Abstract
Elevated mineralocorticoid levels and female sex hormones have been shown to confer opposing effects on renal injury, but their combined effects are still unknown.
Objective
Identify the function of estrogens and of different synthetic progestins on aldosterone salt–mediated renal disease.
Methods
The role of 17β-estradiol, medroxyprogesterone acetate (MPA), and drospirenone during renal injury was studied in Wistar rats subjected to uni-nephrectomy plus aldosterone salt treatment.
Results
Aldo-salt treatment of intact, ovariectomized, and estradiol-treated female rats resulted in remnant kidney hypertrophy without structural damage. Co-treatment with MPA, but not with drospirenone, increased kidney hypertrophy, fluid turnover, sodium retention, and potassium excretion. Medroxyprogesterone acetate also caused glomerular, vascular, tubular, and interstitial lesions that were accompanied by increased blood pressure and enhanced NADPH oxidase (p67phox) and sodium channel (α-ENaC) expression. Drospirenone, a progestin with anti-mineralocorticoid function, and spironolactone prevented kidney hypertrophy, hypertension, and sodium retention. Drospirenone and spironolactone also increased renal angiotensin II type 2 receptor expression and relieved aldosterone-induced suppression of serum angiotensin II levels.
Conclusion
The choice of specific synthetic progestins has profound implications on the development of kidney injury and renal gene expression under conditions of elevated aldosterone serum levels and salt intake.
Keywords: sex, aldosterone, renal injury, progesterone, sex hormones
Introduction
Aldosterone mediates salt and water homeostasis via binding and activation of the mineralocorticoid receptor (MR) in renal epithelial cells. But excessive or disproportional MR activation by aldosterone may cause renal damage including glomerular injury, interstitial inflammation and fibrosis, and tubular damage (Blasi et al. 2003). The sexually dimorphic pattern of nondiabetic renal disease, in which men develop a more rapid decline of renal function compared to women, suggests that male and female sex hormones might modulate the development of renal injury (Donadio et al. 1988; Gretz et al. 1989; Neugarten, Acharya, and Silbiger 2000). This hypothesis is supported by experimental studies that indicate an aggravation of renal injury by androgens and a protective function of estrogens (Antus et al. 2005; Gross et al. 2004; Mulroney et al. 1999; Verzola et al. 1999). Previously, we have shown that 17β-estradiol (E2) attenuates cardiovascular damage and that medroxyprogesterone (MPA), but not drospirenone (DRO), aggravates cardiac hypertrophy and fibrosis in ovariectomized (ovx), aldosterone-salt–treated rats (AST) (Arias-Loza et al. 2006). However, it remains unknown whether estradiol, MPA, and DRO might have also affected the kidney phenotype of female rats that were exposed to long-term aldo-salt treatment.
The synthetic progestin medroxyprogesterone acetate (MPA) binds not only to the progesterone receptor (PR) but also to the mineralocorticoid- (MR), glucocorticoid- (GR), and androgen receptors (AR) (Sitruk-Ware 2002). However, modulation of MR, GR, and AR activity is not required for human hormone replacement therapy (HRT) and may promote undesirable cardiovascular and renal side effects. In addition, it has been speculated that prospective clinical trials on the prevention of cardiovascular disease in postmenopausal women may have failed in part because MPA might have blocked a beneficial function of estrogen substitution (Grady et al. 2002; Manson et al. 2003; Mendelsohn and Karas 2007). In contrast to MPA, DRO acts as a potent PR agonist and MR antagonist (Muhn et al. 1995). Furthermore, DRO is devoid of any GR and AR agonist activity and confers protective cardiovascular effects in experimental and clinical studies (Arias-Loza et al. 2006; Fuhrmann et al. 1996; White et al. 2005).
Based on these observations, we speculated that MPA, but not DRO, might enhance kidney hypertrophy, structural damage, and renal salt uptake, which is required for the development of cardiovascular and renal injury in aldosterone-treated rats. In support of this concept, we demonstrate that DRO is as efficient as the MR antagonist spironolactone (SPIRO) in protecting female rats against aldosterone-mediated renal disease, whereas MPA treatment caused enhanced sodium uptake and extensive renal damage in estrogen-substituted AST rats.
Methods
Animal Treatment
The current study was approved by the local ethics committee and conducted in accordance with the current National Institutes of Health (NIH) guide for the care and use of laboratory animals. Female Wistar rats that were obtained from IFFA CREDO (Lyon, France) at the age of twelve weeks were randomized and received one of the following treatments for two months. Group 1 underwent sham operation for uninephrectomy and ovx and placebo treatment. Group 2 underwent uni-nephrectomy by a left-sided nephrectomy, bilateral ovx, and placebo treatment. Animals in groups 3 to 10 received uni-nephrectomy (npx, groups 4-10) and either sham operation (intact, group 3, n = 10) or ovx (ovx, groups 4-10, n = 10/group). All animals from groups 3 to 10 were subjected to chronic aldosterone-salt treatment (AST; 0.75 μg aldosterone/hour via Alzet pumps plus 1% NaCl added to tap water). Aldosterone-salt treatment groups consisted of groups 3 and 4, placebo; group 5, E2 (2 μg/kg/d); group 6, E2 plus SPIRO (20 mg/kg/d); group 7, E2 plus DRO low dose (3 mg/kg/d); group 8, E2 plus DRO medium dose (9 mg/kg/d); group 9, E2 plus DRO high dose (30 mg/kg/d); group 10, E2 plus MPA (3 mg/kg/d). The 3-mg dosage of MPA and the 9-mg dosage of DRO were chosen because they represent the minimum dosages that are required for pregnancy maintenance in ovx rats. Steroids were dissolved in EtOH and administered by daily subcutaneous injections using either peanut oil (E2) or castor oil (MPA, DRO) as the carrier; SPIRO was supplied with tap water. Animals in groups 1 through 3 received peanut plus castor oil, and animals in group 4 received castor oil injections. Surgical procedures were carried out under isoflurane anesthesia (isoflurane 1.5 vol% supplemented by 0.51L oxygen per minute) following pretreatment with tribromoethanol/amylene hydrate (“Avertin”; 2.5% wt/vol, 6 μL/g body weight, intraperitoneal).
Blood Pressure Measurements and Blood Sampling
Mean blood pressure was calculated from invasive systolic and diastolic blood pressure measurements after eight weeks of continuous treatment according to published protocols (Pelzer et al. 2005). After blood pressure measurements, rats were exsanguinated under deep anesthesia via polyethylene tubing placed in the right common carotid artery. Whole blood samples were placed on ice, and serum was obtained by centrifugation. Serum estradiol and angiotensin II levels were determined by radioimmunoassays (estradiol, DPC-Biermann; angiotensin II, Peninsula). Serum electrolytes (Na+, K+) were measured by routine clinical techniques.
Morphometry
Body weight, absolute kidney weight, and uterus weight were determined following blood pressure analysis.
Renal Pathology
Kidney specimens, originally frozen in TissueTec OCT, were thawed and fixed in 10% neutral buffered formalin and processed for conventional paraffin sectioning. Multiple sections were prepared from each animal for hematoxylin and eosin, Mallory trichrome, or Congo red staining. The trichrome stain was used to confirm the presence of collagen, and the Congo red was used to rule out the presence of amyloid. The renal pathology index included the following criteria: (1) glomerular sclerosis (mesangiolysis, glomerular fibrinoid necrosis and thrombosis), (2) sclerosis of renal arcuate blood vessels (fibrinoid necrosis, thrombosis, segmental myointimal proliferation, perivascular fibrosis), (3) interstitial nephritis (interstitial infiltration of lymphoid cells, interstitial fibrosis), and (4) tubular damage (tubular dilatation, tubular protein casts, tubular regeneration). The subjective grading scales for each parameter consisted of normal (0), minimal (1), mild (2), moderate (3), and marked (4). A renal pathology index was generated by combining all four evaluation parameters. All kidney evaluations were done without knowledge of treatment status.
