Abstract
Objective
Intrathecal IgG synthesis, persistence of bands of oligoclonal IgG, and memory B-cell clonal expansion are well-characterized features of the humoral response in multiple sclerosis (MS). Nevertheless, the target antigen of this response remains enigmatic.
Methods
We produced 53 different human IgG1 monoclonal recombinant antibodies (rAbs) by coexpressing paired heavy-and light-chain variable region sequences of 51 plasma cell clones and 2 B-lymphocyte clones from MS cerebrospinal fluid in human tissue culture cells. Chimeric control rAbs were generated from anti-myelin hybridomas in which murine variable region sequences were fused to human constant region sequences. Purified rAbs were exhaustively assayed for reactivity against myelin basic protein, proteolipid protein, and myelin oligodendrocyte glycoprotein by immunostaining of transfected cells expressing individual myelin proteins, by protein immunoblotting, and by immunostaining of human brain tissue sections.
Results
Whereas humanized control rAbs derived from anti-myelin hybridomas and anti-myelin monoclonal antibodies readily detected myelin antigens in multiple immunoassays, none of the rAbs derived from MS cerebrospinal fluid displayed immuno-reactivity to the three myelin antigens tested. Immunocytochemical analysis of tissue sections from MS and control brain demonstrated only weak staining with a few rAbs against nuclei or cytoplasmic granules in neurons, glia, and inflammatory cells.
Interpretation
The oligoclonal B-cell response in MS cerebrospinal fluid is not targeted to the well-characterized myelin antigens myelin basic protein, proteolipid protein, or myelin oligodendrocyte glycoprotein.
The cause of multiple sclerosis (MS), a chronic inflammatory demyelinating disease of the central nervous system (CNS), is unknown. Competing theories contend that disease is a T-cell–mediated autoimmune response against myelin antigens, infectious, or a virus-triggered immunopathology directed against one or more autoantigens. Clues to the nature of disease may lie in the presence of bands of oligoclonal IgG (OCBs) in MS brain and cerebrospinal fluid (CSF). OCBs are not unique to MS, and among other CNS diseases with intrathecal IgG synthesis and OCBs, all are inflammatory and most are infectious. Importantly, the CSF oligoclonal IgG in these CNS diseases is directed against the causative agent of disease, which provides a rationale for our hypothesis that the OCBs in MS are directed against disease-relevant antigen(s).1 Despite numerous studies, the specificity of oligoclonal IgG in MS CSF remains unknown.
Intrathecal IgG synthesis in MS is concomitant with the increased presence of B lymphocytes and antibody-secreting plasma cells in CSF.2,3 Analysis of immunoglobulin oligoclonal heavy (H)- and light (L)-chain variable (V)-region sequences recovered from MS plaques and CSF demonstrate clonal expansion, intraclonal sequence diversity, somatic hypermutation, and VH-gene segment bias, features consistent with a specific and targeted antibody response.4–10 Furthermore, a strong link has been established between expanded IgG V-region sequences (immunoglobulin transcriptome) amplified from cells in MS CSF and V-region peptide sequences (immunoglobulin proteome) generated from purified CSF OCBs indicating that CSF plasma cells are representative of the intrathecal oligoclonal IgG response.11 B-cell clonal expansion is also seen in brain and CSF of patients with subacute sclerosing panencephalitis (SSPE), a persistent measles virus infection of the brain. To validate their use for antigen identification, we prepared recombinant antibodies (rAbs) from expanded plasma cell clones identified in the brain and CSF of SSPE patients. More than half of rAbs prepared from SSPE brain12 and CSF13 recognize measles virus proteins.
Because immunization of rabbits, multiple rodent species, and primates with myelin basic protein (MBP), proteolipid protein (PLP), or myelin oligodendrocyte glycoprotein (MOG) produces experimental allergic encephalomyelitis, an autoimmune inflammatory demyelinating disorder of the CNS, we analyzed a panel of rAbs prepared from clonally expanded plasma cells and B lymphocytes in MS CSF for binding to MBP, PLP, and MOG to determine whether these antigens are targeted by the intrathecal antibody response in MS.
Subjects and Methods
Multiple Sclerosis and Control Patients
CSF (approximately 20ml) was collected from MS patients (see supplemental Table 1) after informed consent was given. MS diagnosis was made using established international criteria.14,15 CD138+ plasma cells and, in some patients, CD19+ B lymphocytes were sorted, and H- and L-chain V regions were amplified, sequenced, and analyzed as described elsewhere.8,10
Construction of Human IgG1 Recombinant Antibodies
Full-length bivalent IgG1 rAbs expressing an H-chain C-terminal Flag epitope (Supplementary Fig 1) were produced from H- and L-chain V-region sequences of selected CD138+ and CD19+ clones as described previously.16 Cloned V-region inserts were sequenced to ensure fidelity of the rAbs. Recombinant IgG was produced in Freestyle 293 suspension cultures (Invitrogen, Carlsbad, CA) or in human embryonic kidney (HEK) 293 Epstein–Barr virus–associated nuclear antigen (EBNA) cells after cotransfection with EndoFree H- and L-chain plasmid DNA (Qiagen, Valencia, CA), using 293fectin or Lipofectamine 2000 (Invitrogen), respectively. After incubation for 72 to 96 hours, recombinant IgG was affinity-purified from culture supernatants on protein A-Sepharose beads, concentrated to approximately 1ml using Centricon YM 30 Centrifugal Filter Devices (Millipore, Bedford, MA) and dialyzed overnight at 4°C in phosphate-buffered saline (PBS). Antibody was quantified using the BCA Protein Assay Kit (Pierce Chemical, Rockford, IL), supplemented with 0.1% protease-free and IgG-free bovine serum albumin, 0.002% NaN3, and stored at 4°C. Full-length rAbs were confirmed by 10% sodium do-decyl sulfate polyacrylamide gel electrophoresis and immunoblot detection with anti–human IgG antibodies.
Construction of Chimeric Monoclonal Antibodies
Anti–human MBP (5E3 monoclonal antibody [mAb]) and anti–human MOG monoclonal antibodies (6D7 and 2B7 mAbs) were derived from BALB/c mice immunized with purified human MBP17 and with a bacterial recombinant MOG protein containing the extracellular domain (amino acids 1–121) of human MOG refolded from inclusion bodies,18 respectively. Lymph nodes were collected from different sites and lymphocytes were fused with Sp2/0 cells as described elsewhere.19 After screening of culture supernatants for antigen reactivity using standard enzyme-linked immunosorbent assay methods, single-cell clones were isolated by fluorescence-activated cell sorting. Anti-PLP hybridoma (clone P7.6A5)20 was generated after immunization with a PLP peptide comprising amino acids 178 to 191. The class and subclass of each mAb were determined using a commercially available enzyme-linked immunosorbent assay kit (Zymed Laboratory, San Francisco, CA).
