Abstract
Thoracic aortic dissection, one of the major diseases affecting the aorta, carries a very high mortality rate. Improving our understanding of the pathobiology of this disease may help us develop medical treatments to prevent dissection and subsequent aneurysm formation and rupture. Dissection is associated with degeneration of the aortic media. Recent studies have shown increased expression and activation of a family of proteolytic enzymes—called matrix metalloproteinases (MMPs)—in dissected aortic tissue, suggesting that MMPs may play a major role in this disease. Inhibition of MMPs may be beneficial in reducing MMP-mediated aortic damage associated with dissection. This article reviews the recent literature and summarizes our current understanding of the role of MMPs in the pathobiology of thoracic aortic dissection. The potential importance of MMP inhibition as a future treatment of aortic dissection is also discussed.
Thoracic aortic dissection (TAD) begins as a spontaneous tear through either the intima or the adventitia that extends into the media of the aortic wall. Pulsatile blood entering the tear causes the midlayer of the media to tear apart along the length of the vessel. The freshly torn aorta is severely weakened and prone to dilatation and rupture, which is usually fatal. The cornerstones of successful treatment are rapid diagnosis, aggressive anti-impulse therapy, surgical repair for selected patients, and lifelong surveillance imaging.1
Despite medical advances, aortic dissections and aneurysms are a major cause of mortality, being responsible for nearly 14,000 deaths in the United States each year.2 The International Registry of Acute Aortic Dissection indicates that surgical repair of an acute ascending aortic dissection carries a 24% in-hospital mortality rate.3 Even after the initial injury is treated and stabilized, the dissected aorta is prone to late deterioration, leading to progressive aneurysmal dilatation and rupture. Consequently, 10 to 26% of late deaths after ascending aortic dissection repair are caused by rupture of the descending thoracic aorta.4–6 A better understanding of the molecular mechanisms involved in TAD is needed to develop new medical treatments that can prevent dissection formation, expansion, and rupture.
Aortic dissections are associated with degenerative changes in the medial layer of the aortic wall. Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that degrade various components of the extracellular matrix (ECM) and, thus, play an important role in vascular remodeling. Dysregulation of MMP production and activity leads to ECM degradation and medial layer degeneration. The role of MMPs in the pathogenesis of degenerative abdominal and thoracic aortic aneurysms is well established.7–9 Because aneurysmal dilatation is a risk factor for dissection, it is tempting to extrapolate data regarding the pathogenesis of degenerative aneurysms to the development of dissection. Many dissections, however, occur in nondilated aortas, and many aneurysms never result in dissection; therefore, the pathobiology of aneurysms and the development of dissection should be examined independently. Although dissections are studied far less extensively than degenerative aneurysms, mounting evidence supports a role for MMPs in TAD. This article reviews the recent literature and summarizes our current understanding of the role of MMPs in the pathobiology of TAD. In addition, the potential importance of MMP inhibition as a future treatment strategy for TAD is discussed.
ECM Degeneration in TAD
The aorta comprises three layers: an inner intima, a media, and an outer adventitia. The media, a thick layer composed of smooth muscle cells within an ECM, makes the aortic wall extensible. Most dissections are associated with degenerative changes in the medial layer of the aortic wall; the degenerative changes are characterized by a reduction in smooth muscle cells, fragmentation and loss of elastic fibers, and an accumulation of the basophilic-staining ground substance mucopolysaccharide. The cellular components of the aorta are supported and organized by the ECM, a complex combination of structural proteins (elastins, collagens, and fibrillins), adhesive proteins (laminins and fibronectins), and ground substance (glycosaminoglycans and proteoglycans). ECM not only provides mechanical support for the cells but also facilitates and governs cellular activity.
ECM is a dynamic structure constantly undergoing synthesis and degradation. Keeping ECM metabolism in balance is critical for maintaining vascular structure and function. Although low ECM production can weaken the aortic wall, overproduction of a single component can cause wall stiffness, leaving the aorta vulnerable to dissection. Fragmentation of the elastic fibers is one of the most important and early histologic features of dissection tissue.10–12 A decrease in elastin content may further stimulate vascular smooth muscle cell (VSMC) proliferation and fibrosis formation,13 which decreases compliance of the aortic wall and accelerates the development of aortic degeneration. Ultimately, loss of VSMCs and collagen degradation create the catabolic conditions that lead to rupture of the aorta.10
Most of the current information about the cellular and molecular changes associated with TAD is based on analyses of aortic tissue after a dissection has occurred. The specific changes in the aortic wall that precede dissection are largely unknown. However, the changes that occur after dissection are clinically relevant because progressive dilatation of the dissected aorta leads to aneurysm formation and culminates in rupture, unless treated surgically. The ability to interrupt this process in patients with dissection would be a major advance in treatment.
Physiologic Properties of MMPs
Fragmentation of the elastic fibers and aortic dilation are associated with the local release of proteolytic enzymes, including serine proteases, cysteine proteases, and MMPs. These proteolytic enzymes lead to degeneration of the ECM and destruction of the vascular framework. Numerous MMPs are increased in aortic TAD tissue.14–18 Several studies have correlated MMP levels with the severity of the lesion.14–16,18–21 Thus, MMPs have been implicated in TAD formation via their capacity to degrade the particular constituents of the ECM.
Members of the MMP Family
MMPs play an important role in ECM metabolism. Currently, 26 MMPs described in humans can be functionally divided into six groups: collagenases (MMP-1, -8, -13, and -18), gelatinases (MMP-2 and -9), stromelysins (MMP-3, -10, and -11), matrilysins (MMP-7 and -26), and membrane-type matrix metalloproteinases (MT-MMPs; MMP-14 to -17, -24, and -25).
