acids (e.g., triacylglycerol fatty acids) are not effective taste stimuli, whereas free fatty acids may be. Humans can detect free fatty acids varying in chain length (C6–C18) and saturation (PUFA, MUFA, saturated fatty acid) in stimuli designed to minimize nongustatory sensory cues.
Dietary triacylglycerol often enhances the palatability of foods, whereas free fatty acids are aversive. Thus, the taste sensation of fats may primarily serve to inhibit, rather than promote, fat ingestion.
Oral exposure to fat elicits a cascade of responses that occur during food digestion as well as nutrient absorption and utilization. Responses include, but are not limited to, changes of gastric secretions and emptying; pancreatic exocrine and endocrine secretions; absorption and clearance of lipids; and thermogenesis.
Future Issues
The primary obstacle to documenting that free fatty acids are effective taste stimuli is the inability to fully exclude contributions from other sensory systems activated by dietary fats.
There is marked individual variability in detection of free fatty acids. A possible genetic basis warrants further study.
To explore the physiological relevance of oral fat detection, the source, quantities, and nature of oral free fatty acids in humans should be established.
The contribution and clinical significance of oral fat exposure on postprandial lipemia should be clarified.
- Transduction
conversion of a chemical signal to an electrical signal
- Chemical senses
sensory systems mediating taste, smell and chemical irritancy
- Taste primary
a quality sensation not characterized by any combination of other sensations initiated through depolarization of a taste receptor cell by a selected class of chemicals
- Delayed rectifying potassium channels (DRKs)
channels involved in regulation of the electrical properties (excitability) of cells (e.g., neurons, taste receptor cells) and a putative fatty acid transduction mechanism
- G protein–coupled receptor (GPCR)
a protein family of seven transmembrane domain receptors and putative fatty acid taste receptors
- Psychophysics
an experimental approach that measures physiological responses to sensory stimulation
- Cluster of differentiation 36 (CD36)
a cell surface membrane protein that binds free fatty acids and is a putative fatty acid taste receptor
- Cephalic phase
rapid, transient, vagally mediated physiological responses to sensory stimulation
Footnotes
Disclosure Statement: The author is not aware of any biases that might be perceived as affecting the objectivity of this review.
Literature Cited
- 1.Aponte GW, Fink AS, Meyer JH, Tatemoto K, Taylor IL. Regional distribution and release of peptide YY with fatty acids of different chain length. Am J Physiol. 1985;249:G745–50. doi: 10.1152/ajpgi.1985.249.6.G745. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong C. PhD thesis. Purdue Univ.; West Lafayette, IN: 2007. 6-n-Propylthiouracil: orosensory influence on taste, diet and chronic disease risk; p. 275. [Google Scholar]
- 3.Azzazy HM, Pelsers MM, Christenson RH. Unbound free fatty acids and heart-type fatty acid-binding protein: diagnostic assays and clinical applications. Clin Chem. 2006;52:19–29. doi: 10.1373/clinchem.2005.056143. [DOI] [PubMed] [Google Scholar]
- 4.Baillie AG, Coburn CT, Abumrad NA. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J Membr Biol. 1996;153:75–81. doi: 10.1007/s002329900111. [DOI] [PubMed] [Google Scholar]
- 5.Bartoshuk LM. The psychophysics of taste. Am J Clin Nutr. 1978;31:1068–77. doi: 10.1093/ajcn/31.6.1068. [DOI] [PubMed] [Google Scholar]
- 6.Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, et al. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab. 2003;88:3989–92. doi: 10.1210/jc.2003-030630. [DOI] [PubMed] [Google Scholar]
- 7.Bertran E, Blanco M, Coello J, Iturriaga H, Maspoch S, Montoliu I. Determination of olive oil free fatty acid by Fourier transform infrared spectroscopy. J Am Oil Chem Soc. 1999;76:611–16. [Google Scholar]