Chimeric mouse-human rAbs were engineered to contain the murine mAb H- and L-chain V regions fused to the respective human constant regions as described in Table 1. Chimeric rAbs served as positive controls in our assays and required no alternative secondary antibody because constant regions were identical to those of human rAbs. Antibodies were prepared by isolating RNA and synthesizing complementary DNA (cDNA) as described previously.21 H- and L-chain V regions were amplified using primers and methods that Wang and colleagues22 described, or by a 5′ rapid amplification cloning of ends (RACE) protocol (Marathon cDNA kit; Clonetech, Palo Alto, CA) using murine constant region primers To ensure fidelity, we subcloned amplified products into the pCR2.1 vector (Invitrogen) and sequenced. Primers were designed to enable subcloning into the pCEP4 vectors used to express human rAbs.
Table 1.
Positive Control and Chimeric Mouse-Human Monoclonal Antibodies, and Human Heavy- and Light-Chain Constant Regions
| Antibody | Immunogen | Species | Humanized rAba | Human Constant Regions |
|---|---|---|---|---|
| α-MBP | Human MBP | Rabbit polyclonal | ND | — |
| Clone 22 | MBP84–89 | Mouse mAb | ND | — |
| plpc1 | PLP270–276 | Mouse mAb | ND | — |
| P7.6A5 | PLP178–191 | Mouse mAb | Hu P7.6A5 | Human CH and murine Cκ |
| 8-18C5 | rMOG protein | Mouse mAb | ND | — |
| 6D7 | MOG | Mouse mAb | Hu 6D7 | Human CH and murine Cκ |
| 2B7 | MOG | Mouse mAb | Hu 2B7 | Human CH and Cκ |
| 5E3 | MBP | Mouse mAb | Hu 5E3 | Human CH and Cκ |
Humanized chimeric recombinant antibodies (rAbs) were generated from the indicated anti-MBP, -PLP and -MOG mAbs in which a flagged human heavy-chain constant region and, in some constructs, a human heavy- and light-chain constant region were substituted for murine constant regions as indicated. Humanized rAbs served as positive controls and demonstrated the in vitro production of functionally active rAbs.
Immunostaining of Transfected Human Embryonic Kidney 293 Epstein–Barr Virus–Associated Nuclear Antigen Cells
cDNA sequences encoding MBP, MOG, and PLP proteins were amplified from a human brain cDNA library by high-fidelity polymerase chain reaction (Roche Diagnostics, Indianapolis, IN) using gene-specific primers that introduced a 5′ Hind III and 3′ Xho 1 site for insertion into pCEP4. Gene-specific cDNA clones were sequenced and transiently expressed for 24 to 72 hours in HEK 293 EBNA cells propagated on poly-L-lysine–coated glass coverslips. Acetone-fixed coverslips were blocked in 3% bovine serum albumin/PBS, pH 7.4, for 30 minutes at room temperature followed by incubation for 90 minutes with positive control (2–10μg/ml) and CSF-derived rAbs (10μg/ml) diluted in 1% bovine serum albumin/PBS. Coverslips were washed three times in PBS, incubated with a 1:500 dilution of the appropriate horseradish peroxidase–or alkaline-phosphatase–conjugated anti–mouse, anti–rabbit, or anti–human secondary antibody, washed again in PBS, and developed with diaminobenzidine (Vector Labs, Burlingame, CA) or nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) (Roche Diagnostics). Coverslips were counterstained with hematoxylin, rinsed in water, and mounted in aqueous medium for microscopy. rAbs were assayed in pools of two to three rAbs per coverslip. Each set of experiments included staining with an appropriate positive control antibody against the particular myelin protein.
Immunostaining of Multiple Sclerosis and Control Tissue
MS CSF-derived and control rAbs, including a SSPE brain-derived rAb (2B4), were tested blindly on archival, formaldehyde-fixed, and paraffin-embedded brain tissue from MS patients, healthy control subjects, and SSPE brain. MS tissue blocks contained active demyelinating lesions (disease duration between 14 days and 3 months) and active lesions from patients with progressive MS (disease duration 5–10 years).23,24 Antibodies were either tagged with biotin (ImmunoProbe Biotinylation Kit; Sigma, St. Louis, MO) or detected via the Flag epitope as described later.
Human brain sections (5μm) were deparaffinized in graded series of ethanol and assayed both with and without antigen retrieval. Antigen retrieval was conducted by incubation of sections in either citrate buffer (pH 6.0) or EDTA buffer (pH 8.5) in a household steamer for 30 minutes. Nonspecific protein binding was blocked by preincubation with 10% fetal calf serum. Biotinylated or Flag-tagged antibodies (1–10μg/mu;l) were applied in a humidified chamber overnight at 4°C. Depending on the primary label used for detection, sections were incubated in avidin peroxidase (1:70; Sigma) or with a mouse anti-Flag antibody (1:5,000; Nr 200471; Stratagene, San Diego, CA), followed by a 1:200 dilution of biotinylated species-specific anti–mouse antibody (RPN 1001; Amersham, Buckinghamshire, United Kingdom) and avidin peroxidase. Bound antibodies were visualized by development in diaminobenzidine (Fluka, Buchs, Switzerland) and counterstained with hematoxylin.
Protein Lysates
Purified human MBP (>95% pure) was purchased from Research Diagnostics (Flanders, NJ). MS brain protein lysate was prepared from powdered frozen tissue (0.15gm) of an acute MS brain lesion by polytron homogenization in 4ml Tris-buffered saline, pH 7.5, containing protease inhibitors (Complete Mini; Roche Diagnostics). Cell membranes were collected by centrifugation for 60 minutes in a SW60 rotor, and the crude cell pellet was suspended in PBS, quantified, and stored in aliquots at −80°C. Lysates were prepared from pCEP4-MOG and mock pIgG1Flag-transfected HEK 293 EBNA cells. At 48 hours after transfection, cells were scraped from a T75 flask, collected by centrifugation (10 minutes at 1,500gm), washed in 20ml ice-cold PBS, collected, and frozen at −80°C. The cell pellet was thawed on ice, suspended in 1ml PBS containing protease inhibitors, and lysed by several freeze-thaw cycles and sonication. Supernatant was recovered after centrifugation at 1,500g for 10 minutes, quantitated, and stored at −80°C.