MMP Structure
Although functionally distinct, MMPs share a high degree of homology in structure, which consists of a prodomain, a catalytic domain, a hinge region, and a hemopexin domain (Figure 1). MMPs are initially synthesized as inactive zymogens with a prodomain at the N-terminal region. The prodomain has a conserved cysteine switch motif, PRCGXPD. When the switch motif interacts with the zinc (Zn2+) ion in the catalytic domain, the enzyme is kept in an inactive pro-MMP zymogen form, and the substrate is prevented from binding to the enzyme and being cleaved. Some MMPs have a prohormone convertase cleavage site (furin-like). When it is cleaved, the enzyme becomes active.22,23 The prodomain also consists of a signaling peptide that allows the enzyme to be secreted into the endoplasmic reticulum and transported out of the cell.22,23 MMP-23A and MMP-23B have a transmembrane segment in the prodomain.24
Figure 1.
Matrix metalloproteinase (MMP) structures. MMPs consist of a prodomain, a catalytic domain, a hinge region, and a hemopexin domain. The prodomain has a cysteine switch motif, PRCGXPD, that can interact with a zinc ion (Zn2+). The catalytic domain comprises a Zn2+ in its active site and a substrate binding site. The flexible hinge region links the catalytic domain to the hemopexin domain, located on the C-terminal end. Membrane-type MMPs have an additional transmembrane binding domain. Although their substrate profiles differ, collagenases, gelatinases, and stromolysins have a similar basic structure. Two matrilysins are minimal-domain MMPs that lack the hemopexin C-terminal domain.
The catalytic domain has a Zn2+ ion binding site and a substrate binding site, allowing for the binding and subsequent cleavage of a specific substrate. The catalytic domain is connected to the C-terminal domain by a flexible hinge region that allows for the binding of other proteins. The C-terminal domain can alter MMP activity; it has a four-bladed, β-propeller structure that provides a large flat surface for protein–protein interactions and determines substrate specificity. It is also the site that interacts with tissue inhibitors of metalloproteinases (TIMPs). The MT-MMPs have an additional transmembrane binding domain. Two matrilysins are minimal-domain MMPs because they lack the hemopexin C-terminal domain, although they are still potent enzymes involved in many tissue remodeling processes.
MMP Synthesis
MMPs are produced by many cell types, including VSMCs and fibroblasts.23,25 All MMPs are initially synthesized as an enzymatically inactive zymogen (pro-MMPs). Some MMPs are stored within the cell after synthesis (eg, MMP-9 in neutrophil granules), but most are either secreted freely into the extracellular space or anchored to the surface of cell membranes (eg, MT-MMPs).22,23,26,27
MMP Cleavage and Activation
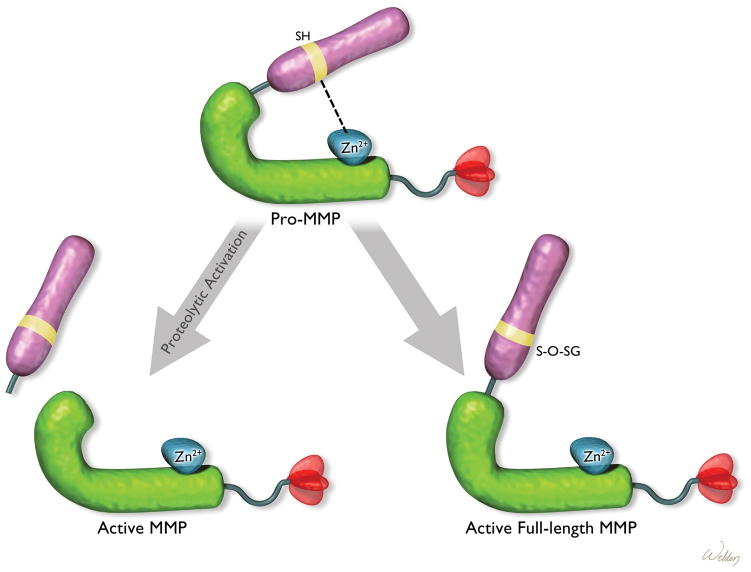
Pro-MMPs can be activated in two ways (Figure 2). First, the inactive proenzymes can be activated by proteolytic cleavage of the inhibitory prodomain by other MMPs or proteinases.22,23 Second, the pro-MMPs can be activated by the dissociation or disturbance of the binding of the cysteine switch motif in the prodomain and the Zn2+ ion in the catalytic domain by thiol-modifying agents, such as oxidized glutathione, sodium dodecyl sulfate, and reactive oxygen species.
Figure 2.
Mechanism of matrix metalloproteinase (MMP) activation. The full-length MMP can be activated by proteolytic removal of the autoinhibitory prodomain or by disruption of the binding of the inhibitory prodomain to the zinc ion (Zn2+) in the catalytic domain. S-O-SG = glutathionylated sulfhydryls (SH groups).
MMP Substrates and Biologic Activities
MMPs function mainly as enzymes that degrade structural components of the ECM. Known substrates include most of the ECM components (eg, elastin, collagen, fibronectin, vitronectin, laminin, entactin, tenascin, aggrecan, myelin basic protein). Although MMPs exhibit substrate specificity, the substrate preference for each MMP is not fully characterized. MMPs can act cooperatively to completely degrade ECM proteins. Recently, increasing evidence suggests that MMPs and their cleaved products can also modify the actions of growth factors, cytokines, chemokines, cell adhesion molecules, tyrosine kinase receptors, and other MMPs,22,23,28,29 thus serving as signaling molecules that affect cell behavior. By cleaving the ECM, intercellular junctions, and the basement membrane and by modifying cell signaling,22 MMPs can change tissue architecture and contribute to vascular remodeling.
MMP Regulation
Rigorous regulation of MMP expression and activity is a crucial part of ECM homeostasis. This regulation occurs primarily at the levels of gene expression, pro-MMP secretion, extracellular localization, zymogen activation, and enzyme inhibition by interaction with endogenous inhibitors such as the TIMPs (Figure 3).
Figure 3.
Matrix metalloproteinase (MMP) regulation. MMPs can be regulated at different levels. MMP production can be controlled during (1) ribonucleic acid (RNA) transcription and (2) protein synthesis; MMP activity can be modulated by pathways for (3) protein secretion, (4) cellular and extracellular localization, (5) zymogen activation, and (6) inhibition by tissue inhibitors of metalloproteinases (TIMPs). ER = endoplasmic reticulum; GPI = glycosylphosphatidylinositol.
