Abstract
Objective:
To report a new American family with hereditary diffuse leukoencephalopathy with spheroids (HDLS), including serial, presymptomatic and symptomatic, cranial MRIs from the proband.
Methods:
We report clinical and genealogic investigations of an HDLS family, sequential brain MRIs of the proband, and autopsy slides of brain tissue from the proband’s father.
Results:
We identified seven affected family members (five deceased). The mean age at symptomatic disease onset was 35 years (range: 20–57), and the mean disease duration was 16 years (range: 3–46). Five affected individuals initially manifested memory disturbance and behavioral changes, whereas two experienced a mood disorder as their presenting symptom. Our proband’s father had been diagnosed clinically with vascular dementia, but his brain autopsy was consistent with HDLS. The proband had a cranial MRI prior to symptom onset, with two subsequent MRIs performed during follow-up. These serial images reveal a progressive, confluent, frontal-predominant leukoencephalopathy with symmetric cortical atrophy.
Conclusions:
The proband of our newly identified hereditary diffuse leukoencephalopathy with spheroids (HDLS) kindred had subtle evidence of an incipient leukoencephalopathy on a presymptomatic cranial MRI. Conceivably, MRI may facilitate identifying affected presymptomatic individuals within known HDLS kindreds, increasing the likelihood of isolating the causative genes.
GLOSSARY
- DLS
= diffuse leukoencephalopathy with spheroids;
- FLAIR
= fluid-attenuated inversion recovery;
- HDLS
= hereditary diffuse leukoencephalopathy with spheroids;
- LENAS
= leukoencephalopathy with neuroaxonal spheroids;
- LFB
= Luxol fast blue;
- NAL
= neuroaxonal leukodystrophy;
- POLD
= pigmentary type of orthochromatic leukodystrophy.
Hereditary diffuse leukoencephalopathy with spheroids (HDLS) is an autosomal dominant disease associated with progressive cognitive and motor dysfunction.1 The original HDLS kindred was identified in Sweden and reported in 1984.2 Currently, eight HDLS families worldwide have been identified in Sweden, Japan, the Netherlands, Australia, and the United States.1–8 Occasionally, sporadic patients have been reported with a similar disease,9–14 and some have found that the pathology of HDLS is indistinguishable from that of the pigmentary type of orthochromatic leukodystrophy (POLD).4,15 The non-hereditary cases have been labeled variably, including as leukoencephalopathy with neuroaxonal spheroids (LENAS)11 and neuroaxonal leukodystrophy (NAL).12 In this report, when both hereditary and sporadic cases are being discussed, they will be linked as diffuse leukoencephalopathy with spheroids (DLS).
The onset of behavioral and cognitive changes in the fourth or fifth decade, progressing to dementia then death within a decade, is typical of HDLS; however, significant intra- and interkindred clinical variability exists.1–8
Cranial MRI findings in DLS are nonspecific and consist of a frontal-predominant leukoencephalopathy with concomitant cerebral atrophy.1,3,6,11,12 As with DLS clinical variability, MRI findings may reveal patchy, asymmetric white matter abnormalities,6 or confluent, symmetric lesions.1,3,11,12 Heretofore, longitudinal MRIs of a patient with HDLS, including a presymptomatic study, have not been available. Recently, we have identified another HDLS kindred.16 The proband underwent a cranial MRI in 1999 as part of a headache evaluation, which was interpreted as normal. Since developing cognitive and personality changes in 2004, this patient has had two additional cranial MRIs. Below, we describe the patient’s history and neurologic examination; demonstrate neuropathologic specimens from her father and describe his clinical presentation; show serial brain MR images of the patient; and provide this kindred’s current pedigree.
METHODS
Clinical and genealogic studies.
A written informed consent approved by the Mayo Clinic Ethics Committee was obtained from all participants. Family members were assessed by two of us (Z.K.W. and J.A.V.G.) with the use of a standardized medical history; the Mayo Clinic neurologic examination form; the Unified PD Rating Scale; the Kokmen short test of mental status17; and the Mini-Mental State Examination. Extant historical material, family records, and medical records were collected. These were supplemented by eliciting histories of probable-affected kindred members from available, familial sources.