Results
Generation of Functional Recombinant Antibodies from V-Region Sequences
We generated recombinant IgG1 antibodies by coex-pressing paired H- and L-chain V-region sequences from individual cells of expanded CSF CD138+ plasma cell clones and two CD19+ B-lymphocyte clones in mammalian tissue culture cells (see Supplementary Fig 1). Fifty-three different rAbs were derived from MS CSF at various stages of disease. Table 2 lists the features of CSF-derived rAbs including MS patient, family germline segment, degree of somatic mutation, and frequency of detection in the CSF repertoire. As positive controls, chimeric rAbs expressing human constant regions were generated from V-region sequences of murine hybridomas producing mAbs to the myelin proteins MBP, PLP, and MOG.
Table 2.
Recombinant Antibodies Derived From Multiple Sclerosis Cerebrospinal Fluid Plasma Cells
| CSF rAba | V Regions | Frequency | |||
|---|---|---|---|---|---|
| VH | % | VL | % | ||
| MS02-19 | |||||
| 19–11 | 4–39 | 95.7 | L6 | 98.2 | 2/23 |
| 19–15 | 2–70 | 96.3 | L12 | 96.4 | 2/23 |
| 19–17 | 4–39 | 93 | O12 | 95.8 | 2/23 |
| 19–21 | 4–59 | 95.9 | B3 | 96 | 3/23 |
| 19–25 | 4–39 | 93.3 | L4 | 96.5 | 2/23 |
| 19–28 | 4–34 | 87.6 | O12 | 93 | 1/23 |
| 19–42 | 4–39 | 92.2 | L6 | 97.2 | 4/23 |
| 19–52 | 4–39 | 91.6 | L12 | 93.7 | 2/23 |
| 19–53 | 4–59 | 92.4 | L12 | 95.4 | 2/23 |
| MS03-1b | |||||
| 1–2 | 4–34 | 95.2 | 7b | 95.6 | 8/78 |
| 1–3 | 3–30.3 | 95 | O18 | 95.4 | 2/78 |
| 1–5 | 2–70 | 93.9 | L2 | 96.4 | 3/78 |
| 1–10 | 3–09 | 95.6 | B3 | 98 | 2/78 |
| 1–13 | 3–09 | 94.9 | 1b | 97.7 | 17/78 |
| 1–15 | 3–09 | 92.9 | O12 | 95.3 | 8/78 |
| 1–17 | 4–04 | 93.3 | B3 | 95.6 | 2/78 |
| 1–22 | 3–72 | 95 | L1 | 97.1 | 5/78 |
| 1–31 | 4–59 | 91.3 | A19 | 99 | 2/78 |
| 1–37 | 4–30.4 | 94.3 | O12 | 95.1 | 5/78 |
| 1–57c | 3–23 | 95.3 | O12 | 93.7 | 1/78 |
| 1–64 | 4–59 | 95.9 | A27 | 98.3 | 2/78 |
| 1–69c | 3–53 | 94.2 | A27 | 95.1 | 1/78 |
| 1–86 | 3–30.3 | 93.3 | L1 | 95.4 | 2/78 |
| MS04-2 | |||||
| 2–9 | 4–b | 93.2 | L6 | 96.8 | 2/66 |
| 2–11 | 1–46 | 87.2 | O12 | 90.9 | 5/66 |
| 2–13 | 5–51 | 92.2 | L6 | 95.2 | 2/66 |
| 2–30 | 4–04 | 92.4 | O12 | 96.4 | 2/66 |
| 2–33 | 4–59 | 87.4 | L8 | 92.8 | 2/66 |
| 2–63 | 3–09 | 93.9 | A17 | 95.8 | 4/66 |
| 2–80 | 3–23 | 91.6 | B3 | 94.7 | 2/66 |
| 2–92 | 5–51 | 91.2 | L8 | 95.3 | 2/66 |
| MS03-7 | |||||
| 7–10 | 3–07 | 91.2 | B3 | 98 | 2/39 |
| 7–13 | 4–31 | 95.2 | A3 | 96 | 11/39 |
| MS04-3b | |||||
| 3–1 | 4–61 | 93.3 | A19 | 99 | 38/40 |
| ON03-3b | |||||
| 3–6d | 3–74 | 94.5 | 3r | 96 | 11/56 |
| 3–52 | 4–34 | 90 | L8 | 94 | 2/18 |
| 3–55d | 1–08 | 93 | 1c | 96 | 3/56 |
| 3–69 | 1–e | 89 | L2 | 92 | 4/18 |
| 3–77 | 1–69 | 91 | A19 | 92 | 2/18 |
| ON03-5b | |||||
| 5–17 | 4–39 | 94 | A2 | 97 | 7/41 |
| 5–61 | 2–70 | 99 | A2 | 98 | 3/41 |
| 5–81e | 4–39 | 97 | A2 | 98 | 7/41 |
| ON04-7b | |||||
| 7–18 | 3–30 | 90.1 | 1b | 96.1 | 9/61 |
| 7–23 | 4–39 | 94.8 | L6 | 97.2 | 6/61 |
| 7–26 | 3–07 | 93.4 | O2 | 95.4 | 5/61 |
| 7–57 | 2–05 | 95.9 | L2 | 96.1 | 7/76 |
| 7–59 | 4–31 | 93.3 | O2 | 94.4 | 10/61 |
| 7–78 | 3–30 | 89.1 | L12 | 92.2 | 2/61 |
| 7–94 | 2–05 | 94.9 | L2 | 96.6 | 7/61 |
| ON04-8b | |||||
| 8–10 | 4–04 | 96.6 | L6 | 97.5 | 4/25 |
| 8–11 | 4–04 | 90.5 | O2 | 93.2 | 2/25 |
| 8–21 | 3–48 | 87.8 | L12 | 91.1 | 2/25 |
| 8–74 | 3–33 | 93.8 | B3 | 94.3 | 2/25 |
| SSPE 83f | |||||
| 2B4 | 3–30 | 92.4 | B3 | 96.1 | ND |
Columns indicate features of individual rAbs generated from CSF plasma blast clones and include donor CSF and rAb number, heavy-(VH) and light-chain V-region (VL) germline (designated by locus), the percentage homology of each V region to germline, and frequency in the CSF CD138+ or CD19+ IgG repertoire.