Regulation of MMP Production
MMP messenger ribonucleic acid (mRNA) levels can be affected by several factors, including hormones, growth factors (eg, transforming growth factor β [TGF-β]), cytokines (eg, tumor necrosis factor α and interleukin-1), vascular factors (eg, angiotensin II and endothelin-1), ECM proteins, hypoxia, mechanical stretch, and shear stress.25,30 These factors can alter MMP gene expression by activating signaling pathways and transcription factors that bind specific response elements on MMP promoters. Signaling molecules, such as the extracellular signal-regulated kinases, the c-Jun N-terminal kinases (JNKs), and the p38 kinases, may be involved in the regulation of MMP transcription through activation of activated protein 1 family members, JunB and FosB. The inflammatory nuclear factor kappa B (NF-κB) pathway has also been reported to be involved in MMP regulation, and several MMP promoters contain NF-κB binding sites.25,30
Regulation of MMP Activation
The activation of MMPs by proteolytic cleavage of the prodomain is tightly regulated by the levels and activities of MMPs, other proteinases, and endogenous MMP inhibitors.22,23 On the other hand, MMPs are activated when the Zn2+ ion in the catalytic domain is exposed. The full-length MMP is also proteolytically active during oxidative stress. MMP-1, -8, and -9 can be activated by the oxidant species peroxynitrite (ONOO−), without removal of the inhibitory prodomain.31,32 In addition, extensive modifications and activation of MMP-2 by S-glutathiolation, hydroxylation, and nitration have been shown by mass spectrometric analysis.33
Protein phosphorylation, which has not previously been considered a potential modulator of MMP activity, may participate in regulation of MMP functions. For example, MMP-2 contains 29 potential phosphorylation sites and has been shown to be phosphorylated on several sites by protein kinase C.34 Several kinases, including protein kinase A,35 protein kinase C,36 glycogen synthase kinase 3,37 and phosphatidylinositol 3-kinase,38 can phosphorylate MMP-2 and modulate its activity.
Regulation of MMP Activity by Endogenous Inhibitors
MMP activity is inhibited by plasma proteins (eg, α2-macroglobulin) and endogenous TIMPs.39–42 TIMPs comprise a family of four protease inhibitors: TIMP-1, TIMP-2, TIMP-3, and TIMP-4. They are small (≈23 kDa), cysteine-rich proteins with a large N-terminal domain and a smaller C-terminal domain.39 All four TIMPs are expressed in the heart and in the vascular wall,25 and their expression can be induced by a variety of stimuli, including inflammatory cytokines43–45 and angiotensin II.46–48
TIMPs inhibit MMP activity by binding to the C-terminal domain of MMPs (see Figure 3).39,49,50 TIMPs demonstrate some degree of target specificity for particular MMPs. For example, TIMP-2 has a preference for MMP-2 and membrane type 1 matrix metalloproteinase (MT1-MMP).25,51 However, the substrate specificity for each TIMP is not fully characterized, and the structural basis for its binding specificity is not completely understood.49 In addition to MMP inhibition, TIMPs act as signaling molecules and have direct effects on the proliferation, migration, and death of VSMCs52 and fibroblasts.53
MMPs in TAD
MMPs contribute to many important physiologic processes, such as normal vessel development and wound healing, by regulating VSMC migration and ECM metabolism. Dysregulation of MMP activity can result in adverse vascular remodeling in many pathologic conditions. Increased MMP activity has been reported in various systemic vascular diseases, such as hypertension, pre-eclampsia, atherosclerotic plaque destabilization, and abdominal aortic aneurysms.7 Several studies focusing on degenerative thoracic aortic disease (without dissection) have suggested that MMPs play a role in the formation of thoracic aortic aneurysms.9,14–16,18,19,54
In contrast to the large number of studies of MMPs in degenerative aortic aneurysms, relatively few studies have focused on the role of MMPs in TAD (Table 1). In a study of seven patients with Marfan syndrome (three of whom had aortic dissections), Segura and colleagues, using immunohistochemical analysis, showed a specific pattern of MMP expression in ascending aortic tissue.14 MMPs or TIMPs showed little expression in the areas of “cystic” medial degeneration in the aorta of Marfan syndrome patients; however, all MMPs, especially MMP-2 and MMP-9, were more highly expressed in the smooth muscle cells at the borders of the areas of medial degeneration than in other regions. MMP-2 and MMP-9 were also expressed at the surfaces of disrupted elastic fibers, indicating the potential role of MMPs in elastin degradation. In 2000, Tamura and colleagues reported increased MMP-9 expression in macrophages at the site of the intimal tear in six patients with atherosclerosis-related dissections.55 Similarly, Ishii and Asuwa reported immunohistochemical evidence of MMP-2 and -9 at the initial tear in 21 postmortem acute dissection specimens.56 Using in situ hybridization for MMP-9 mRNA, Schneiderman and colleagues demonstrated MMP-9 involvement in four subacute dissections.57 A complementary deoxyribonucleic acid (cDNA) array analysis of tissue from six acute aortic dissections recently revealed upregulation of inflammatory and proteolytic genes and downregulation of ECM, adhesion, and cytoskeletal proteins, suggesting a degradative process.58 A semiquantitative study by Koullias and colleagues showed significant increases in MMP-1 and -9 in dissection tissue when compared with control tissue from cadavers.15 In a recent study, the activities and distributions of MMP-2, MT1-MMP, and MMP-9 were evaluated by gelatin zymography, immunohistochemistry, and in situ hybridization; the results suggested that MMP-2 and MT1-MMP may be involved not only in the degeneration of aortic tissue but also in tissue remodeling, which may be associated with the healing process.21 In contrast, a study by Manabe and colleagues showed that MMP-2 and TIMP-2 were significantly lower in aortic samples from patients with acute aortic dissection than in control aortic samples from patients undergoing coronary artery bypass, suggesting that low TIMP-2 to MMP-2 and TIMP-2 to MMP-9 ratios might play an important role in aortic dissection.20
Table 1.