Neuroimaging.
MRI scans were performed using standard techniques. The same neuroradiologist (D.F.B.) examined all MRI films with another of the authors (J.A.V.G.).
Pathology.
Formalin fixed tissue, including cerebral cortex, thalamus, midbrain, pons, medulla, and cerebellum from the proband’s father (figure 1, individual IV–V), was available for further study. The tissue was stained with hematoxylin and eosin, thioflavin-S, and Luxol fast blue, and immunostained for ubiquitin (Ubi-1), neurofilament (SMI-31), αB-crystallin, HLA-DR (LN3) for microglia, tau (CP13), α-synuclein (NACP), and amyloid precursor protein (APP; 22C11) using standard methods.
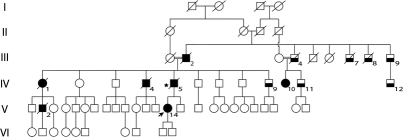
Figure 1 Simplified pedigree of our hereditary diffuse leukoencephalopathy with spheroids kindred
Generations are numbered with Roman numerals, individuals with Arabic numerals. Circles represent females and squares represent males. Solid symbols indicate affected members, lower half solid symbols indicate possibly affected. Proband: individual V-14 (arrowed). Star sign: individual who underwent brain autopsy. Slash indicates deceased.
RESULTS
Clinical and genealogic studies.
Examination of the proband (V-14).
A 37-year-old, right-handed white woman presented with insidiously progressive memory loss and personality changes over 2 years. Her father and a paternal uncle had had a similar neurologic illness, beginning in their sixth decade, leading to progressive incapacitation and death within approximately 6 years. Our patient had a flat affect, was mildly disinhibited, and scored 31/38 on the Kokmen short test of mental status. Her cognitive difficulties included executive dysfunction, short-term memory loss, and dyscalculia. Frontal release signs were present. Slight motor impersistence was evident on saccade testing. Speech, language, coordination, gait/station, strength, myotatic stretch reflexes, and plantar responses were unremarkable.
Pedigree.
We examined 35 subjects from this 65-member pedigree with six generations (figure 1). Of the seven affected subjects over three generations (five deceased), we directly examined one (figure 1, individual V-14). The pedigree displays apparent autosomal dominant inheritance (figure 1). However, two marriages between close relatives occurred in generation III.
Clinical data on the proband’s father (IV-5).
At age 57, this patient began manifesting personality and behavioral changes. He had a longstanding history of poorly controlled hypertension and diabetes; thus, the white matter changes on his brain MRI from that time were interpreted as consistent with a microvascular leukoencephalopathy. Over the next 2 years, the patient became more withdrawn, “speaking little” and demonstrating a “flat affect.” A year later, he was “more forgetful” and had “difficulty putting his shoes on.” He also ambulated around aimlessly, and exhibited perseverative speech, as well as bowel and bladder incontinence. A repeat cranial MRI showed confluent, deep white matter changes and generalized atrophy, suggestive of a vascular origin. By the next year, the patient could not “follow any commands.” He died of complications of pneumonia at age 63, approximately 6 years after the onset of his neurologic illness. The available medical records do not suggest that abrupt changes occurred neurologically; rather, the process appears to have been insidiously progressive.
Clinical data on other family members.
Besides the proband (individual V-14) and her father (individual IV-5), the rating of other family members as affected (individuals III-2, IV-1, IV-4, IV-10, V-2) was based on history provided by relatives, deemed by the authors to be consistent with HDLS. These individuals are all deceased, except for patient IV-10, who resides in a nursing home. Individual IV-10 is not competent to provide informed consent to participate in our study, and her legal guardian refused to allow even an examination of her medical records. Examined kindred members rated as possibly affected (individuals III-4, III-7, III-8, III-9, IV-9, IV-11, IV-12) demonstrated varying combinations of frontal release signs, impaired executive function, and mood dysfunction. Except for the proband and her father, brain MRIs were not available of any of the kindred’s affected or possibly affected members. Thus far, none of the possibly affected kindred members has assented to undergoing a screening, cranial MRI.