Metabolic Measurements
After seven weeks of treatment, fluid consumption and excretion were analyzed by placing rats in metabolic cages for a twenty-four-hour adjustment period followed by a twenty-four-hour sampling period with continued treatment and liquids as well as food ad libitum (Cordaillat et al. 2005).
Protein Expression
Renal protein expression was analyzed by Western blotting of crude kidney extracts generated from the remnant right kidney. The following antibodies were employed: anti-α-ENaC (Abcam, Cambridge, UK, 1:2000 rabbit polyclonal), anti-rac-1 (BD Transduction L, California, USA, 1:500 mouse monoclonal), antip67phox (BD Transduction L, California, USA, 1:500 mouse monoclonal), anti-MnSOD (Upstate, Chemicon; Schwalbach, Germany, 1:2000 rabbit polyclonal), anti-angiotensin-II type 1 receptor (AT1R, Santa Cruz, Heidelberg, Germany, 1:100 rabbit polyclonal) and anti-angiotensin-II type 2 receptor (AT2R, Alpha Diagnostics International, Herford, Germany, 1:250 rabbit polyclonal). Crude renal extracts were subjected to SDS PAGE gel electrophoresis and transferred to nylon membranes, and immunoreactive proteins were visualized by HRP-coupled antibodies (Amersham, Freiburg, Germany) and ECL. The ImageQuant software Scan Pack 3.0 (Biometra, Goettingen, Germany 1998) was used for densitometric analysis based on peak area. Specificity of the AT2R antibody was determined by Western blots in the presence or absence of a specific blocking peptide (16 ng/μL AT21-P, Alpha Diagnostics International). Equal loading was assessed by reprobing membranes with a GAPDH antibody (Chemicon, 1:3000 mouse monoclonal).
Dihydroethidium Fluorescent Microphotography
Dihydroethidium (DHE) was used to evaluate the in situ production of superoxide according to published protocols (Socha et al. 2007). In brief, nonfixed and frozen renal samples were cut into 10-μm-thick sections and placed on glass slides. Slides were incubated in the presence of DHE (0.1 μM; D1168; Molecular Probes, Karlsruhe, Germany) in a light-protected humidified chamber (37°C, twenty minutes) and washed in phosphate-buffered saline for five minutes before co-staining with the DNA-specific dye SYTOX Green (Invitrogen, Karlsruhe, Germany). After PBS washing, sections were mounted with the VectaShield mounting medium (Vector Laboratories, Otzberg, Germany), and imaging was performed on a fluorescence imaging system (Keyence/Biozero, Neu-Isenburg, Germany). Local ROS generation, which oxidizes DHE to ethidium, yielded a bright red nuclear fluorescence signal that was easily discriminated from non-oxidized DHE, which gives a blue cytosolic fluorescence signal. Dihydroethidium staining was quantified by measuring pixel density of 1600 to 2000 random nuclei per animal (n = 4/group) using Scion software (Version Alpha 4.03.2; NIH, Maryland-USA 1995). Red ethidium fluorescence was normalized using the Sytox green fluorescence staining of all nuclei. Negative controls included renal sections that were pre-incubated for sixty minutes with superoxide dismutase (SOD, 120 U/mL; Sigma-Aldrich, Germany), catalase (100 U/mL), and apocynin (300 μmol/L; Sigma-Aldrich).
Nitrotyrosine and PCNA Immunofluorescence
Cryosections (10 μm thick) were fixed in 4% paraformaldehyde (Nitrotyrosin) or cold methanol:acetone solution (PCNA) and subsequently stained with specific primary antibodies (anti-nitrotyrosin monoclonal antibody HM11, Biomol, at a 1:100 dilution; anti-proliferating cell nuclear antigen F-2, Santa Cruz, at a 1:10 dilution) followed by incubation with a donkey anti-mouse IgG Alexa Fluor 488 conjugate (1:250 dilution; Invitrogen). 4,6-Diamidino-2-phenylindole dihydrochloride (DAPI; Sigma-Aldrich) was used to stain nuclei and control cellular density. The negative control consisted either of nonrelevant primary antibody or anti-nitrotyrosin primary antibody that was pre-incubated with 3-nitro-tyrosin solution (10 mmol/L; Sigma-Aldrich).
PCNA Immunofluorescence Quantification
Digitalized pictures of the PCNA fluorescence signal were transformed into grayscale and loaded in the Image J (Version 1.42; NIH, Maryland-USA 1997) program (four pictures per animal, four animals per group). Images were threshold to standard values, and an analysis of particle area and intensity was performed. Total particle signal for the PCNA signal of a picture was normalized against the corresponding total particle signal for DAPI.
Statistics
Results are presented as mean ± SEM. Multigroup comparisons were done by one-way or two-way analysis of variance followed by Student-Newman-Keuls post hoc all pairwise testing using SigmaStat 2.03 software, version 2.03 California, USA 1996, and p values < .05 were considered significant.
Results
Global, Hemodynamic, and Metabolic Parameters
Absolute kidney weight was comparable among intact, ovx, and E2-substituted AST rats and was significantly greater than that of uni-nephrectomized female rats not receiving AST treatment (Table 1). Co-treatment with the medium or the high dose of DRO or SPIRO attenuated kidney hypertrophy. Remnant kidney mass was significantly greater in AST rats receiving E2 plus MPA compared to all other groups. Although all dosages of DRO as well as SPIRO attenuated blood pressure levels in E2-treated AST rats, MPA co-treatment resulted in blood pressure levels that were similar to those observed in ovx AST rats.
Table 1.
Cardiac weight and serum markers of renal toxicity.
| Sham (n = 10) | Ovx/npx (n = 5) | Intact/npx AST placebo (n = 10) | Ovx/npx AST placebo (n = 10) | Ovx/npx AST + E2 (n = 10) | Ovx/npx AST + E2 + spiro (n = 10) | Ovx/npx AST + E2 + dro 3mg (n = 10) | Ovx /npx AST + E2 + dro 9mg (n = 10) | Ovx/npx AST + E2 + dro 30mg (n = 10) | Ovx/npx AST + E2 + MPA (n = 10) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Heart weight (mg) | 637 ± llac | 709 ± 21ac | 809 ± 11c | 922 ± 35c | 762 ± 20ac | 788 ± 18c | 838 ± 68c | 743 ± 32ac | 752 ± 16ac | 1082 ± 81 |
| Heart weight/tibia length (mg/cm) | 167 ± 3ac | 187 ± 6ac | 213 ± 3c | 242 ± 9c | 200 ± 5ac | 207 ± 5c | 221 ± 19c | 194 ± 8ac | 192 ± 4ac | 279 ± 21 |
| Serum creatinine (mg/dL) | 0.4 ± 0.01 | 0.5 ± 0.04 | 0.4 ± 0.02 | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.4 ± 0.03 | 0.4 ± 0.02 | 0.3 ± 0.03 | 0.4 ± 0.02 | 0.4 ± 0.04 |
| Serum urea (mg/dL) | 59.1 ± 7 | 70.4 ± 6 | 41.2 ± 2 | 49 ± 2 | 55 ± 5 | 59 ± 8 | 50.4 ± 5 | 46.3 ± 2 | 62.1 ± 6 | 43.1 ± 5 |
| Serum albumin (g/dL) | 3.6 ± 0.30 | 3.5 ± 0.06 | N.A. | 3.2 ± 0.15b | 3.9 ± 0.17abc | 4.0 ± 0.03abc | 3.8 ± 0.09abc | 3.9 ± 0.13abc | 3.8 ± 0.16abc | 2.7 ± 0.25b |
Note: Values are mean ± SEM. Significances:
p < .05 vs. ovx/npx AST placebo
p < .05 vs. intact/npx AST + placebo
p < .05 vs. ovx/npx AST + E2 + mpa
§ p < .05 vs. ovx/npx AST + E2
‡ < .05 vs ovx/npx
∥ p < .05 vs. ovx/npx and sham.