Single-cell repertoire analysis was performed on CSF obtained during first clinical event. Each patient subsequently experienced development of relapsing-remitting multiple sclerosis (MS) within a 6-month period.
Although this sequence was detected only once in the CD138+ repertoire, it was considered clonal because the same VH and VL rearrangements were also observed in the CD19+ repertoire of this donor cerebrospinal fluid (CSF).
rAbs were derived from expanded CSF CD19+ B-cell clones of this patient.
This rAb was derived from a clonal variant of rAb 5–17 from ON03-5 CSF.
rAb 2B4 was initially obtained by panning a Fab antibody library derived from subacute sclerosing panencephalitis (SSPE) 83 brain tissue on measles virus protein lysates.25
ND = not done.
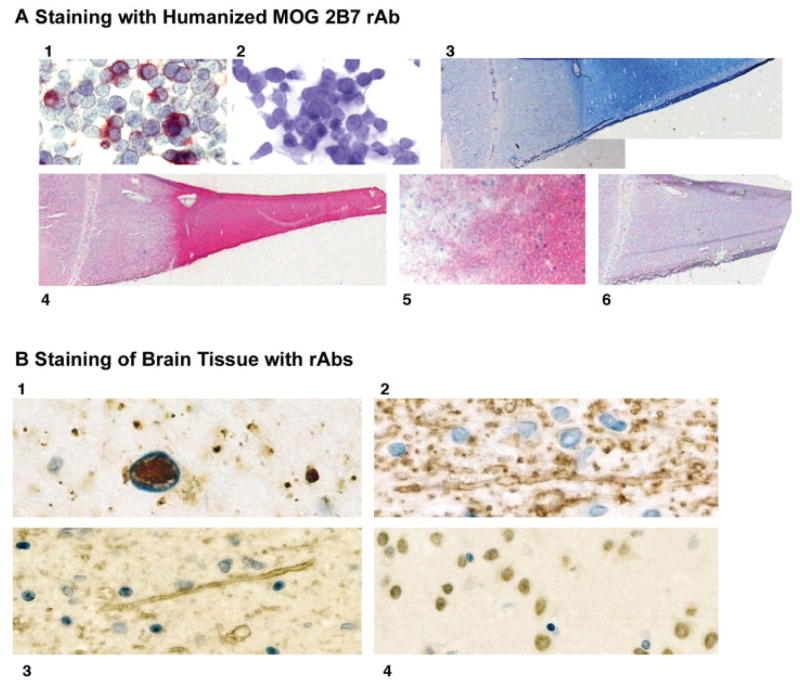
Bivalent rAbs produced in tissue culture cells readily duplicated the antigen specificity of parent hybridomas. Immunostaining with Hu2B7 rAb labeled MOG-transfected cells (see Fig 1A, panels 1 and 2), and clearly demarcated plaque from normal-appearing white matter in MS lesions (see Fig 1A, panels 4 and 5). Positive myelin staining of brain (see Figs 1B, panels 2 and 3) and of HEK 293 EBNA cells expressing the appropriate target myelin protein (Figs 2A, panel 3, 2B, panel 4, and 2C, panel 4) were also seen with humanized rAbs against MOG, PLP and MBP (myelin staining of brain not shown for Hu5E3 rAb).
Fig 1.
(A) Identification of MOG protein by immunostaining with chimeric MOG 2B7 rAb. (1) MOG-transfected and (2) mock-transfected HEK 293 EBNA cells incubated with Hu 2B7 rAb (2μg/ml). (3) Luxol fast blue staining of active MS lesion. (4) Staining of acetone fixed-frozen section of an active MS lesion with Hu 2B7 rAb (2μg/ml) via the Flag epitope. (5) Staining at the plaque border of an active MS lesion shown in (3) with Hu 2B7 rAb. (6) Background control staining of active MS lesion with mouse anti-Flag (1:1,000) secondary and horse anti–mouse-alkaline phosphatase tertiary antibody (1:1,000) using Vector Red as chromogenic substrate. (B) Staining of formalin-fixed, paraffin-embedded brain tissue with control and MS rAbs. (1) SSPE lesion stained with rAb 2B4; note the strong immunoreactivity within nuclear inclusion and some granular cytoplasmic reactivity in measles-virus–infected cell. (2) Selective and strong staining of myelin at the edge of an MS lesion incubated with Hu P7.6AT rAb (reactive with PLP). (3) A similar staining of myelin with Hu 6D7 (reactive with MOG. (4) Nuclear staining of neurons in MS tissue with rAb 8–74. Similar weak nuclear staining was also observed with rAb 1–69 (not shown). Original magnification ×400 (A, panels 1, 2); ×20(B, panels 3, 4); ×200 (A, panels 5, 6); ×1,000 (B, panel 1); ×600 (B, panels 2–4).
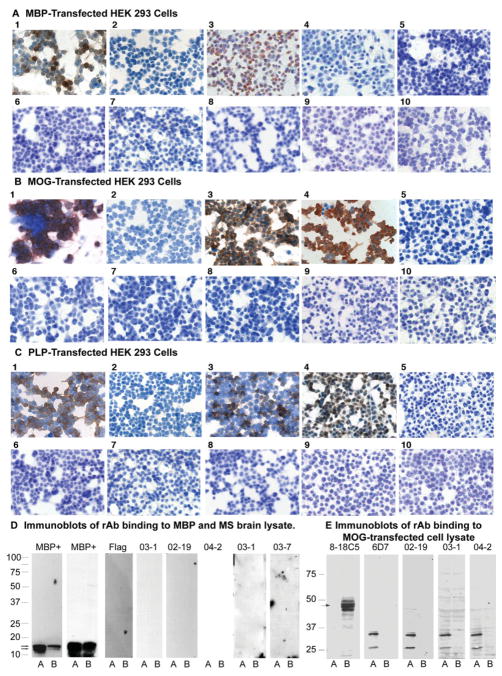
Fig 2.