- 8.Bisulco S, Slotnick B. Olfactory discrimination of short chain fatty acids in rats with large bilateral lesions of the olfactory bulbs. Chem Senses. 2003;28:361–70. doi: 10.1093/chemse/28.5.361. [DOI] [PubMed] [Google Scholar]
- 9.Breslin PA, Beauchamp GK, Pugh EN., Jr Monogeusia for fructose, glucose, sucrose, and maltose. Percept Psychophys. 1996;58:327–41. doi: 10.3758/bf03206809. [DOI] [PubMed] [Google Scholar]
- 10.Breslin P, Huang L. Human taste: peripheral anatomy, taste transduction, and coding. In: Hummel T, Welge-Lussen A, editors. Taste and Smell: An Update. Basel: Karger; 2006. pp. 152–90. [DOI] [PubMed] [Google Scholar]
- 11.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 12.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–19. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 13.Carriere F, Barrowman JA, Verger R, Laugier R. Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology. 1993;105:876–88. doi: 10.1016/0016-5085(93)90908-u. [DOI] [PubMed] [Google Scholar]
- 14.Chale-Rush A, Burgess JR, Mattes RD. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem Senses. 2007;32:423–31. doi: 10.1093/chemse/bjm007. [DOI] [PubMed] [Google Scholar]
- 15.Chale-Rush A, Burgess JR, Mattes RD. Multiple routes of chemosensitivity to free fatty acids in humans. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1206–12. doi: 10.1152/ajpgi.00471.2006. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci. 2000;3:113–19. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- 17.Che Man YB, Moh MH, Van de Voort FR. Determination of free fatty acids in crude palm oil and refined-bleached-deodorized palm olein using Fourier transform infrared spectroscopy. J Am Oil Chem Soc. 1999;76:485–90. [Google Scholar]
- 18.Chow C. Fatty Acids in Foods and Their Health Implications. New York: Mercel Dekker; 1992. [Google Scholar]
- 19.Coburn CT, Abumrad NA. Structure-function of CD36 and evidence for its role in facilitating membrane fatty acid transport. In: Duttaroy A, Spener F, editors. Cellular Proteins and Their Fatty Acids in Health and Disease. Weinheim, Germany: Wiley-VCH; 2003. pp. 3–29. [Google Scholar]
- 20.Cometto-Muniz JE, Cain WS, Abraham MH. Nasal pungency and odor of homologous aldehydes and carboxylic acids. Exp Brain Res. 1998;118:180–88. doi: 10.1007/s002210050270. [DOI] [PubMed] [Google Scholar]
- 21.Cometto-Muniz JE, Cain WS, Abraham MH, Gola JM. Ocular and nasal trigeminal detection of butyl acetate and toluene presented singly and in mixtures. Toxicol Sci. 2001;63:233–44. doi: 10.1093/toxsci/63.2.233. [DOI] [PubMed] [Google Scholar]
- 22.Crystal SR, Teff KL. Tasting fat: cephalic phase hormonal responses and food intake in restrained and unrestrained eaters. Physiol Behav. 2006;89:213–20. doi: 10.1016/j.physbeh.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Damak S, Le-Coutre J, Bezencon C, Cartoni C. U.S. Patent Application No. WO2007/014824A1 2007
- 24.Davis JD, Kung TM, Rosenak R. Interaction between orosensory and postingestional stimulation in the control of corn oil intake by rats. Physiol Behav. 1995;57:1081–87. doi: 10.1016/0031-9384(95)00008-7. [DOI] [PubMed] [Google Scholar]
- 25.De Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci. 2004;24:3086–93. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delwiche J. The impact of perceptual interactions on perceived flavor. Food Qual Pref. 2004;15:137–46. [Google Scholar]
- 27.DeSimone JA. Focus on “rapid entry of bitter and sweet tastants into liposomes and taste cells: implications for signal transduction.”. Am J Physiol Cell Physiol. 2000;278:C13–16. doi: 10.1152/ajpcell.2000.278.1.C13. [DOI] [PubMed] [Google Scholar]
- 28.Erickson RP. Studies on the perception of taste: Do primaries exist? Physiol Behav. 1982;28:57–62. doi: 10.1016/0031-9384(82)90102-0. [DOI] [PubMed] [Google Scholar]
- 29.Erickson RP, Covey E. On the singularity of taste sensations: What is a taste primary? Physiol Behav. 1980;25:527–33. doi: 10.1016/0031-9384(80)90117-1. [DOI] [PubMed] [Google Scholar]
- 30.Esteller M, Orlandi de Lima A, Caetano da Silva Lannes S. Color measurement in hamburger buns with fat and sugar replacers. LWT Food Sci Technol. 2006;39:184–87. [Google Scholar]
