Abstract
Objective:
To describe factors associated with survival in Alzheimer disease (AD) in a multiethnic, population-based longitudinal study.
Methods:
AD cases were identified in the Washington Heights Inwood Columbia Aging Project, a longitudinal, community-based study of cognitive aging in Northern Manhattan. The sample comprised 323 participants who were initially dementia-free but developed AD during study follow-up (incident cases). Participants were followed for an average of 4.1 (up to 12.6) years. Possible factors associated with shorter lifespan were assessed using Cox proportional hazards models with attained age as the time to event (time from birth to death or last follow-up). In subanalyses, median postdiagnosis survival durations were estimated using postdiagnosis study follow-up as the timescale.
Results:
The mortality rate was 10.7 per 100 person-years. Mortality rates were higher among those diagnosed at older ages, and among Hispanics compared to non-Hispanic whites. The median lifespan of the entire sample was 92.2 years (95% CI: 90.3, 94.1). In a multivariable-adjusted Cox model, history of diabetes and history of hypertension were independently associated with a shorter lifespan. No differences in lifespan were seen by race/ethnicity after multivariable adjustment. The median postdiagnosis survival duration was 3.7 years among non-Hispanic whites, 4.8 years among African Americans, and 7.6 years among Hispanics.
Conclusion:
Factors influencing survival in Alzheimer disease include race/ethnicity and comorbid diabetes and hypertension.
GLOSSARY
- AD
= Alzheimer disease;
- NDI
= National Death Index;
- WHICAP
= Washington Heights Inwood Columbia Aging Project.
Recent estimates of survival in Alzheimer disease (AD) range from 3 to 9 years.1–12 Most of these studies examined patients with prevalent AD, with survival calculated either from study entry3,4,7,9,13,14 or from retrospectively estimated dates of disease onset.12 For a summary of recent population-based studies of mortality in AD, see table 1. Estimating survival time using prevalent disease cohorts is problematic because of diversity in baseline disease severity. Including cases of advanced disease can lead to underestimation of survival. On the other hand, survival can be overestimated when patients who die rapidly after diagnosis are not recruited into studies. This has been called “survivor” or “length” bias.12
Table 1 Comparison of previous community-based studies of survival in participants diagnosed with AD: 1990–present
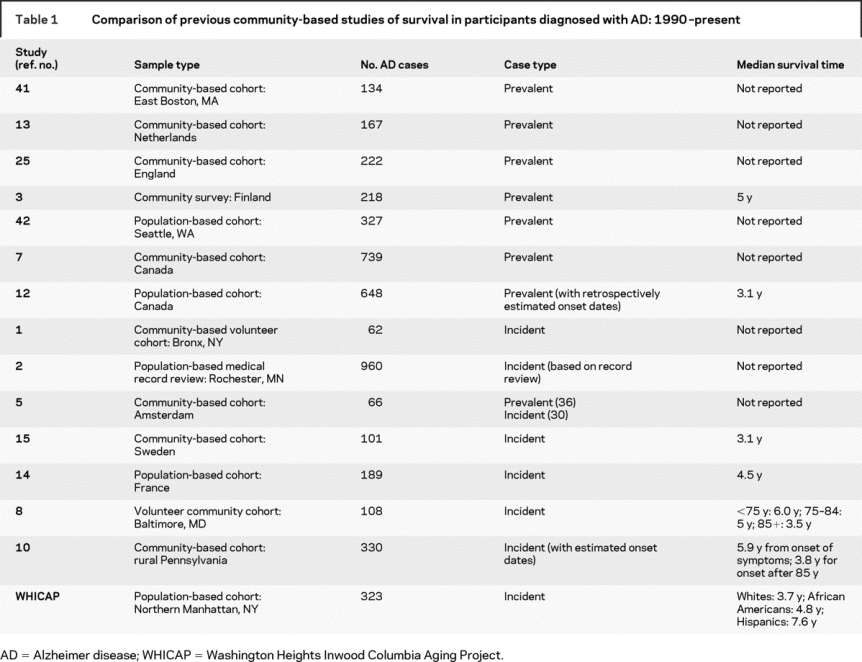
Prospective, population-based studies that start with dementia-free participants and actively screen for incident disease provide the most unbiased estimates of mortality and survival duration, yet few such studies have been undertaken in recent years,1,5,8,10,15 and these have been limited either by small sample sizes1,5,8,15 or ethnic homogeneity.5,8,10,15 The current report is from the Washington Heights Inwood Columbia Aging Project (WHICAP), a multiethnic, population-based, prospective study of cognitive aging in Northern Manhattan. Herein we provide ethnicity-specific statistics for 323 incident AD cases, taking age, sex, education, APOE-ɛ4 status, and major medical comorbidities into account.
