Abstract
Eosinophils are multifunctional leukocytes implicated in the pathogenesis of asthma and in immunity to certain organisms. Associations between exposure to an environmental fungus, such as Alternaria, and asthma have been recognized clinically. Protease-activated receptors (PARs) are G protein-coupled receptors that are cleaved and activated by serine proteases, but their roles in innate immunity remain unknown. We previously found that human eosinophils respond vigorously to Alternaria organisms and to the secretory product(s) of Alternaria with eosinophils releasing their pro-inflammatory mediators. Herein, we investigated the roles of protease(s) produced by Alternaria and of PARs expressed on eosinophils in their immune responses against fungal organisms. We found that Alternaria alternata produces aspartate protease(s) and that human peripheral blood eosinophils degranulate in response to the cell-free extract of A. alternata. Eosinophils showed an increased intracellular calcium concentration ([Ca2+]i) in response to Alternaria that was desensitized by peptide and protease ligands for PAR-2 and inhibited by a PAR-2 antagonistic peptide. Alternaria-derived aspartate protease(s) cleaved PAR-2 to expose “neo-ligands”; these neo-ligands activated eosinophil degranulation in the absence of proteases. Finally, treatment of Alternaria extract with aspartate protease inhibitors, which are conventionally used for HIV-1 and other microbes, attenuated the eosinophils’ responses to Alternaria. Thus, fungal aspartate protease and eosinophil PAR-2 appear critical for the eosinophils’ innate immune response to certain fungi, suggesting a novel mechanism for pathologic inflammation in asthma and for host-pathogen interaction.
INTRODUCTION
Eosinophils are multifunctional leukocytes implicated in the pathogenesis of various inflammatory processes, including allergic diseases, bronchial asthma, helminth and viral infections, tumor immunity, and tissue injury (1, 2). Activated eosinophils produce several proinflammatory cytokines, such as IL-4, IL-13 and TGF-α/β, chemokines, and lipid mediators (1, 2). Furthermore, toxic granule proteins, such as major basic protein (MBP)3 and eosinophil peroxidase (EPO), released by activated eosinophils may cause tissue damage and dysfunction (3). However, the mechanisms for eosinophil activation and release of these proinflammatory and immunomodulatory mediators are largely unknown.
Epidemiological and clinical reports recognize associations between the presence of or exposure to certain airborne fungi, such as Alternaria (in North America), Cladosporium and Aspergillus (in Europe) (4), and asthma and allergic diseases. For example, increased fungal spore counts and fungal Ag levels correlate with allergic symptoms (5–7). In a large multi-country and cross-sectional study, sensitization to Alternaria or Cladosporium, but not to pollens or cats, is a significant risk factor for severe asthma (odds ratio 2.34) (8), and exposure to Alternaria is a risk factor for respiratory arrest in patients with asthma (9). Thus, a major question remains as to the immunological mechanisms underlying the association between fungal exposure and asthma and allergic diseases.
Drosophila detects fungal infections by recognizing PAMPs and by monitoring the effects of fungal virulence factors. Specifically, the receptor Gram-negative binding protein 3 (GNBP3) on Drosophila recognizes the fungal cell wall β-(1,3)-glucan, and the secreted fungal virulence factor PR1 protease cleaves Drosophila Persephone, activating the downstream immune response to the fungi (10). Because fungi use the PR1 protease to break down the protective cuticle of the insect and allow infection (11), the Drosophila Persephone may act as a sensor to monitor the fungal protease activity and integrity of the cuticle. Whether humans have analogous sensor systems to recognize fungal virulence factors remains unknown.
Herein we used the fungus, Alternaria, as a model microbe relevant to human asthma, to investigate the molecular mechanisms involved in the immune recognition of ubiquitous environmental allergen(s). Human eosinophils are activated by live Alternaria alternata organisms, release their granule proteins, and kill the fungi (12). Eosinophils, but not neutrophils, responded to secreted products from A. alternata (13). We found that eosinophils are equipped with innate cellular activation machinery that responds to the extracellular aspartate protease activity secreted by Alternaria. A novel mechanism to activate protease-activated receptor (PAR)-2, as compared to serine protease activation of PAR-2, is likely involved. Thus, human eosinophils may recognize certain danger signals or virulence factors produced by fungi and respond with inflammatory reactions against these organisms. Dysregulation of such an innate immune mechanism may play roles in the pathophysiology of human diseases, such as asthma.
MATERIAL AND METHODS
Materials
Culture extracts from A. alternata are derived from the fungi’s growth media; as fungi grow, they excrete proteins into the media; the media liquid is dialyzed and lyophilized (Greer Laboratories, Lenoir, NC). EGTA, trypsin, chymostatin, 4-Amidinophenylmethanesulfonyl fluoride hydrochloride (APMSF), and trans-exysuccinyl-L-leucylamide (4-guanidino) butane were from Sigma Aldrich Chemical Co. (St. Louis, MO). Ionomycin, alkalo-thermophilic Bacillus aspartate protease inhibitor (ATBI), Indo-1/AM and PMA were from Calbiochem (San Diego, CA). Platelet-activating factor (PAF) was from BIOMOL International (Plymouth Meeting, PA). Agarose beads conjugated with the aspartate protease inhibitor, pepstatin A, and control agarose beads were from PIERCE (Rockford, IL). Ritonavir, an HIV aspartate protease retropepsin inhibitor, was kindly provided by Dr. Andrew Badley, Mayo Clinic. The 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) was from Fluka (Sigma). Tethered ligand peptides specific for human PAR-2 were made at Mayo Clinic Rochester: PAR-2 agonistic peptide, Ser-Leu-Ile-Gly-Lys-Val-NH2 (SLIGKV), PAR-2 antagonistic peptide, Leu-Ser-Ile-Gly-Lys-Val-NH2 (LSIGKV), and control PAR-2 scrambled peptide, Gly-Leu-Ile-Val-Lys-Ser-NH2 (GLIVKS) (14, 15). The PAR-2 peptides, which represent novel N-terminus sequences generated by A. alternata extract’s cleavage of PAR-2 peptide, including Leu-Ile-Gly-Lys-Val-Asp-NH2 (LIGKVD) and Ile-Gly-Lys-Val-Asp-Gly-NH2 (IGKVDG), were also made at Mayo Clinic Rochester. The internally-quenched, synthetic, fluorogenic peptide substrate Abz-33Ser-Lys-Gly-Arg-Ser-Leu-Ile-Gly-Lys41(Dnp)-Asp (Abz-SKGRSLIGKdD), corresponding to the amino acid sequence near the trypsin cleavage site (i.e. 36Arg-37Ser) of PAR-2 was from JPT Peptides (Berlin, Germany). The quenched, synthetic, fluorogenic peptide substrate for malaria aspartate protease, DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS [(DABCYL)-ERNleFLSFP(EDANS)], was from Bachem (Torrence, CA). Blocking anti-human TLR2 and anti-human TLR4 mAbs were from eBioscience (San Diego, CA). Isotype control mouse IgG1 and mouse IgG2a were from BD Biosciences (San Jose, CA).
Eosinophil isolation
Human eosinophils were isolated from normal volunteers or patients with histories of asthma or allergic rhinitis by Percoll density gradient centrifugation and magnetic cell sorting using MACSR® anti-CD16 microbeads as described earlier (16); eosinophil purity was >98%. The Mayo Clinic Rochester Institutional Review Board approved the protocol to obtain blood from volunteers; all provided informed consent.
Eosinophil degranulation assays
To monitor eosinophil function in response to Alternaria extract, trypsin, PAR-2 peptides, PAF or PMA, we measured eosinophil degranulation by quantitating EDN released into supernatants as described earlier (17). In brief, freshly isolated eosinophils were suspended in HBSS with 10 mM HEPES and 0.01% gelatin at 5×105 cells/ml and were incubated with Alternaria extract, 1 nM trypsin, 50 and 200 μM PAR-2 peptides, 1 μM PAF, or 10 ng/ml PMA in 96-well tissue culture plates for 3 h at 37 °C and 5% CO2. Cell-free supernatants were stored at −20 °C before EDN was measured by RIA (17). To examine the calcium dependency of the eosinophil degranulation response to Alternaria, cells were preincubated with 1 mM EGTA for 15 min at 37 °C before stimulation. To investigate the role of PAR-2, eosinophils were preincubated with 100 μM PAR-2 antagonist (LSIGKV), 100 μM scrambled peptide (GLIVKS), or medium for 30 min at room temperature before stimulation. To investigate the roles of TLR2 and TLR4, eosinophils were preincubated with 10 μg/ml anti-TLR2, anti-TLR4, isotype control mouse IgG1 or IgG2a, or medium for 30 min at room temperature before stimulation. Fractions of Alternaria extract were also analyzed for their ability to induce eosinophil degranulation. Alternaria extract was separated by DEAE anion-exchange chromatography (GE HealthSciences, Piscataway, NJ) (Buffer A, 20 mM Tris pH 7.5; Buffer B, 20 mM Tris 1M NaCl pH 7.5), and forty-five 1 ml fractions were collected; 2 μl per fraction was tested for eosinophil degranulation.