Assay of control and representative multiple sclerosis (MS) recombinant antibodies (rAbs) for immunoreactivity with human myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG), and proteolipid protein (PLP). (A) Human embryonic kidney (HEK) 293 cells, transfected with pCEP-4 plasmid DNA expressing the full-length protein coding region for MBP or mock pCEP4 vector DNA, were immunostained with positive control antibodies and cerebrospinal fluid (CSF)–derived rAbs. (1) MBP+ cells stained with anti-MBP84–89 monoclonal antibody (mAb). (2) Mock-transfected cells stained with anti-MBP84–89 mAb. (3) MBP+ cells stained with anti-MBP 5E3 rAb. (4) Mock-transfected cells stained with anti-MBP 5E3 rAb. (5) MBP+ cells stained with MS02-19 #21 and #52 rAbs. (6) MBP+ cells stained with MS03-1 #5 and #15 rAbs. (7) MBP+ cells stained with MS04-2 #13 and #63 rAbs. (8) MBP+ cells stained with MS03-7 #10 and #13 rAbs. (9) MBP+ cells stained with ON04-7 #94 and ON04-8 #10 rAbs. (10) MBP+ cells stained with ON04-7 #18 and #57 rAbs. (B) HEK 293 cells, transfected with pCEP-4 plasmid DNA expressing the full-length protein coding region for MOG or mock vector pCEP-4 DNA, were immunostained with positive control antibodies and CSF-derived rAbs. (1) MOG+ cells stained with 8-18C5 anti-MOG mAb. (2) Mock-transfected cells stained with 8-18C5 mAb. (3) MOG+ cells stained with anti-MOG 6D7 mAb. (4) MOG+ cells stained with anti-MOG 6D7 rAb. (5) MOG+ cells stained with MS02-19 #28 and #53 rAbs. (6) MOG+ cells stained with MS03-1 #5 and #15 rAbs. (7) MOG+ cells stained with MS04-2 #30 and #33 rAbs. (8) MOG+ cells stained with MS03-7 #10 and #13 rAbs. (9) MOG+ cells stained with ON03-3 #69 and #77 rAbs. (10) MOG+ cells stained with ON04-7 #18 and #57 rAbs. (C) HEK 293 cells, transfected with pCEP-4 plasmid DNA expressing the full-length protein coding region for PLP or mock pCEP4 vector DNA, were immunostained with positive control antibodies and CSF-derived rAbs. (1) PLP+ cells stained with plpc1 anti-PLP mAb. (2) Mock-transfected cells stained with plpc1 anti-PLP mAb. (3) PLP+ cells stained with P7.6A5 anti-PLP mAb. (4) PLP+ cells stained with P7.6A5 anti-PLP rAb. (5) PLP+ cells stained with MS02-19 #15 and #17 rAbs. (6) PLP+ cells stained with MS03-1 #5 and #15 rAbs. (7) PLP+ cells stained with MS04-2 #13 and #63 rAbs. (8) PLP+ cells stained with MS03-7 #10 and #13 rAbs. (9) PLP+ cells stained with ON04-8 #11 and #21 rAbs. (10) PLP+ cells stained with ON03-5 #17 and #61 rAbs. (D) Protein blots of purified human MBP (lanes A, 2 μg) or acute MS brain homogenate (lanes B, 20μg) were incubated with MBP+ control antibodies or two to three pooled MS CSF-derived recombinant antibodies (rAbs): (first panel) rabbit polyclonal MBP sera; (second panel) anti-MBP mAb; (third panel) anti-Flag and anti–mouse secondary antibodies only; (fourth panel) MS03-1 (#22, 57, and 64); (fifth panel) MS02-19 (#15,17, and 25); (sixth panel) MS04-2 (#13, 30, and 33); (seventh panel) MS03-1 (#48, 69, and 86); and (eight panel) MS03-7 (#10, 13, and MS04-3 #1) CSF. Binding of anti-MBP Abs was detected by chemiluminescence (Super Signal West Fempto Substrate; Pierce Chemical, Rockford, IL) followed by incubation with the appropriate horseradish peroxidase (HRP)–conjugated anti–rabbit or anti–mouse secondary IgG. Binding of MS rAbs was assayed via the Flag epitope and included sequential incubations with anti-Flag mouse mAb (1μg/ml) and HRP-conjugated anti–mouse IgG (1:10,000). (E) Protein lysates (20μg protein per lane) of mock- or MOG cDNA-transfected HEK 293 EBNA cells (lanes A and B, respectively) were separated in a 12.5% sodium dodecyl sulfate gel, transferred to nitrocellulose filters and probed with 8-18C5 positive control mAb, MOG 6D7 mouse–human chimeric rAb and pooled MS CSF rAbs from patients MS02-19 (#17, 25, 28, and 52), MS03-1 (#3, 17, 22, and 31), and MS04-2 (#9, 13, 30, and 92). Binding was visualized with the appropriate alkaline-phosphatase–conjugated anti–mouse or anti–human secondary antibody (1:500) followed by detection with NBT/BCIP chromogenic substrate.
Binding of Cerebrospinal Fluid–Derived Recombinant Antibodies to Myelin Proteins
MS CSF-derived rAbs assessed for binding to HEK 293 EBNA cells transfected with full-length MBP, MOG, or PLP cDNA demonstrated no representative staining (see Figs 2A–C), whereas all positive control antibodies and humanized rAbs (see Table 1 and Figure 2) stained transfected cells expressing their target protein, but not mock-transfected cells.
Immunoblotting to assess binding of control and MS rAbs to purified MBP and MS brain lysate (see Fig 2D) indicated reactivity of control anti-MBP antibody with purified MBP (see Fig 2D, lane A) and with a similar-sized protein (approximately 18kDa) in brain homogenate (see Fig 2D, lane B), whereas no binding of any MS rAb to MBP or other brain proteins was demonstrated, even after longer exposure (10-fold) of the film.
Immunoblotting of MOG and mock-transfected HEK 293 cell lysates (see Fig 2E) showed that control 8-18C5 mAb recognized an approximately 50kDa protein in MOG-transfected lysates (see Fig 2E, lane B) but not control lysates (see Fig 2E, lane A), corresponding in size to MOG dimers. MOG was not detected under denaturing conditions with either the 6D7 mAb or Hu6D7 rAb, but was detected with Hu2B7 anti-MOG rAb (not shown). None of the CSF-derived rAbs recognized MOG in protein immunoblots. Lower-molecular-weight bands observed in mock control lanes are human IgG1 protein fragments produced from the pIgG1Flag vector DNA used for mock transfections based on their detection with anti–human secondary antibody alone.