METHODS
Subjects.
Participants in WHICAP come from two population-based cohorts of Medicare enrollees. Recruitment for the first cohort began in 1992. The study area was the 14 census tracts in Manhattan between (approximately) 155th and 181st Streets. Lists of all Medicare or Medicaid recipients in the study area were obtained from the Health Care Financing Administration. Potential study subjects were then drawn by systematic random sampling into one of six strata based on ethnicity (Hispanics, non-Hispanic blacks, non-Hispanic whites) and age (65–74, 75+). Most of the Hispanics living in Northern Manhattan are of Caribbean descent. A total of 2,125 subjects were interviewed at baseline. A “refreshment” cohort of 2,183 additional participants was formed in 1999 using generally similar methods, with several exceptions: new lists of beneficiaries were obtained but those drawn into the 1992 cohort were excluded; subjects who reported being diagnosed with dementia in the course of arranging for the initial evaluation were excluded; the study area was extended to encompass all of Manhattan north of 145th Street.
Of participants from the above two cohorts, 458 individuals developed dementia over the course of the follow-up period (incident cases). Of these, 417 were diagnosed with AD, 323 of whom had available follow-up data. Of the 94 participants with AD who lacked follow-up data, 92 had not yet been seen for the next assessment cycle (recently diagnosed incident cases), leaving 2 lost to follow-up. Those without follow-up data or lost to follow-up did not vary from the analysis sample in race/ethnicity, gender, APOE- ɛ4 status, and most medical comorbidities (hypertension, stroke, or malignancy), but were more likely to have a history of heart disease (p = 0.03), and were older at AD diagnosis (p = 0.003).
The study was reviewed and approved by the Columbia University institutional review board, and written informed consent was obtained from all subjects.
Assessment of incident AD.
Physician-administered physical and neurologic examinations, along with a standardized neuropsychological battery,16 were used to diagnose AD. All assessments were administered at baseline and at subsequent follow-up visits, which occurred at approximately 18-month intervals. Evaluations were conducted in either English or Spanish, based on participant preference. All available ancillary information, including medical charts and imaging studies, was considered in the evaluations.
Consensus diagnosis of dementia/no dementia was made at diagnostic conferences attended by neurologists and neuropsychologists, using results from the neuropsychological battery as well as evidence of social or occupational function deficits. The type of dementia was subsequently determined based on Diagnostic and Statistical Manual of Mental disorders, Revised Third Edition criteria. Diagnosis of probable or possible AD was made based on criteria suggested by the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association. Only participants identified as having possible or probable AD were included in our analysis.
Assessment of mortality status and covariates.
Mortality was tracked through follow-up interviews every 18 months and submission of identifying information to the National Death Index. Demographic information (age, sex, ethnicity, years of education) and major medical comorbidities (heart disease, hypertension, stroke, diabetes, and malignancy) up to dementia incidence were considered in analyses. Stroke was defined according to the World Health Organization criteria.17 Medical condition diagnoses were assessed based on questioning each participant (or a proxy if necessary) about the presence of physician-diagnosed conditions. Diagnostic assessments were supplemented by results of a neurologic examination and review of medical records. Diabetes and hypertension were defined as a reported history of or documented treatment of either disorder at any time up to AD diagnosis. Heart disease was defined as a history of myocardial infarction, congestive heart failure, or angina pectoris at any time prior to or including the AD diagnosis visit.
Ethnicity was based on 1990 US Census designations. Education was dichotomized based on a cutpoint of 12 years (high school education). The Clinical Dementia Rating18 was used to assess the severity of dementia and at the incidence visit.
Statistical methods.