Abbreviations: AST, aldosterone salt treatment; MPA, medroxyprogesterone acetate; E2, 17β-estradiol; ovx, ovarectomy; npx, uni-nephrectomy; dro, drospirenone; spiro, spironolactone.
The physiological effects of hormone supplementation were controlled by measuring uterus mass, which was significantly decreased in ovx rats and was recovered by E2 supplementation. Drospirenone, but not SPIRO, attenuated the increase of uterine mass that was observed in E2-substituted rats. Increased body mass among estrogen-depleted compared to intact rats was normalized by substituting E2 and not altered by DRO, MPA, or SPIRO treatment. Serum estradiol concentrations were lower in ovx rats receiving placebo compared to intact or estrogen substituted animals. Serum angiotensin II concentrations, which were suppressed in AST compared to uni-nephrectomized rats, did not differ among intact and ovx AST rats receiving placebo, E2, or E2 plus MPA (Table 1). However, angiotensin II serum levels increased gradually and significantly in estrogen-substituted AST rats receiving increasing dosages of DRO or SPIRO. Serum potassium concentrations were lowest in AST rats treated with placebo or E2 plus MPA and increased significantly in animals receiving the 30 mg dose of DRO. Water and sodium uptake (not shown) as well as fluid excretion were greater in AST rats receiving E2 plus MPA compared to all other groups. Sodium uptake and renal fluid excretion were lower in E2-substituted rats receiving additional DRO or SPIRO treatment.
Kidney Histology
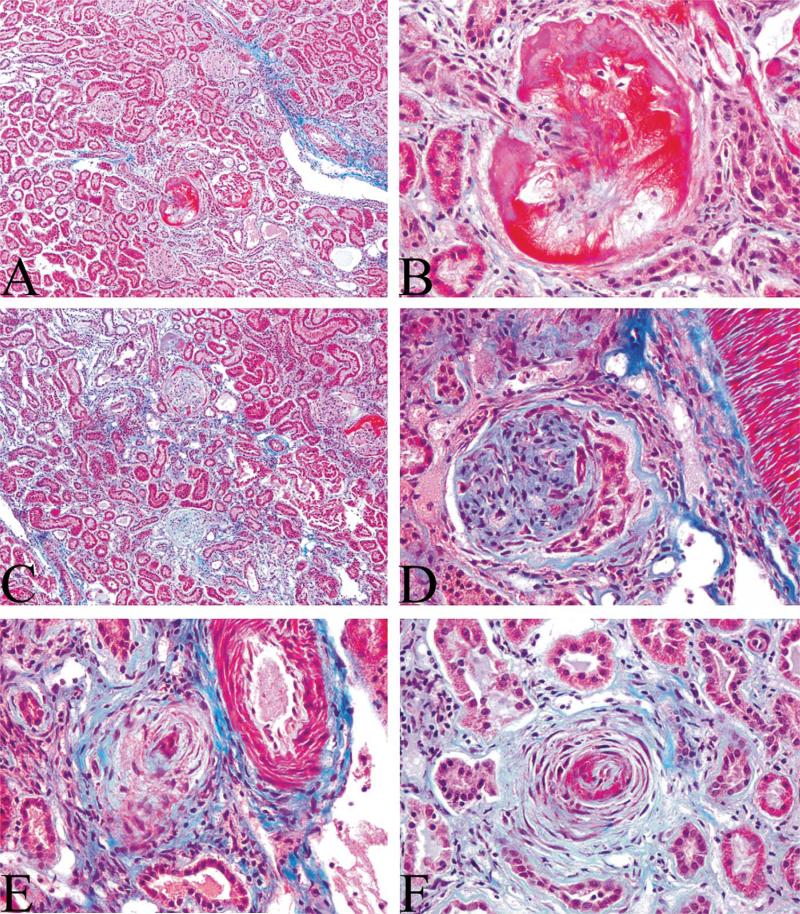
Kidney morphological changes were within normal limits among all groups, with the exception of AST rats receiving MPA plus E2, which showed extensive renal damage including generalized glomerular sclerosis, fibrinoid necrosis, mesangiolysis, and periglomerular fibrosis (Figure 1; ovx AST E2 MPA: grade 4+). Renal pathology also included extensive vascular lesions as well as tubular and interstitial lesions. Myointimal proliferation and sclerosis affected a large number of small cortical arteries, leading to complete occlusion of several vessels. Perivascular fibrosis was also present around occasional cortical vessels. Multifocal to generalized tubular degeneration and necrosis accompanied by interstitial lymphocyte infiltration and fibroplasia was also observed in AST rats receiving MPA. Dilated tubules frequently contained eosinophilic protein material that most likely resulted from glomerular damage. No such effects were observed in any other treatment group (all grade 1+). Representative kidney sections from all treatment groups are available in the form of an online supplement (please see online supplement [http://tpx.sagepub.com/supplemental]).
Figure 1.
Photomicrographs of trichrome-stained renal sections from AST rats receiving combined treatment with estradiol and medroxyprogesterone acetate illustrate marked structural damage (grade 4+) including glomerular mesangiolysis and fibrinoid necrosis (A and B); glomerulosclerosis and periglomerular fibrosis (C and D); and vascular myointimal proliferation, sclerosis, and perivascular fibrosis (E and F). A full set of representative kidney sections from all treatment groups is available in the online supplement.