Changes in Matrix Metalloproteinases in Thoracic Aortic Dissection
| MMP | Alternative Names | Major Substrates | Changes in Dissection |
|---|---|---|---|
| MMP-1 | Collagenase-1; interstitial collagenase | Fibrillar collagens, fibronectin | Increased15 |
| MMP-2 | Gelatinase A, 72 kDa; a gelatinase | Denatured collagen, fibronectin, elastin | Increased14,15,21,56 Decreased20 |
| MMP-9 TIMP-2 |
Gelatinase B, 92 kDa; a gelatinase | Denatured collagen, fibronectin, elastin | Increased15,21,55–57 Decreased20 |
MMP = matrix metalloproteinase; TIMP = tissue inhibitor of metalloproteinase.
Two important caveats related to interpreting the results of these studies deserve emphasis. First, the substantial variability in study design makes direct comparison of results difficult; key differences between the studies include the type of underlying aortic pathology being investigated (Marfan syndrome, atherosclerosis, and idiopathic medial degeneration), the source of the diseased aortic tissue (samples obtained postmortem versus those obtained during surgical repair), the stage of the disease (acute, subacute, and chronic), and the source of control tissues (organ donors, cadavers, and patients undergoing coronary artery bypass surgery). These differences in study design can contribute to seemingly disparate results, such as those described above for MMP-2. Therefore, evaluating how each of these factors affects the pattern of MMP expression within the aortic wall is an important step toward improving our understanding of the role of MMPs in dissection. Second, it is not clear whether changes in MMP expression precede (and potentially cause) the acute dissection event or whether they merely occur in response to the local trauma caused by the dissection. Despite the variability in study design and regardless of when abnormal MMP expression begins, the accumulating data generally support the paradigm that the initial tissue injury during acute aortic dissection triggers a cycle of inflammation, MMP overproduction, and progressive destruction of the aortic wall, ultimately leading to expansion and rupture (Figure 4).
Figure 4.
Matrix metalloproteinases (MMPs) in thoracic aortic dissections (TADs). This drawing illustrates a paradigm under investigation for the role of MMPs in TAD. The initial tissue injury from the dissection (1) causes local release of chemokines (2) and recruitment of an inflammatory infiltrate (3). The release of proteases, especially MMPs, from macrophages and smooth muscle cells (4) may cause further destruction of the aortic wall matrix (5), ultimately leading to expansion and rupture of the aorta (6).
Genetic variations affecting MMP expression and activity may increase the risk of TAD. Genetic disorders that affect upstream regulators of ECM metabolism, such as TGF-β, are well-established causes of TAD,59–61 and upregulation of MMPs may be an important component of the aortic manifestations. In a genetic association study, we recently identified a single nucleotide polymorphism (–8202A/G) in the MMP-9 gene that was associated with TAD (adjusted odds ratio 4.26, 95% confidence interval 1.70–10.66).62,63 Further studies are needed to replicate this observation and to investigate the functional role of the –8202A/G variant in MMP-9 expression.
Inhibition of MMPs: Potential Medical Therapies for Aortic Dissection?
Efforts to elucidate the role of MMPs in TAD are driven, in part, by the increasing availability of pharmacologic inhibitors of MMPs that have potential as therapeutic agents. Medical treatment would be particularly useful in two clinical situations. The first is in the prevention of TAD in patients who are determined to be at high risk, such as those with connective tissue disorders. The second is in the prevention of aortic expansion in patients with dissections involving the descending thoracic aorta. Although MMP inhibitors have not been studied in the context of aortic dissections, it is worthwhile to briefly review these agents, given their future potential.
Doxycycline
One class of MMP inhibitors that may be of clinical use is the tetracycline class of antibiotics. Doxycycline, the tetracycline most widely studied for inhibiting aneurysms, binds the zinc-containing active site of MMPs and nonselectively inhibits MMP activity. Doxycycline potently inhibits a broad range of MMPs. Systemic administration of doxycycline inhibits aneurysm formation in rat and mouse models.64–67 Local delivery improves the efficacy of doxycycline.68–70 A recent study showed that doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome.71 To date, doxycycline is the only MMP inhibitor approved for clinical use by the US Food and Drug Administration. Clinical trials in patients with aortic aneurysms have demonstrated that doxycycline decreases plasma levels of MMP-972 and inhibits aneurysm progression.72–75
Statins
The 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, or statins, are well-studied drugs that exert many beneficial effects on the cardiovascular system, independent of their lipid-lowering properties. In cultured cells, statins reduce MMP secretion by macrophages and VSMCs.76–78 Statins can directly suppress MMP production and modulate the structure and function of the aortic wall. In a mouse model of abdominal aortic aneurysm, statins reduced the expression of MMP-9 and increased the expression of TIMP-1 in the aortic wall, and inhibited aneurysmal expansion and medial destruction.69 Although there is no evidence that statins can prevent or stabilize TAD, it seems reasonable to control lipid levels with statins in patients with aortic dissections. This is particularly true in patients with a penetrating atherosclerotic ulceration, a variant of aortic dissection caused by unstable atherosclerosis of the aorta.
Recombinant TIMPs
The use of TIMPs, through gene therapy or direct protein injection, for the treatment of vascular diseases is in the early stages of development.79 Studies have demonstrated that overexpression of TIMPs by gene transfer can suppress MMP activity,80–84 reduce intimal thickening,80–82 and attenuate atherosclerotic plaque development and instability.83,84 Local expression of TIMP-1 has been shown to prevent progression and rupture of aneurysms in a rat model of abdominal aortic aneurysm.85 The use of gene transfer to increase local levels of TIMPs may be a potential treatment for TAD. However, expressing wild-type TIMPs could have drawbacks because multiple MMPs may be inhibited, underscoring the importance of both understanding the structural basis of TIMP substrate specificity49 and developing target-specific recombinant TIMPs.86
MMP Inhibitory Peptides and Neutralizing Antibodies
Inhibitory peptides that may bind to the hydrophobic substrate pocket of MMPs have been used to selectively inhibit MMP-2 and -9.87,88 In experimental models, MMP-2 neutralizing antibodies have shown protective effects in hearts exposed to proinflammatory cytokines89 and ischemia reperfusion injury.90,91 However, these agents have not been tested in aortic dissection models. It is unclear whether these peptides and antibodies could achieve sufficient distribution throughout the aortic media to effectively suppress MMPs.