Measurement of intracellular Ca2+ Concentration ([Ca2+]i)
Real time changes in [Ca2+]i were measured by flow cytometry (18) using the calcium indicator, Indo-1/AM (19). To load the eosinophils with Indo-1, a 1 ml suspension (1 to 2 × 106 cells/ml) was incubated with 3 mM Indo-1/AM in phenol red-free HBSS with 10% alpha calf serum and 10 mM HEPES for 30 min at 37 °C. After washing, cells were suspended in HBSS with 0.1% HSA, 10 mM HEPES and 1.2 mM calcium. To measure [Ca2+]i, cells were stimulated with agonists, including Alternaria extract, PAF, and ionomycin, and fluorescence was analyzed by a FACS analyzer with an ion-argon laser (Becton Dickinson). [Ca2+]i was monitored for 600 s based on the ratio of the fluorescence of the calcium-bound Indo-1/AM emission (401 nm) and the free Indo-1/AM emission (475 nm). To examine the dependency of the eosinophil [Ca2+]i response on extracellular calcium, cells were preincubated with 3 mM EGTA for 15 min at 37 °C before stimulation. To investigate the roles of PAR-2 in the eosinophils’ [Ca2+]i response to Alternaria, we used both desensitization and PAR-2 antagonist approaches. Eosinophils, loaded with Indo-1 as above, were first incubated with 1 nM trypsin, 100 μM PAR-2 agonist peptide (SLIGKV), or 100 μM control peptide (GLIVKS) at 20 s and then stimulated with 50 μg/ml Alternaria extract or 1 μM ionomycin. Alternatively, eosinophils were preincubated with PAR-2 antagonistic peptide (LSIGKV) or control peptide (GLIVKS), and then stimulated with Alternaria extract.
Quantitation of PAR-2 cleavage activity and aspartate protease activity
The enzymatic activities of proteases were measured using synthetic, fluorogenic peptide substrates where cleavage of the internal sequence of the peptide substrate generates fluorescence by fluorescence resonance energy transfer (20). An N-terminus peptide from human PAR-2, Abz-SKGRSLIGKdD, and the substrate for malaria aspartate protease, (DABCYL)-ERNleFLSFP(EDANS) (21) were used to measure protease activities. Alternaria extract (50 and 100 μg/ml) and 1 nM of trypsin in HBSS with 10 mM HEPES and 0.01% gelatin (pH 7.4) were mixed with 10 mM of substrate. The time course of enzymatic activity was measured spectrofluorometrically (λex =360 nm; λem = 460 nm) on a CytoFluor Multi-Well Plate Reader (PerSeptive Biosystems, Framingham, Mass).
Role of aspartate protease in PAR-2 cleavage and eosinophil activation
To investigate the role(s) of aspartate protease in PAR-2 cleavage and eosinophil activation, several approaches were taken. First, Alternaria extract was treated with a half volume of Pepstatin A gel® (PIERCE, 0020215), Reacti-gel® (PIERCE, 0020260 as control agarose) or medium for 15 min. After centrifugation at 1000 ×g for 5 min, the supernatants were assayed for EDN release, PAR-2 cleavage activity, and [Ca2+]i response. As controls, trypsin, PMA, or PAF were treated similarly to the Alternaria extract. The final concentrations were as follows: Alternaria extract 50 μg/ml, trypsin 6 nM, PMA 1 ng/ml, and PAF 1 μM. Second, to investigate other protease inhibitors, stimulants were treated with ATBI, ritonavir, APMSF or medium alone for 30 min at room temperature. These mixtures were incubated with eosinophils, and EDN release, PAR-2 cleavage, and [Ca2+]i were measured.
Production of PAR-2 cleaving protease(s) by Alternaria cultured with mucin
The kinetics of Alternaria’s growth and production of PAR-2 activating enzyme(s) were examined. A. alternata (ATCC11680) was purchased from ATCC (Manassas, VA) and cultured on potato agarose media (Difco, Kansas City, MO). The fungi were tagged with GFP gene as previously described (22). Spores of GFP-transformed A. alternata (1,000 spore/well) were suspended in HBSS medium supplemented with or without bovine submaxillary gland mucin (Sigma). After 24 h or 48 h at 30 °C, fungal growth was measured by the GFP fluorescence intensities using a CytoFluor Multi-Well Plate Reader. Supernatants of fungal cultures were collected, and the PAR-2 activating proteases in the supernatants were measured by PAR-2 fluorogenic peptide substrates, Abz-SKGRSLIGKdD, as described above.
Capillary reverse-phase HPLC with tandem mass spectrometric detection (LC-MS/MS) analysis of the PAR-2 cleavage site(s) by Alternaria extract
To identify the PAR-2 cleavage site(s) used by Alternaria extract, we analyzed cleavage products of the peptide substrate, Abz-SKGRSLIGKdD, which contains a sequence near the N-terminus of human PAR-2. The peptide substrate was incubated with Alternaria extract (50 and 100 μg/ml) or 1 nM trypsin (as a positive control) for 30 min. To examine specificity, the proteases were also preincubated with pepstatin A gel, control gel, or APMSF, as described above, before incubation with the peptide substrate. The peptide products were subjected to capillary reverse-phase LC using a Cap LC system (Waters Corp., Milton, MA). Ten μl from each incubation was injected and trapped on a Targa C18 cartridge column (5 μM, 2.5 × 0.5 mm, Higgins Analytical, Inc., Mountain View, CA) before separation on a Targa C18 column (5 μM, 50 × 0.150 mm, Higgins Analytical). Peptides were separated using a gradient starting at 95% mobile phase A (98% water: 1% acetonitrile:1% n-propanol:0.2% formic acid, v/v) and going to 55% B (10% water:80% acetonitrile:10% n-propanol:0.2% formic acid; v/v) over 35 min. The flow rate was 12 μl/min and was split pre-column allowing ~400 nl/min into the nano-electrospray ionization source on a Micromass Q-TOF API-US quadrupole time-of-flight mass spectrometer (Waters Corp.). MS and MS/MS spectra were collected in positive mode using a precursor ion scan range of 100 to 1800 m/z. To identify the peptides, the experimental peptide masses were compared to the expected masses from the known peptide substrate sequences.
Analysis of a fraction from DEAE anion-exchange chromatography
An active fraction from DEAE anion-exchange chromatography, namely fraction 18, was further characterized by SDS-PAGE and by proteomic analysis. Briefly, fraction 18 was purified by hydroxyapatite chromatography (BioRad, Hercules, CA) and the DuoFlow Fast Performance Liquid Chromatography system (BioRad) (Buffer A, 50 mM phosphate pH 6.8; buffer B, 500 mM phosphate pH.6.8). Alternaria crude extract and partially-purified fraction 18 were electrophoresed with SDS-PAGE and stained with silver stain. Fraction 18 was also trypsin digested, and the resulting peptides were analyzed by LC-MS/MS as described above. The experimental peptide masses were Blasted/searched against several databases (GenBank NR, a set of predicted proteins derived from the Alternaria brassicicola whole genome sequence, and Alternaria ESTs) to identify fungal genes encoding potential immunostimulatory proteins.
Statistics
Data from ≥ three experiments from different donors were summarized and presented as mean +/− SEM. A one-way ANOVA with repeated measures, student t-test, or Mann Whitney-U test were used to analyze statistical significance. Significance was established at the P < 0.05 level.
