Abstract
Objective:
To investigate the combined ability of hippocampal volumes, 1H magnetic resonance spectroscopy (MRS) metabolites, and cerebrovascular disease to predict the risk of progression to dementia in mild cognitive impairment (MCI).
Methods:
We identified 151 consecutively recruited subjects with MCI from the Mayo Clinic Alzheimer’s Disease Research Center and Patient Registry who underwent MRI and 1H MRS studies at baseline and were followed up with approximately annual clinical examinations. A multivariable proportional hazards model that considered all imaging predictors simultaneously was used to determine whether hippocampal volumes, posterior cingulate gyrus 1H MRS metabolites, white matter hyperintensity load, and presence of cortical and subcortical infarctions are complementary in predicting the risk of progression from MCI to dementia.
Results:
Seventy-five subjects with MCI progressed to dementia by last follow-up. The model that best predicted progression to dementia included age, sex, hippocampal volumes, N-acetylaspartate (NAA)/creatine (Cr) on 1H MRS, and cortical infarctions. Based on age- and sex-adjusted Kaplan–Meier plots, we estimated that by 3 years, 26% of the MCI patients with normal hippocampal volumes, NAA/Cr ratios >1 SD, and no cortical infarctions will progress to dementia, compared with 78% of the MCI patients with hippocampal atrophy, low NAA/Cr (≤1 SD), and cortical infarction.
Conclusions:
Multiple magnetic resonance (MR) markers of underlying dementia pathologies improve the ability to identify patients with prodromal dementia over a single MR marker, supporting the concept that individuals with multiple brain pathologies have increased odds of dementia compared with individuals with a single pathology.
GLOSSARY
- AD
= Alzheimer disease;
- ADPR
= Alzheimer’s Disease Patient Registry;
- ADRC
= Alzheimer’s Disease Research Center;
- AIC
= Akaike Information Criteria;
- aMCI
= amnestic mild cognitive impairment;
- CDR
= Clinical Dementia Rating;
- Cho
= choline;
- CI
= confidence interval;
- Cr
= creatine;
- DLB
= dementia with Lewy bodies;
- DSM
= Diagnostic and Statistical Manual of Mental Disorders;
- FLAIR
= fluid-attenuated inversion recovery;
- FTLD
= frontotemporal lobar degeneration;
- HR
= hazard ratio;
- MCI
= mild cognitive impairment;
- mI
= myoinositol;
- MMSE
= Mini-Mental State Examination;
- MR
= magnetic resonance;
- MRS
= magnetic resonance spectroscopy;
- NAA
= N-acetylaspartate;
- naMCI
= nonamnestic mild cognitive impairment;
- NIA
= National Institute on Aging;
- WMH
= white matter hyperintensity.
People with amnestic mild cognitive impairment (aMCI) have an increased risk of developing Alzheimer disease (AD) compared with their cognitively normal peers.1,2 Recently, the concept of mild cognitive impairment (MCI) has been broadened to include patients with nonamnestic MCI (naMCI). There is accumulating evidence that patients with naMCI also have an increased risk of progressing to dementia3–6 relative to cognitively normal elderly subjects. The most common pathologies underlying dementia in the community are AD, cerebrovascular disease, and Lewy body disease. Noninvasive imaging markers of the pathologic substrates of dementia in MCI defined broadly to include the amnestic and nonamnestic subtypes would be useful for early diagnosis and treatment of dementia.
Quantitative magnetic resonance (MR)–based predictors of prodromal AD in aMCI include decreased hippocampal volumes on MRI and decreased neuronal integrity marker N-acetylaspartate (NAA)/creatine (Cr) on 1H magnetic resonance spectroscopy (MRS). The effects of various categories of cerebrovascular disease such as cortical and subcortical infarctions and white matter hyperintensities (WMHs) reported in the literature have been nuanced. Infarctions and WMHs have been associated with an increased risk of cognitive decline in normal people.7–10 In other reports, subcortical infarctions and WMHs did not predict progression to dementia in MCI11,12 and did not correlate with cognitive function in AD,13,14 suggesting that the effects of subcortical vascular disease on risk of future cognitive decline is more significant in people with normal cognitive function than those who are cognitively impaired at baseline.