Baseline characteristics by survival status were compared using χ2 or t tests. Case-fatality rates were stratified by age group (<75, 75–84, 85+), sex, and race/ethnic group. Age group–specific (<85, 85+) case-fatality rates were then calculated within race strata. Rates were calculated by dividing the number of deceased participants by person-years of study follow-up, then multiplying by 100. Case-fatality rates differ from mortality rates as they are calculated using data only from cases, rather than using data from cases and non-cases from the same cohort.
Survival analyses used lifespan (attained age) as the time to event scale.19,20 Attained age was measured as birth date to 1) date of death or 2) date of last follow-up. Death dates were determined via study follow-up and by an audit of the National Death Index (NDI) through December 31, 2002. If we learned of cases who died following this audit date, such cases were also included as deaths. For cases defined as living, last follow-up was defined as last clinical assessment or NDI audit date, whichever came last.
Kaplan-Meier curves and log-rank statistics were used to determine median lifespans and to compare cumulative survival between groups.21 Cox proportional hazards regression models were used to assess the effects of baseline characteristics on survival.22 Cox models were stratified on median age at AD diagnosis. The unadjusted effects of baseline characteristics (sex, race/ethnicity, education, heart disease, stroke, hypertension, diabetes) on survival were assessed in separate bivariate Cox models. Multivariable Cox models were used to evaluate the simultaneous effects of the above mentioned baseline characteristics, as well as study follow-up time, on survival.
Several supplementary analyses were completed. In a subsample of 288 participants with available genotyping data, we explored the effect of APOE-ɛ4 on survival. We also examined survival separately in a subsample of patients with AD with concomitant stroke. Additionally, for comparison to previous studies, we calculated postdiagnosis survival duration. In Kaplan-Meier models that used postdiagnosis follow-up as the timescale, we estimated median postdiagnosis survival by age at diagnosis (<75, 75–84, 85+ years), sex, and ethnicity.
Finally, we compared our postdiagnosis survival duration to expected survival in the US population by 5-year age groups based on US life table estimates that included Hispanics.23
Analysis of Martingale residuals was used to verify the validity of the proportional hazards assumption.24 All analyses were done using SPSS version 12.0.
RESULTS
Characteristics of study population.
The mean age of the sample at baseline was 87.0 years (range 67 to 100 years). The sample was 70% women, 55% Hispanic (mainly of Caribbean ancestry) and 33% African American.
Differences by mortality status.
Those who died were more likely to be white (non-Hispanic) or African American. Additionally, those who died tended to be more highly educated (table 2).
Table 2 Characteristics of the sample by mortality status
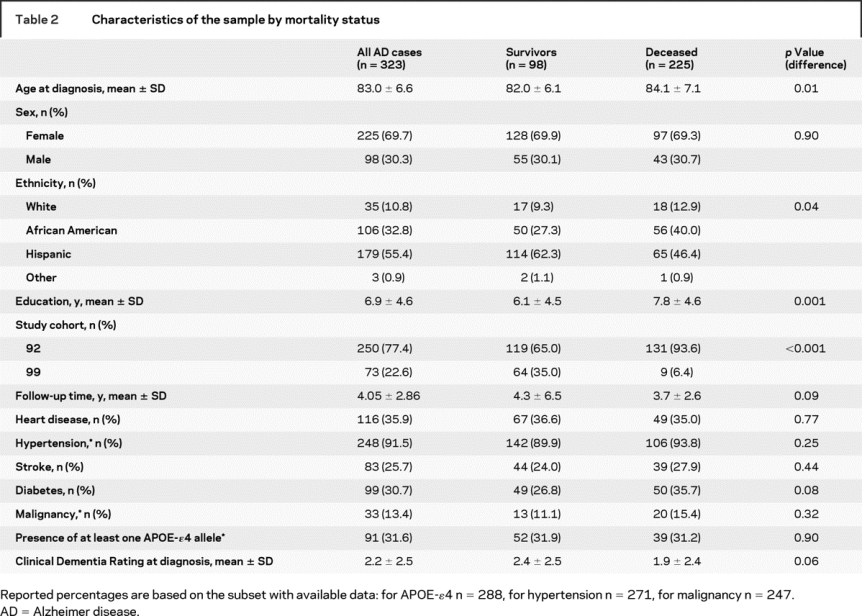
Differences by ethnicity.