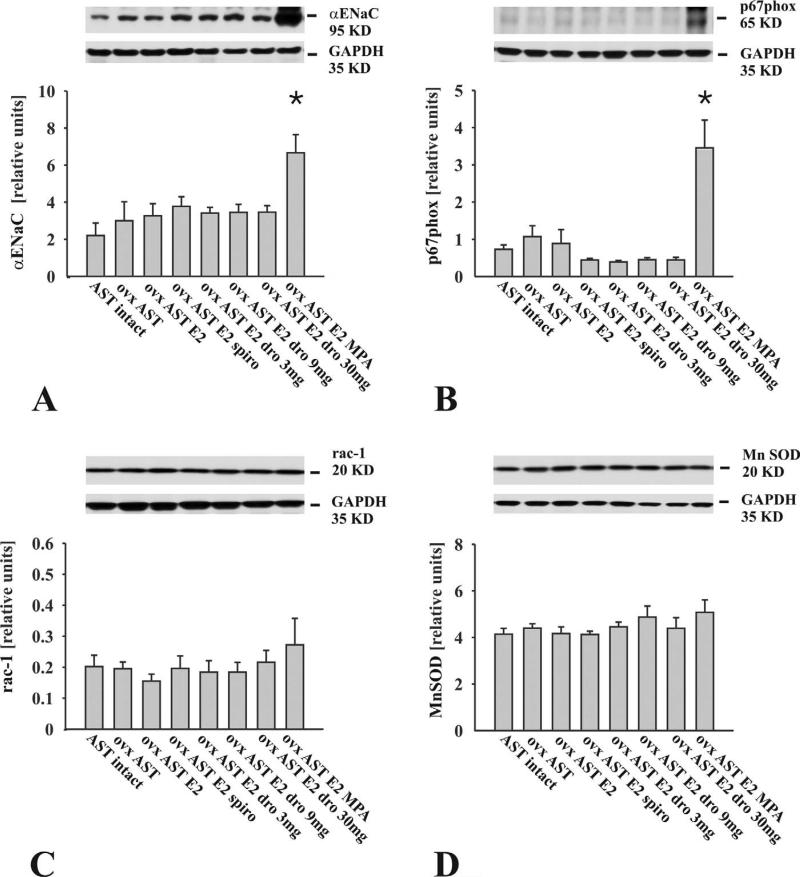
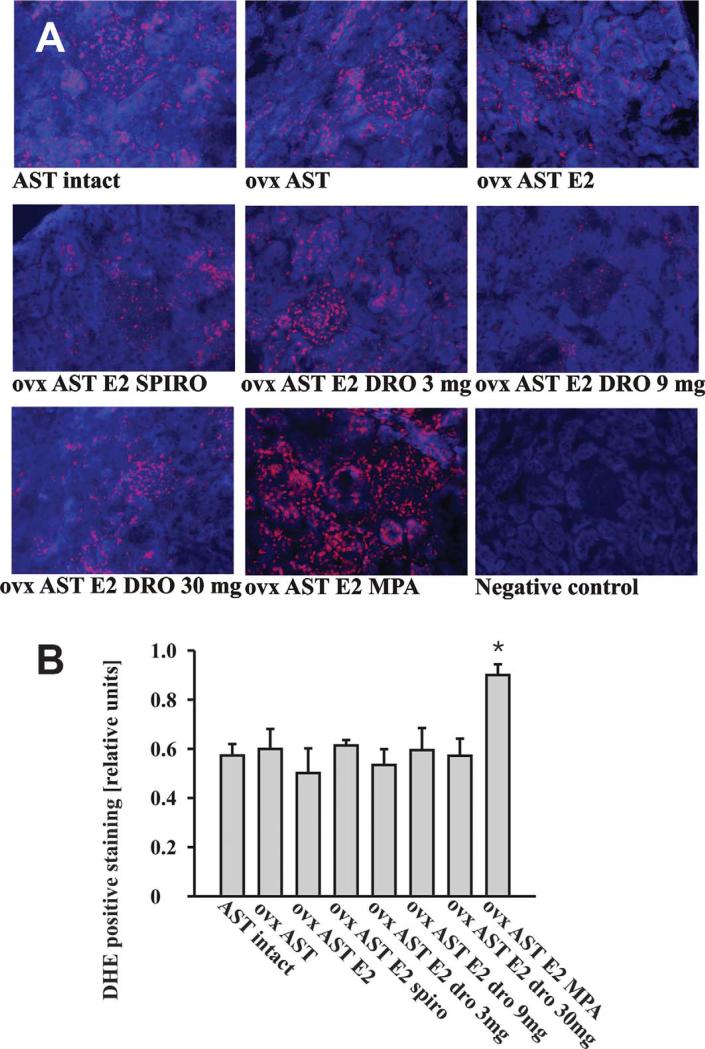
Renal α-ENaC Expression and Oxidative Stress
Expression levels of the α-epithelial Na+ channel (α-ENaC) were significantly elevated in AST rats receiving MPA plus E2 compared to all other treatment groups (Figure 2A). Nuclear (red) DHE fluorescence indicating increased local superoxide production was enhanced only in renal sections from animals receiving MPA plus E2 (Figure 3). Nitrotyrosine immunoreactivity was detected only in glomeruli of animals receiving MPA (Figure 4). Renal expression levels of the stimulatory NADPH-oxidase subunit p67phox were significantly elevated in AST rats receiving MPA plus E2 (Figure 2B). Estradiol, DRO, or SPIRO treatment did not alter the abundance of α-ENaC and p67phox. Rac1 and manganese-dependent superoxide dismutase (MnSOD) were expressed at comparable levels among all animals (Figure 2C and 2D).
Figure 2.
Representative Western blots and bar graphs illustrate the expression of the α-epithelial Na+ channel α-ENaC, the NADPH subunits p67phox, rac1, and manganese-dependent superoxide dismutase in unfractionated kidney extracts. (A) α-ENaC abundance was significantly increased only in AST rats receiving medroxyprogesterone plus estradiol. (B) p67phox abundance was significantly increased only in AST rats receiving medroxyprogesterone plus estradiol. (C, D) Rac1 and MnSOD expression levels were comparable among all animals. (n = 10 animals/group; *p < .05 vs. all groups).
Figure 3.
Representative renal sections stained with the fluorescent dye DHE (A) and quantification of nuclear ethidium staining (B) illustrate increased renal reactive oxygen species generation in medroxyprogesterone acetate–treated AST rats. Reactive oxygen species-dependent DHE oxidation yields a red fluorescence signal that localizes to nuclei. Nonoxidized DHE appears as a blue cytosolic fluorescence signal. (n= 4 animals/group; *p < 0.05 vs. all groups). Abbreviations: ovx (ovarectomy); AST (aldosterone-salt treatment); DRO (drospirenone); MPA (medroxyprogesterone acetate); E2 (17β-estradiol); SPIRO (spironolactone).
Figure 4.
Representative photomicrographs of renal sections illustrate increased protein nitrosylation in aldosterone-salt–treated rats receiving medroxyprogesterone acetate. Tyrosin-nitrosylation is indicated by a green fluorescent signal that localized predominantly to the glomerular compartment and was observed only in renal sections in aldosterone-salt–treated rats receiving medroxyprogesterone acetate (n = 10 animals). Abbreviations: ovx (ovarectomy); AST (aldosterone-salt treatment); DRO (drospirenone); MPA (medroxyprogesterone acetate); E2 (17β-estradiol); SPIRO (spironolactone).
Renal Angiotensin II Type 1 and Type 2 Receptor Expression
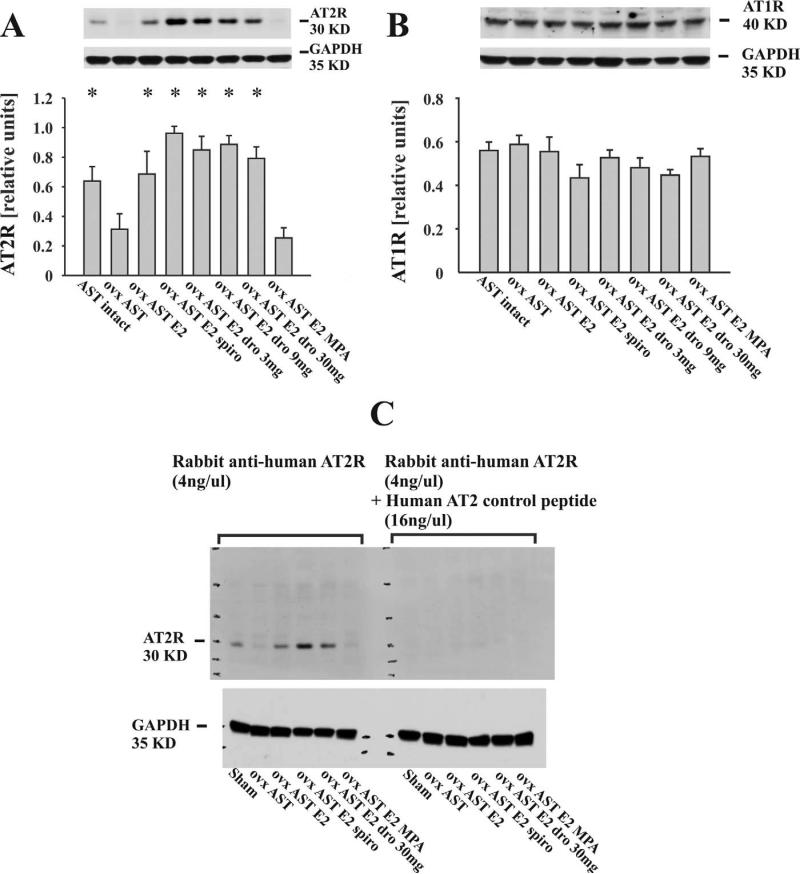
Lower AT2R expression in ovx compared to intact AST rats was restored by E2 (Figure 5A). Co-treatment with DRO, or with SPIRO further increased renal AT2R expression in E2-substituted AST rats. Medroxyprogesterone acetate blocked the induction of AT2R expression that was observed in estradiol treated animals. Expression of AT1R receptor was uniform among all treatment groups (Figure 5B).