In this study, we hypothesized that MR abnormalities that are independently associated with underlying dementia pathologies in different brain regions may predict future dementia in MCI more accurately than a single MR abnormality. We investigated whether hippocampal atrophy, 1H MRS metabolite abnormalities, and cerebrovascular disease are complementary in predicting the risk of progression to dementia in MCI defined broadly to include the amnestic and nonamnestic subtypes.
METHODS
Recruitment of subjects.
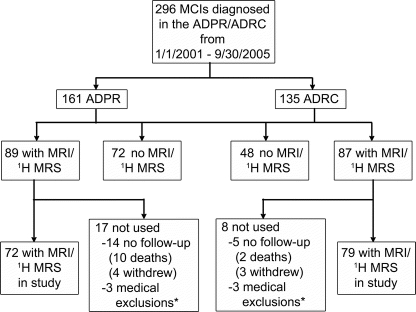
From January 2001 through September 2005, 296 patients with MCI were recruited to the Mayo Clinic Alzheimer’s Disease Research Center (ADRC)/Alzheimer’s Disease Patient Registry (ADPR).15 One hundred fifty-one of these patients with MCI agreed to participate in MRI and 1H MRS studies, and were followed up with annual clinical examinations until December 2008 (figure 1). Subjects were censored at the point when they progressed to dementia, died, or were lost to follow-up. This study was approved by the Mayo institutional review board, and informed consent for participation was obtained from every subject or an appropriate surrogate.
Figure 1 Flowchart indicating the steps involved in defining the study cohort and the number of individuals involved in each step
Subjects who did not undergo MRI and magnetic resonance spectroscopy (MRS) examinations were either not willing to participate or could not undergo magnetic resonance scanning because of safety (e.g., having a pacemaker, claustrophobia). *Medical exclusions were related to alcohol addiction (n = 2), cerebral contusion (n = 1), epilepsy (n = 1), Parkinson disease (n = 1), or amyotrophic lateral sclerosis (n = 1). MCI = mild cognitive impairment; ADPR = Alzheimer’s Disease Patient Registry; ADRC = Alzheimer’s Disease Research Center.
Individuals participating in the ADRC/ADPR studies undergo approximately annual neurologic examinations, structural brain MRI and 1H MRS, routine laboratory tests, and a battery of neuropsychological tests. At the completion of the evaluation, a consensus committee meeting is held involving the behavioral neurologists, neuropsychologists, nurses, and geriatrician who evaluated the subjects.
The operational definition of MCI was based on clinical judgment through a history from the patient and almost always a collateral source without reference to MRI using the criteria for the broad definition of MCI16: cognitive complaint, cognitive function not normal for age, decline in cognition, essentially normal functional activities, and without dementia. Patients with MCI were further classified into one of the two MCI subtypes: aMCI, if the impairment included the memory domain, or naMCI, if the impairment was in one or more nonmemory domains with relative preservation of memory. We grouped all subjects with MCI in this study because of the small number of patients with naMCI (n = 21). Patients with structural abnormalities that could impair cognitive function other than cerebrovascular lesions were excluded. Subjects were not excluded for the presence of infarctions and leukoaraiosis; thus, the full range of ischemic cerebrovascular disease was included.
During follow-up evaluations, a diagnosis of dementia was based on the DSM-III-R criteria.17 The clinical diagnoses of AD, vascular dementia, dementia with Lewy bodies (DLB), and frontotemporal lobar degeneration (FTLD) were made using published consensus criteria.18 The endpoint in time to event analysis was the diagnosis of dementia. Although different dementia diagnoses were noted, they were not considered as separate endpoints.
MRI and 1H MRS.