There were no differences in age at study baseline or at diagnosis by race/ethnicity. Whites had the most education (mean 10.5 years) followed by blacks (8.5 years) and Hispanics (5.1 years) (p < 0.0001). There were no race/ethnic differences in APOE-ɛ4 status, sex, or history of stroke, diabetes, hypertension, or malignancy (data not shown).
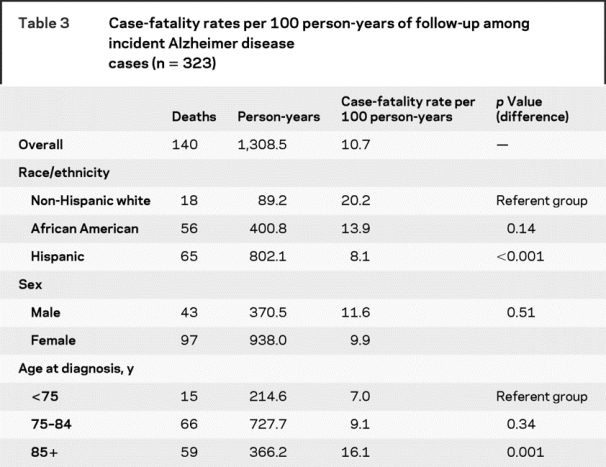
Case-fatality rates.
Person-year-adjusted case-fatality rates are presented in table 3. No sex differential in case-fatality was found. The case-fatality rate among non-Hispanic whites was more than twice as high as the rate among Hispanics. Case-fatality rates for the oldest age group (85 years or older) were more than double that of the youngest group (less than 75 years).
Table 3 Case-fatality rates per 100 person-years of follow-up among incident Alzheimer disease cases (n = 323)
Influence of baseline characteristics on survival.
In Cox models using lifespan as the time scale, only hypertension and diabetes were independently associated with shorter lifespan (table 4). In supplementary analyses that used postdiagnosis follow-up as the time scale, median survival after diagnosis was 6.0 years (95% CI 5.0, 7.1). For those younger than age 75 at diagnosis, median postdiagnosis survival was 9.9 years (95% CI 6.8, 13.0); for those 75–84 at diagnosis, 6.9 years (95% CI 5.4, 8.5); and for those ≥85 at diagnosis, 4.4 years (95% CI 3.5, 5.3) (log-rank statistic = 20.1; p < 0.0001). Postdiagnosis survival was shortest among non-Hispanic whites (3.7 years; 95% CI 1.5, 5.9), followed by African Americans (4.8 years; 95% CI 4.0, 5.7) and Hispanics (7.6 years; 95% CI 6.4, 8.7) (log-rank statistic = 20.8, p < 0.0001) (figure). No sex differences were found.
Table 4 Median survival time (attained age) and hazard of shorter lifespan
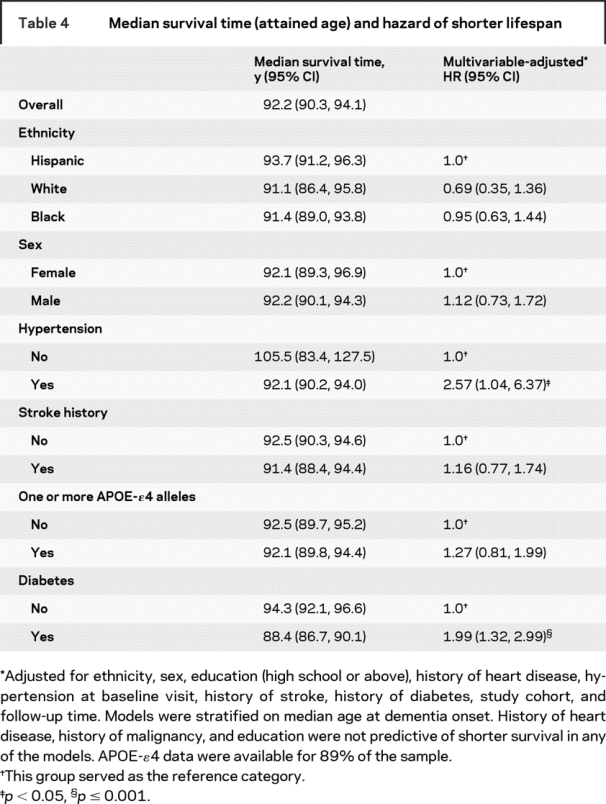
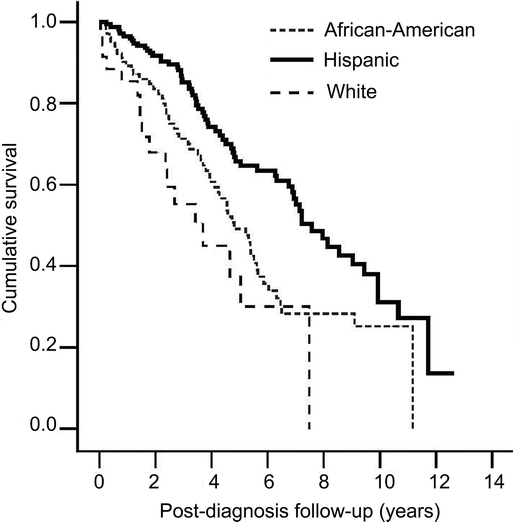
Figure Kaplan-Meier curves depicting postdiagnosis survival by race/ethnic group
Lifespan among those with AD with concomitant vascular disease did not differ from those with “pure” AD (log-rank statistic = 1.30, p = 0.24). Survival among individuals with incident AD was shorter than what would be expected based on US census-based life table estimates.23 The median survival of those with incident AD was 3 years less than the overall population for those diagnosed at 70–75 years old, and 1–2 years less than the overall population for those diagnosed at older ages.