- 31.Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–62. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 32.Fernel J. Therapeutices Universalis. Frankfurt, Germany: Andream Wechelum; 1581. [Google Scholar]
- 33.Fielding BA, Callow J, Owen RM, Samra JS, Matthews DR, Frayn KN. Postprandial lipemia: the origin of an early peak studied by specific dietary fatty acid intake during sequential meals. Am J Clin Nutr. 1996;63:36–41. doi: 10.1093/ajcn/63.1.36. [DOI] [PubMed] [Google Scholar]
- 34.Forss DA. Odor and flavor compounds from lipids. Prog Chem Fats Other Lipids. 1972;13:177–258. doi: 10.1016/0079-6832(73)90007-4. [DOI] [PubMed] [Google Scholar]
- 35.Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett. 2003;554:381–88. doi: 10.1016/s0014-5793(03)01196-7. [DOI] [PubMed] [Google Scholar]
- 36.Fukuwatari T, Kawada T, Tsuruta M, Hiraoka T, Iwanaga T, et al. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 1997;414:461–64. doi: 10.1016/s0014-5793(97)01055-7. [DOI] [PubMed] [Google Scholar]
- 37.Fukuwatari T, Shibata K, Iguchi K, Iwata T, Tani K, et al. Role of gustation in the recognition of oleate and triolein in anosmic rats. Physiol Behav. 2003;78:579–83. doi: 10.1016/s0031-9384(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 38.Fushiki T, Kawai T. Chemical reception of fats in the oral cavity and the mechanism of addiction to dietary fat. Chem Senses. 2005;30(Suppl 1):i184–85. doi: 10.1093/chemse/bjh175. [DOI] [PubMed] [Google Scholar]
- 39.Gagliardo A, Ioale P, Savini M, Wild JM. Having the nerve to home: trigeminal magnetoreceptor versus olfactory mediation of homing in pigeons. J Exp Biol. 2006;209:2888–92. doi: 10.1242/jeb.02313. [DOI] [PubMed] [Google Scholar]
- 40.Gaillard D, Laugerette F, Darcel N, El-Yassimi A, Passilly-Degrace P, et al. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–68. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- 41.Gilbertson TA. Gustatory mechanisms for the detection of fat. Curr Opin Neurobiol. 1998;8:447–52. doi: 10.1016/s0959-4388(98)80030-5. [DOI] [PubMed] [Google Scholar]
- 42.Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 1997;272:C1203–10. doi: 10.1152/ajpcell.1997.272.4.C1203. [DOI] [PubMed] [Google Scholar]
- 43.Gilbertson TA, Liu L, Kim I, Burks CA, Hansen DR. Fatty acid responses in taste cells from obesity-prone and -resistant rats. Physiol Behav. 2005;86:681–90. doi: 10.1016/j.physbeh.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 44.Glasbrenner B, Bruckel J, Gritzmann R, Adler G. Cephalic phase of pancreatic polypeptide release: a valid test of autonomic neuropathy in diabetics? Diabetes Res Clin Pract. 1995;30:117–23. doi: 10.1016/0168-8227(95)01153-6. [DOI] [PubMed] [Google Scholar]
- 45.Gopala Krishna AG, Hemakumar KH, Khatoon S. Acidity of Oryzanol and its contribution to free fatty acids value in vegetable oils. J Am Oil Chem Soc. 2006;83:999–1005. [Google Scholar]
- 46.Greenberg D, Kava RA, Lewis DR, Greenwood MR, Smith GP. Time course for entry of intestinally infused lipids into blood of rats. Am J Physiol. 1995;269:R432–36. doi: 10.1152/ajpregu.1995.269.2.R432. [DOI] [PubMed] [Google Scholar]
- 47.Guichard E. Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Rev Int. 2002;18:49–70. [Google Scholar]
- 48.Hajnal A, Takenouchi K, Norgren R. Effect of intraduodenal lipid on parabrachial gustatory coding in awake rats. J Neurosci. 1999;19:7182–90. doi: 10.1523/JNEUROSCI.19-16-07182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halpern BP. What's in a name? Are MSG and umami the same? Chem Senses. 2002;27:845–46. doi: 10.1093/chemse/27.9.845. [DOI] [PubMed] [Google Scholar]
- 50.Hamilton JA, Johnson RA, Corkey B, Kamp F. Fatty acid transport: the diffusion mechanism in model and biological membranes. J Mol Neurosci. 2001;16:99–108. 151–57. doi: 10.1385/JMN:16:2-3:99. discussion. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton JA, Kamp F. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes. 1999;48:2255–69. doi: 10.2337/diabetes.48.12.2255. [DOI] [PubMed] [Google Scholar]