All subjects underwent MRI and 1H MRS studies on a 1.5-tesla scanner (Signa, General Electric Medical Systems, Milwaukee, WI). Hippocampal volume measurements were derived from a T1-weighted three-dimensional volumetric spoiled gradient-recalled echo sequence. Hippocampal volumes were measured by manually tracing their anatomic boundaries for each image slice sequentially from posterior to anterior.19 The intrarater, test–retest coefficient of variation of hippocampal volume measurements is 0.97% for the image analyst (M.M.S.) who manually traced each hippocampus and was blinded to all clinical information. Volumes were adjusted for age, sex, and head size normal percentiles based on a previous study.20
A fluid-attenuated inversion recovery (FLAIR) sequence was used for the assessment for cerebrovascular disease. A radiologist (K.K.) blinded to all clinical information assessed WMHs and hemispheric cortical and lacunar infarctions. WMH volume was estimated by visually comparing the subject’s FLAIR images to a bank of 10 FLAIR image templates with increasing WMH volumes (from 1 to 100 cm3) determined with an automated image segmentation algorithm.21 A continuous scale with a slider bar was used to estimate subject’s WMH volume by matching to the WMH templates.22 The WMH volume estimation algorithm was previously validated against quantification using automated image segmentation of the WMH volume (concordance correlation coefficient = 0.88, 95% confidence interval [CI] 0.83–0.94).23 Intrarater reliability for assessment of the white matter hyperintensities is excellent (intraclass correlation coefficient = 0.98, 95% CI 0.97–0.98). Hemispheric cortical infarctions were defined as areas of elevated signal intensity involving the cortical gray mantle and immediately subjacent white matter, which exceed 1 cm in largest diameter on FLAIR images. Subcortical infarctions were defined as discrete subcortical lesions >3 mm in diameter with intensity that is equivalent to CSF on FLAIR images and accompanying hyperintense gliotic rim. Intrarater reliability for assessment of subcortical infarctions (proportion in agreement = 0.94) and cortical infarctions (proportion in agreement = 0.98) is excellent.
T1-weighted images in the sagittal plane were obtained for localizing the 1H MRS voxel. Point-resolved spectroscopy pulse sequence with repetition time/echo time = 2,000/30 msec were used for the examinations. An 8-cm3 (2 × 2 × 2-cm) voxel, prescribed on a midsagittal T1-weighted image, included right and left posterior cingulate gyri and inferior precunei.24 We quantified metabolite intensities by referencing to an internal standard, the Cr peak, to correct for coil loading, relaxation times and intersubject differences in atrophy (i.e., partial volume averaging of the MRS voxel).
Statistical analysis.
We used Cox proportional hazards models to evaluate the effect of imaging predictors on the hazard of progression from MCI to dementia. Time zero was considered the date of the MR scan with the event time defined as the date of the first diagnosis of dementia. All predictors were standardized to have a mean of 0 and an SD of 1. Because white matter hyperintensity volume was skewed, before standardizing it we used the natural logarithm transformation.
We used two approaches to evaluate imaging predictors of progression to dementia. We first evaluated each imaging predictor by itself and after adjusting for age, sex, and education. These adjustment variables were chosen because of a priori expectations that they would be related to progression. These models provide estimates of the univariate and age-, sex-, and education-adjusted effect of the imaging predictor on the hazard of progression from MCI to dementia.
We next fit a multivariable Cox model that considered age, sex, education, and all imaging predictors simultaneously. To protect against overfitting and provide a parsimonious model, we used the so-called L1 penalization method, which returns estimated coefficients that maximize the partial log likelihood conditional on a constraint imposed on the sum of the absolute value of these coefficients.25,26 This method can be interpreted as shrinking, or penalizing, some coefficients toward zero, with some coefficients shrunk to exactly zero and essentially excluded from the model. This penalization approach has been used recently to evaluate a relatively large number of clinical and imaging predictors of progression from aMCI to dementia in a logistic regression model.27
Because the L1 penalization approach provides estimated coefficients that depend on the level of the constraint on the coefficients, setting this constraint is important and left to the analyst. In this analysis, we chose the constraint that optimized the Akaike Information Criteria (AIC). We report 95% CIs based on replicating this process based on 1,000 bootstrap samples.