RESULTS
Alternaria induces eosinophil degranulation that depends on [Ca2+]i
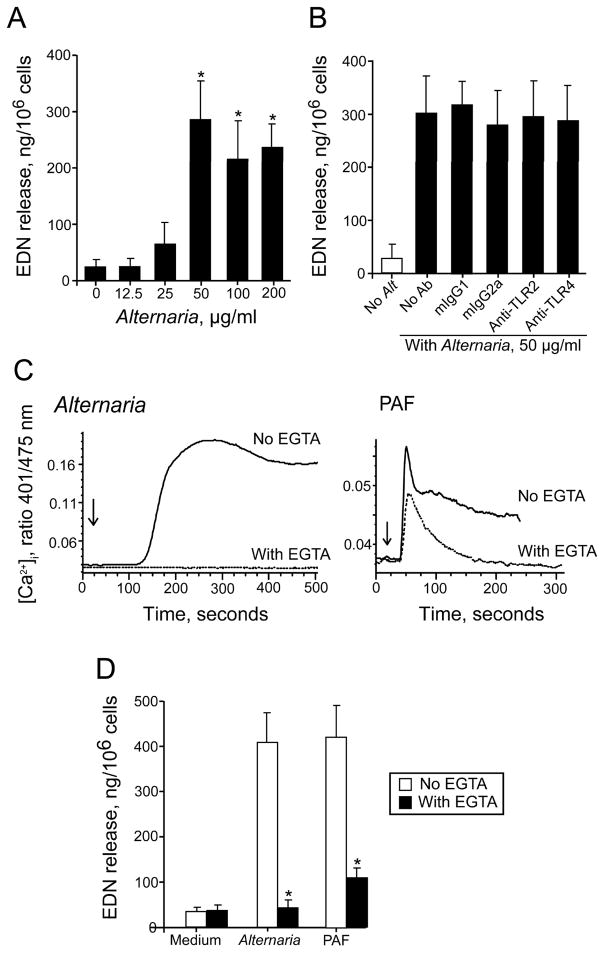
To examine the effects of Alternaria exposure on eosinophil activation and degranulation, eosinophils were incubated with increasing concentrations of A. alternata extract in vitro. Alternaria extract induced degranulation (as measured by EDN release) of human eosinophils in a concentration-dependent manner (Figure 1A). The effects reached a plateau at 50 μg/ml Alternaria. This degranulation increased with time (results not shown), and after 3 h incubation, Alternaria (50 μg/ml) induced maximal EDN release, about 30% of total cellular EDN. To investigate whether eosinophils recognize Alternaria products through TLR, we used blocking Abs, including anti-TLR2 and anti-TLR4, and observed minimal (≤10%) inhibition of Alternaria-induced degranulation (Figure 1B).
FIGURE 1.
Alternaria induces eosinophil degranulation that depends on [Ca2+]i, but not on TLRs. (A) Human eosinophils were incubated with Alternaria extract for 3 h at 37 °C. EDN levels in the cell-free supernatants were measured by RIA. Results show the mean±SEM from six different eosinophil preparations. *; significant differences compared with medium alone (p<0.05). (B) Eosinophils were preincubated with medium alone, anti-TLR2, anti-TLR4 or isotype controls for 30 minutes and stimulated with Alternaria extract for 3 h at 37 °C. EDN levels were measured by RIA. Results show the mean±SEM from four different eosinophil preparations. (C) Eosinophils were loaded with the calcium-sensitive fluorescent dye, indo-1-AM, and stimulated after 20 s (denoted by arrows) with 75 μg/ml Alternaria extract or 1 μM PAF in the presence or absence of 3 mM EGTA. [Ca2+]i was analyzed by FACS. (D) Eosinophils were incubated with 50 μg/ml Alternaria extract or 1 μM PAF for 3 h at 37 °C in the presence or absence of 1 mM EGTA. EDN levels were measured by RIA. *; significant differences compared with medium alone (p<0.05).
The molecular mechanisms for eosinophil degranulation are incompletely understood, but increased [Ca2+]i plays a pivotal role (2, 23). Therefore, we examined whether exposure to Alternaria extract induces increased [Ca2+]i. Eosinophils incubated with 75 μg/ml Alternaria extract showed gradual increases in [Ca2+]i by 150 s with a peak response between 200 to 350 s that was maintained up to 500 s (Figure 1C). The Alternaria-induced [Ca2+]i response was abolished when extracellular calcium was chelated with EGTA, suggesting that calcium influx mainly mediates the response. In contrast, an authentic lipid agonist for eosinophils, which stimulates a seven transmembrane G protein-coupled PAF receptor (24), rapidly increased [Ca2+]i by 50 s, followed by gradual decrease for 250 s. EGTA modestly affected the rapid phase, but abolished the plateau phase, suggesting that PAF induces the initial release of calcium ion from the intracellular stores followed by influx from extracellular milieu. EGTA also inhibited the Alternaria- and PAF-induced EDN release by 97% (p<0.01; n=4) and 85% (p<0.01; n=4), respectively (Figure 1D). Thus, eosinophils exposed to Alternaria extract show a robust [Ca2+]i response and degranulate, and the [Ca2+]i response plays a critical role in degranulation.
Eosinophil response to Alternaria involves PAR-2
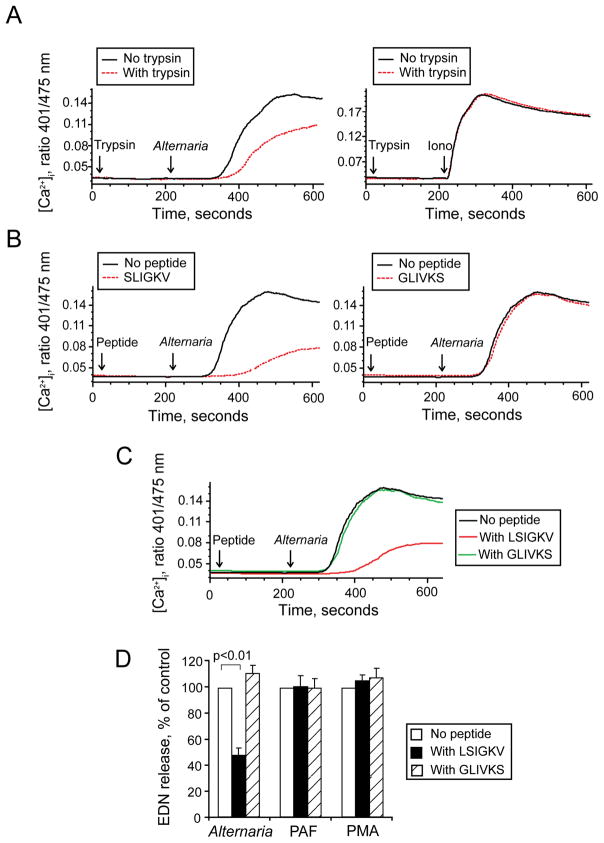
Fungi produce large quantities of proteases (4). Heat treatment of Alternaria extract at 56 °C for 30 min destroys its ability to induce eosinophil degranulation (13), suggesting protease-like activity. A four-member family of seven transmembrane G protein-coupled receptors, PARs, is activated by proteases, in particular serine proteases (25, 26); in general, PAR-1, PAR-3 and PAR-4 respond to thrombin, and PAR-2 responds to trypsin and trypsin-like serine proteases. With human eosinophils, trypsin induces cellular activation and triggers degranulation through PAR-2 (27, 28); other PARs are probably not involved in eosinophil activation. Therefore, we suspected a role for PAR-2 in the Alternaria-induced [Ca2+]i response and subsequent degranulation of human eosinophils. To test the hypothesis, we examined whether an authentic enzymatic agonist for PAR-2 (i.e. trypsin) would desensitize the eosinophils’ [Ca2+]i response to Alternaria extract. Eosinophils were exposed to trypsin at 20 s and then stimulated with agonists, Alternaria extract or ionomycin (a negative control) at 210 s. Trypsin partially decreased the Alternaria-induced [Ca2+]i response (Figure 2A). The ionomycin-induced [Ca2+]i response was not affected by trypsin pretreatment, suggesting specificity for trypsin’s desensitizing effects.
FIGURE 2.
Alternaria-induced [Ca2+]i response and degranulation from eosinophils depends on PAR-2. (A) Eosinophils were loaded with indo-1-AM, incubated with 1 nM trypsin or medium at 20 s (first arrow), and then stimulated with 50 μg/ml Alternaria extract (left) or 1 μM ionomycin (right) at 210 s (second arrow). (B) Indo-1-AM-loaded eosinophils were incubated with 100 μM PAR-2 agonist peptide [SLIGKV (left)], 100 μM scrambled peptide [GLIVKS (right)], or medium at 20 s (first arrow), and then stimulated with Alternaria extract at 210 s (second arrow). (C) Indo-1-AM-loaded eosinophils were incubated with 100 μM PAR-2 antagonist peptide (LSIGKV), 100 μM scrambled peptide (GLIVKS), or medium at 20 s (first arrow), and then stimulated with Alternaria extract at 210 s (second arrow). (D) Eosinophils were preincubated with 100 μM LSIGKV, GLIVKS or medium for 30 min and stimulated with Alternaria extract, PAF or PMA for 3 h at 37 °C. EDN concentrations were measured by RIA. Results show the ratio of control (%) with no peptides and mean±SEM from five different eosinophil preparations.
Trypsin cleaves the extracellular N-terminus of PAR-2 between the R36 and S37 and exposes a tethered “neo-ligand” (i.e. S37LIGKV-) that, in turn, binds intramolecularly to PAR-2 and triggers receptor activation (25, 26). Synthetic peptides, such as SLIGKV, corresponding to the sequence of the tethered “neo-ligand,” can also bind to uncleaved PARs; these peptides are valuable tools to study the consequences of ligand binding to PAR-2 without exogenous proteases. Similarly to desensitization with trypsin, the incubation of eosinophils with PAR-2 peptide agonist, SLIGKV, desensitized eosinophil PAR-2 and markedly decreased the Alternaria-induced [Ca2+]i response (Figure 2B); incubation with a scrambled peptide, GLIVKS, did not desensitize the PAR-2.