DISCUSSION
This study provides survival statistics for a multiethnic sample of incident AD cases. Patients with AD in this study, who were on average 83 years old at diagnosis, had a median lifespan of 92.2 years. Although this longevity may seem remarkable, it is still 1–3 years less than the expected conditional lifespan based on population-wide life table estimates, depending on age at diagnosis. Factors independently associated with shorter lifespan among patients with AD included history of hypertension and diabetes.
An advantage to this study is that we included only participants with incident AD. Previous investigators reported that survival may be overestimated in studies of prevalent disease. This could occur when cases of rapidly progressing disease are not identified before death and are thus omitted from studies.12 However, there is also the competing risk that survival may be underestimated, since individuals are captured later in the disease course, instead of at the point of disease onset.
As in other recent studies of incident AD,8,12,14 we found that older age at AD diagnosis was associated with significantly shorter postdiagnosis survival. Those in the youngest age group at diagnosis (<75 years) survived more than twice as long as those in the oldest group (85+), probably because older patients are more likely to die of other causes. In contrast with most3–5,9,25,26 but not all8 recent studies, we did not find a survival advantage among women. Our sample was predominately Hispanic. According to US life table estimates,23 in the overall non-Hispanic white population, life expectancy is markedly higher in women compared to men among those aged 80 and older. In contrast, there are negligible differences by sex in this age group among Hispanics. Thus our finding of a lack of a sex differential (and perhaps previous studies’ findings of a female advantage) may reflect sex-related life expectancy in the background populations studied.
The mortality rate was highest among non-Hispanic whites—approximately twice as high as the rate among Hispanics. The mortality rate among African Americans was lower than that of non-Hispanic whites and higher than that of Hispanics.