- 52.Hansen DR, McKenna L, Shah BP, Gilbertson TA. Expression of fatty acid activated G protein coupled receptors in chemosensory cells. Proc. ACHEMS 28th Annu. Meet.; Sarasota, FL. 2006. Abstr. [Google Scholar]
- 53.Heath RB, Jones R, Frayn KN, Robertson MD. Vagal stimulation exaggerates the inhibitory ghrelin response to oral fat in humans. J Endocrinol. 2004;180:273–81. doi: 10.1677/joe.0.1800273. [DOI] [PubMed] [Google Scholar]
- 54.Hiraoka T, Fukuwatari T, Imaizumi M, Fushiki T. Effects of oral stimulation with fats on the cephalic phase of pancreatic enzyme secretion in esophagostomized rats. Physiol Behav. 2003;79:713–17. doi: 10.1016/s0031-9384(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 55.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 56.Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–51. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- 57.Hopman WP, Jansen JB, Rosenbusch G, Lamers CB. Effect of equimolar amounts of long-chain triglycerides and medium-chain triglycerides on plasma cholecystokinin and gallbladder contraction. Am J Clin Nutr. 1984;39:356–59. doi: 10.1093/ajcn/39.3.356. [DOI] [PubMed] [Google Scholar]
- 58.Ibrihimi A, Sfeir Z, Magharaie H, Amri E, Grimaldi P, Abumrad NA. Expression of the CD36 homolog (FAT) in fibroblast cells: effects on fatty acid transport. Proc Natl Acad Sci USA. 1996;93:2646–51. doi: 10.1073/pnas.93.7.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibrahimi A, Abumrad NA. Role of CD36 in membrane transport of long chain fatty acids. Curr Opin Clin Nutr Metab Care. 2002;5:139–45. doi: 10.1097/00075197-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Ikeda K. New seasonings. Chem Senses. 2002;27:847–49. doi: 10.1093/chemse/27.9.847. [DOI] [PubMed] [Google Scholar]
- 61.Imaizumi M, Takeda M, Fushiki T. Effects of oil intake in the conditioned place preference test in mice. Brain Res. 2000;870:150–56. doi: 10.1016/s0006-8993(00)02416-1. [DOI] [PubMed] [Google Scholar]
- 62.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujji R, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–76. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 63.Jackson KG, Robertson MD, Fielding BA, Frayn KN, Williams CM. Second meal effect: modified sham feeding does not provoke the release of stored triacylglycerol from a previous high-fat meal. Br J Nutr. 2001;85:149–56. doi: 10.1079/bjn2000226. [DOI] [PubMed] [Google Scholar]
- 64.Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. Multidimensional chemotropic responses to n-aliphatic acid odorants in the rat olfactory bulb. J Comp Neurol. 1999;409:529–48. [PubMed] [Google Scholar]
- 65.Jones SA. Physical, chemical and sensory aspects of fat replacement. In: Roller S, Jones S, editors. Handbook of Fat Replacers. Boca Raton, FL: CRC Press; 1996. pp. 59–86. [Google Scholar]
- 66.Kadohisa M, Verhagen JV, Rolls ET. The primate amygdala: neuronal representations of the viscosity, fat texture, temperature, grittiness and taste of foods. Neuroscience. 2005;132:33–48. doi: 10.1016/j.neuroscience.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Kajiura SM, Holland KN. Electroreception in juvenile scalloped hammerhead and sandbar sharks. J Exp Biol. 2002;205:3609–21. doi: 10.1242/jeb.205.23.3609. [DOI] [PubMed] [Google Scholar]
- 68.Kamp F, Hamilton JA. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot Essent Fatty Acids. 2006;75:149–59. doi: 10.1016/j.plefa.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Kamphuis MM, Saris WH, Westerterp-Plantenga MS. The effect of addition of linoleic acid on food intake regulation in linoleic acid tasters and linoleic acid nontasters. Br J Nutr. 2003;90:199–206. doi: 10.1079/bjn2003858. [DOI] [PubMed] [Google Scholar]
- 70.Kaplan JM, Siemers W, Grill HJ. Effect of oral versus gastric delivery on gastric emptying of corn oil emulsions. Am J Physiol. 1997;273:R1263–70. doi: 10.1152/ajpregu.1997.273.4.R1263. [DOI] [PubMed] [Google Scholar]
- 71.Katschinski M. Nutritional implications of cephalic phase gastrointestinal responses. Appetite. 2000;34:189–96. doi: 10.1006/appe.1999.0280. [DOI] [PubMed] [Google Scholar]