While no small molecule inhibitors for PAR-2 are available, a modified PAR-2-derived peptide, LSIGKV, does interact with a tethered ligand-binding site on PAR-2 and inhibits trypsin-induced activation of PAR-2 (14). Thus, eosinophils were exposed at 20 s to LSIGKV, a scrambled peptide (GLIVKS), or medium, and then stimulated with Alternaria at 210 s. LSIGKV, but not GLIVKS, decreased the Alternaria-induced [Ca2+]i response (Figure 2C). Altogether, PAR-2 is likely involved in the eosinophils’ [Ca2+]i response to Alternaria extract.
To examine effects of the modified PAR-2 peptide on eosinophil degranulation, eosinophils were incubated with LSIGKV or a scrambled GLIVKS for 30 min and stimulated with Alternaria extract, PAF or PMA for 3 h. The LSIGKV peptide partially but significantly inhibited Alternaria-induced EDN release by 54%, (p<0.01; n=5) (Figure 2D). The LSIGKV peptide had no effect on PAF- and PMA-induced eosinophil degranulation. In contrast, a control peptide, GLIVKS, showed no effects on Alternaria-induced eosinophil degranulation.
Aspartate protease activity from Alternaria activates human PAR-2
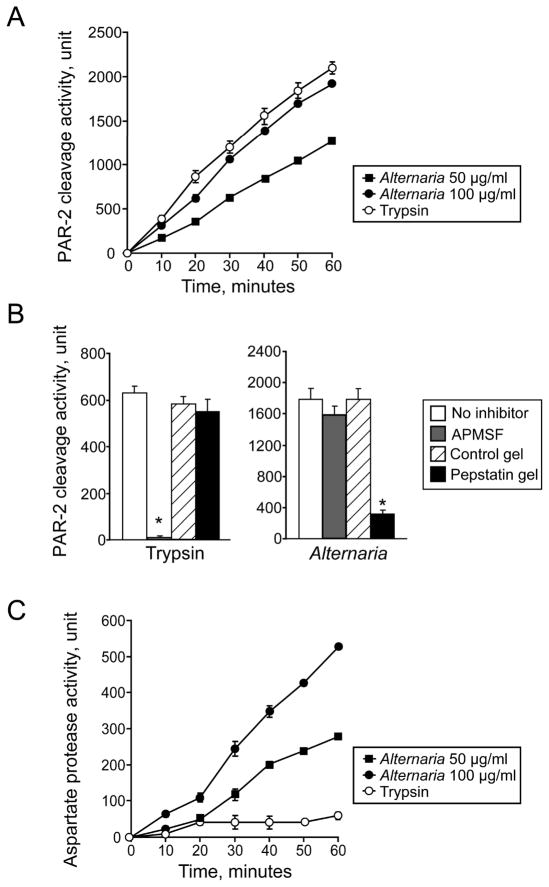
To characterize the protease activity in Alternaria extract that cleaves and activates human PAR-2, we used a fluorogenic peptide substrate, Abz-SKGRSLIGKdD, which corresponds to the four amino acids on either side of the trypsin cleavage site of human PAR-2 (i.e.36Arg/37Ser) (29). The Abz group fluoresces only after release of the Lys(Dnp) group, following cleavage of internal peptides. As expected, the PAR-2 peptide was cleaved by trypsin, resulting in a time-dependent increase in the fluorescence intensity (Figure 3A). Thrombin, an agonist for PAR-1, PAR-3 and PAR-4, did not cleave the peptide (data not shown). Alternaria extract cleaved the PAR-2 peptide in a concentration-dependent manner with kinetics of peptide cleavage similar to trypsin.
FIGURE 3.
Aspartate protease(s) in Alternaria extract cleaves the N-terminus of PAR-2. (A) The fluorogenic PAR-2 peptide substrate, Abz-SKGRSLIGKdD, which contains a sequence near the N-terminus of human PAR-2, was incubated with 1 nM trypsin or Alternaria extract (50 and 100 μg/ml). Cleavage of the peptide was monitored spectrofluorometrically for 60 min. (B) Alternaria extract (50 μg/ml) and trypsin (6 nM) were treated with 200 μM APMSF or medium control for 15 min. Alternatively, Alternaria extract and trypsin were treated with pepstatin A gel (aspartate protease inhibitor) or control gel for 60 min. Activities of the treated proteases to cleave the PAR-2 fluorogenic peptide, Abz-SKGRSLIGKdD, were monitored for 60 min. Results show the mean±SEM from five different experiments. *; significant differences compared with no inhibitors (p<0.01). (C) The fluorogenic malaria aspartate protease substrate, (DABCYL)-ERNleFLSFP(EDANS), was incubated with 1 nM trypsin or Alternaria extract (50 and 100 μg/ml). Cleavage of the peptide was monitored spectrofluorometrically for 60 min.
Serine proteases, such as trypsin and trypsin-like proteases (e.g. mast cell tryptase, tissue kallikreins, and coagulation factors VIIa and Xa), cleave and activate PAR-2 (25, 26). The ability of cysteine proteases, such as house dust mite Der p 1, to activate PAR-2 has been controversial (30, 31). To characterize the proteases in Alternaria extract responsible for the PAR-2 cleavage, we used protease inhibitors. As expected, PAR-2 cleavage by trypsin was abolished by an irreversible serine protease inhibitor, APMSF (Figure 3B). In contrast, PAR-2 cleavage by Alternaria extract was not inhibited by APMSF, suggesting that proteases other than serine proteases are likely involved. Other serine protease inhibitors, including chymostatin and AEBSF, and a cysteine protease inhibitor, trans-epoxysuccinyl-L-leucylamide (4-guanidino) butane, did not inhibit Alternaria’s PAR-2 cleavage activity (data not shown). However, an authentic inhibitor for aspartate protease, pepstatin A (32), decreased PAR-2 cleavage by Alternaria extract by 80% (p<0.01, n=5). Pepstatin A showed no effects on trypsin-mediated PAR-2 cleavage, demonstrating the inhibitor’s specificity. Furthermore, aspartate protease activity, as examined by using a fluorogenic peptide substrate for malaria aspartate protease, (DABCYL)-ERNleFLSFP(EDANS) (21), was clearly detected in Alternaria extract, but not in trypsin (Figure 3C). Thus, aspartate protease activity in Alternaria extract, but not serine protease activity, appears to cleave and activate PAR-2.
During fungal germination and growth, the production of so-called “allergens” by fungi increases markedly (33). We used live A. alternata to examine the growth and production of PAR-2 cleavage activity. In HBSS liquid medium supplemented with bovine mucin, A. alternata spores germinated and grew in a mucin-dependent manner with extensive growth between 24 and 48 h (Figure 4A). Similarly, PAR-2 cleavage activity in the A. alternata supernatants increased with mucin concentration (Figure 4B). Thus, Alternaria produces and releases PAR-2-activating enzyme(s) extracellularly during germination and active growth.
FIGURE 4.
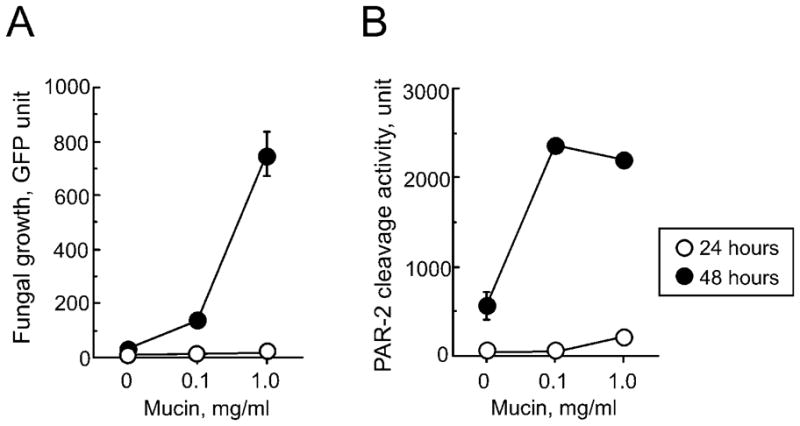
Airway mucin promotes growth of A. alternata and production of PAR-2-activating enzyme(s). Spores of GFP-transformed A. alternata (1,000 spore/well of 96-well tissue culture plates) were cultured in HBSS medium supplemented with different concentrations of bovine mucin. (A) Fungal growth was quantitated after 24 or 48 hours by measuring the intensity of GFP fluorescence in each well. (B) Production of PAR-2-activating proteases by fungi into the supernatants was measured at 24 or 48 h by using a PAR-2 fluorogenic peptide, Abz-SKGRSLIGKdD. Data are mean±SEM from a triplicate experiment, a representative of three experiments showing similar findings.