Imaging data of the proband (V-14).
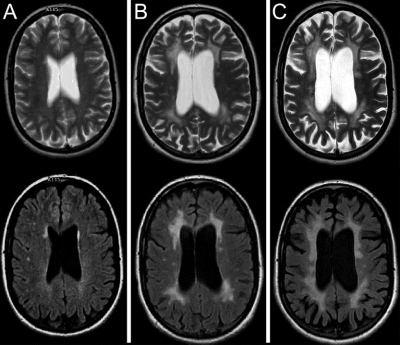
The 1999 cranial MRI, performed at age 29 for headache evaluation, was originally interpreted as normal. However, review of T2-weighted and fluid-attenuated inversion recovery (FLAIR) images reveals scattered asymmetric hyperintense foci in the bifrontal periventricular white matter (figure 2A). Increased confluent and more symmetric T2 hyperintensities in the bifrontal white matter, new confluent T2 hyperintensities in the biparietal periventricular white matter, and moderate diffuse atrophy are present on the 2006 examination (age 36, figure 2B). The 2007 examination shows progression of both T2 hyperintensities and diffuse atrophy (age 37, figure 2C).
Figure 2 Sequential MRI study of the proband (individual V-14)
Upper row, T2-weighed images; lower row, fluid-attenuated inversion recovery images. (A) Presymptomatic images (age: 29 years) show subtle right-predominant frontal white matter signal changes. (B) Symptomatic images (age: 36 years) show significant patchy and confluent bifrontal and biparietal periventricular white matter lesions, with moderate cortical and subcortical atrophy. (C) Symptomatic images (age: 37 years) show severe symmetric confluent bifrontal and biparietal white matter lesions, with marked cortical and subcortical atrophy.
Pathologic study of the proband’s father (IV-5).
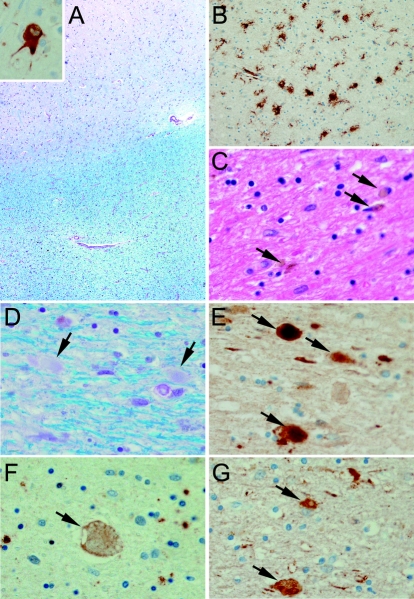
The most significant pathology was in the cerebral white matter, affecting the centrum semiovale, the periventricular white matter, and the arcuate fibers. These areas had rarefaction and vacuolation of myelin (figure 3A), with myelinated fiber loss, gliosis, lipid-laden macrophages, and diffuse microglial activation (figure 3B). Some of the macrophages contained brown granular pigment (figure 3C). There was a paucity of microglial activation in the overlying cortex. Axons within the demyelinated foci were swollen (figure 3D) and intensely immunostained with APP (figure 3E) and neurofilament (figure 3G) antibodies and to a lesser extent with ubiquitin antibodies (figure 3F). The overlying cortex had some axonal spheroids. The spheroids were negative for αB-crystallin, but this immunostain revealed ballooned neurons in the overlying cortex. The αB-crystallin immunostain also revealed bizarre astrocytes in the white matter pathology. The white matter tracts in the brainstem, including the medullary pyramids, were unaffected. Immunostaining for tau and α-synuclein was negative. The findings were characteristic of HDLS.