- 72.Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R447–54. doi: 10.1152/ajpregu.00729.2002. [DOI] [PubMed] [Google Scholar]
- 73.Keast R, Breslin P. An overview of binary taste-taste interactions. Food Qual Pref. 2002;14:111–24. [Google Scholar]
- 74.Kirkmeyer SV, Tepper BJ. Understanding creaminess perception of dairy products using free-choice profiling and genetic responsivity to 6-n-propylthiouracil. Chem Senses. 2003;28:527–36. doi: 10.1093/chemse/28.6.527. [DOI] [PubMed] [Google Scholar]
- 75.Koriyama T, Kohata T, Watanabe K, Abe H. Effects of docosahexaenoic acid content in triacylglycerol on human taste perception. J Food Sci. 2002;67:2352–56. [Google Scholar]
- 76.Kreiss E, Schmitz H, Gebhardt M. Electrophysiological characterization of the infrared organ of the Australian “Little Ash Beetle” Acanthocnemus nigricans (Coleoptera, Acanthocnemidae) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193:729–39. doi: 10.1007/s00359-007-0228-8. [DOI] [PubMed] [Google Scholar]
- 77.Larue C. Oral cues involved in the rat's selective intake of fats. Chem Senses. 1978;3:1–6. [Google Scholar]
- 78.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–84. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–89. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Garcia-Gonzalez DL, van de Voort FR. Determination of free fatty acids in edible oils with the use of a variable filter array IR spectrometer. J Am Oil Chem Soc. 2008;85:599–604. [Google Scholar]
- 81.Madsen PT, Kerr I, Payne R. Echolocation clicks of two free-ranging oceanic delphinids with different food preferences: false killer whales Pseudorca crassidens and Risso's dolphins Grampus griseus. J Exp Biol. 2004;207:1811–23. doi: 10.1242/jeb.00966. [DOI] [PubMed] [Google Scholar]
- 82.Mashek DG, Coleman RA. Cellular fatty acid uptake: the contribution of metabolism. Curr Opin Lipidol. 2006;17:274–78. doi: 10.1097/01.mol.0000226119.20307.2b. [DOI] [PubMed] [Google Scholar]
- 83.Matsumura S, Mizushige T, Yoneda T, Tsuziki S, Inoue K, et al. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res. 2007;28:49–55. doi: 10.2220/biomedres.28.49. [DOI] [PubMed] [Google Scholar]
- 84.Mattes RD. Oral fat exposure alters postprandial lipid metabolism in humans. Am J Clin Nutr. 1996;63:911–17. doi: 10.1093/ajcn/63.6.911. [DOI] [PubMed] [Google Scholar]
- 85.Mattes RD. Physiologic responses to sensory stimulation by food: nutritional implications. J Am Diet Assoc. 1997;97:406–13. doi: 10.1016/S0002-8223(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 86.Mattes RD. The taste of fat elevates postprandial triacylglycerol. Physiol Behav. 2001;74:343–48. doi: 10.1016/s0031-9384(01)00578-9. [DOI] [PubMed] [Google Scholar]
- 87.Mattes RD. Oral exposure to butter, but not fat replacers elevates postprandial triacylglycerol concentration in humans. J Nutr. 2001;131:1491–96. doi: 10.1093/jn/131.5.1491. [DOI] [PubMed] [Google Scholar]
- 88.Mattes RD. Oral fat exposure increases the first phase triacylglycerol concentration due to release of stored lipid in humans. J Nutr. 2002;132:3656–62. doi: 10.1093/jn/132.12.3656. [DOI] [PubMed] [Google Scholar]
- 89.Mattes RD. Effects of linoleic acid on sweet, sour, salty, and bitter taste thresholds and intensity ratings of adults. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1243–48. doi: 10.1152/ajpgi.00510.2006. [DOI] [PubMed] [Google Scholar]
- 90.Mattes RD. Oral detection of short-, medium-, and long-chain free fatty acids in humans. Chem Senses. 2009 doi: 10.1093/chemse/bjn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mattes RD. Brief oral stimulation, but especially oral fat exposure, elevates serum triglycerides in humans. Am J Physiol. 2009 doi: 10.1152/ajpgi.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCance R. Fatty Acids Seventh Supplement to the Fifth Edition of McCance and Widdowson's The Composition of Foods. Cambridge, UK: Royal Soc. Chem.; 1998. p. 209. [Google Scholar]