We summarize effect sizes with hazard ratios for a 2-SD change in the predictor. This change can be interpreted as comparing patients who are 1 SD above the mean with those who are 1 SD below it. We also graphically present effect of predictors with Kaplan–Meier type plots in which the survivorship function for certain covariate levels is estimated from the Cox model and the Breslow estimate of the baseline cumulative hazard.28 All analyses were performed using R version 2.7.1 and the “survival” and “glmpath” packages.29
RESULTS
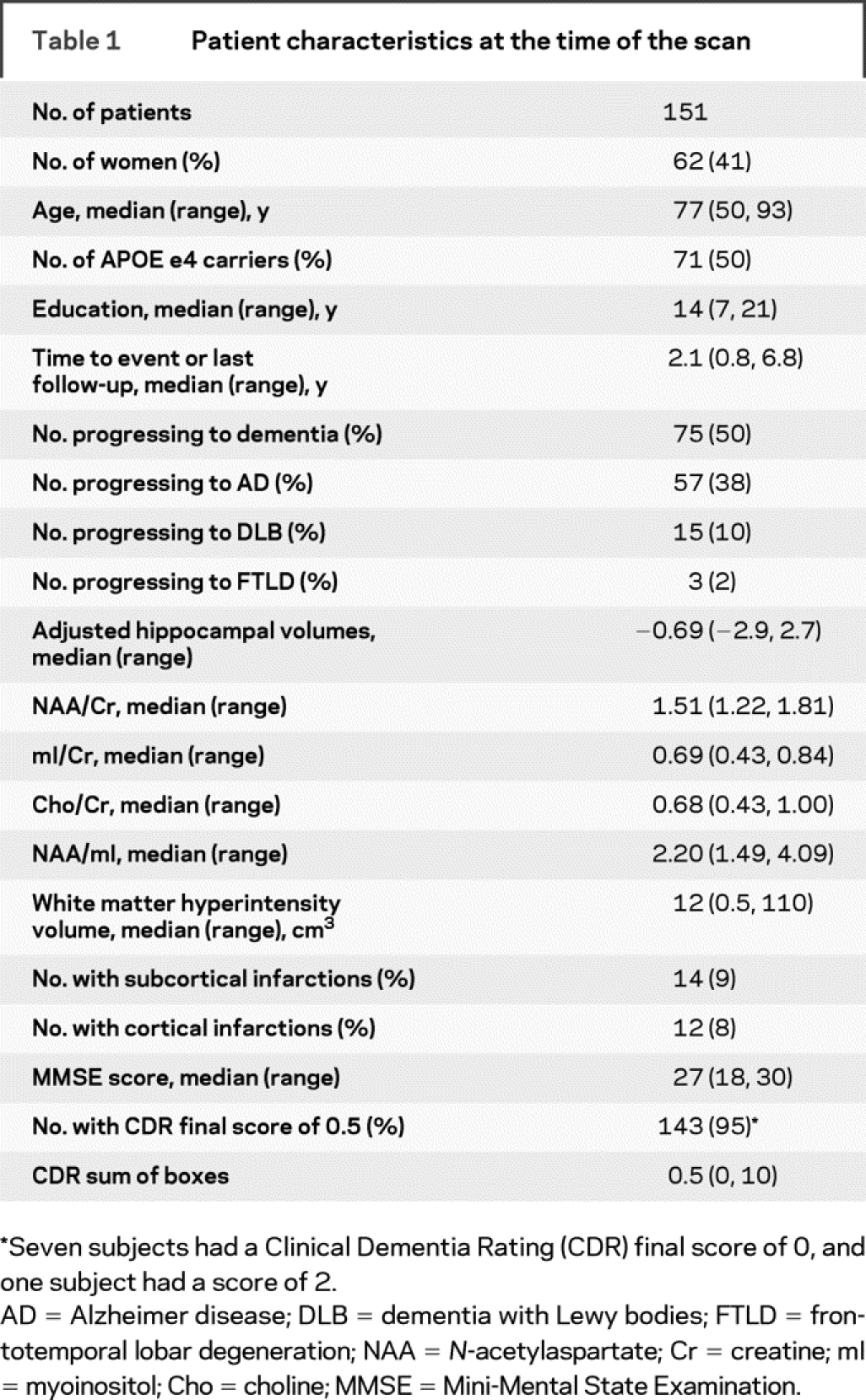
The demographic aspects of the patients with MCI who were included in the imaging study are compared with those of the patients who did not participate or were excluded because of medical reasons in table e-1 on the Neurology® Web site at www.neurology.org. In the ADPR cohort, subjects with MCI who were included in the current study had on average lower Clinical Dementia Rating sum of boxes scores and higher Mini-Mental State Examination scores than those who were not included (p < 0.01). In the ADRC cohort, subjects with MCI who were included in the current study had on average higher education than those who were not included (p = 0.04). Seventy-five (50%) of the 151 subjects with MCI progressed to dementia by the last follow-up (figure 1). The annual rate of progression to dementia was 19% (95% CI 15%–24%). Table 1 lists the demographic aspects and clinical and MR findings in subjects with MCI at baseline.