Kinase Inhibitors
Recent evidence indicates that inhibition of JNK, a signaling molecule crucial for MMP-2 and MMP-9 production and secretion, prevents the development of abdominal aortic aneurysms and even causes regression of existing aortic aneurysms in mouse models.92 Rapamycin, a TOR inhibitor, decreased MMP-9 levels and aortic expansion in a rat model of abdominal aortic aneurysm.93 These findings suggest that targeting signaling molecules involved in MMP expression, secretion, and activation may be an effective therapeutic strategy in the treatment of TAD.
Other MMP Inhibitors
Several rationally designed MMP inhibitors, such as o-phenanthroline, batimastat, marimastat, GM-6001 (ilomastat or gelardin), and PD-166793, have been developed. These molecules inhibit MMPs by chelating the Zn2+ active site in the catalytic domain.94,95 Although animal studies have shown some promise in the treatment of pathologies in which MMPs are suspected to be involved,96–100 most of these inhibitors have performed poorly in clinical trials. They failed to show expected results and had unanticipated side effects, such as tendonitis-like fibromyalgia, which appeared to be unrelated to MMP inhibition.94,95 Nonselective, broad-spectrum MMP inhibition is thought to be responsible for the low efficiency and toxic effects of these inhibitors.94,95 More selective inhibitors, such as compounds that are designed to interact with various binding pockets on the MMPs, are desired.
Summary
Initial progress in understanding the production and activation of MMPs in TAD suggests that MMPs play a significant role in vascular remodeling and TAD pathobiology. Future investigation of MMPs and their inhibitors in TAD will likely lead to advancements in the diagnosis, prognosis, and treatment of this deadly disease. Inhibition of MMP-mediated matrix degradation may be a promising therapeutic direction for TAD. In addition, inhibiting MMP production and activity via anti-MMP therapy using selective MMP inhibitors, kinase inhibitors, or TIMP gene therapy may eventually be part of the prevention and treatment of TAD.
Acknowledgments
Dr. LeMaire is supported by a Thoracic Surgery Foundation for Research and Education/National Heart, Lung, and Blood Institute Co-sponsored Mentored Clinical Scientist Development Award (K08 HL080085).
We wish to thank Hilary D. Marks, PhD, and Rebecca Bartow, PhD, for their editorial assistance and Scott A. Weldon, MA, CMI, for creating the illustrations.
Footnotes
The authors have no commercial relationships with manufacturers of products or providers of services discussed in this article.
References
- 1.Wong DR, LeMaire SA, Coselli JS. Managing dissections of the thoracic aorta. Am Surg. 2008;74:364–80. [PMC free article] [PubMed] [Google Scholar]
- 2.Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:33. [PubMed] [Google Scholar]
- 3.Rampoldi V, Trimarchi S, Eagle KA, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83:55–61. doi: 10.1016/j.athoracsur.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Lai DT, Robbins RC, Mitchell RS, et al. Does profound hypothermic circulatory arrest improve survival in patients with acute type a aortic dissection? Circulation. 2002;106:I218–28. [PubMed] [Google Scholar]
- 5.Bachet J, Goudot B, Dreyfus GD, et al. Surgery for acute type A aortic dissection: the Hopital Foch experience (1977–1998) Ann Thorac Surg. 1999;67:2006–9. doi: 10.1016/s0003-4975(99)00433-6. [DOI] [PubMed] [Google Scholar]
- 6.Kawahito K, Adachi H, Yamaguchi A, Ino T. Early and late surgical outcomes of acute type A aortic dissection in patients aged 75 years and older. Ann Thorac Surg. 2000;70:1455–9. doi: 10.1016/s0003-4975(00)01934-2. [DOI] [PubMed] [Google Scholar]
- 7.Hobeika MJ, Thompson RW, Muhs BE, et al. Matrix metalloproteinases in peripheral vascular disease. J Vasc Surg. 2007;45:849–57. doi: 10.1016/j.jvs.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 8.Pearce WH, Shively VP. Abdominal aortic aneurysm as a complex multifactorial disease: interactions of polymorphisms of inflammatory genes, features of autoimmunity, and current status of MMPs. Ann N Y Acad Sci. 2006;1085:117–32. doi: 10.1196/annals.1383.025. [DOI] [PubMed] [Google Scholar]
- 9.Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139:292–307. doi: 10.1016/j.jss.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Dobrin PB, Mrkvicka R. Failure of elastin or collagen as possible critical connective tissue alterations underlying aneurysmal dilatation. Cardiovasc Surg. 1994;2:484–8. [PubMed] [Google Scholar]
- 11.Sakalihasan N, Heyeres A, Nusgens BV, et al. Modifications of the extracellular matrix of aneurysmal abdominal aortas as a function of their size. Eur J Vasc Surg. 1993;7:633–7. doi: 10.1016/s0950-821x(05)80708-x. [DOI] [PubMed] [Google Scholar]
- 12.Baxter BT, McGee GS, Shively VP, et al. Elastin content, cross-links, and mRNA in normal and aneurysmal human aorta. J Vasc Surg. 1992;16:192–200. [PubMed] [Google Scholar]
- 13.Satta J, Juvonen T, Haukipuro K, et al. Increased turnover of collagen in abdominal aortic aneurysms, demonstrated by measuring the concentration of the aminoterminal propeptide of type III procollagen in peripheral and aortal blood samples. J Vasc Surg. 1995;22:155–60. doi: 10.1016/s0741-5214(95)70110-9. [DOI] [PubMed] [Google Scholar]
- 14.Segura AM, Luna RE, Horiba K, et al. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with Marfan’s syndrome. Circulation. 1998;98:II331–8. [PubMed] [Google Scholar]
- 15.Koullias GJ, Ravichandran P, Korkolis DP, et al. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg. 2004;78:2106–11. doi: 10.1016/j.athoracsur.2004.05.088. [DOI] [PubMed] [Google Scholar]