- 93.McCormack DN, Clyburn VL, Pittman DW. Detection of free fatty acids following a conditioned taste aversion in rats. Physiol Behav. 2006;87:582–94. doi: 10.1016/j.physbeh.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 94.Mela DJ. Sensory assessment of fat content in fluid dairy products. Appetite. 1988;10:37–44. doi: 10.1016/s0195-6663(88)80031-x. [DOI] [PubMed] [Google Scholar]
- 95.Mela DJ, Christensen C. Sensory assessment of oiliness in a low moisture food. J Sens Stud. 1987;2:273–81. [Google Scholar]
- 96.Mela DJ, Langley KR, Martin A. No effect of oral or sample temperature on sensory assessment of fat content. Physiol Behav. 1994;56:655–58. doi: 10.1016/0031-9384(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 97.Mendeloff AI. The effects of eating and of sham feeding upon the absorption of vitamin A palmitate in man. J Clin Invest. 1954;33:1015–21. doi: 10.1172/JCI102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Metzger K, Angres G, Maier H, Lehmann WD. Lipoxygenase products in human saliva: patients with oral cancer compared to controls. Free Radic Biol Med. 1995;18:185–94. doi: 10.1016/0891-5849(94)00108-v. [DOI] [PubMed] [Google Scholar]
- 99.Miettinen SM, Hyvonen L, Tuorila H. Timing of intensity perception of a polar vs nonpolar aroma compound in the presence of added vegetable fat in milk. J Agric Food Chem. 2003;51:5437–43. doi: 10.1021/jf0342066. [DOI] [PubMed] [Google Scholar]
- 100.Mindell S, Smith GP, Greenberg D. Corn oil and mineral oil stimulate sham feeding in rats. Physiol Behav. 1990;48:283–87. doi: 10.1016/0031-9384(90)90314-t. [DOI] [PubMed] [Google Scholar]
- 101.Nasser JA, Kissileff HR, Boozer CN, Chou CJ, Pi-Sunyer FX. PROP taster status and oral fatty acid perception. Eat Behav. 2001;2:237–45. doi: 10.1016/s1471-0153(01)00031-9. [DOI] [PubMed] [Google Scholar]
- 102.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 103.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–90. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 104.Orthoefer FT, Gurkin S, Liu L. Dynamics of frying. In: Perkins EG, Erickson MD, editors. Deep Frying: Chemistry, Nutrition, and Practical Applications. Champaign, IL: AOCS Press; 1996. pp. 223–44. [Google Scholar]
- 105.Paderborn F. Aristoteles Uber die Seele. Paderborn, Germany: Herstellung: Ferdinand Schoningh; 1961. [Google Scholar]
- 106.Parks EJ. Oral fat sensory exposure elevates chylomicron-TG and delays subsequent glucose metabolism. Am. Diabetes Assoc. 68th Annu. Meet.; San Francisco. 2008. [Google Scholar]
- 107.Peri I, Mamrud-Brains H, Rodin S, Krizhanovsky V, Shai Y, et al. Rapid entry of bitter and sweet tastants into liposomes and taste cells: implications for signal transduction. Am J Physiol Cell Physiol. 2000;278:C17–25. doi: 10.1152/ajpcell.2000.278.1.C17. [DOI] [PubMed] [Google Scholar]
- 108.Picard F, Naimi N, Richard D, Deshaies Y. Response of adipose tissue lipoprotein lipase to the cephalic phase of insulin secretion. Diabetes. 1999;48:452–59. doi: 10.2337/diabetes.48.3.452. [DOI] [PubMed] [Google Scholar]
- 109.Pittman D, Crawley ME, Corbin CH, Smith KR. Chorda tympani nerve transection impairs the gustatory detection of free fatty acids in male and female rats. Brain Res. 2007;1151:74–83. doi: 10.1016/j.brainres.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 110.Pittman DW, Labban CE, Anderson AA, O'Connor HE. Linoleic and oleic acids alter the licking responses to sweet, salt, sour, and bitter tastants in rats. Chem Senses. 2006;31:835–43. doi: 10.1093/chemse/bjl026. [DOI] [PubMed] [Google Scholar]
- 111.Powley TL. The ventromedial hypothalamic syndrome, satiety, and a cephalic phase hypothesis. Psychol Rev. 1977;84:89–126. [PubMed] [Google Scholar]
- 112.Raats M, Shepherd R. Free-choice profiling of milks and other products prepared with milks of different fat contents. J Sens Stud. 1992;7:179–203. [Google Scholar]
- 113.Ramirez I. Oral stimulation alters digestion of intragastric oil meals in rats. Am J Physiol. 1985;248:R459–63. doi: 10.1152/ajpregu.1985.248.4.R459. [DOI] [PubMed] [Google Scholar]
- 114.Ramirez I. Chemoreception for fat: Do rats sense triglycerides directly? Appetite. 1992;18:193–206. doi: 10.1016/0195-6663(92)90197-e. [DOI] [PubMed] [Google Scholar]