Table 1 Patient characteristics at the time of the scan
Proportional hazards univariate modeling on each one of the MR predictors indicated that lower adjusted hippocampal volume was a significant predictor of the risk of progression to dementia in MCI. There was a trend for increased risk of dementia in patients with decreased NAA/Cr (p = 0.06) after adjusting for age, sex, and education (table 2).
Table 2 Effect of imaging measures on hazard of progression to dementia
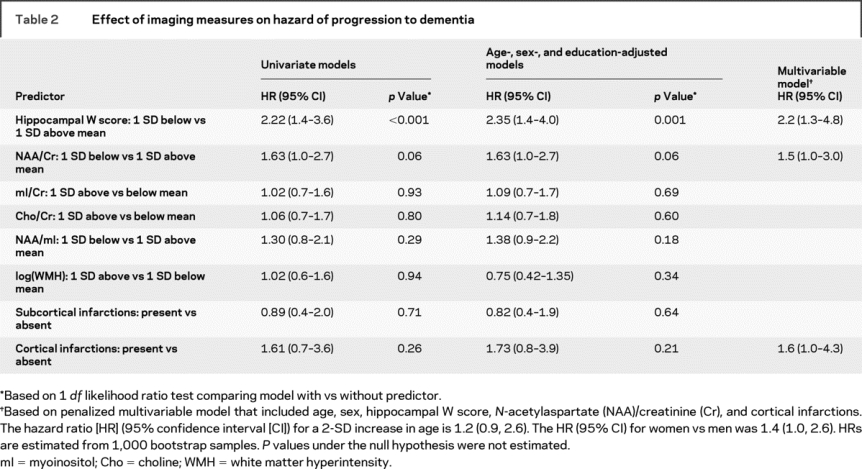
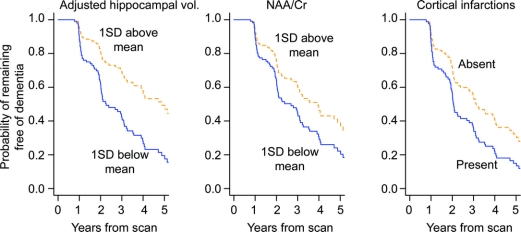
To protect against overfitting and provide a parsimonious multivariable Cox model, we considered age, sex, education, and all imaging predictors simultaneously using the L1 penalization method. The L1 penalization penalty term λ ranged from λ = 0, corresponding to the full model with no penalty and highest degree of overfitting, to the fully constrained null model, where all coefficients are constrained to sum to zero (λ >29). The final multivariable model selected minimizes AIC at λ = 4.3 and included age, sex, adjusted hippocampal volume, NAA/Cr, and cortical infarctions (figure e-1). According to the multivariable Cox model, women with MCI had a higher risk of progressing to dementia. The risk of dementia increased with increasing age, decreasing hippocampal volume and NAA/Cr, and presence of cortical infarctions (table 2). We graphically illustrate the univariate effects of these MR variables in predicting progression to dementia in MCI with individual Kaplan–Meier plots in figure 2.
Figure 2 Estimates of the probability of remaining free of dementia over time based on age-, sex-, and education-adjusted Cox models having a single imaging predictor
Estimates assume a 76-year-old patient with 14.6 years of education and represent a weighted average of the curves for men and women where the weights are the proportion of men and women in our sample. NAA/Cr = N-acetylaspartate/creatine.
The additive effects of the three independent MR predictors are demonstrated with a Kaplan–Meier plot obtained from the multivariate Cox model (figure 3). Estimates shown represent a weighted average of the curves for men and women where the weights are the proportion of men and women in our sample. Based on this multivariable model, we estimate that by 3 years from baseline, 26% of MCI patients without cortical infarctions and having the relative protection of hippocampal volumes and NAA/Cr ratio each 1 SD above the MCI average will have progressed to dementia. This is compared with 78% of MCI patients with cortical infarctions and having hippocampal volumes and NAA/Cr each 1 SD below the MCI average progressing to dementia.