Aspartate protease activity in Alternaria extract induces [Ca2+]i response and degranulation of human eosinophils
To characterize the eosinophil-activating aspartate protease activity in Alternaria extract further, we used DEAE anion exchange column chromatography to fractionate Alternaria extract. The column fractions were tested for their aspartate protease activities by using (DABCYL)-ERNleFLSFP(EDANS) and for their activities to induce eosinophil degranulation by EDN release. The most potent eosinophil degranulation and aspartate protease activities were detected in fractions 17 through 19 (Figure 5); other fractions contained minimal or no activity. These active fractions from 17 to 19 also induced the [Ca2+]i response, similar to the unfractionated Alternaria extract (data not shown). Fraction 18 contained aspartate protease-like protein by proteomic analysis (Supplemental Figure and Table). Thus, the aspartate protease activity in Alternaria extract is a likely candidate to induce eosinophil degranulation.
FIGURE 5.
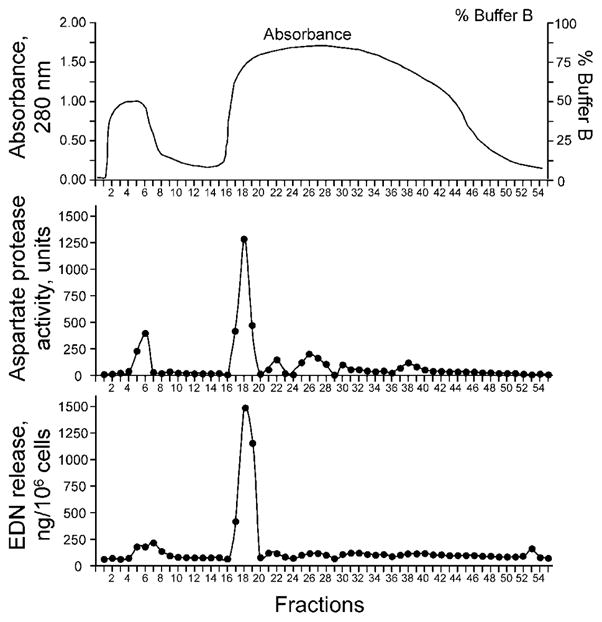
Partially purified proteins in Alternaria extract show aspartate protease activity and activate eosinophils. Alternaria extract was separated by DEAE anion-exchange chromatography; the elution profile shows absorbance at 280 nm and addition of buffer B. DEAE fractions were analyzed for their aspartate protease activities by using the malaria aspartate protease substrate, [(DABCYL)-ERNleFLSFP(EDANS)], and for eosinophil degranulation activity by EDN release.
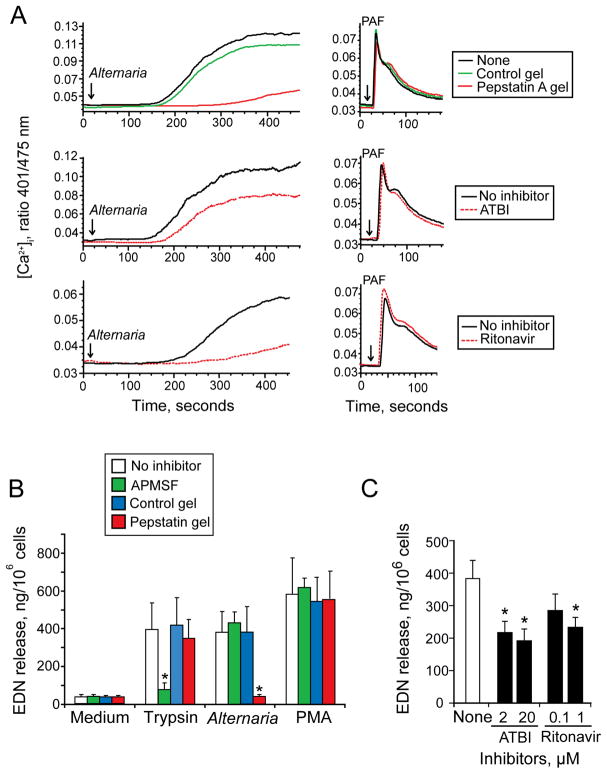
To verify this finding, we investigated the effects of aspartate protease inhibitors on the Alternaria extract-induced [Ca2+]i response and degranulation. Treatment of Alternaria extract with pepstatin A gel, but not control gel, inhibited the extract’s [Ca2+]i response (Figure 6A). This pepstatin A gel inhibition is likely specific because neither pepstatin A nor control gel inhibited the PAF-induced [Ca2+]i response. Two other classes of aspartate protease inhibitors, namely ritonavir and ATBI, which inhibit HIV-1 aspartate protease retropepsin and secreted aspartate proteases of Candida albicans (34–36), respectively, partially inhibited the Alternaria-induced [Ca2+]i response (Figure 6A). In contrast, these same inhibitors did not inhibit the PAF-induced [Ca2+]i response.
FIGURE 6.
Aspartate protease inhibitors suppress Alternaria-induced eosinophil [Ca2+]i response and degranulation. (A) Alternaria extract, 50 μg/ml, was pretreated with pepstatin A gel and control gel (top) for 60 min or with aspartate protease inhibitors, 20 μM ATBI (middle) and 1 μM ritonavir (bottom), for 15 min. PAF (1 μM), which was treated similarly, was used as a control. Treated Alternaria extract (left panels) or PAF (right panels) was added to eosinophils and the [Ca2+]i response was measured. (B) Trypsin (6 nM), Alternaria extract (50 μg/ml), and PMA (1 ng/ml) were pretreated with pepstatin A gel and control gel for 60 minutes or with 200 μM APMSF for 15 minutes. Eosinophils were incubated with treated stimuli for 3 hours, and EDN release was measured. Results show the mean±SEM from five different experiments. *; significant differences compared with no inhibitors (p<0.01). (C) Alternaria extract was pretreated with indicated concentrations of ATBI and ritonavir for 15 minutes and incubated with eosinophils for 3 hours; EDN release was measured by RIA. Results show the mean±SEM from five different experiments. *; significant differences compared with no inhibitors (p<0.01).
For eosinophil degranulation, a serine protease inhibitor, APMSF, inhibited the trypsin-induced degranulation by ~85%; trypsin treated with pepstatin A gel or control gel retained its ability to induce degranulation (Figure 6B). In contrast, pepstatin A gel, but not the control gel, inhibited the Alternaria-induced eosinophil degranulation by >90% (p<0.01, n=5). APMSF showed no effects on Alternaria-induced degranulation. Neither pepstatin A gel nor APMSF inhibited eosinophil degranulation induced by PMA. Both ATBI and ritonavir partially but significantly inhibited Alternaria extract-induced EDN release by ~60%, (p<0.01, n=5) and by ~50% (p<0.01, n=5) at optimal concentrations, respectively (Figure 6C). Thus, the aspartate protease activity in Alternaria extract appears to be involved in the eosinophil’s [Ca2+]i response and degranulation.
Aspartate protease in Alternaria cleaves human PAR-2 peptide at novel sites
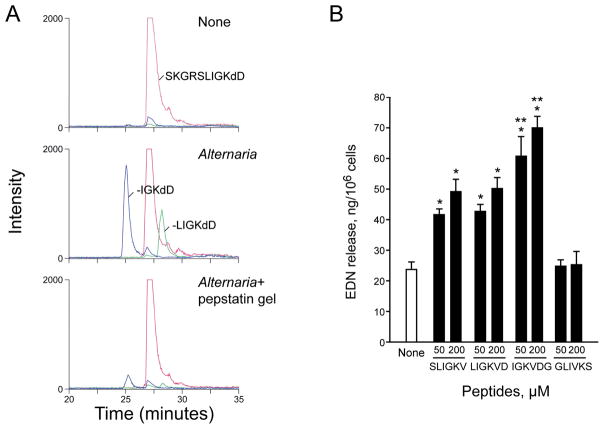
PAR-2 is cleaved by trypsin or trypsin-like proteases at a specific site, namely between Arg36 and Ser37; however, there are no reports for the effects of aspartate proteases on PAR-2. Therefore, we asked whether the Alternaria aspartate protease(s) cleaves PAR-2 at the same site as trypsin and whether the “neo-ligand” exposed by this reaction acts as a ligand for PAR-2. We used the peptide substrate, Abz-SKGR36S37LIGKdD, which corresponds to the four amino acids on either side of the PAR-2’s trypsin cleavage site. The Abz-SKGRSLIGKdD substrate was incubated with Alternaria extract, with or without pretreatment with protease inhibitor, and peptide fragments from each incubation were analyzed by LC-MS/MS. As expected, trypsin cleaved between Arg36 and Ser37 and produced the SLIGKdD fragment (25, 26) (Table 1); trypsin pretreated with APMSF did not produce the SLIGKdD fragment (data not shown). In contrast, Alternaria cleaved the substrate and produced two new fragments, namely LIGKdD and IGKdD (Table 1 and Figure 7A); Alternaria did not cleave the substrate to generate the SLIGKdD fragment. When Alternaria was treated with pepstatin A gel, no new peptide fragments were produced, but neither the control gel nor APMSF inhibited the Alternaria extract’s ability to generate new peptide fragments (Table 1, Figure 7A). Thus, compared to trypsin, the aspartate protease(s) in Alternaria cleaves human PAR-2 at unique peptide sequence sites (Ser37/Leu38, Leu38/Ile39).