Immunostaining of Multiple Sclerosis Lesions with Cerebrospinal Fluid–Derived Recombinant Antibodies
Binding of rAbs to formaldehyde-fixed, paraffin-embedded brain sections was also evaluated. A positive control measles-virus–specific 2B4 rAb selectively stained intranuclear inclusions in SSPE brain cells (see Fig 1B, panel a). Occasional granular cytoplasmic immunoreactivity was also seen in measles-virus–infected cells. No staining was seen when 2B4 was applied to MS or control brain (data not shown). Anti-PLP P7.6A5 and anti-MOG 6D7 Hu rAbs selectively and strongly stained myelin sheaths in normal brain and in MS lesions (see Fig 1B, panels b and c); in active MS lesions, reactivity was also seen in myelin degradation products within macrophages. Nearly all rAbs derived from MS CSF plasma cells failed to react with MS or control brain (see Supplementary Table 1). A few rAbs reacted weakly with granules in the cytoplasm of neurons, glial cells, and inflammatory cells in MS and control brain; several rAbs reacted weakly with neuronal but not glial cell nuclei in brain (see Fig 1B, panel d), and one rAb (see Supplementary Table 2, 1–17) reacted moderately at the edge of an MS plaque (not shown).
Discussion
We produced and tested 53 rAbs derived from clonally expanded plasma cells and B lymphocytes in MS CSF for binding to well-characterized myelin antigens. Importantly, all anti-MBP–, anti-PLP–, and anti-MOG–positive control mAbs, humanized chimeric rAbs, and polyclonal sera (see Supplementary Table 1) stained HEK 293 cells expressing the relevant myelin protein, demonstrating the strength of this immunoassay for identifying antibodies that recognize either linear or conformational epitopes. Indeed, immunostaining of measles-virus–infected cells or cells transfected with individual measles virus genes had demonstrated that immunostaining is a reliable and sensitive tool for identifying low-affinity rAbs not always captured by immunoblotting or immunoprecipitation.12,13,26 An important question is whether the repertoire of antibodies being assayed is fully representative of the disease to generate definitive conclusions regarding the specificity of CSF OCBs for white matter proteins. MS CSF was obtained from some patients during their first clinical event, and in others during relapsing and progressive phases of disease. We chose to study some patients in the early stages of disease because antibody specificities may be more likely targeted to an inciting antigen and not confounded by potentially different specificities that could possibly occur in established MS patients because of epitope spreading and tissue destruction. Nonetheless, plasma blast repertoires generated from established MS patients, including those with disease for more than 20 years, show the same features as clinically isolated syndrome patients including VH4 bias, indicating that once established, the driving force for B-cell clonal expansion also persists.10,27 In Patients MS02-19, MS03-1, and ON04-7, rAbs represented most of the unique plasma cells identified in CSF (87, 77, and 75%, respectively), providing a comprehensive representation of potential antigenic specificities. Although it was not feasible to prepare rAbs from every clonally expanded plasma cell population among the many MS patients studied, the recognition of myelin proteins by each positive control antibody and humanized chimeric rAb demonstrated here indicate that such specificities, if prominent in the MS CSF oligoclonal immunoglobulin response, should have been detected. Furthermore, the high percentage (15/22 rAbs) of anti-measles virus rAbs generated from SSPE CSF13 and brain plasma cells12,26 attest to dominance of disease-specific plasma cells in inflammatory CNS and to the faithful reproduction of their antigen specificities by the bivalent IgG1 rAbs. Importantly, we have also constructed a pool of rAbs from expanded clonal populations recovered from neuromyelitis optica CSF. More than half (6/11) of the rAbs tested to date bind the aquaporin 4 water channel, the putative primary autoantigen in disease (Dr Jeffrey Bennett, unpublished data). Thus, if MBP, PLP, or MOG were the primary antigens in MS, they should have reacted with at least some of the 53 MS CSF-derived rAbs assayed in this study. It also appears unlikely that myelin-specific plasma cells would have been negatively selected by our single-cell polymerase chain reaction strategy because we have previously demonstrated that VH amplification did not introduce significant experimental error into the observed B-lymphocyte and plasma cell repertoires, and that peripheral blood B-cell repertoires from both healthy adult control subjects and MS patients yielded VH family distributions consistent with observations obtained by other independent studies.10
The occurrence of anti-MBP and -MOG antibody responses has been analyzed extensively in MS serum and CSF. Multiple studies have produced discordant results regarding antibody prevalence in MS and control patients,28–31 and whether their presence is a significant predictor of conversion to clinically definite MS.32,33 Interestingly, sensitive solution-phase radio-immunoassays designed to detect high-affinity anti-MBP34 or anti-MOG29,35 antibodies have identified a significant humoral response in patients with acute disseminated encephalomyelitis but not in MS.35 Thus, the failure of MS CSF-derived rAbs to bind MBP, PLP, and MOG is not surprising and confirms that targets of the clonally expanded plasma cell antibody response in MS CSF are not the major white matter proteins. This does not preclude the possible presence of lower abundance antimyelin plasma cells in CSF and brain of a subset of MS patients that may contribute to disease pathogenesis.30 Analysis of recombinant Fab fragments prepared from clonally expanded MS CSF CD19+ B cells demonstrated binding of some Fabs to MBP, whereas other Fabs showed polyreactivity against several brain proteins.36 Most recently, immunofluorescent analysis of rAbs prepared from clonally expanded plasma cells in MS CSF demonstrated some staining at plaque borders, but not in normal brain white matter,37 as was seen with our rAb 1–17, and rAbs produced in both studies did not bind to MOG or MBP. Although anti-MOG antibodies have been demonstrated in autopsy material of a few MS brain lesions,18,38 our studies demonstrate that the targets of most MS CSF plasma cell clones remain unknown.
Antibody specificity in MS is confounding, and a wide array of seemingly unrelated viral and autoreactive specificities have been reported in both serum and CSF. Consequently, the intrathecal IgG response in MS is often viewed as a nonspecific epiphenomenon.39 This view contrasts with CSF repertoire analyses showing prominent clonal expansion of somatically mutated CSF plasma cells and VH4 family gene segment bias. Because VH4 bias in CSF does not mirror VH use by either naive peripheral blood B cells or circulating class-switched memory B cells,8 it is unlikely that expanded MS CSF plasma cell populations are attributable to random B-cell activation alone. Although chronic inflammation in MS could lead to polyclonal bystander memory B-cell activation40 and production of some of the disparate viral antibody specificities observed in MS CSF, antigens recognized by OCBs remain unknown. We predict that clonally expanded plasma cells in MS CSF represent the intrathecal oligoclonal IgG response and are a consequence of direct antigen stimulation and clonal selection within the CNS. Supporting this view is a strong link between expanded IgG V-region sequences (immunoglobulin transcriptome) amplified from cells in MS CSF and V-region peptide sequences (immunoglobulin proteome) generated from purified CSF OCBs.11 Furthermore, IgG produced by cultured MS CSF lymphocytes in the presence of CpG DNA oligonucleotides yielded an oligoclonal banding pattern almost identical to that observed in donor MS CSF (data not shown). Finally, most expanded SSPE CSF plasma cell clones, like those recovered from brain, were measles virus-specific, indicating that the majority of plasma cells in CSF represent the intrathecal response to virus.