- 16.Taketani T, Imai Y, Morota T, et al. Altered patterns of gene expression specific to thoracic aortic aneurysms: microarray analysis of surgically resected specimens. Int Heart J. 2005;46:265–77. doi: 10.1536/ihj.46.265. [DOI] [PubMed] [Google Scholar]
- 17.Jones JA, Barbour JR, Lowry AS, et al. Spatiotemporal expression and localization of matrix metalloproteinas-9 in a murine model of thoracic aortic aneurysm. J Vasc Surg. 2006;44:1314–21. doi: 10.1016/j.jvs.2006.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung AW, Au Yeung K, Sandor GG, et al. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ Res. 2007;101:512–22. doi: 10.1161/CIRCRESAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- 19.Ikonomidis JS, Jones JA, Barbour JR, et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation. 2006;114:I365–70. doi: 10.1161/CIRCULATIONAHA.105.000810. [DOI] [PubMed] [Google Scholar]
- 20.Manabe T, Imoto K, Uchida K, et al. Decreased tissue inhibitor of metalloproteinase-2/matrix metalloproteinase ratio in the acute phase of aortic dissection. Surg Today. 2004;34:220–5. doi: 10.1007/s00595-003-2683-3. [DOI] [PubMed] [Google Scholar]
- 21.Akiyama M, Ohtani H, Sato E, et al. Up-regulation of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase were coupled with that of type I procollagen in granulation tissue response after the onset of aortic dissection. Virchows Arch. 2006;448:811–21. doi: 10.1007/s00428-006-0194-5. [DOI] [PubMed] [Google Scholar]
- 22.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–59. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei D, Kang T, Qi H. Cysteine array matrix metalloproteinase (CA-MMP)/MMP-23 is a type II transmembrane matrix metalloproteinase regulated by a single cleavage for both secretion and activation. J Biol Chem. 2000;275:33988–97. doi: 10.1074/jbc.M006493200. [DOI] [PubMed] [Google Scholar]
- 25.Chow AK, Cena J, Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol. 2007;152:189–205. doi: 10.1038/sj.bjp.0707344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 27.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 28.Hamano Y, Zeisberg M, Sugimoto H, et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Jilani SM, Nikolova GV, et al. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–91. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211:19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto T, Akaike T, Sawa T, et al. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276:29596–602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto T, Akaike T, Nagano T, et al. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch Biochem Biophys. 1997;342:261–74. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 33.Viappiani S, Schulz R. Detection of specific nitrotyrosine-modified proteins as a marker of oxidative stress in cardiovascular disease. Am J Physiol Heart Circ Physiol. 2006;290:H2167–8. doi: 10.1152/ajpheart.00128.2006. [DOI] [PubMed] [Google Scholar]
- 34.Sariahmetoglu M, Crawford BD, Leon H, et al. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007;21:2486–95. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- 35.Inserte J, Garcia-Dorado D, Ruiz-Meana M, et al. Ischemic preconditioning attenuates calpain-mediated degradation of structural proteins through a protein kinase A-dependent mechanism. Cardiovasc Res. 2004;64:105–14. doi: 10.1016/j.cardiores.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Murphy S, Frishman WH. Protein kinase C in cardiac disease and as a potential therapeutic target. Cardiol Rev. 2005;13:3–12. doi: 10.1097/01.crd.0000124914.59755.8d. [DOI] [PubMed] [Google Scholar]
- 37.Juhaszova M, Zorov DB, Kim SH, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–49. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellerbroek SM, Halbleib JM, Benavidez M, et al. Phosphatidylinositol 3-kinase activity in epidermal growth factor-stimulated matrix metalloproteinase-9 production and cell surface association. Cancer Res. 2001;61:1855–61. [PubMed] [Google Scholar]
- 39.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–83. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 40.Woessner JF., Jr That impish TIMP: the tissue inhibitor of metalloproteinases-3. J Clin Invest. 2001;108:799–800. doi: 10.1172/JCI13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagase H, Brew K. Designing TIMP (tissue inhibitor of metalloproteinases) variants that are selective metalloproteinase inhibitors. Biochem Soc Symp. 2003:201–12. doi: 10.1042/bss0700201. [DOI] [PubMed] [Google Scholar]
- 42.Clark IM, Swingler TE, Young DA. Acetylation in the regulation of metalloproteinase and tissue inhibitor of metalloproteinases gene expression. Front Biosci. 2007;12:528–35. doi: 10.2741/2079. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, McCluskey K, Fujii K, Wahl LM. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J Immunol. 1998;161:3071–6. [PubMed] [Google Scholar]
- 44.Krizanac-Bengez L, Hossain M, Fazio V, et al. Loss of flow induces leukocyte-mediated MMP/TIMP imbalance in dynamic in vitro blood-brain barrier model: role of pro-inflammatory cytokines. Am J Physiol Cell Physiol. 2006;291:C740–9. doi: 10.1152/ajpcell.00516.2005. [DOI] [PubMed] [Google Scholar]
- 45.Ries C, Egea V, Karow M, et al. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–63. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 46.Chua CC, Hamdy RC, Chua BH. Angiotensin II induces TIMP-1 production in rat heart endothelial cells. Biochim Biophys Acta. 1996;1311:175–80. doi: 10.1016/0167-4889(95)00205-7. [DOI] [PubMed] [Google Scholar]
- 47.Castoldi G, Di Gioia CR, Pieruzzi F, et al. ANG II increases TIMP-1 expression in rat aortic smooth muscle cells in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H635–43. doi: 10.1152/ajpheart.00986.2001. [DOI] [PubMed] [Google Scholar]