Our finding of a 3.7-year postdiagnosis survival among non-Hispanic whites is consistent with previous studies of incident AD with predominantly Caucasian samples. The Baltimore Longitudinal Study of Aging found that the median survival duration among men was 3.8 years and 4.4 years among women.8 The French PAQUID study reported a median survival duration of 3.7 years for men and 5.2 for women.14 The Canadian Study of Health and Aging found median survival of 2.8 years among those with prevalent AD, after adjusting for length bias.12
We found markedly longer postdiagnosis survival among Hispanic participants, yet no race/ethnic differences in lifespan. This might be explained by assessment bias brought about by the lower educational attainment among Hispanics compared to the other ethnic groups. Those with less education often perform slightly worse on standardized cognitive tests despite comparable disease pathology. Thus, the Hispanics in this study might have been identified as having AD earlier than those in other ethnic groups; nonetheless, the mean age at diagnosis did not vary by ethnicity. We considered the possibility that ethnic differences in medical comorbidity might explain differences in survival—yet the prevalence of diabetes and hypertension was higher, not lower, among Hispanics than in the other ethnicities. There is a growing literature supporting a survival advantage among Hispanics in the United States compared to other race/ethnic groups, which may be explained by ethnic differences in health-related behaviors, family networks, and social support.27,28 Thus, our findings among Hispanics may reflect comparatively longer survival among all US Hispanics. Our findings are consistent with a recent large study of patients enrolled in US Alzheimer’s Disease Centers, which found that survival (measured from first ADC visit) was longer among Hispanic and African American patients with AD compared to non-Hispanic whites, with Hispanics surviving the longest.29
Like others,9 we found that diabetes was independently associated with shorter survival. In contrast to previous findings9 we additionally found that hypertension was associated with shorter survival. In contrast to most previous studies,3,9,25 we found no link between survival duration and a history of heart disease or malignancy among patients with AD.
We did not find a differential effect of the presence of at least one APOE-ɛ4 allele on survival. The APOE-ɛ4 genotype has been associated with earlier disease onset,30 and some31,32 but not all33,34 studies have demonstrated more rapid disease progression among ɛ4 carriers. Indeed, in a separate analysis of this cohort, we demonstrated faster cognitive decline among incident cases who were ɛ4 carriers.35 Our failure to demonstrate an ɛ4-associated difference in survival may reflect a differential effect of ɛ4 in the earlier stages of disease that is eclipsed by other factors (medical, social, or disease-related factors) later in the disease course.
Results of previous studies that considered the effect of APOE-ɛ4 on survival in AD have been conflicting. One small study found shorter survival in male, but not female, ɛ4-carriers.36 We found no differential ɛ4 effect by sex. Others found longer survival among ɛ4 carriers,37,38 and still others found no association.39,40
Several issues should be considered in interpreting our findings. First, our follow-up visits were roughly separated by 1.5 years, possibly affecting the precision of our incidence dates. Second, 96 participants with AD had not yet been seen for the next assessment cycle. These individuals may be at higher risk of dying and may have skewed our results toward longer survival time. Third, our sample differs from the general US population in that it consists of 55% Caribbean Hispanics and is mostly women (70%). Also, subjects under age 65 were not assessed, thus we likely excluded some individuals who developed AD at a relatively young age and died prior to study entry. These factors may limit the generalizability of our findings. Finally, we were unable to report particular causes of death among patients with AD since this information was not available for most participants.
Despite these possible limitations, confidence in our findings is strengthened by the fact that the WHICAP study is by design an investigation of cognitive aging. All participants were seen for frequent follow-up assessments by neurologists with experience in dementia. In lieu of cognitive screening instruments used in some studies, we administered a comprehensive battery of neuropsychological tests at each follow-up assessment, and case status was determined after careful consideration in consensus conferences attended by study neurologists and neuropsychologists. The fact that our sample is mainly population-based reduces biases associated with the use of convenience samples (disease registries, hospital- or clinic-based samples) that may not accurately reflect the course of the disease in the general population.
Most recent community-based studies have been limited by the study of only prevalent cases.3,7,12,13,25,41,42 Of the studies that actively screened dementia-free individuals for incident AD, most were limited by small samples1,5,8,14,15 (table 1). Our large sample of persons with incident AD was drawn from a representative sample of a multiethnic community, thus our findings extend those of other recent community-based studies that included mostly white participants.
ACKNOWLEDGMENT
The authors thank Dr. M. Maria Glymour for helpful suggestions.
Address correspondence and reprint requests to Dr. Yaakov Stern, Columbia University Medical Center, 630 West 168th Street, P&S Box 16, New York, NY, 10032 ys11@columbia.edu
Supported by federal grants P 01-AG07232, AG00261, RR00645, 5T32NS007153-22, and the Taub Institute for Research in Alzheimer’s Disease and the Aging Brain.
Disclosure: The authors report no disclosures.