Clinical and pathological findings in MS indicate that B cells are relevant to disease pathogenesis. Breij and colleagues’41 recent study showed antibody and complement-mediated myelin phagocytosis to be the dominant mode of demyelination in essentially all active MS lesions sampled from established MS autopsy specimens. More importantly, two recently published clinical trials showed that rituximab, an anti–B-cell monoclonal antibody, substantially reduced relapses and new magnetic resonance imaging lesion formation in relapsing-remitting patients.42,43 Thus, the identification of disease-relevant antigens is important for determining whether the primary role of B cells in MS is through antibody production, antigen presentation to T cells, or in the production of regulatory cytokines.
Our finding that MS CSF-derived plasma cell clones are not directed against the well-characterized myelin proteins MBP, PLP, and MOG, and that they do not readily stain MS lesions raises an interesting conundrum regarding their specificity and the search for antigenic targets of the CSF oligoclonal bands. Antigen identification in MS tissue may be compromised in immunoassays by several factors including antibody affinity, sensitivity to fixation, and antigen abundance. The list of potential antigen targets is extensive and includes self proteins such as αB-crystallin44 or neurofascin,45 glycolipids, or foreign pathogens. The search for viral antigens with MS CSF rAbs is a high priority because epidemiological studies have long suggested an environmental agent in disease susceptibility (reviewed in Ascherio and Munger’s46 article), and OCBs are found in multiple chronic viral infections of the CNS.1 If MS exacerbations are triggered by a virus, its expression may be transient and abundance quite low. Thus, viral antigen may not be detectable after the immune response occurs. Although productive varicella zoster virus infection was recently found in MS CSF during relapses,47 our rAbs do not bind to varicella zoster virus–infected cells and proteins.48 Because numerous studies have also suggested that Epstein–Barr virus plays a role in the pathogenesis of MS,49,50 we are currently examining our rAbs for immunoreactivity against Epstein–Barr virus antigens. Importantly, the procedures described herein allow an unlimited production of MS-CSF–derived monoclonal rAbs, which will be available to other investigators studying antigen identification in MS.
Acknowledgments
This work was supported by the Public Health Service (NS32623, D.G., G.P.O.; EY014573, J.L.B.; NS041549, M.P.B.) and National Multiple Sclerosis Society (RG3908, J.L.B.).
We thank Dr C. Bernard for 8-18C5 anti-MOG antibody, Dr V. Kuchroo for anti-PLP hybridoma (clone P7.6A5), M. Hoffman for editorial review, and C. Allen for manuscript preparation.
Footnotes
Gregory Owens and Jeffrey Bennett contributed equally to this work.
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2006;4:195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cepok S, Rosche B, Grummel V, et al. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128:1667–1676. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- 3.Corcione A, Casazza S, Ferretti E, et al. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 2004:11064–11069. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens GP, Kraus H, Burgoon MP, et al. Restricted use of VH4 germline segments in an acute multiple sclerosis brain. Ann Neurol. 1998;43:236–243. doi: 10.1002/ana.410430214. [DOI] [PubMed] [Google Scholar]
- 5.Qin Y, Duquette P, Zhang Y, et al. Clonal expansion and somatic hypermutation of VH genes of B cells from cerebrospinal fluid in multiple sclerosis. J Clin Invest. 1998;102:1045–1050. doi: 10.1172/JCI3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baranzini SE, Jeong MC, Butunoi C, et al. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J Immunol. 1999;163:5133–5144. [PubMed] [Google Scholar]
- 7.Colombo M, Dono M, Gazzola P, et al. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J Immunol. 2000;164:2782–2789. doi: 10.4049/jimmunol.164.5.2782. [DOI] [PubMed] [Google Scholar]
- 8.Ritchie AM, Gilden DH, Williamson RA, et al. Comparative analysis of the CD19+ and CD138+ cell antibody repertoires in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol. 2004;173:649–656. doi: 10.4049/jimmunol.173.1.649. [DOI] [PubMed] [Google Scholar]
- 9.Monson NL, Brezinchek HP, Brezinchek R, et al. Receptor revision and atypical mutational characteristics in clonally expanded B cells from the cerebrospinal fluid of recently diagnosed multiple sclerosis patients. J Neuroimmunol. 2005;158:170–181. doi: 10.1016/j.jneuroim.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Owens GP, Winges KM, Ritchie AM, et al. VH4 gene segments dominate the intrathecal humoral immune response in multiple sclerosis. J Immunol. 2007;179:6343–6351. doi: 10.4049/jimmunol.179.9.6343. [DOI] [PubMed] [Google Scholar]
- 11.Obermeier B, Mentele R, Malotka J, et al. Matching of the oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med. 2008;6:688–693. doi: 10.1038/nm1714. [DOI] [PubMed] [Google Scholar]
- 12.Burgoon MP, Keays KM, Owens GP, et al. Laser capture microdissection of plasma cells from SSPE brain reveals intrathecal disease-relevant antibodies. Proc Natl Acad Sci U S A. 2005;102:7245–7250. doi: 10.1073/pnas.0502323102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owens GP, Ritchie AM, Gilden DH, et al. Measles virus-specific plasma cells are prominent in SSPE CSF. Neurology. 2007;68:1815–1819. doi: 10.1212/01.wnl.0000262036.56594.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Eden G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 16.Yu X, Gilden DH, Ritchie AM, et al. Specificity of recombinant antibodies generated from multiple sclerosis cerebrospinal fluid probed with a random peptide library. J Neuroimmunol. 2006;172:121–131. doi: 10.1016/j.jneuroim.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Deibler GE, Martenson RE, Kies MW. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2:139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor KC, Appel H, Bregoli L, et al. Antibodies from inflamed central nervous system tissue recognize myelin oligodendrocyte glycoprotein. J Immunol. 2005;175:1974–1982. doi: 10.4049/jimmunol.175.3.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponath PD, Kassam N, Qin S. Monoclonal antibodies to chemokine receptors. Methods Mol Biol. 2000;138:231–242. doi: 10.1385/1-59259-058-6:231. [DOI] [PubMed] [Google Scholar]