Figure 5.
Representative Western blots and bar graphs illustrate renal angiotensin-II receptor expression. (A) Lower renal angiotensin II type 2 receptor (AT2R) expression in ovx compared to intact aldosterone-salt–treated (AST) rats was restored by estrogen treatment. Medroxyprogesterone acetate (MPA) blocked the increase of AT2R expression in estradiol-treated rats. Drospirenone and SPIRO further enhanced renal AT2R expression in estradiol-treated animals. (B) Renal AT1R expression was comparable among all animals (n = 10 animals/group; *p < .05 vs. ovx AST placebo and ovx AST + E2 + MPA). (C) Antibody competition assay illustrating the specificity of the AT2R antibody for its cognate polypeptide. Renal extracts (100 μg/lane) from six independent animals were subjected to Western blotting and probing with the polyclonal anti-AT2R antibody after pre-incubation with a specific blocking peptide or buffer. Note the disappearance of the 30 KD band representing the rat renal AT2R upon pre-incubation of the AT2R antibody with the blocking peptide.
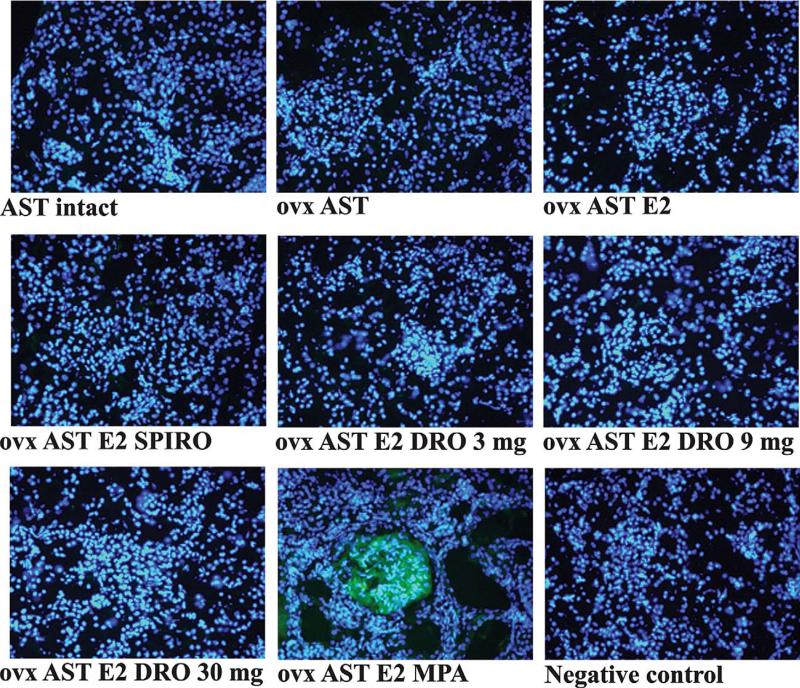
Proliferating Cell Nuclear Antigen Expression in the Kidney
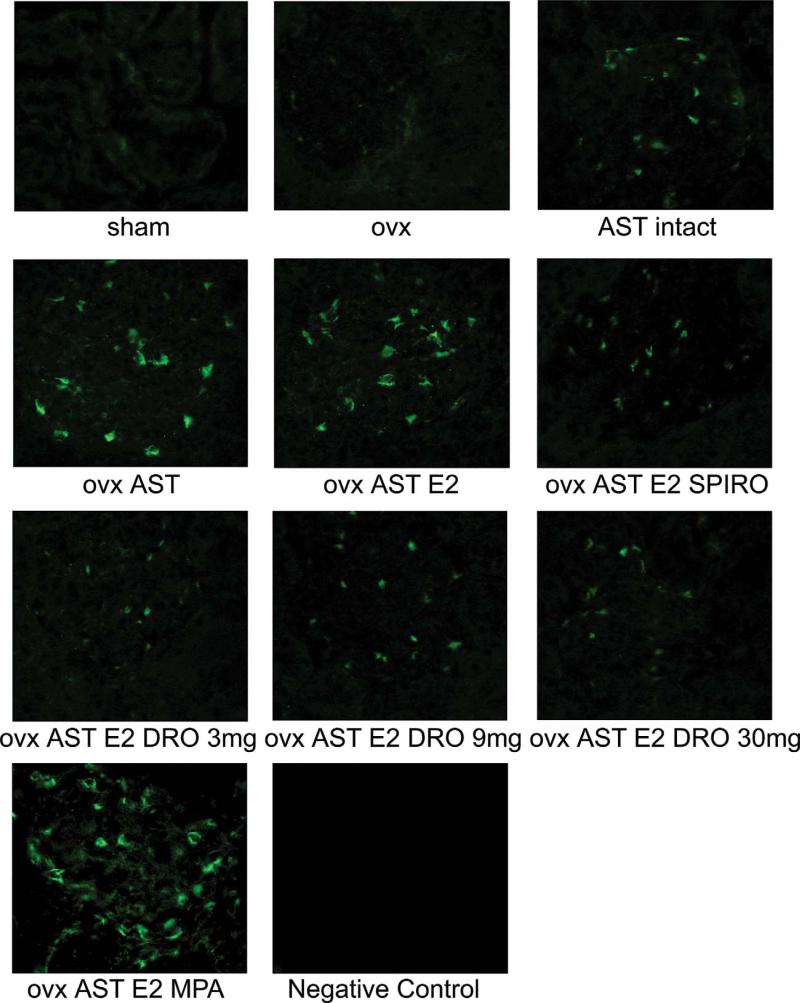
The number of renal proliferating cell nuclear antigen (PCNA)-positive cells was higher in rats receiving AST in comparison to sham-operated rats. Although E2 supplementation did not affect PCNA expression, addition of MPA resulted in a further PCNA staining increase, as opposed to the MR blockade by SPIRO and drospirenone, which reduced the renal PCNA staining (Figure 6).
Figure 6.
Renal immunofluorescence staining for PCNA. Pictures correspond to representative staining of kidney sections, a consistent increase in the number of PCNA positive cells was observed in the glomeruli of animals receiving AST. PCNA staining was unaffected by the estradiol status of the animal and was further increased by MPA treatment. Spironolactone and drospirenone treatment lowered the PCNA staining in AST rats. Abbreviations: ovx (ovarectomy); AST (aldosterone-salt treatment); DRO (drospirenone); MPA (medroxyprogesterone acetate); E2 (17β-estradiol); SPIRO (spironolactone).