Figure 3 Pathology of the proband’s father (IV-5)
(A) Low power image of cortex stained with Luxol fast blue (LFB) shows gray-white junction with staining pallor of deep white matter and sparing of U-fibers. Inset shows a ballooned cortical neuron immunostained for αB-crystallin. (B) Low power image of white matter immunostained for HLA-DR shows diffuse microglial activation. (C) High power image stained with hematoxylin and eosin shows brown granular pigment in white matter macrophages (arrows). (D) High power image of white matter stained with LFB shows pale staining axonal spheroids (arrows). (E) High power image of white matter immunostained for amyloid precursor protein shows axonal spheroids (arrows). (F) High power image of white matter immunostained for ubiquitin shows only a few axonal spheroids (arrow). (G) High power image of white matter immunostained for neurofilament protein shows axonal spheroids (arrows).
DISCUSSION
Subtle frontal white matter changes on a brain MRI of our newly identified HDLS proband were evident some 5 years before she manifested behavioral changes and cognitive dysfunction. This initial MRI study was almost certainly presymptomatic, since headache has not been reported in HDLS patients so far. Contrary to some reports stipulating that the typical white matter changes in DLS are “patchy,”6,18 the MR images in publications of familial and nonfamilial cases of DLS usually reveal confluent changes.1,3,11,12 The presymptomatic MRI of our patient showed asymmetric focal white matter changes; these later became confluent and symmetric. This suggests that white matter lesions in DLS may initially be asymmetric and patchy and then become confluent with disease progression, hence the variable imaging patterns reported; however, the MR images of Patient 1 in a German-Dutch kindred revealed patchy white matter changes after 2 years of symptomatic disease.6 Thus, as with the well-known inter- and intrakindred clinical variability of HDLS, as well as sporadic DLS, the MRI changes are also apparently heterogeneous.
Our family displays apparent autosomal dominant inheritance of the disease, in accordance with previous reports.1–8 However, two marriages occurred between close relatives in generation III (figure 1), a pattern that may have suggested a pseudodominant recessive inheritance. Disease transmission in generations IV and V, however, strongly argues against this hypothesis and rather supports a dominant inheritance.
HDLS and sporadic DLS are likely frequently unrecognized. Indeed, our proband’s father was diagnosed with vascular dementia, given his longstanding, poorly controlled diabetes and hypertension. In nonfamilial patients with DLS, misdiagnosis is likely even more common, in light of the nonspecific MRI findings and commonality of cerebrovascular disease. Currently, an accurate diagnosis of HDLS depends on a histopathologic evaluation, because the genes causing HDLS remain unknown.1,15,18 MRI may be a useful screening tool for identifying presymptomatic individuals in known HDLS kindreds, particularly in those without obvious risk factors for microvascular leukoencephalopathy, which potentially could facilitate isolating the causative genes. However, caution is advised since the condition is currently untreatable, and diagnosis is dependent on the exclusion of other causes of white matter disease.
ACKNOWLEDGMENT
The authors thank all family members who participated in the study. The authors thank Jessica Young and Audrey Strongosky for their help in investigating the family.
Address correspondence and reprint requests to Dr. Z.K. Wszolek, Mayo Clinic, Neurology, 4500 San Pablo Rd., Jacksonville, FL 32224 wszolek.zbigniew@mayo.edu
C.W. is supported by the Swiss National Science Foundation, Parkinson Switzerland, and the Robert and Clarice Smith Fellowship program. Z.K.W., J.A.V.G., and D.W.D. are supported by the Morris K. Udall Center of Excellence for PD Research (P50-NS40256). D.W.D. is supported by the Mayo Clinic ADRC grant P50-AG16574. Z.K.W. and D.W.D. are supported by the Pacific Alzheimer Research Foundation (PARF) grant C06-01.
Disclosure: The authors report no disclosures.
Received March 31, 2008. Accepted in final form June 12, 2008.