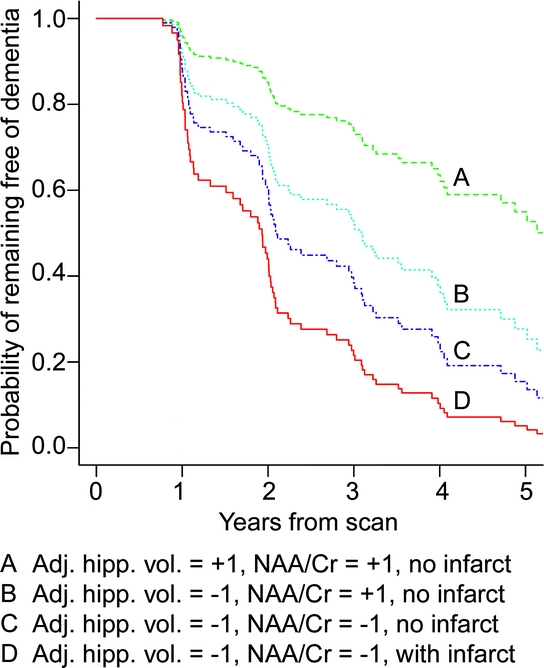
Figure 3 Estimates of the probability of remaining free of dementia for four patient groups with increasingly negative prognoses
Group A has adjusted hippocampal volume and N-acetylaspartate/creatine (NAA/Cr) 1 SD above mild cognitive impairment (MCI) average and no cortical infarctions. Group B has adjusted hippocampal volume 1 SD below MCI average with NAA/Cr 1 SD above MCI average and no cortical infarctions. Group C has adjusted hippocampal volume and NAA/Cr both 1 SD below MCI average and no cortical infarctions. Group D has adjusted hippocampal volume and NAA/Cr both 1 SD below MCI average and cortical infarctions. Estimates shown represent a weighted average of the curves for men and women where the weights are the proportion of men and women in our sample.
DISCUSSION
The data from this longitudinal MR study in MCI indicate that small hippocampal volumes, decreased neuronal marker NAA/Cr, and the presence of cortical infarctions additively increase the risk of progression to dementia in patients with MCI. Hippocampal volume is one of the most investigated imaging markers for early diagnosis of AD and has been a strong predictor of the risk of progression to AD in aMCI.30 In the current study, hippocampal atrophy increased the risk of future progression to dementia in a broadly defined MCI cohort of both aMCI and naMCI subjects. Furthermore, decreased posterior cingulate gyrus NAA/Cr increased the risk of progression to dementia in MCI patients with hippocampal atrophy. This is consistent with cross-sectional studies showing the added value of 1H MRS and hippocampal volumes for discriminating cognitively impaired individuals without dementia from cognitively normal subjects.31 Hippocampal volumes and NAA/Cr levels have also been shown to be independent and complementary predictors of verbal memory on neuropsychometric testing in older adults without dementia, demonstrating that verbal memory depends on both structural and metabolic integrity of the hippocampus.32
NAA is synthesized in the neuronal mitochondria, and NAA levels are thought to reflect the integrity of neuronal mitochondrial metabolism.33 The volume of the hippocampus, on the other hand, is closely associated with hippocampal neuronal density.34 Our data suggest that the risk of progression to dementia in MCI increases when neuronal loss in the hippocampus is accompanied by impairment in neuronal metabolic integrity in the posterior cingulate gyrus. These MR findings from two different regions of the brain may be related to a common pathologic mechanism reflecting incremental involvement with the neurodegenerative pathology from hippocampus to the posterior cingulate gyrus as patients with MCI progress to AD. Another possible explanation, however, is the association between the neuronal changes in these regions and different pathologic mechanisms that are somewhat related but collectively increase the risk of dementia in MCI. For example, decreased glucose metabolism in the posterior cingulate gyrus on PET, which is thought to reflect decreased synaptic activity in this region, is a predictor of future progression to AD in MCI.35,36 Decreased neuronal metabolic integrity measured with NAA/Cr in the posterior cingulate gyrus may be related to a similar pathologic mechanism such as loss of synaptic integrity that increases the risk of dementia in MCI patients with hippocampal atrophy.