Renal Nuclear Hormone Receptor Expression
Expression levels of both estrogen receptor isoforms, ERα and ERβ; the mineralocorticoid receptor; and the glucocorticoid receptor were not significantly different among all treatment groups (please see online supplement).
Discussion
The present study is the first to evaluate the effect of E2 and of synthetic progestins with different pharmacological profiles on renal injury in rats with an excess activation of the mineralocorticoid receptor. We demonstrate that a progestin, which is devoid of partial AR and GR activities and that acts as a potent MR antagonist (DRO) confers protective functions, whereas MPA caused adverse effects on renal structure and function and on gene expression in female AST rats.
Mineralocorticoids and sex hormones affect gene expression and kidney morphology and function via specific nuclear hormone receptors for estrogens, progestins, glucocorticoids, and androgens. In models of hypertension employing male rats, activation of the mineralocorticoid receptor by MR agonists contributes to kidney damage, and administration of MR antagonists or adrenalectomy attenuates renal injury (Rocha et al. 1999, 2000). In addition, AST treatment in male rats is characterized by vascular and glomerular sclerosis, fibrinoid necrosis, tubular damage, and increased inflammation markers in addition to increased cellular proliferation, which is detectable by PCNA staining (Blasi et al. 2003; Peng et al. 2001).
Although it is conceivable that sex differences in renal disease might be explained in part by different sex hormone levels, chronic AST, which causes kidney hypertrophy and injury in male rodents, has so far not been studied in female rats. Here, we describe the renal effects of AST treatment on female rats. In contrast to male animals, aldosterone did not cause the degree of extensive renal damage that has been reported for male rats. Although kidney mass is lower in female versus male rats, AST treatment caused a similar extent of kidney hypertrophy as that observed previously in male AST rats. Kidney weight: 1404 mg female AST vs. 711 mg female control; 3618 mg male AST vs. 2192 mg male control as reported by Peng (2001). Sex-associated differences in aggravation of renal hemodynamics in response to angiotensin II have been analyzed in single nephrons from male and female rats, showing that male nephrons present lower preglomerular resistance (Baylis 1994). Similar to what has been reported in male rats (Peng et al. 2001), the increase of kidney weight in female AST rats was accompanied by augmented PCNA staining, suggesting that aldosterone induced increased cellular proliferation. However, the onset of inflammatory processes and renal fibrosis appears to be delayed in female compared to male rats as, except for MPA-treated animals, we were not able to detect a significant inflammation and renal fibrosis in the kidneys of female AST rats. In line with this hypothesis, Caron et al. (2006) reported on a sexual dimorphism with a delayed onset of inflammation in female adrenomedullin one-copy:Ren transgenic mice. Furthermore, testosterone is known to increase plasma IL-1B concentrations (Machowska et al. 2004). Interestingly, kidney hypertrophy and hypertension were differentially affected by estradiol substitution, because blood pressure, but not kidney mass, responded favorably to estrogen treatment. These observations are in agreement with previous studies on blood pressure regulation in estrogen-treated AST rats and match with persistent kidney hypertrophy in estrogen-treated stroke-prone SHR rats (Arias-Loza et al. 2006, 2007; Gross et al. 2004). Esqueda, Craig, and Hinojosa-Laborde (2007) recently reported that estrogen status affects renal ERα and ERβ expression in salt-sensitive and salt-resistant Dahl rats. Therefore, it is important to note that global ERα and ERβ content did not differ among kidneys samples from all treatment groups.
The current findings therefore substantiate the hypothesis that sex differences also play an important role in the development of aldosterone-salt–induced renal injury similar to what is already known in cardiovascular disease (Kim and Levin 2006; Meyer, Haas, and Barton 2006; Reckelhoff 2001). But it appears unlikely that estrogens play the dominant role in this process, since estrogen depletion did not aggravate the renal phenotype of AST rats. Instead, varying androgen levels or genetic factors may act as more important mediators of sex-specific differences in renal injury owing to chronic MR activation. Aldosterone plays an important role in both cardiac and renal disease, and patients with chronic renal failure face an increased incidence of concomitant heart disease (Herzog, Ma, and Collins 1998; Silberg et al. 1989). Of note, the current effects of E2, DRO, and MPA on renal disease in AST rats are generally in good agreement with our recent observations on cardiac and vascular injury in the very same animal model (Arias-Loza et al. 2006). However, increased kidney mass persisted in E2-treated rats despite a significant reduction in blood pressure.
A second and novel finding of this study is the observation that MPA aggravated several key features of renal injury that have been previously attributed to excess mineralocorticoid receptor activity such as increased renal mass, excessive fluid uptake, increased renal sodium absorption, and potassium loss. These observations were accompanied by extensive structural damage that included the vascular, glomerular, tubular, and interstitial compartments of the remnant kidney and decreased serum albumin levels (please see online supplement). In particular, co-treatment of AST rats with MPA plus E2 caused myointimal proliferation of the small cortical arterioles with occasional perivascular fibrosis, including occasional complete occlusion of the vessels. There was generalized glomerular sclerosis necrosis and mesangiolysis, tubular degeneration and necrosis, and interstitial collagen and lymphocyte accumulations. No such alterations were observed in intact or in ovx AST rats receiving placebo, E2, DRO, or SPIRO. Therefore it is likely that MPA treatment also promotes renal injury by mechanisms that are blood pressure independent.
Because chronic aldosterone infusion in the absence of a high-salt diet causes only a very mild degree of hypertension and cardiovascular damage, enhanced sodium uptake might provide another rationale to explain excessive renal damage in MPA-treated rats (Brilla and Weber 1992; Silberg et al. 1989; Yoshida et al. 2005). In support of this concept, sodium uptake reached maximum levels in MPA-treated animals. Renal sodium absorption in the collecting duct is facilitated via the amiloride-sensitive Na+ channel (ENaC), which consists of three subunits, α, β, and γ. Moreover, the mineralocorticoid receptor plays a critical role in regulating ENaC activity because aldosterone up-regulates α-ENaC expression via the MR and because MR knockout mice exhibit severely impaired ENaC activity that is accompanied by excessive renal sodium wasting (Berger et al. 2000; Masilamani et al. 1999). However, renal MR expression was not different among all rats, which is in line with previous observations in male AST rats (Silvestre et al. 2000). Interestingly, Thomas, Liu, and Vats (2006) have recently reported that MPA up-regulates α-ENaC promoter activity in renal collecting duct epithelia via binding and trans-activating mineralocorticoid and glucocorticoid receptors. Although not formally tested here, it appears conceivable that a very similar mechanism might have also been operational in MPA-treated AST rats, which would provide a mechanistic concept to explain increased sodium uptake and thus renal injury in female rats exposed to chronic AST.
Excess MR activity is associated not only with altered fluid, potassium, and sodium homeostasis, but also with increased oxidative stress (Nishiyama et al. 2004). Medroxyprogesterone acetate treatment of AST rats enhanced renal nuclear DHE fluorescence and glomerular protein tyrosin-nitrosylation, and elevated the expression levels of the NADPH-oxidase subunit p67phox (Touyz 2004). Collectively, these observations suggest that oxidative stress may have aggravated structural injury in the kidneys of MPA-treated rats. In support of this concept, enhanced oxidative stress has recently been identified as a mechanism for early podocyte damage in male rats receiving AST treatment (Shibata et al. 2007). However, future and more specific studies will be required to prove this hypothesis, the clarification of which was beyond the scope of this article.