REFERENCES
- 1.Baba Y, Ghetti B, Baker MC, et al. Hereditary diffuse leukoencephalopathy with spheroids: clinical, pathologic and genetic studies of a new kindred. Acta Neuropathol (Berl) 2006;111:300–311. [DOI] [PubMed] [Google Scholar]
- 2.Axelsson R, Roytta M, Sourander P, Akesson HO, Andersen O. Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl 1984;314:1–65. [PubMed] [Google Scholar]
- 3.Hancock N, Poon M, Taylor B, McLean C. Hereditary diffuse leucoencephalopathy with spheroids. J Neurol Neurosurg Psychiatry 2003;74:1345–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marotti JD, Tobias S, Fratkin JD, Powers JM, Rhodes CH. Adult onset leukodystrophy with neuroaxonal spheroids and pigmented glia: report of a family, historical perspective, and review of the literature. Acta Neuropathol (Berl) 2004;107:481–488. [DOI] [PubMed] [Google Scholar]
- 5.Terada S, Ishizu H, Yokota O, et al. An autopsy case of hereditary diffuse leukoencephalopathy with spheroids, clinically suspected of Alzheimer’s disease. Acta Neuropathol (Berl) 2004;108:538–545. [DOI] [PubMed] [Google Scholar]
- 6.van der Knaap MS, Naidu S, Kleinschmidt-Demasters BK, Kamphorst W, Weinstein HC. Autosomal dominant diffuse leukoencephalopathy with neuroaxonal spheroids. Neurology 2000;54:463–468. [DOI] [PubMed] [Google Scholar]
- 7.Yazawa I, Nakano I, Yamada H, Oda M. Long tract degeneration in familial sudanophilic leukodystrophy with prominent spheroids. J Neurol Sci 1997;147:185–191. [DOI] [PubMed] [Google Scholar]
- 8.Itoh K, Shiga K, Shimizu K, Muranishi M, Nakagawa M, Fushiki S. Autosomal dominant leukodystrophy with axonal spheroids and pigmented glia: clinical and neuropathological characteristics. Acta Neuropathol (Berl) 2006;111:39–45. [DOI] [PubMed] [Google Scholar]
- 9.Browne L, Sweeney BJ, Farrell MA. Late-onset neuroaxonal leucoencephalopathy with spheroids and vascular amyloid. Eur Neurol 2003;50:85–90. [DOI] [PubMed] [Google Scholar]
- 10.Goodman LE, Dickson DW. Nonhereditary diffuse leucoencephalopathy with spheroids presenting as early-onset rapidly progressive dementia. J Neuropathol Exp Neurol 1995;54:471. Abstract.
- 11.Moro-de-Casillas ML, Cohen ML, Riley DE. Leucoencephalopathy with neuroaxonal spheroids (LENAS) presenting as the cerebellar subtype of multiple system atrophy. J Neurol Neurosurg Psychiatry 2004;75:1070–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mascalchi M, Gavazzi C, Morbin M, et al. CT and MR imaging of neuroaxonal leukodystrophy presenting as early-onset frontal dementia. Am J Neuroradiol 2006;27:1037–1039. [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita M, Yamamoto T. Neuroaxonal leukoencephalopathy with axonal spheroids. Eur Neurol 2002;48:20–25. [DOI] [PubMed] [Google Scholar]
- 14.Keegan BM, Giannini C, Parisi JE, Lucchinetti CF, Boeve BF, Josephs KA. Sporadic adult-onset leukoencephalopathy with neuroaxonal spheroids mimicking cerebral MS. Neurology 2008;70:1128–1133. [DOI] [PubMed] [Google Scholar]
- 15.Ali ZS, Van Der Voorn JP, Powers JM. A comparative morphologic analysis of adult onset leukodystrophy with neuroaxonal spheroids and pigmented glia–a role for oxidative damage. J Neuropathol Exp Neurol 2007;66:660–672. [DOI] [PubMed] [Google Scholar]
- 16.Brown LA, Van Gerpen JA, Wider C, Uitti RJ, Dickson DW, Wszolek ZK. A new American kindred with hereditary diffuse leukoencephalopathy with spheroids (HDLS). Mov Disord 2007;22(suppl 16):A835. Abstract.
- 17.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol 1991;48:725–728. [DOI] [PubMed] [Google Scholar]
- 18.Lyon G, Fattal-Valevski A, Kolodny EH. Leukodystrophies: clinical and genetic aspects. Top Magn Reson Imaging 2006;17:219–242. [DOI] [PubMed] [Google Scholar]