Presence of a cortical infarction increased the risk of progression to dementia in MCI when considered together with decreased hippocampal volumes and NAA/Cr levels. Autopsy studies indicate that people with infarctions are more likely to have dementia and can develop dementia with fewer plaques and tangles than those without infarctions.37,38 Furthermore, in a recent community-based autopsy cohort, older persons with multiple brain pathologies such as AD, infarctions, and Lewy body disease were more likely to have dementia than those who had only one of these pathologies.39 In the current study, hippocampal atrophy was a strong predictor of future dementia in MCI by itself. However, the presence of cortical infarctions increased the risk of dementia in those patients with hippocampal atrophy, demonstrating that patients with MCI progress to dementia faster if they have cortical infarctions along with findings associated with AD on MRI.40 This is in agreement with clinical–pathologic studies indicating that individuals with multiple brain pathologies have increased odds of dementia compared with individuals with a single pathology.39 Conversely, in our study subcortical infarctions did not predict progression to dementia in MCI, consistent with a previous longitudinal study in MCI,11 but not with other clinical–pathologic studies.38
WMH volume did not have an effect on the risk of dementia in patients with MCI in the multivariable model. WMH has been implicated as an important surrogate for subcortical ischemic disease in the CNS, influencing cognitive function. WMH is associated with an increased risk of cognitive decline in cognitively normal elderly.7–10 However, several studies have found no relationship between WMH and future cognitive decline in patients with MCI and AD,11–14 and our findings are consistent with this. A way to synthesize our findings in WMH and risk of progression from MCI to dementia in a manner that is consistent with the literature is the following: We speculate that the presence of WMHs is associated with a mild disturbance in cognitive function, but it is not a progressive pathology that by itself will lead to dementia. Therefore, the presence of WMHs may increase the odds of an individual receiving a diagnosis of MCI but does not increase the likelihood of progressing from MCI to dementia. That is, WMHs tend to drive people into the diagnostic category of MCI but does not drive them out of MCI to dementia. In contrast, AD pathology (manifested as hippocampal atrophy and decreased NAA/Cr) increases both the likelihood of receiving a diagnosis of MCI and of progressing from MCI to dementia. This set of circumstances leads to the result we found here that higher WMH does not increase the risk of progression from MCI to AD in a cohort of subjects with both WMHs and neurodegenerative pathology.
This study was designed to identify MRI and 1H MRS predictors of progression to dementia in MCI. Although there are well-known genetic and clinical features that increase the risk of progression to dementia in MCI, they were not included in this study because of the large number of imaging variables we investigated. Larger cohorts are required to determine the complementary role of genetic, clinical, and imaging variables on the risk of progression to dementia.27 We also note that the subjects of this study were consecutive patients with MCI recruited from community and dementia clinics, and on average had less cognitive impairment and were more educated than the patients with MCI who did not participate in the current study. Therefore, these findings should be confirmed in a population-based cohort. Based on the multivariate modeling, we estimated that in 3 years, three times as many (78% vs 26%) MCI patients with hippocampal atrophy, low posterior cingulate gyrus NAA/Cr, and at least one cortical infarction will progress to dementia, compared with MCI patients with preserved hippocampal volumes, higher-than-average posterior cingulate NAA/Cr levels, and no infarctions. Each one of the three MR predictors contributed to the risk of dementia independently as the risk of dementia increased with the number of MR abnormalities.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by S.D. Weigand, S.A. Przybelski, and P.C. O’Brien.
Supplementary Material
Address correspondence and reprint requests to Dr. Kejal Kantarci, Mayo Clinic, 200 First St. SW, Rochester, MN 55905 kantarci.kejal@mayo.edu
Supplemental data at www.neurology.org
Supported by the Paul B. Beeson Career Development Award in Aging K23 AG030935 (NIH/National Institute on Aging [NIA]), Alzheimer’s Association New Investigator Research Grant 03-4842, and the NIH Roadmap Multidisciplinary Clinical Research Career Development Award KL2 RR024151 (NIH/NCRR) to K.K.; P50 AG16574 (NIH/NIA) and U01 AG06786 (NIH/NIA) to R.C.P.; R01 AG11378 (NIH/NIA); the Alexander Family Professorship in Alzheimer’s Research to C.R.J.; and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program.
Disclosure: The authors report no disclosures.
Received September 15, 2008. Accepted in final form February 3, 2009.