In contrast to MPA, DRO not only acts as a potent progestogen, but it also possesses a strong MR-antagonist activity (Muhn et al. 1995; White et al. 2006). Therefore, it is interesting to note that the medium and high dosages of DRO were as potent as SPIRO in attenuating kidney hypertrophy in E2-treated AST rats. The fact that compared to the medium dose of DRO, the high dose did not cause a further reduction of kidney mass and blood pressure is most likely explained by a maximum effect of DRO that is achieved already with the medium dose of the drug. In addition, increasing dosages of DRO gradually relieved the suppression of systemic angiotensin II serum concentrations that occurred in response to aldosterone-salt treatment. An identical effect was observed in AST rats receiving SPIRO treatment. Perhaps more important, renal pathology was completely normal among all rats receiving DRO or SPIRO. Together, these observations provide the first evidence for a possible renal-protective function of DRO in female AST rats that most likely relates to the very different pharmacological profiles and partial agonist activities of DRO and MPA.
Estrogens, progestins, and aldosterone interact with the renin angiotensin aldosterone system (RAAS). The very different effects of MPA and DRO on renal morphology and function might thus also relate to differential interactions with the RAAS. Serum angiotensin II concentrations are decreased in response to chronic AST treatment and MR blockade with SPIRO or DRO relieved this suppression, which by itself does not explain the reduction of blood pressure in DRO- or SPIRO-treated AST rats (Arias-Loza et al. 2006, 2007). Instead, the expression pattern and the divergent function of both angiotensin II receptor subtypes, AT1R and AT2R, may provide an additional explanation for the blood pressure–lowering effect of DRO and SPIRO. Renal AT1R activation by angiotensin II causes vasoconstriction and increased blood pressure (Blume Kaschina, and Unger 2001; Matsubara 1998). But although female sex hormones down-regulate AT1R expression in vascular cells, AT1R expression levels were not different among all groups, which is unlikely to explain the protective function of DRO and SPIRO (Nickenig et al. 1998). The function of the AT2R largely opposes those of the AT1R, since pharmacological activation or genetic manipulation of AT2R expression revealed important natriuretic and blood pressure–lowering effects. Activation of AT2R by Ang II thus counteracts and balances AT1R activity (Hein et al. 1995; Inagami et al. 1997; Lo et al. 1995). Up-regulation of renal AT2R expression in E2-substituted rats may indicate a protective function, but the constant suppression of serum AII levels may have limited the extent of AT2R activation. However, co-treatment with DRO or SPIRO not only caused an additional increase of renal AT2R expression in E2-treated rats, but it also alleviated the suppression of serum AII levels. Although we have not formally tested the hypothesis that enhanced renal AT2R activation promoted increased renal sodium excretion and lower blood pressure in AST rats receiving combined treatment with E2 plus DRO (or SPIRO), such a concept is supported by recent studies by Gross et al. (2000), who reported that deletion of the AT2R results in a threefold reduction of renal sodium and water excretion. Together, these data provide novel and mechanistic hypotheses that might explain the protective function of DRO and SPIRO on kidney hypertrophy and renal sodium excretion.
In conclusion, the choice of specific synthetic progestins has profound implications on the development of hypertension, kidney injury, and renal gene expression under conditions of elevated aldosterone serum levels and salt intake. Clinical and experimental data indicate that mineralocorticoid and estrogen receptors do functionally interact in the development of vascular and renal injury. The current report shows that different synthetic progestins that are currently employed for HRT confer either adverse (MPA) or protective (DRO) effects on aldosterone-induced renal injury. The present information could be useful to enhance the pharmacological safety of HRT in female patients who are at risk to develop or may already be diagnosed with hypertension and coexisting renal disease.
Acknowledgments
The assistance of C. Wanner (Department of Nephrology, Medicine I, University of Würzburg, Germany) in preparing the manuscript is greatly appreciated. We are grateful to Ms. Teenette Jones of the NIEHS for histology support.
T. Pelzer received support from the Interdisciplinary Center for Clinical Research “IZKF” Würzburg (Z4-70, E83-N). This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. T. Pelzer has received financial support toward the evaluation of novel steroid hormone receptor ligands from Schering AG Berlin.
Abbreviations
- AST
aldosterone-salt treatment
- α-ENAC
α subunit of the epithelial sodium channel
- AT1R
angiotensin-II type 1 receptor
- AT2R
angiotensin-II type 2 receptor
- DRO
drospirenone
- E2
17β-estradiol
- GR
glucocorticoid receptor
- HRT
hormone replacement therapy
- MPA
medroxyprogesterone acetate
- MR
mineralocorticoid receptor
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate oxidase
- ROS
reactive oxygen species
- SPIRO
spironolactone
References
- Antus B, Liu S, Yao Y, Zou H, Song E, Lutz J, Heemann U. Effects of progesterone and selective oestrogen receptor modulators on chronic allograft nephropathy in rats. Nephrol Dial Transplant. 2005;20:329–35. doi: 10.1093/ndt/gfh602. [DOI] [PubMed] [Google Scholar]
- Arias-Loza PA, Hu K, Schafer A, Bauersachs J, Quaschning T, Galle J, Jazbutyte V, Neyses L, Ertl G, Fritzemeier KH, Hegele-Hartung C, Pelzer T. Medroxyprogesterone acetate but not drospirenone ablates the protective function of 17β-estradiol in aldosterone salt-treated rats. Hypertension. 2006;48:994–1001. doi: 10.1161/01.HYP.0000242482.57186.e8. [DOI] [PubMed] [Google Scholar]
- Arias-Loza PA, Hu K, Dienesch C, Mehlich AM, Konig S, Jazbutyte V, Neyses L, Hegele-Hartung C, Heinrich Fritzemeier K, Pelzer T. Both estrogen receptor subtypes, alpha and beta, attenuate cardiovascular remodeling in aldosterone salt-treated rats. Hypertension. 2007;50:432–38. doi: 10.1161/HYPERTENSIONAHA.106.084798. [DOI] [PubMed] [Google Scholar]
- Baylis C. Age-dependent glomerular damage in the rat. Dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. J Clin Invest. 1994;94:1823–29. doi: 10.1172/JCI117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Bleich M, Schmid W, Greger R, Schutz G. Mineralocorticoid receptor knockout mice: Lessons on Na+ metabolism. Kidney Int. 2000;57:1295–98. doi: 10.1046/j.1523-1755.2000.00965.x. [DOI] [PubMed] [Google Scholar]
- Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- Blume A, Kaschina E, Unger T. Angiotensin II type 2 receptors: Signalling and pathophysiological role. Curr Opin Nephrol Hypertens. 2001;10:239–46. doi: 10.1097/00041552-200103000-00013. [DOI] [PubMed] [Google Scholar]
- Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120:893–901. [PubMed] [Google Scholar]
- Caron K, Hagaman J, Nishikimi J, Kim H-S, Smithies O. Adrenomedullin gene expression differences in mice do not affect blood pressure but modulate hypertension-induced pathology in males. PNAS. 2006;104:3420–25. doi: 10.1073/pnas.0611365104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaillat M, Rugale C, Casellas D, Mimran A, Jover B. Cardiorenal abnormalities associated with high sodium intake: Correction by spironolactone in rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1137–43. doi: 10.1152/ajpregu.00154.2005. [DOI] [PubMed] [Google Scholar]