Received April 22, 2008. Accepted in final form August 1, 2008.
REFERENCES
- 1.Aronson MK, Ooi WL, Geva DL, Masur D, Blau A, Frishman W. Dementia: age-dependent incidence, prevalence, and mortality in the old old. Arch Intern Med 1991;151:989–992. [DOI] [PubMed] [Google Scholar]
- 2.Beard CM, Kokmen E, O’Brien PC, Kurland LT. Are patients with Alzheimer’s disease surviving longer in recent years? Neurology 1994;44:1869–1871. [DOI] [PubMed] [Google Scholar]
- 3.Molsa PK, Marttila RJ, Rinne UK. Long-term survival and predictors of mortality in Alzheimer’s disease and multi-infarct dementia. Acta Neurol Scand 1995;91:159–164. [DOI] [PubMed] [Google Scholar]
- 4.Heyman A, Peterson B, Fillenbaum G, Pieper C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part XIV: demographic and clinical predictors of survival in patients with Alzheimer’s disease. Neurology 1996;46:656–660. [DOI] [PubMed] [Google Scholar]
- 5.Geerlings MI, Deeg DJ, Schmand B, Lindeboom J, Jonker C. Increased risk of mortality in Alzheimer’s disease patients with higher education? A replication study. Neurology 1997;49:798–802. [DOI] [PubMed] [Google Scholar]
- 6.Aevarsson O, Svanborg A, Skoog I. Seven-year survival rate after age 85 years: relation to Alzheimer disease and vascular dementia. Arch Neurol 1998;55:1226–1232. [DOI] [PubMed] [Google Scholar]
- 7.Ostbye T, Hill G, Steenhuis R. Mortality in elderly Canadians with and without dementia: a 5-year follow-up. Neurology 1999;53:521. [DOI] [PubMed] [Google Scholar]
- 8.Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Arch Neurol 2002;59:1764–1767. [DOI] [PubMed] [Google Scholar]
- 9.Larson EB, Shadlen M-F, Wang L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med 2004;140:501–509. [DOI] [PubMed] [Google Scholar]
- 10.Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer disease and mortality: a 15-year epidemiological study. Arch Neurol 2005;62:779–784. [DOI] [PubMed] [Google Scholar]
- 11.Waring SC, Doody RS, Pavlik VN, Massman PJ, Chan W. Survival among patients with dementia from a large multi-ethnic population. Alzheimer Dis Assoc Disord 2005;19:178–183. [DOI] [PubMed] [Google Scholar]
- 12.Wolfson C, Wolfson DB, Asgharian M, et al. A reevaluation of the duration of survival after the onset of dementia. N Engl J Med 2001;344:1111–1116. [DOI] [PubMed] [Google Scholar]
- 13.Heeren TJ, van Hemert AM, Rooymans HGM. A community-based study of survival in dementia. Acta Psychiatr Scand 1992;85:415–418. [DOI] [PubMed] [Google Scholar]
- 14.Helmer C, Joly P, Letenneur L, Commenges D, Dartigues JF. Mortality with dementia: results from a French prospective community-based cohort. Am J Epidemiol 2001;2001:642–648. [DOI] [PubMed] [Google Scholar]
- 15.Aguero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winblad B. Mortality from dementia in advanced age: a 5-year follow-up study of incident dementia cases. J Clin Epidemiol 1999;52:737–743. [DOI] [PubMed] [Google Scholar]
- 16.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol 1992;49:453–460. [DOI] [PubMed] [Google Scholar]
- 17.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 1976;54:541–553. [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 19.Korn EL, Garaubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 1997;145:72–80. [DOI] [PubMed] [Google Scholar]
- 20.Lamarca R, Alonso J, Gomez G, Munoz A. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci 1998;53:M337–343. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 22.Cox D. Regression models and life tables. J R stat Soc Series (B) 1972;34:187–220. [Google Scholar]
- 23.Guend A, Swallen KC. Abridged Life Tables by Sex, Race, and Hispanic Ethnicity; US Population 1989–1991. Madison, WI: Center for Demography and Ecology University of Wisconsin-Madison; 2002. [Google Scholar]