- 115.Ramirez I. Role of olfaction in starch and oil preference. Am J Physiol. 1993;265:R1404–9. doi: 10.1152/ajpregu.1993.265.6.R1404. [DOI] [PubMed] [Google Scholar]
- 116.Ramirez I. Chemosensory similarities among oils: Does viscosity play a role? Chem Senses. 1994;19:155–68. doi: 10.1093/chemse/19.2.155. [DOI] [PubMed] [Google Scholar]
- 117.Rice HB, Greenberg D, Corwin RL. Different preferences for oils with similar fatty acid profiles. Physiol Behav. 2000;68:755–59. doi: 10.1016/s0031-9384(99)00255-3. [DOI] [PubMed] [Google Scholar]
- 118.Richardson-Harman N, Stevens R, Walker S, Gamble J, Miller M, et al. Mapping consumer perceptions of creaminess and liking for liquid dairy products. Food Qual Pref. 2000;11:239–46. [Google Scholar]
- 119.Roberts DD, Pollien P, Antille N, Lindinger C, Yeretzian C. Comparison of nosespace, headspace, and sensory intensity ratings for the evaluation of flavor absorption by fat. J Agric Food Chem. 2003;51:3636–42. doi: 10.1021/jf026230+. [DOI] [PubMed] [Google Scholar]
- 120.Robertson MD, Parkes M, Warren BF, Ferguson DJP, Jackson KG, et al. Mobilisation of enterocyte fat stores by oral glucose in humans. Gut. 2003;52:834–39. doi: 10.1136/gut.52.6.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rolls ET. Smell, taste, texture, and temperature multimodal representations in the brain, and their relevance to the control of appetite. Nutr Rev. 2004;62:S193–204. S224–41. doi: 10.1111/j.1753-4887.2004.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 122.Rolls ET, Critchley HD, Browning AS, Hernadi I, Lenard L. Responses to the sensory properties of fat of neurons in the primate orbitofrontal cortex. J Neurosci. 1999;19:1532–40. doi: 10.1523/JNEUROSCI.19-04-01532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rolls ET, Verhagen JV, Kadohisa M. Representations of the texture of food in the primate orbitofrontal cortex: neurons responding to viscosity, grittiness, and capsaicin. J Neurophysiol. 2003;90:3711–24. doi: 10.1152/jn.00515.2003. [DOI] [PubMed] [Google Scholar]
- 124.Rukunudin IH, White PJ, Bern CJ, Bailey TB. A modified method for determining free fatty acids from small soybean oil sample sizes. J Am Oil Chem Soc. 1998;75:563–68. [Google Scholar]
- 125.Sawano S, Imaizumi M, Takeda M, Mori T, Fushiki T. Effects of taste and smell on preference for corn oil in mice. Chem Senses. 2001;26:316. [Google Scholar]
- 126.Schafmayer A, Nustede R, Pompino A, Kohler H. Vagal influence on cholecystokinin and neurotensin release in conscious dogs. Scand J Gastroenterol. 1988;23:315–20. doi: 10.3109/00365528809093872. [DOI] [PubMed] [Google Scholar]
- 127.Schiffman SS, Graham BG, Sattely-Miller EA. Orosensory perception of dietary fat. Curr Dir Psychol Sci. 1998;7(5):137–43. [Google Scholar]
- 128.Schiffman SS, McElroy AE, Erickson RP. The range of taste quality of sodium salts. Physiol Behav. 1980;24:217–24. doi: 10.1016/0031-9384(80)90077-3. [DOI] [PubMed] [Google Scholar]
- 129.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1823–32. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 130.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol. 2005;289:R712–20. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 131.Siitari H, Honkavaara J, Viitala J. Ultraviolet reflection and female, mate choice in the pied flycatcher. J Am Oil Chem Soc. 2002;63:97–102. [Google Scholar]
- 132.Smeets AJ, Westerterp-Plantenga MS. Satiety and substrate mobilization after oral fat stimulation. Br J Nutr. 2006;95:795–801. doi: 10.1079/bjn20051725. [DOI] [PubMed] [Google Scholar]
- 133.Smith JC, Fisher EM, Maleszewski V, McClain B. Orosensory factors in the ingestion of corn oil/sucrose mixtures by the rat. Physiol Behav. 2000;69:135–46. doi: 10.1016/s0031-9384(00)00197-9. [DOI] [PubMed] [Google Scholar]
- 134.Smith L, Clifford A, Hamblin C, Creveling T. Changes in physical and chemical properties of shortenings used for commercial deep fat frying. J Am Oil Chem Soc. 1986;63:1017–23. [Google Scholar]