- Donadio JV, Jr., Torres VE, Velosa JA, Wagoner RD, Holley KE, Okamura M, Ilstrup DM, Chu CP. Idiopathic membranous nephropathy: The natural history of untreated patients. Kidney Int. 1988;33:708–715. doi: 10.1038/ki.1988.56. [DOI] [PubMed] [Google Scholar]
- Esqueda ME, Craig T, Hinojosa-Laborde C. Effect of ovariectomy on renal estrogen receptor-alpha and estrogen receptor-beta in young salt-sensitive and -resistant rats. Hypertension. 2007;50:768–72. doi: 10.1161/HYPERTENSIONAHA.107.095265. [DOI] [PubMed] [Google Scholar]
- Fuhrmann U, Krattenmacher R, Slater EP, Fritzemeier KH. The novel progestin drospirenone and its natural counterpart progesterone: Biochemical profile and antiandrogenic potential. Contraception. 1996;54:243–51. doi: 10.1016/s0010-7824(96)00195-3. [DOI] [PubMed] [Google Scholar]
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- Gretz N, Zeier M, Geberth S, Strauch M, Ritz E. Is gender a determinant for evolution of renal failure? A study in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1989;14:178–83. doi: 10.1016/s0272-6386(89)80068-x. [DOI] [PubMed] [Google Scholar]
- Gross V, Schunck WH, Honeck H, Milia AF, Kargel E, Walther T, Bader M, Inagami T, Schneider W, Luft FC. Inhibition of pressure natriuresis in mice lacking the AT2 receptor. Kidney Int. 2000;57:191–202. doi: 10.1046/j.1523-1755.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- Gross ML, Adamczak M, Rabe T, Harbi NA, Krtil J, Koch A, Hamar P, Amann K, Ritz E. Beneficial effects of estrogens on indices of renal damage in uninephrectomized SHRsp rats. J Am Soc Nephrol. 2004;15:348–58. doi: 10.1097/01.asn.0000105993.63023.d8. [DOI] [PubMed] [Google Scholar]
- Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377:744–47. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339:799–805. doi: 10.1056/NEJM199809173391203. [DOI] [PubMed] [Google Scholar]
- Inagami T, Eguchi S, Tsuzuki S, Ichiki T. Angiotensin II receptors AT1 and AT2—new mechanisms of signaling and antagonistic effects of AT1 and AT2. Jpn Circ J. 1997;61:807–13. doi: 10.1253/jcj.61.807. [DOI] [PubMed] [Google Scholar]
- Kim JK, Levin ER. Estrogen signaling in the cardiovascular system. Nucl Recept Signal. 2006;4:e013. doi: 10.1621/nrs.04013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M, Liu KL, Lantelme P, Sassard J. Subtype 2 of angiotensin II receptors controls pressure-natriuresis in rats. J Clin Invest. 1995;95:1394–97. doi: 10.1172/JCI117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machowska A, Szlachcic A, Pawlik M, Brzozowski T, Konturek SJ, Pawlik WW. The role of female and male sex hormones in the healing process of preexisting lingual and gastric ulcerations. J Physiol Pharmacol. 2004;55(Suppl 2):91–104. [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res. 1998;83:1182–91. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356:2639–41. doi: 10.1056/NEJMe078072. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: New perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–26. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- Muhn P, Fuhrmann U, Fritzemeier KH, Krattenmacher R, Schillinger E. Drospirenone: A novel progestogen with antimineralocorticoid and antiandrogenic activity. Ann N Y Acad Sci. 1995;761:311–35. doi: 10.1111/j.1749-6632.1995.tb31386.x. [DOI] [PubMed] [Google Scholar]
- Mulroney SE, Woda C, Johnson M, Pesce C. Gender differences in renal growth and function after uninephrectomy in adult rats. Kidney Int. 1999;56:944–53. doi: 10.1046/j.1523-1755.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol. 2000;11:319–29. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Bohm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation. 1998;97:2197–201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–48. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- Pelzer T, Loza PA, Hu K, Bayer B, Dienesch C, Calvillo L, Couse JF, Korach KS, Neyses L, Ertl G. Increased mortality and aggravation of heart failure in estrogen receptor-beta knockout mice after myocardial infarction. Circulation. 2005;111:1492–98. doi: 10.1161/01.CIR.0000159262.18512.46. [DOI] [PubMed] [Google Scholar]
- Peng H, Carretero OA, Raij L, Yang F, Kapke A, Rhaleb NE. Antifibrotic effects of N-acetyl-seryl-aspartyl-Lysyl-proline on the heart and kidney in aldosterone-salt hypertensive rats. Hypertension. 2001;37:794–800. doi: 10.1161/01.hyp.37.2.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- Rocha R, Chander PN, Zuckerman A, Stier CT. Role of aldosterone in renal vascular injury in stroke prone spontaneously hypertensive rats. Hypertension. 1999;33:232–37. doi: 10.1161/01.hyp.33.1.232. [DOI] [PubMed] [Google Scholar]
- Rocha R, Stier CT, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: A mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–78. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: Roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–64. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- Silberg JS, Barre PE, Prichard SS, Snidermann SD. Impact of left ventricular hyperthrophy on survival in end-stage renal disease. Kidney Int. 1989;36:286–90. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Robert V, Escoubet B, Heymes C, Oubenaissa A, Desopper C, Swynghedauw B, Delcayre C. Different regulation of cardiac and renal corticosteroid receptors in aldosterone-salt treated rats: Effect of hypertension and glucocorticoids. J Mol Cell Cardiol. 2000;32:1249–63. doi: 10.1006/jmcc.2000.1159. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R. Progestogens in hormonal replacement therapy: New molecules, risks, and benefits. Menopause. 2002;9:6–15. doi: 10.1097/00042192-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Socha MJ, Manhiani M, Said N, Imig JD, Motamed K. Secreted protein acidic and rich in cysteine deficiency ameliorates renal inflammation and fibrosis in angiotensin hypertension. Am J Pathol. 2007;171:1104–12. doi: 10.2353/ajpath.2007.061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Liu KZ, Vats HS. Medroxyprogesterone acetate binds the glucocorticoid receptor to stimulate alpha-ENaC and sgk1 expression in renal collecting duct epithelia. Am J Physiol Renal Physiol. 2006;290:F306–12. doi: 10.1152/ajprenal.00062.2005. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension. 2004;44:248–52. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- Verzola D, Gandolfo MT, Salvatore F, Villaggio B, Gianiorio F, Traverso P, Deferrari G, Garibotto G. Testosterone promotes apoptotic damage in human renal tubular cells. Kidney Int. 2004;65:1252–61. doi: 10.1111/j.1523-1755.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- White WB, Pitt B, Preston RA, Hanes V. Antihypertensive effects of drospirenone with 17beta-estradiol, a novel hormone treatment in postmenopausal women with stage 1 hypertension. Circulation. 2005;112:1979–84. doi: 10.1161/CIRCULATIONAHA.104.501502. [DOI] [PubMed] [Google Scholar]
- White WB, Hanes V, Chauhan V, Pitt B. Effects of a new hormone therapy, drospirenone and 17-β-estradiol, in postmenopausal women with hypertension. Hypertension. 2006;48:246–53. doi: 10.1161/01.HYP.0000232179.60442.84. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kim-Mitsuyama S, Wake R, Izumiya Y, Izumi Y, Yukimura T, Ueda M, Yoshiyama M, Iwao H. Excess aldosterone under normal salt diet induces cardiac hypertrophy and infiltration via oxidative stress. Hypertens Res. 2005;28:447–55. doi: 10.1291/hypres.28.447. [DOI] [PubMed] [Google Scholar]