Table 1.
Peptide fragments produced from a PAR-2 N-terminus peptide by Alternaria extract
| Enzymes | Inhibitor pretreatment | Generated peptide and fragments |
|---|---|---|
| None | None | SKGRSLIGKdD |
| Trypsin | None | -SLIGKdD |
| Alternaria | None | SKGRSLIGKdD, -LIGKdD, -IGKdD |
| Alternaria | APMSF | SKGRSLIGKdD, -LIGKdD, -IGKdD |
| Alternaria | Pepstatin gel | SKGRSLIGKdD |
| Alternaria | Control gel | SKGRSLIGKdD, -LIGKdD, -IGKdD |
The peptide SKGRSLIGKdD containing a N-terminus sequence of human PAR-2, from Ser33 to Lys41, was incubated with trypsin and Alternaria extract treated with protease inhibitors indicated above for 60 minutes. The generated peptide fragments were analyzed by LC-MS/MS.
FIGURE 7.
Aspartate protease in Alternaria cleaves human PAR-2 peptide, producing novel peptides that activate eosinophils. (A) Ion chromatograms from LC-MS/MS analyses of peptide fragments from an N-terminus peptide of human PAR-2. The peptide Abz-SKGRSLIGKdD containing a N-terminus sequence of human PAR-2, from Ser33 to Lys41, was incubated with medium alone, Alternaria extract or Alternaria extract treated with pepstatin A gel for 60 min and analyzed by LC-MS/MS. Chromatograms display ions at m/z 673.3 (27 min, SKGRSLIGKdD, +2 charge state), 711.3 (28 min, -LIGKdD, +1 charge state), and 598.29 (25 min, -IGKdD, +1 charge state). (B) Eosinophils were incubated with indicated concentrations of synthetic peptides representing novel peptides produced by aspartate protease from Alternaria (LIGKVD and IGKVDG), conventional PAR-2 agonist peptide (SLIGKV), or scrambled peptide (GLIVKS) for 3 hours. Eosinophil degranulation was measured by EDN RIA. Results show the mean±SEM from 7 different experiments. *; significant differences compared to medium alone (i.e. none, p<0.01). **; significant differences compared to SLIGKV (p<0.05).
When cleaved by trypsin, a “neo-ligand” of human PAR-2, namely S37LIGKV-, serves as a ligand for PAR-2 itself (25, 26). Synthetic peptides, such as SLIGKV, that correspond to the sequence of the tethered “neo-ligand” can also bind to and activate uncleaved PAR-2. Therefore, we next investigated whether synthetic peptides, which mimic those produced by aspartate protease(s) from Alternaria, can represent “neo-ligands” of human PAR-2 and stimulate PAR-2 without proteases. Eosinophils were incubated with the synthetic peptides, L38IGKVD43 and I39GKVDG44, representing “neo-ligands” produced by the Alternaria extract. The peptide, S37LIGKV42, representing the “neo-ligand” generated by trypsin, and its scrambled peptide, GLIVKS, served as positive and negative controls, respectively. SLIGKV as well as LIGKVD and IGKVDG induced EDN release from eosinophils (Figure 7B, p<0.01, n=7); IGKVDG induced significantly more EDN release than SLIGKV (at both 50 and 200 μM, p<0.05, n=7). A control peptide, GLIVKS, showed no effect. Overall, these results suggest a novel mechanism for PAR-2 activation and cellular activation in human eosinophils stimulated by aspartate protease(s) from Alternaria.
DISCUSSION
In this study, we found that the PAR-2-mediated recognition of aspartate protease activity secreted by the actively growing fungus, Alternaria, triggers human eosinophils to become activated and degranulate. These conclusions are based on the following observations. 1) A PAR-2 agonist enzyme and PAR-2 ligand peptide desensitized the Alternaria-induced [Ca2+]i response, and a modified PAR-2 peptide inhibited the [Ca2+]i response. 2) Aspartate protease activity present in extracts of growing A. alternata, not serine protease activity, cleaved and activated human PAR-2. 3) Various aspartate protease inhibitors, but not serine or cysteine protease inhibitors, reduced the Alternaria-induced PAR-2 activation, [Ca2+]i response, and EDN release in eosinophils. 4) Alternaria aspartate protease(s) cleaved PAR-2 to expose novel “neo-ligands” (e.g. IGKVDG- and LIGKVD-), which were distinct from neo-ligands generated by trypsin; these neo-ligands activated eosinophil degranulation without proteases. Although we used both biochemical and cell biological methods to demonstrate the role of PAR-2, our study has a potential limitation in that we were unable (for technical reasons) to verify our observations with molecular biological methods, such as small interfering RNA for PAR-2.
Although humans are normally exposed to many airborne proteins and microorganisms, only a small fraction contributes to asthma. How innate immune receptors discriminate between pathogenic and non-pathogenic molecules or microorganisms remains a fundamental immunological question. Because both pathogenic and non-pathogenic organisms likely have similar PAMPS (37, 38), the recognition of PAMPs by TLRs and other pattern-recognition receptors (PRRs) may not fully explain the discrimination between these organisms. Also, mammalian TLRs and PRRs lack the receptor diversity to match the microbial and environmental diversity (39, 40). Plants respond to infection using a two-branched innate immune system: one recognizes and responds to molecules common to many microbes, including non-pathogens, and the other one responds to pathogen virulence factors (41). In Drosophila, the detection of fungal infections relies both on PAMP recognition and on monitoring the effects of virulence factor protease(s) (10). In mammals, the roles for TLRs and other PRRs, such as TLR2, TLR4, TLR9, IL-1R1, and dectin-1, to recognize fungal or yeast infections are well established (42). Thus, like plants and Drosophila, our findings suggest that receptors, such as PAR-2, are activated by endogenous and exogenous proteases and may act like sensors to monitor fungal protease activities or putative virulence factors and provoke immune and inflammatory responses. Mammalian TLRs have likely evolved to survey exogenous products from microorganisms (e.g. LPS) and also the host’s endogenous tissue degradation products (e.g. oligosaccharides of hyaluronan (43) and molecules released from necrotic cell death, such as high-mobility group box 1 protein (44)). Similarly, mammalian PARs may have evolved to survey both exogenous products from microorganisms (e.g. fungal aspartate protease(s)) and endogenous products (e.g. trypsin).
Several reports implicate a role for PAR, especially PAR-2, in airway inflammation and asthma. In mouse airways in vivo, co-administration of PAR-2 agonist peptide and an experimental antigen, ovalbumin (OVA), enhanced Th2-type sensitization to OVA, while administration of OVA alone induced tolerance (45). In patients with asthma, PAR-2 is overexpressed in airway epithelial cells (46), but natural ligands for PAR-2 in human airways are not fully understood. In human epithelial cells, PAR-2 recognizes serine protease allergens, such as Der p 3, Der p 9, Pen c 13; this induces production of proinflammatory cytokines and chemokines (31, 47, 48). Arginine-specific (trypsin-like) cysteine proteinases, the gingipains, as produced by a periodontopathic bacterium, Porphyromonas gingivalis, also activate PAR-2 (49–51). Furthermore, exogenous chitinase from a bacterium, Streptomyces griseus, cleaves human PAR-2 peptide and induces a PAR-2-dependent [Ca2+]i response (52). Thus, PAR-2 can recognize both conventional trypsin-like proteases and perhaps other proteases and glycosidases derived from microbes, fungi and insects. Further studies will be needed to elucidate whether and how PARs are involved in inflammation and perhaps tissue repair and remodeling in human asthma.