- 135.Spielman S, D'Abundo R, Field R, Schmale H. Protein analysis of human von Ebner saliva and a method for its collection from the foliate papillae. J Dent Res. 1993;72:1331–35. doi: 10.1177/00220345930720091301. [DOI] [PubMed] [Google Scholar]
- 136.Stratford JM, Curtis KS, Contreras RJ. Chorda tympani nerve transection alters linoleic acid taste discrimination by male and female rats. Physiol Behav. 2006;89:311–19. doi: 10.1016/j.physbeh.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 137.Suzuki A, Yamane T, Fushiki T. Inhibition of fatty acid beta-oxidation attenuates the reinforcing effects and palatability to fat. Nutrition. 2006;22:401–7. doi: 10.1016/j.nut.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 138.Tepper BJ, Nurse RJ. Fat perception is related to PROP taster status. Physiol Behav. 1997;61:949–54. doi: 10.1016/s0031-9384(96)00608-7. [DOI] [PubMed] [Google Scholar]
- 139.Tittelbach TJ, Mattes RD. Oral stimulation influences postprandial triacylglycerol concentrations in humans: nutrient specificity. J Am Coll Nutr. 2001;20:485–93. doi: 10.1080/07315724.2001.10719057. [DOI] [PubMed] [Google Scholar]
- 140.Tso P. Gastrointestinal digestion and absorption of lipid. Adv Lipid Res. 1985;21:143–86. doi: 10.1016/b978-0-12-024921-3.50011-3. [DOI] [PubMed] [Google Scholar]
- 141.Tsuruta M, Kawada T, Fukuwatari T, Fushiki T. The orosensory recognition of long-chain fatty acids in rats. Physiol Behav. 1999;66:285–88. doi: 10.1016/s0031-9384(98)00299-6. [DOI] [PubMed] [Google Scholar]
- 142.Tuorila H. An application of the repertory grid method in food acceptance research. Lebensm Wiss Technol. 1986;19:344–45. [Google Scholar]
- 143.Verhagen JV, Rolls ET, Kadohisa M. Neurons in the primate orbitofrontal cortex respond to fat texture independently of viscosity. J Neurophysiol. 2003;90:1514–25. doi: 10.1152/jn.00320.2003. [DOI] [PubMed] [Google Scholar]
- 144.Wan PJ, Pakarinen DR, Wakelyn PJ. Concerns for the determination of free fatty acid in cottonseed. J Am Oil Chem Soc. 1998;75:1321–24. [Google Scholar]
- 145.Weiss T. Food Oils and Their Uses. Westport, CT: Avi Publ.; 1970. [Google Scholar]
- 146.Wisen O, Bjorvell H, Cantor P, Johansson C, Theodorsson E. Plasma concentrations of regulatory peptides in obesity following modified sham feeding (MSF) and a liquid test meal. Regul Pept. 1992;39:43–54. doi: 10.1016/0167-0115(92)90007-h. [DOI] [PubMed] [Google Scholar]
- 147.Wojdemann M, Olsen O, Norregaard P, Sternby B, Rehfeld JF. Gastric lipase secretion after sham feeding and cholinergic blockade. Dig Dis Sci. 1997;42:1070–75. doi: 10.1023/a:1018805623669. [DOI] [PubMed] [Google Scholar]
- 148.Wojdemann M, Traberg P, Stadil F, Sternby B, Larsen S, et al. Effect of sham feeding and acute suppression of acid secretion on human gastric lipase secretion. Am J Gastroenterol. 1998;93:244–48. doi: 10.1111/j.1572-0241.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- 149.Woods SC, D'Alessio DA, Tso P, Rushing PA, Clegg DJ, et al. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83:573–78. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 150.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101:1045–50. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yu T, Shah B, Liu P, Hansen DR, Gilbertson TA. Fatty acid-induced changes in intracellular calcium in somatosensory cells; mechanisms underlying the textural perception of fat. Intl. Symp. Olfaction Taste XV; San Francisco, CA. 2008. Abstr. [Google Scholar]
- 152.Yu T, Shah BP, Hansen DR, Gilbertson TA. Fatty acid responses in rat trigeminal neurons, textural cues for dietary fat. Keystone Symp.; Snowbird, UT. 2007. Abstr. [Google Scholar]
- 153.Zafra MA, Molina F, Puerto A. The neural/cephalic phase reflexes in the physiology of nutrition. Neurosci Biobehav Rev. 2006;30:1032–44. doi: 10.1016/j.neubiorev.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 154.Zubare-Samuelov M, Shaul ME, Peri I, Aliluiko A, Tirosh O, Naim M. Inhibition of signal termination-related kinases by membrane-permeant bitter and sweet tastants: potential role in taste signal termination. Am J Physiol Cell Physiol. 2005;289:C483–92. doi: 10.1152/ajpcell.00547.2004. [DOI] [PubMed] [Google Scholar]