- 48.Castoldi G, di Gioia CR, Travaglini C, et al. Angiotensin II increases tissue-specific inhibitor of metalloproteinase-2 expression in rat aortic smooth muscle cells in vivo: evidence of a pressure-independent effect. Clin Exp Pharmacol Physiol. 2007;34:205–9. doi: 10.1111/j.1440-1681.2007.04573.x. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Catalan C, Bode W, Huber R, et al. Crystal structure of the complex formed by the membrane type 1-matrix metalloproteinase with the tissue inhibitor of metalloproteinases-2, the soluble progelatinase A receptor. EMBO J. 1998;17:5238–48. doi: 10.1093/emboj/17.17.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgunova E, Tuuttila A, Bergmann U, Tryggvason K. Structural insight into the complex formation of latent matrix metalloproteinase 2 with tissue inhibitor of metalloproteinase 2. Proc Natl Acad Sci U S A. 2002;99:7414–9. doi: 10.1073/pnas.102185399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275:26411–5. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101:1478–87. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corcoran ML, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinase-2 stimulates fibroblast proliferation via a cAMP-dependent mechanism. J Biol Chem. 1995;270:13453–9. doi: 10.1074/jbc.270.22.13453. [DOI] [PubMed] [Google Scholar]
- 54.LeMaire SA, Wang X, Wilks JA, et al. Matrix metalloproteinases in ascending aortic aneurysms: bicuspid versus trileaflet aortic valves. J Surg Res. 2005;123:40–8. doi: 10.1016/j.jss.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Tamura K, Sugisaki Y, Kumazaki T, Tanaka S. [Atherosclerosis-related aortic dissection] Kyobu Geka. 2000;53:194–201. [PubMed] [Google Scholar]
- 56.Ishii T, Asuwa N. Collagen and elastin degradation by matrix metalloproteinases and tissue inhibitors of matrix metalloproteinase in aortic dissection. Hum Pathol. 2000;31:640–6. doi: 10.1053/hupa.2000.7642. [DOI] [PubMed] [Google Scholar]
- 57.Schneiderman J, Bordin GM, Adar R, et al. Patterns of expression of fibrinolytic genes and matrix metalloproteinase-9 in dissecting aortic aneurysms. Am J Pathol. 1998;152:703–10. [PMC free article] [PubMed] [Google Scholar]
- 58.Muller BT, Modlich O, Prisack HB, et al. Gene expression profiles in the acutely dissected human aorta. Eur J Vasc Endovasc Surg. 2002;24:356–64. doi: 10.1053/ejvs.2002.1731. [DOI] [PubMed] [Google Scholar]
- 59.LeMaire SA, Pannu H, Tran-Fadulu V, et al. Severe aortic and arterial aneurysms associated with a TGFBR2 mutation. Nat Clin Pract Cardiovasc Med. 2007;4:167–71. doi: 10.1038/ncpcardio0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loeys BL, Pannu H, Tran-Fadula V, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–98. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 61.Pannu H, Tran-Fadula V, Avidan N, Milewicz DM. Genetic basis of thoracic aortic aneurysms and aortic dissections. Am J Med Genet C Semin Med Genet. 2006;1085:242–55. doi: 10.1196/annals.1383.024. [DOI] [PubMed] [Google Scholar]
- 62.Chen L, Wang X, Carter SA, et al. A single nucleotide polymorphism in the matrix metalloproteinase 9 gene (–8202A/G) is associated with thoracic aortic aneurysms and thoracic aortic dissection. J Thorac Cardiovasc Surg. 2006;131:1045–52. doi: 10.1016/j.jtcvs.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmad U, Javed MA, Fatimi SH. Candidate gene association analysis of thoracic aortic aneurysm and dissection. J Thorac Cardiovasc Surg. 2006;132:988. doi: 10.1016/j.jtcvs.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 64.Petrinec D, Liao S, Holmes DR, et al. Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: preservation of aortic elastin associated with suppressed production of 92 kD gelatinase. J Vasc Surg. 1996;23:336–46. doi: 10.1016/s0741-5214(96)70279-3. [DOI] [PubMed] [Google Scholar]
- 65.Curci JA, Petrinec D, Liao S, et al. Pharmacologic suppression of experimental abdominal aortic aneurysms: acomparison of doxycycline and four chemically modified tetracyclines. J Vasc Surg. 1998;28:1082–93. doi: 10.1016/s0741-5214(98)70035-7. [DOI] [PubMed] [Google Scholar]
- 66.Boyle JR, McDermott E, Crowther M, et al. Doxycycline inhibits elastin degradation and reduces metalloproteinase activity in a model of aneurysmal disease. J Vasc Surg. 1998;27:354–61. doi: 10.1016/s0741-5214(98)70367-2. [DOI] [PubMed] [Google Scholar]
- 67.Pyo R, Lee JK, Shipley JM, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–9. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sho E, Chu J, Sho M, et al. Continuous periaortic infusion improves doxycycline efficacy in experimental aortic aneurysms. J Vasc Surg. 2004;39:1312–21. doi: 10.1016/j.jvs.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 69.Steinmetz EF, Buckley C, Shames ML, et al. Treatment with simvastatin suppresses the development of experimental abdominal aortic aneurysms in normal and hypercholesterolemic mice. Ann Surg. 2005;241:92–101. doi: 10.1097/01.sla.0000150258.36236.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bartoli MA, Parodi FE, Chu J, et al. Localized administration of doxycycline suppresses aortic dilatation in an experimental mouse model of abdominal aortic aneurysm. Ann Vasc Surg. 2006;20:228–36. doi: 10.1007/s10016-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 71.Xiong W, Knispel RA, Dietz HC, et al. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg. 2008;47:166–72. doi: 10.1016/j.jvs.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baxter BT, Pearce WH, Waltke EA, et al. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (phase II) multicenter study. J Vasc Surg. 2002;36:1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- 73.Thompson RW, Liao S, Curci JA. Therapeutic potential of tetracycline derivatives to suppress the growth of abdominal aortic aneurysms. Adv Dent Res. 1998;12:159–65. doi: 10.1177/08959374980120011301. [DOI] [PubMed] [Google Scholar]