While the PAR-2 peptides, corresponding to the “neo-ligands” produced by Alternaria, induced eosinophil degranulation, the amounts of EDN release were smaller than those induced by Alternaria extract (compare Figures 1 and 7). Perhaps the peptide ligands have lower affinities compared to the natural ligands. However, other explanations are possible. Alternaria extract contains a mixture of various biomolecules, including proteases, glycosidases, and carbohydrates (Supplemental Figure and Table). Human PAR-2 possesses two N-linked glycosylation sequons, and the wild-type molecule is highly glycosylated (53). Deglycosylation of PAR-2 increased its sensitivity to tryptase and decreased its sensitivity to trypsin (53). Moreover, our Alternaria extract contained β-glucan (44 μg/mg extract), which is one of the ligands for eosinophil β2-integrin CD11b, and the interaction between β-glucan and CD11b is implicated in eosinophil activation in response to live Alternaria organisms (12). Furthermore, synergistic molecular interactions between PAMPs and PARs, such as TLR4 and PAR-2, have been recognized (54). Thus, the Alternaria extract’s ability to induce robust EDN release, as compared to PAR-2 peptides, may be explained by the effects of other enzymes in the extract on PAR-2 as well as the presence of carbohydrate molecules, which may act synergistically with PAR-2-activating enzymes.
The Alternaria aspartate protease(s) is likely sensitive to an authentic aspartate protease inhibitor, pepstatin A, and also to inhibitors for aspartate proteases from other microbes, such as C. albicans and HIV-1 (i.e. ATBI and ritonavir). These inhibitors suppressed PAR-2 cleavage (Figure 3) and also suppressed the eosinophils’ [Ca2+]i response and degranulation (Figure 6) induced by Alternaria aspartate protease(s). With a molecular mass of approximately 50~60 kDa (13), the Alternaria aspartate protease(s) is distinct from known Alternaria allergens. Little is known about the biology of Alternaria aspartate protease(s), but aspartate proteases secreted by Candida albicans are virulence factors (55–57). These C. albicans secreted aspartic proteases are likely critical for infection by breaking down tissue barriers during invasion, destroying host defense molecules, and providing nutrition (57). A novel class of fungal cell wall aspartate proteases, the yapsins, is also implicated in fungal cell wall assembly and integrity (58). Increased understanding about how Alternaria aspartate protease(s) and other exogenous fungus-derived PAR-activating proteases affect immune cells could explain the interactions between fungi and immune responses and their roles in disease. Recent advances in fungal genomics could facilitate the process (59).
In summary, we discovered that aspartate protease activities secreted by Alternaria induce activation and EDN release from human eosinophils through PAR-2. Thus, a novel communication network may exist involving pathogens, immune cells, proteases and their putative receptors. A recent study suggests that the lipid-binding property of Der p 2, which mimics MD-2 in the TLR4 signaling complex, provides intrinsic adjuvant activity and makes this molecule highly allergenic (60). Thus, certain allergens could have structural or functional intrinsic characteristics that facilitate interactions with the innate immune system; these characteristics could be pivotal for allergenicity and for the development and exacerbation of allergic diseases. An important, but poorly investigated question, concerns how natural exogenous and endogenous proteases activate PARs on mucosal immune cells under physiological and pathophysiological conditions in humans. A better understanding of airway mucosal immunity and the mechanisms involved in the development of asthma and allergic diseases will create novel strategies to prevent and to treat these diseases.
Supplementary Material
Acknowledgments
The authors thank Ms. Cheryl Adolphson for editorial assistance and Ms. LuRaye Eischens for secretarial assistance.
ABBREVIATIONS
- 3MBP
major basic protein
- Abz-SKGRSLIGKdD
Abz-33Ser-Lys-Gly-Arg-Ser-Leu-Ile-Gly-Lys41(Dnp)-Asp
- AEBSF
4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride
- APMSF
4-Amidinophenylmethanesulfonyl fluoride hydrochloride
- ATBI
alkalo-thermophilic Bacillus aspartate protease inhibitor
- [Ca2+]i
intracellular calcium concentration
- (DABCYL)-ERNleFLSFP(EDANS)
DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS
- DCs
dendritic cells
- E64
l-trans-epoxysuccinylleucylamide (4-guanidino) butane-N-[N-(l-3-trans-carboxyirane-2-carbonyl)-l-leucyl] agimatine
- EDN
eosinophil-derived neurotoxin
- EPO
eosinophil peroxidase
- GLIVKS
Gly-Leu-Ile-Val-Lys-Ser-NH2
- IGKVDG
Ile-Gly-Lys-Val-Asp-Gly-NH2
- LC-MS/MS
capillary reverse-phase HPLC with tandem mass spectrometric detection
- LIGKVD
Leu-Ile-Gly-Lys-Val-Asp-NH2
- LSIGKV
Leu-Ser-Ile-Gly-Lys-Val-NH2
- OVA
ovalbumin
- PAF
platelet-activating factor
- PAMP
pathogen-associated molecular pattern
- PRR
pattern-recognition receptor
- SLIGKV
Ser-Leu-Ile-Gly-Lys-Val-NH2
- TLR
toll-like receptor
Footnotes
Supported in part by Grants AI34486 and AI49235 from the National Institutes of Health and by the Mayo Foundation
References
- 1.Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- 2.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 3.Gleich GJ, Adolphson CR, Leiferman KM. The biology of the eosinophilic leukocyte. Annu Rev Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- 4.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 5.Dill I, Niggemann B. Domestic fungal viable propagules and sensitization in children with IgE mediated allergic diseases. Pediatr Allergy Immunol. 1996;7:151–155. doi: 10.1111/j.1399-3038.1996.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 6.Li CS, Hsu LY. Airborne fungus allergen in association with residential characteristics in atopic and control children in a subtropical region. Arch Environ Health. 1997;52:72–79. doi: 10.1080/00039899709603804. [DOI] [PubMed] [Google Scholar]
- 7.Aas K, Leegaard J, Aukrust L, Grimmer O. Immediate type hypersensitivity to common moulds. Comparison of different diagnostic materials. Allergy. 1980;35:443–451. doi: 10.1111/j.1398-9995.1980.tb01791.x. [DOI] [PubMed] [Google Scholar]
- 8.Zureik M, Neukirch C, Leynaert B, Liard R, Bousquet J, Neukirch F. Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. BMJ. 2002;325:411–414. doi: 10.1136/bmj.325.7361.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O’Connell EJ, Ballard DJ, Sachs MI. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med. 1991;324:359–363. doi: 10.1056/NEJM199102073240602. [DOI] [PubMed] [Google Scholar]
- 10.Gottar M, Gobert V, Matskevich AA, Reichhart JM, Wang C, Butt TM, Belvin M, Hoffmann JA, Ferrandon D. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:1425–1437. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng J, An K, Deng C, Li W, Bao X, Qiu D. Cloning a cuticle-degrading serine protease gene with biologic control function from Beauveria brongniartii and its expression in Escherichia coli. Curr Microbiol. 2006;53:124–128. doi: 10.1007/s00284-005-5336-5. [DOI] [PubMed] [Google Scholar]
- 12.Yoon J, Ponikau JU, Lawrence CB, Kita H. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J Immunol. 2008;181:2907–2915. doi: 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue Y, Matsuwaki Y, Shin SH, Ponikau JU, Kita H. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J Immunol. 2005;175:5439–5447. doi: 10.4049/jimmunol.175.8.5439. [DOI] [PubMed] [Google Scholar]
- 14.Al-Ani B, Saifeddine M, Wijesuriya SJ, Hollenberg MD. Modified proteinase-activated receptor-1 and -2 derived peptides inhibit proteinase-activated receptor-2 activation by trypsin. J Pharmacol Exp Ther. 2002;300:702–708. doi: 10.1124/jpet.300.2.702. [DOI] [PubMed] [Google Scholar]
- 15.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansel TT, I, De Vries J, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 17.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J Immunol. 1994;152:5457–5467. [PubMed] [Google Scholar]
- 18.Rabinovitch PS, June CH, Grossmann A, Ledbetter JA. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986;137:952–961. [PubMed] [Google Scholar]
- 19.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 20.Gershkovich AA, V, Kholodovych V. Fluorogenic substrates for proteases based on intramolecular fluorescence energy transfer (IFETS) J Biochem Biophys Methods. 1996;33:135–162. doi: 10.1016/s0165-022x(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 21.Istvan ES, Goldberg DE. Distal substrate interactions enhance plasmepsin activity. J Biol Chem. 2005;280:6890–6896. doi: 10.1074/jbc.M412086200. [DOI] [PubMed] [Google Scholar]