REFERENCES
- 1.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia—mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1133–1142. [DOI] [PubMed] [Google Scholar]
- 2.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol 2001;58:397–405. [DOI] [PubMed] [Google Scholar]
- 3.Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 2006;67:2176–2185. [DOI] [PubMed] [Google Scholar]
- 4.Di Carlo A, Lamassa M, Baldereschi M, et al. CIND and MCI in the Italian elderly: frequency, vascular risk factors, progression to dementia. Neurology 2007;68:1909–1916. [DOI] [PubMed] [Google Scholar]
- 5.Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 2007;68:288–291. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord 2006;22:312–319. [DOI] [PubMed] [Google Scholar]
- 7.De Groot JC, De Leeuw FE, Oudkerk M, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 2002;52:335–341. [DOI] [PubMed] [Google Scholar]
- 8.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 9.Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 2008;71:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol 2003;60:1394–1399. [DOI] [PubMed] [Google Scholar]
- 11.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology 2004;63:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith EE, Egorova S, Blacker D, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol 2008;65:94–100. [DOI] [PubMed] [Google Scholar]
- 13.Hirono N, Kitagaki H, Kazui H, Hashimoto M, Mori E. Impact of white matter changes on clinical manifestation of Alzheimer’s disease: a quantitative study. Stroke 2000;31:2182–2188. [DOI] [PubMed] [Google Scholar]
- 14.Pettersen JA, Sathiyamoorthy G, Gao FQ, et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol 2008;65:790–795. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC, Kokmen E, Tangalos E, Ivnik RJ, Kurland LT. Mayo Clinic Alzheimer’s Disease Patient Registry. Aging Clin Exp Res 1990;2:408–415. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC. Mild cognitive impairment. Continuum 2004;10:9–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 18.Knopman DS, Boeve BF, Petersen RC. Essentials of the proper diagnoses of mild cognitive impairment, dementia, and major subtypes of dementia. Mayo Clin Proc 2003;78:1290–1308. [DOI] [PubMed] [Google Scholar]
- 19.Jack CR Jr, Bentley MD, Twomey CK, Zinsmeister AR. MR imaging-based volume measurements of the hippocampal formation and anterior temporal lobe: validation studies. Radiology 1990;176:205–209. [DOI] [PubMed] [Google Scholar]
- 20.Jack CR Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology 1997;49:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jack CR Jr, O’Brien PC, Rettman DW, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging 2001;14:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu CC, Mungas D, Petkov CI, et al. Brain structure and cognition in a community sample of elderly Latinos. Neurology 2002;59:383–391. [DOI] [PubMed] [Google Scholar]
- 23.Murray ME, Shiung MM, Weigand SD, et al. Mayo MRI visual grading scale: validation studies. Abstracts of the Annual Meeting of the Society for Neuroscience, San Diego, CA. Available at: http://sfn.scholarone.com/2007;889.6/O2.
- 24.Kantarci K, Jack CR Jr, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: a 1H MRS study. Neurology 2000;55:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med 1997;16:385–395. [DOI] [PubMed] [Google Scholar]
- 26.Park MY, Hastie T. L1-regularization path algorithm for generalized linear models. J R Stat Soc B Stat Methodol 2007;69:659–677. [Google Scholar]
- 27.Fleisher AS, Sun S, Taylor C, et al. Volumetric MRI vs clinical predictors of Alzheimer disease in mild cognitive impairment. Neurology 2008;70:191–199. [DOI] [PubMed] [Google Scholar]
- 28.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Berlin, Germany: Springer-Verlag; 2000. [Google Scholar]
- 29.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 30.Jack CR Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999;52:1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao LL, Schuff N, Kramer JH, et al. Reduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patients. Neurology 2005;64:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmerman ME, Pan JW, Hetherington HP, et al. Hippocampal neurochemistry, neuromorphometry, and verbal memory in nondemented older adults. Neurology 2008;70:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benarroch EE. N-acetylaspartate and N-acetylaspartylglutamate: neurobiology and clinical significance. Neurology 2008;70:1353–1357. [DOI] [PubMed] [Google Scholar]
- 34.Bobinski M, Wegiel J, Tarnawski M, et al. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J Neuropathol Exp Neurol 1997;56:414–420. [DOI] [PubMed] [Google Scholar]
- 35.Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology 2003;60:1374–1377. [DOI] [PubMed] [Google Scholar]
- 36.Mosconi L, Perani D, Sorbi S, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology 2004;63:2332–2340. [DOI] [PubMed] [Google Scholar]
- 37.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 2005;64:834–841. [DOI] [PubMed] [Google Scholar]
- 38.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA 1997;277:813–817. [PubMed] [Google Scholar]
- 39.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204. [DOI] [PubMed] [Google Scholar]
- 40.Jagust WJ, Zheng L, Harvey DJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol 2008;63:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.