- 74.Curci JA, Mao D, Bohner DG, et al. Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J Vasc Surg. 2000;31:325–42. doi: 10.1016/s0741-5214(00)90163-0. [DOI] [PubMed] [Google Scholar]
- 75.Mosorin M, Juvonen J, Biancari F, et al. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg. 2001;34:606–10. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- 76.Bellosta S, Via D, Canavesi M, et al. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler Thromb Vasc Biol. 1998;18:1671–8. doi: 10.1161/01.atv.18.11.1671. [DOI] [PubMed] [Google Scholar]
- 77.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23:769–75. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 78.Furman C, Copin C, Kandoussi M, et al. Rosuvastatin reduces MMP-7 secretion by human monocyte-derived macrophages: potential relevance to atherosclerotic plaque stability. Atherosclerosis. 2004;174:93–8. doi: 10.1016/j.atherosclerosis.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–27. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 80.Forough R, Koyama N, Hasenstab D, et al. Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ Res. 1996;79:81220. doi: 10.1161/01.res.79.4.812. [DOI] [PubMed] [Google Scholar]
- 81.George SJ, Baker AH, Angelini GD, Newby AC. Gene transfer of tissue inhibitor of metalloproteinase-2 inhibits metalloproteinase activity and neointima formation in human saphenous veins. Gene Ther. 1998;5:1552–60. doi: 10.1038/sj.gt.3300764. [DOI] [PubMed] [Google Scholar]
- 82.Lamfers ML, Grimbergen JM, Aalders MC, et al. Gene transfer of the urokinase-type plasminogen activator receptor-targeted matrix metalloproteinase inhibitor TIMP-1. ATF suppresses neointima formation more efficiently than tissue inhibitor of metalloproteinase-1. Circ Res. 2002;91:945–52. doi: 10.1161/01.res.0000041418.51906.57. [DOI] [PubMed] [Google Scholar]
- 83.Rouis M, Adamy C, Duverger N, et al. Adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-1 reduces atherosclerotic lesions in apolipoprotein E-deficient mice. Circulation. 1999;100:533–40. doi: 10.1161/01.cir.100.5.533. [DOI] [PubMed] [Google Scholar]
- 84.Johnson JL, Baker AH, Oka K, et al. Suppression of atherosclerotic plaque progression and instability by tissue inhibitor of metalloproteinase-2: involvement of macrophage migration and apoptosis. Circulation. 2006;113:2435–44. doi: 10.1161/CIRCULATIONAHA.106.613281. [DOI] [PubMed] [Google Scholar]
- 85.Allaire E, Forough R, Clowes M, et al. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J Clin Invest. 1998;102:1413–20. doi: 10.1172/JCI2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei S, Chen Y, Chung L, et al. Protein engineering of the tissue inhibitor of metalloproteinase 1 (TIMP-1) inhibitory domain. In search of selective matrix metalloproteinase inhibitors. J Biol Chem. 2003;278:9831–4. doi: 10.1074/jbc.M211793200. [DOI] [PubMed] [Google Scholar]
- 87.Koivunen E, Arap W, Valtanen H, et al. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol. 1999;17:768–74. doi: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- 88.Higashi S, Miyazaki K. Identification of a region of beta-amyloid precursor protein essential for its gelatinase A inhibitory activity. J Biol Chem. 2003;278:14020–8. doi: 10.1074/jbc.M212264200. [DOI] [PubMed] [Google Scholar]
- 89.Gao CQ, Sawicki G, Suarez-Pinzon WL, et al. Matrix metallo-proteinase-2 mediates cytokine-induced myocardial contractile dysfunction. Cardiovasc Res. 2003;57:426–33. doi: 10.1016/s0008-6363(02)00719-8. [DOI] [PubMed] [Google Scholar]
- 90.Cheung PY, Sawicki G, Wozniak M, et al. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation. 2000;101:1833–9. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- 91.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001;89:201–10. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- 92.Yoshimura K, Aoki H, Ikeda Y, et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med. 2005;11:1330–8. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]
- 93.Lawrence DM, Singh RS, Franklin DP, et al. Rapamycin suppresses experimental aortic aneurysm growth. J Vasc Surg. 2004;40:334–8. doi: 10.1016/j.jvs.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 94.Peterson JT. Matrix metalloproteinase inhibitor development and the remodeling of drug discovery. Heart Fail Rev. 2004;9:63–79. doi: 10.1023/B:HREV.0000011395.11179.af. [DOI] [PubMed] [Google Scholar]
- 95.Peterson JT. The importance of estimating the therapeutic index in the development of matrix metalloproteinase inhibitors. Cardiovasc Res. 2006;69:677–87. doi: 10.1016/j.cardiores.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 96.Beaudeux JL, Giral P, Bruckert E, et al. Matrix metalloproteinases, inflammation and atherosclerosis: therapeutic perspectives. Clin Chem Lab Med. 2004;42:121–31. doi: 10.1515/CCLM.2004.024. [DOI] [PubMed] [Google Scholar]
- 97.Mendes O, Kim HT, Stoica G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis. 2005;22:237–46. doi: 10.1007/s10585-005-8115-6. [DOI] [PubMed] [Google Scholar]
- 98.Brown RD, Jones GM, Laird RE, et al. Cytokines regulate matrix metalloproteinases and migration in cardiac fibroblasts. Biochem Biophys Res Commun. 2007;362:200–5. doi: 10.1016/j.bbrc.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Treharne GD, Boyle JR, Goodall S, et al. Marimastat inhibits elastin degradation and matrix metalloproteinase 2 activity in a model of aneurysm disease. Br J Surg. 1999;86:1053–8. doi: 10.1046/j.1365-2168.1999.01196.x. [DOI] [PubMed] [Google Scholar]
- 100.Paraskevas KI, Mikhailidis DP, Perrea D. Experimental models of abdominal aortic aneurysms: an overview. Curr Pharm Des. 2008;14:325–37. [PubMed] [Google Scholar]