- 20.Greenfield EA, Reddy J, Lees A, et al. Monoclonal antibodies to distinct regions of human myelin proteolipid protein simultaneously recognize central nervous system myelin and neurons of many vertebrate species. J Neurosci Res. 2006;83:415–431. doi: 10.1002/jnr.20748. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor KC, Nguyen K, Stollar BD. Recognition of DNA by VH and Fv domains of an IgG anti-poly(dC) antibody with a singly mutated VH domain. J Mol Recognit. 2001;14:18–28. doi: 10.1002/1099-1352(200101/02)14:1<18::AID-JMR515>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Murisiku R, Howard M, et al. Universal PCR amplification of mouse immunoglobulin gene variable regions: the design of degenerate primers and an assessment of the effect of DNA polymerase 3′ to 5′ exonuclease activity. J Immunol Methods. 2000;233:167–177. doi: 10.1016/s0022-1759(99)00184-2. [DOI] [PubMed] [Google Scholar]
- 23.Marik C, Felts PA, Bauer J, et al. Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain. 2007;130:2800–2815. doi: 10.1093/brain/awm236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutzneligg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 25.Burgoon MP, Williamson RA, Owens GP, et al. Cloning the antibody response in humans with inflammatory CNS disease: isolation of measles virus-specific antibodies from phage display libraries of a subacute sclerosing panencephalitis brain. J Neuroimmunol. 1999;94:204–211. doi: 10.1016/s0165-5728(98)00243-4. [DOI] [PubMed] [Google Scholar]
- 26.Burgoon MP, Caldas YA, Keays KM, et al. Recombinant antibodies generated from both clonal and less abundant plasma cell IgG sequences in SSPE brain are directed against measles virus. J Neurovirol. 2006;12:398–402. doi: 10.1080/13550280600957414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett JL, Haubold K, Ritchie AM, et al. CSF IgG heavy-chain bias in patients at the time of a clinically isolated syndrome. J Neuroimmunol. 2008;199:126–132. doi: 10.1016/j.jneuroim.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reindl M, Linington C, Brehm U, et al. Antibodies against the myelin oligodendrocyte glycoprotein and myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain. 1999;22:2047–2056. doi: 10.1093/brain/122.11.2047. [DOI] [PubMed] [Google Scholar]
- 29.Lampasona V, Franciotta D, Furlan R, et al. Similar low frequency of anti-MOG and IgM in MS patients and healthy subjects. Neurology. 2004;62:2092–2094. doi: 10.1212/01.wnl.0000127615.15768.ae. [DOI] [PubMed] [Google Scholar]
- 30.Zhou D, Srivastava R, Nessler S, et al. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103:19057–19062. doi: 10.1073/pnas.0607242103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalive PH, Mengue T, Delarasse C, et al. Antibodies to native myelin oligodendrocyte glycoprotein are serologic markers of early inflammation in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103:2280–2285. doi: 10.1073/pnas.0510672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger T, Rubner P, Schautzer F, et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349:139–145. doi: 10.1056/NEJMoa022328. [DOI] [PubMed] [Google Scholar]
- 33.Kuhle J, Pohl C, Mehling M, et al. Lack of association between antimyelin antibodies and progression to multiple sclerosis. N Engl J Med. 2007;356:371–378. doi: 10.1056/NEJMoa063602. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor KC, Chitnis T, Griffin DE, et al. Myelin basic protein-reactive autoantibodies in the serum and cerebrospinal fluid of multiple sclerosis patients are characterized by low-affinity interactions. J Neuroimmunol. 2003;136:140–148. doi: 10.1016/s0165-5728(03)00002-x. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor KC, McLaughlin KA, DeJager PL, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. 2007;13:211–217. doi: 10.1038/nm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambracht-Washington D, O’Connor KC, Jowdry A, et al. Antigen specificity of clonally expanded and receptor edited cere-brospinal fluid B cells from patients with relapsing remitting MS. J Neuroimmunol. 2007;186:164–176. doi: 10.1016/j.jneuroim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Büdingen H-C, Harrer MD, Kuenzle S, et al. Clonally expanded plasma cells in the cerebrospinal fluid of MS patients produce myelin-specific antibodies. Eur J Immunol. 2008;38:2014–2023. doi: 10.1002/eji.200737784. [DOI] [PubMed] [Google Scholar]
- 38.Genain C, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- 39.Reder AT, Oger JJ. Anti-myelin oligodendrocyte glycoprotein antibodies in multiple sclerosis. Neurology. 2004;62:1922–1923. doi: 10.1212/01.wnl.0000130069.55343.06. [DOI] [PubMed] [Google Scholar]
- 40.Bernasconi N, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 41.Breij ECW, Brink BP, Veerhuis R, et al. Homogeneity of active demyelinating lesions in established MS patients. Ann Neurol. 2008;63:16–25. doi: 10.1002/ana.21311. [DOI] [PubMed] [Google Scholar]
- 42.Hauser SL, Waubant E, Arnold DL, et al. B cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 43.Bar-Or A, Calabresi PAJ, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week open-label, phase 1 trial. Ann Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 44.Ousman SS, Tamooka BH, van Noort J, et al. Protective and therapeutic role for αB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 45.Mathey EK, Derfuss T, Storch MK, et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med. 2007;204:2363–2372. doi: 10.1084/jem.20071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ascherio A, Munger KL. Environment risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–289. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 47.Sotelo J, Martinez-Palomo A, Ordoñez G, Pineda B. Varicella-zoster virus in cerebrospinal fluid at relapses of multiple sclerosis. Ann Neurol. 2008;63:303–311. doi: 10.1002/ana.21316. [DOI] [PubMed] [Google Scholar]
- 48.Burgoon MP, Cohrs RJ, Bennett JL, et al. Varicella zoster virus is not a disease-relevant antigen in multiple sclerosis. Ann Neurol. 2009;65:474–479. doi: 10.1002/ana.21605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cepok S, Zhou D, Srivastava R, et al. Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. J Clin Invest. 2005;115:1352–1360. doi: 10.1172/JCI23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serafini B, Rosicarelli B, Franciotta D, et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007;204:2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]