- 22.Cho Y, Davis JW, Kim KH, Wang J, Sun QH, Cramer RA, Jr, Lawrence CB. A high throughput targeted gene disruption method for Alternaria brassicicola functional genomics using linear minimal element (LME) constructs. Mol Plant Microbe Interact. 2006;19:7–15. doi: 10.1094/MPMI-19-0007. [DOI] [PubMed] [Google Scholar]
- 23.Kernen P, Wymann MP, von Tscharner V, Deranleau DA, Tai PC, Spry CJ, Dahinden CA, Baggiolini M. Shape changes, exocytosis, and cytosolic free calcium changes in stimulated human eosinophils. J Clin Invest. 1991;87:2012–2017. doi: 10.1172/JCI115230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishii S, Nagase T, Shimizu T. Platelet-activating factor receptor. Prostaglandins Other Lipid Mediat. 2002;68–69:599–609. doi: 10.1016/s0090-6980(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 25.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008;153(Suppl 1):S263–282. doi: 10.1038/sj.bjp.0707507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miike S, McWilliam AS, Kita H. Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J Immunol. 2001;167:6615–6622. doi: 10.4049/jimmunol.167.11.6615. [DOI] [PubMed] [Google Scholar]
- 28.Bolton SJ, McNulty CA, Thomas RJ, Hewitt CR, Wardlaw AJ. Expression of and functional responses to protease-activated receptors on human eosinophils. J Leukoc Biol. 2003;74:60–68. doi: 10.1189/jlb.0702351. [DOI] [PubMed] [Google Scholar]
- 29.Lourbakos A, Chinni C, Thompson P, Potempa J, Travis J, Mackie EJ, Pike RN. Cleavage and activation of proteinase-activated receptor-2 on human neutrophils by gingipain-R from Porphyromonas gingivalis. FEBS Lett. 1998;435:45–48. doi: 10.1016/s0014-5793(98)01036-9. [DOI] [PubMed] [Google Scholar]
- 30.Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002;169:4572–4578. doi: 10.4049/jimmunol.169.8.4572. [DOI] [PubMed] [Google Scholar]
- 31.Adam E, Hansen KK, Astudillo Fernandez O, Coulon L, Bex F, Duhant X, Jaumotte E, Hollenberg MD, Jacquet A. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J Biol Chem. 2006;281:6910–6923. doi: 10.1074/jbc.M507140200. [DOI] [PubMed] [Google Scholar]
- 32.Umezawa H. Structures and activities of protease inhibitors of microbial origin. Methods Enzymol. 1976;45:678–695. doi: 10.1016/s0076-6879(76)45058-9. [DOI] [PubMed] [Google Scholar]
- 33.Green BJ, Mitakakis TZ, Tovey ER. Allergen detection from 11 fungal species before and after germination. J Allergy Clin Immunol. 2003;111:285–289. doi: 10.1067/mai.2003.57. [DOI] [PubMed] [Google Scholar]
- 34.Dash C, Rao M. Interactions of a novel inhibitor from an extremophilic Bacillus sp. with HIV-1 protease: implications for the mechanism of inactivation. J Biol Chem. 2001;276:2487–2493. doi: 10.1074/jbc.M005662200. [DOI] [PubMed] [Google Scholar]
- 35.Pichova I, Pavlickova L, Dostal J, Dolejsi E, Hruskova-Heidingsfeldova O, Weber J, Ruml T, Soucek M. Secreted aspartic proteases of Candida albicans, Candida tropicalis, Candida parapsilosis and Candida lusitaniae. Inhibition with peptidomimetic inhibitors. Eur J Biochem. 2001;268:2669–2677. doi: 10.1046/j.1432-1327.2001.02152.x. [DOI] [PubMed] [Google Scholar]
- 36.Lea AP, Faulds D. Ritonavir. Drugs. 1996;52:541–546. doi: 10.2165/00003495-199652040-00007. discussion 547–548. [DOI] [PubMed] [Google Scholar]
- 37.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 40.Johnson GB, Brunn GJ, Tang AH, Platt JL. Evolutionary clues to the functions of the Toll-like family as surveillance receptors. Trends Immunol. 2003;24:19–24. doi: 10.1016/s1471-4906(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 41.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 42.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4:1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 43.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 45.Ebeling C, Lam T, Gordon JR, Hollenberg MD, Vliagoftis H. Proteinase-activated receptor-2 promotes allergic sensitization to an inhaled antigen through a TNF-mediated pathway. J Immunol. 2007;179:2910–2917. doi: 10.4049/jimmunol.179.5.2910. [DOI] [PubMed] [Google Scholar]
- 46.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, Thompson PJ. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 47.Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol. 2001;167:1014–1021. doi: 10.4049/jimmunol.167.2.1014. [DOI] [PubMed] [Google Scholar]
- 48.Chiu LL, Perng DW, Yu CH, Su SN, Chow LP. Mold allergen, pen C 13, induces IL-8 expression in human airway epithelial cells by activating protease-activated receptor 1 and 2. J Immunol. 2007;178:5237–5244. doi: 10.4049/jimmunol.178.8.5237. [DOI] [PubMed] [Google Scholar]
- 49.Lourbakos A, Potempa J, Travis J, D’Andrea MR, Andrade-Gordon P, Santulli R, Mackie EJ, Pike RN. Arginine-specific protease from Porphyromonas gingivalis activates protease-activated receptors on human oral epithelial cells and induces interleukin-6 secretion. Infect Immun. 2001;69:5121–5130. doi: 10.1128/IAI.69.8.5121-5130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tancharoen S, Sarker KP, Imamura T, Biswas KK, Matsushita K, Tatsuyama S, Travis J, Potempa J, Torii M, Maruyama I. Neuropeptide release from dental pulp cells by RgpB via proteinase-activated receptor-2 signaling. J Immunol. 2005;174:5796–5804. doi: 10.4049/jimmunol.174.9.5796. [DOI] [PubMed] [Google Scholar]
- 51.Uehara A, Muramoto K, Imamura T, Nakayama K, Potempa J, Travis J, Sugawara S, Takada H. Arginine-specific gingipains from Porphyromonas gingivalis stimulate production of hepatocyte growth factor (scatter factor) through protease-activated receptors in human gingival fibroblasts in culture. J Immunol. 2005;175:6076–6084. doi: 10.4049/jimmunol.175.9.6076. [DOI] [PubMed] [Google Scholar]
- 52.Hong JH, Hong JY, Park B, Lee SI, Seo JT, Kim KE, Sohn MH, Shin DM. Chitinase activates protease-activated receptor-2 in human airway epithelial cells. Am J Respir Cell Mol Biol. 2008;39:530–535. doi: 10.1165/rcmb.2007-0410OC. [DOI] [PubMed] [Google Scholar]
- 53.Compton SJ, Sandhu S, Wijesuriya SJ, Hollenberg MD. Glycosylation of human proteinase-activated receptor-2 (hPAR2): role in cell surface expression and signalling. Biochem J. 2002;368:495–505. doi: 10.1042/BJ20020706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rallabhandi P, Nhu QM, Toshchakov VY, Piao W, Medvedev AE, Hollenberg MD, Fasano A, Vogel SN. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. J Biol Chem. 2008;283:24314–24325. doi: 10.1074/jbc.M804800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Staib F. Proteolysis and pathogenicity of Candida albicans strains. Mycopathol Mycol Appl. 1969;37:345–348. doi: 10.1007/BF02129881. [DOI] [PubMed] [Google Scholar]
- 56.Macdonald F, Odds FC. Virulence for mice of a proteinase-secreting strain of Candida albicans and a proteinase-deficient mutant. J Gen Microbiol. 1983;129:431–438. doi: 10.1099/00221287-129-2-431. [DOI] [PubMed] [Google Scholar]
- 57.Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gagnon-Arsenault I, Tremblay J, Bourbonnais Y. Fungal yapsins and cell wall: a unique family of aspartic peptidases for a distinctive cellular function. FEMS Yeast Res. 2006;6:966–978. doi: 10.1111/j.1567-1364.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- 59.Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, Abe K, Archer DB, Bermejo C, Bennett J, Bowyer P, Chen D, Collins M, Coulsen R, Davies R, Dyer PS, Farman M, Fedorova N, Fedorova N, Feldblyum TV, Fischer R, Fosker N, Fraser A, Garcia JL, Garcia MJ, Goble A, Goldman GH, Gomi K, Griffith-Jones S, Gwilliam R, Haas B, Haas H, Harris D, Horiuchi H, Huang J, Humphray S, Jimenez J, Keller N, Khouri H, Kitamoto K, Kobayashi T, Konzack S, Kulkarni R, Kumagai T, Lafon A, Latge JP, Li W, Lord A, Lu C, Majoros WH, May GS, Miller BL, Mohamoud Y, Molina M, Monod M, Mouyna I, Mulligan S, Murphy L, O’Neil S, Paulsen I, Penalva MA, Pertea M, Price C, Pritchard BL, Quail MA, Rabbinowitsch E, Rawlins N, Rajandream MA, Reichard U, Renauld H, Robson GD, Rodriguez de Cordoba S, Rodriguez-Pena JM, Ronning CM, Rutter S, Salzberg SL, Sanchez M, Sanchez-Ferrero JC, Saunders D, Seeger K, Squares R, Squares S, Takeuchi M, Tekaia F, Turner G, Vazquez de Aldana CR, Weidman J, White O, Woodward J, Yu JH, Fraser C, Galagan JE, Asai K, Machida M, Hall N, Barrell B, Denning DW. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005;438:1151–1156. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- 60.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.