- 24.Therneau TM, Grambsch PM, Fleming TR. Martingale-based residuals for survival models. Biometrika 1990;77:147–160. [Google Scholar]
- 25.Jagger CJ, Clarke M, Stone A. Predictors of survival with Alzheimer’s disease: a community-based study. Psychol Med 1995;25:171–177. [DOI] [PubMed] [Google Scholar]
- 26.Stern Y, Tang M-X, Albert MS, et al. Predicting time to nursing home care and death in individuals with Alzheimer’s disease. JAMA 1997;277:806–812. [PubMed] [Google Scholar]
- 27.Franzini L, Ribble JC, Keddie AM. Understanding the Hispanic paradox. Ethnicity Dis 2001;11:496–518. [PubMed] [Google Scholar]
- 28.Jasso G, Massey DS, Rosenzweig RS, Smith JP. Immigrant health, selectivity and acculturation. In: Anderson NB, Bulatao RA, Cohen B, eds. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. National Academy Press; 2005. [Google Scholar]
- 29.Mehta KM, Yaffe K, Perez-Stable EJ, et al. Race/ethnic differences in AD survival in US Alzheimer’s Disease Centers. Neurology 2008;70:1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer MR, Tschanz JT, Norton MC, et al. APOE genotype predicts when—not whether—one is predisposed to develop Alzheimer disease. Nat Genet 1998;19:321–322. [DOI] [PubMed] [Google Scholar]
- 31.Martins CAR, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology 2005;65:1888–1893. [DOI] [PubMed] [Google Scholar]
- 32.Craft S, Teri L, Edland SD, et al. Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer’s disease. Neurology 1998;51:149–153. [DOI] [PubMed] [Google Scholar]
- 33.Hoyt BD, Massman PJ, Schatschneider C, Cooke N, Doody RS. Individual growth curve analysis of APOE ɛ4-associated cognitive decline in Alzheimer disease. Arch Neurol 2005;62:454–459. [DOI] [PubMed] [Google Scholar]
- 34.Frisoni GB, Govoni S, Geroldi C, et al. Gene dose of the e4 allele of apolipoprotein E and disease progression in sporadic late-onset Alzheimer’s disease. Ann Neurol 1995;37:596–604. [DOI] [PubMed] [Google Scholar]
- 35.Cosentino S, Scarmeas N, Helzner E, et al. APOE ɛ4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology 2008;70:1842–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dal Forno G, Carson KA, Brookmeyer R, Troncoso J, Kawas CH, Brandt J. APOE genotype and survival in men and women with Alzheimer’s disease. Neurology 2002;58:1045–1050. [DOI] [PubMed] [Google Scholar]
- 37.Stern Y, Brandt J, Albert M, et al. The absence of an apolipoprotein epsilon4 allele is associated with a more aggressive form of Alzheimer’s disease. Ann Neurol 1997;41:615–620. [DOI] [PubMed] [Google Scholar]
- 38.van Duijn CM, de Knijff P, Wehnert A, et al. The apolipoprotein E epsilon 2 allele is associated with an increased risk of early-onset Alzheimer’s disease and a reduced survival. Ann Neurol 1995;37:605–610. [DOI] [PubMed] [Google Scholar]
- 39.Slooter AJC, Houwing-Duistermaat JJ, van Harskamp F, et al. Apolipoprotein E genotype and progression of Alzheimer’s disease: the Rotterdam Study. J Neurol 1999;246:304–308. [DOI] [PubMed] [Google Scholar]
- 40.Koivisto AM, Lempiainen P, Koivisto K, et al. Apolipoprotein E phenotype alone does not influence survival in Alzheimer’s disease: a population-based longitudinal study. Neuroepidemiology 2000;19:327–332. [DOI] [PubMed] [Google Scholar]
- 41.Evans DA, Smith LA, Scherr PA, Albert MS, Funkenstein HH, Hebert LE. Risk of death from Alzheimer’s disease in a community population of older persons. Am J Epidemiol 1991;134:403–412. [DOI] [PubMed] [Google Scholar]
- 42.Bowen JD, Malter AD, Sheppard L, et al. Predictors of mortality in patients diagnosed with probable Alzheimer’s disease. Neurology 1996;47:433–439. [DOI] [PubMed] [Google Scholar]


