Abstract
The family of NADPH oxidase (NOX) genes produces reactive oxygen species (ROS) pivotal for both cell signalling and host defense. To investigate whether NOX and NOX accessory gene expression might be a factor common to specific human tumour types, this study measured the expression levels of NOX genes 1–5, dual oxidase 1 and 2, as well as those of NOX accessory genes NoxO1, NoxA1, p47phox, p67phox and p22phox in human cancer cell lines and in tumour and adjacent normal tissue pairs by quantitative, real-time RT-PCR. The results demonstrate tumour-specific patterns of NOX gene expression that will inform further studies of the role of NOX activity in tumour cell invasion, growth factor response and proliferative potential.
Keywords: NADPH oxidase (NOX), reactive oxygen species (ROS), human cancer, human tumour cell lines, hydrogen peroxide
Introduction
The recently-discovered epithelial NADPH oxidases (NOXs) mediate critical physiological and pathological processes including cell signalling, inflammation and mitogenesis through the generation of reactive oxygen species (ROS) [1,2]. The role of the ROS produced by these enzymes in specific tissue types and distinct cellular compartments is a matter of intense current investigation [3,4].
Cancer cells, like non-malignant tissues, produce ROS; in tumours, reactive oxygen metabolites can act as signalling molecules to promote cell survival over apoptosis [5,6]. Nox1-generated hydrogen peroxide can trigger an ‘angiogenic switch’ that includes the induction of angiogenic factors, such as the vascular endothelial growth factor (VEGF), that promote tumour cell vascularization and proliferation [7]. Nox4-mediated ROS have been shown to prevent apoptosis and promote tumour cell growth in pancreatic cancer cells [8,9]; and Nox5 has been implicated in protein tyrosine phosphorylation-dependent activation of B cells in patients with hairy cell leukaemia [10]. However, despite these studies, our understanding of the role(s) of the NOX family of genes in the development and growth of human cancer is limited [11-13].
The first NOX family member identified, Nox2 (originally named gp91phox), is a membrane-bound glycoprotein expressed in phagocytes that generates ROS in response to bacterial and fungal infection after interaction with a series of cytoplasmic and membrane-associated proteins [14]. The physiologic functions of the Nox2 homologues identified in nonphagocytic cells are not as well defined, but their restricted expression in different cell types and requirement for different cofactors suggests considerable functional specificity. Nox1, in addition to colon epithelial cells, has been found at lower levels in the stomach and the uterus and in vascular smooth muscle cells (VSMCs); it is involved in host defense, cytokine signalling and oxidant stress-dependent vascular hypertrophy [15-17]. Nox3 expression is restricted to the cochlear and vestibular sensory epithelial cells of the inner ear, where it is involved in maintaining balance [11,18]. Nox4 is expressed in the kidney, osteoclasts, VSMCs, endothelial cells and pancreatic cancer cell lines [8,11,16,19]. Nox5 is predominantly found in the adult testis, prostate cancer cell lines, as well as areas of the spleen and lymph nodes that contain mature B- and T-lymphocytes, respectively [11,20,21].
The final two members of the NOX family are the dual oxidases (Duox) 1 and 2; originally discovered in the normal thyroid gland, these two homologues appear to be involved in host defense in the lung and along the entire course of the gastrointestinal tract, as well as in the production of thyroid hormone [22-24].
Nox1, 2, 3 and 4 all must have direct interaction with p22phox to form an active, ROS-generating complex [25]. Homologues of the p47phox and p67phox members of the NOX complex found in leukocytes have been named NADPH oxidase organizer 1 (NoxO1) and NADPH oxidase activator 1 (NoxA1), respectively [26]. Nox1-mediated generation of ROS requires the interaction of both NoxO1 and NoxA1 [27]. Nox4 is constitutively active in the presence of p22phox and does not require either NoxA1 or NoxO1 for activity [25].
To investigate the potential for NOX family members and cofactors to play a role in human cancer, we measured levels of NOX gene expression in tumours and in adjacent normal tissues obtained from surgical resection samples of patients with melanoma or breast, brain, colon, liver, lung, head and neck, kidney, prostate, testicular, ovarian or stomach cancer and chronic myelogenous leukaemia and in 47 human tumour cell lines (including both parental and drug-resistant derivatives). We also measured the expression of NoxO1, NoxA1, p67phox, p47phox and p22phox in a sub-set of these lines. The purpose of these studies was to develop a more detailed understanding of the tumour-specific expression levels of the individual members of the NOX family of genes.
Materials and methods
Human cells and tumours and adjacent normal tissues
Samples of flash-frozen, surgically-resected, primary (non-metastatic) malignant tumours and the adjacent non-malignant tissues from the same patients were obtained from the City of Hope Comprehensive Cancer Center Pathology Core Facility. All samples utilized in this study had been examined by a faculty member of the City of Hope Division of Anatomic Pathology; light microscopic evaluation revealed that the tumour specimens contained a predominance of a specific malignant cell type and that the specimens characterized as ‘normal’ were histologically free of tumour cells. The tumour types studied were chosen in part based on current knowledge of the presence of NOX isoforms in some human tumours (Nox1 in colon cancer, for example), the relative availability of matched pairs of histologically-confirmed tumour/normal pairs in the Core Facility and the attempt to examine a sufficient number of separate tumour samples from each disease so that an initial, pilot examination of the presence of NOX gene family members could be performed and form the basis for further, more detailed studies. All of the tissues used in this study were ‘de-identified’ samples of surgical ‘discard material’ which, when collected, did not require individual patient consent for research purposes. The use of these tumours and adjacent tissues was reviewed and approved by the City of Hope National Medical Center Institutional Review Board. Polymorphonuclear leukocytes collected from healthy volunteers were provided by the City of Hope National Medical Center and National Institutes of Health Blood Banks.
Cell culture
Unless otherwise specified, tumour cell lines were purchased from the American Type Culture Collection (ATCC; Manassas, VA). Cells were grown in the appropriate media with 10–20% foetal bovine serum, according to ATCC recommendations. Culture media and supplements were obtained from Irvine Scientific (Santa Ana, CA) and Mediatech (Herndon, VA). Cells were cultured in a humidified 37°C incubator in an atmosphere of 5% CO2 in air. Cells were harvested during logarithmic phase growth for estimation of NOX expression levels. The origin of each cell line and the specific propagation conditions are listed in the ATCC product descriptions (www.atcc.org).
Four of the cell lines studied were derived from the parental MCF-7 breast cancer line. MCF-7/ADR cells were originally obtained from Dr Kenneth Cowan of the National Cancer Institute and were maintained in minimal essential medium (MEM) with sodium pyruvate and 2% heat-inactivated foetal calf serum (HI-FCS), insulin/transferrin (1:1000) and doxorubicin (1 μM) [28]. The MCF-7/ADR cells were subsequently adapted to growth in DMEM/F12 medium containing 0.5% HI-FCS to which was added either 0 (MR-0 cells) or 30 (MR-30 cells) nM sodium selenite. The JD-MCF-7/ADR cell line was separately derived by the development of resistance to stepwise addition of increasing concentrations of doxorubicin to parental MCF-7 cells up to a level of 2.5 μM of the anthracycline; these cells were passaged in DMEM/F12 medium with 2% HI-FCS. COH-BR6 human breast cancer cells were maintained in Eagle's MEM with 5% foetal bovine serum as previously described [29]. The hydroxyurea-resistant KB cell line KB-HUR was passaged in RPMI medium with 10% HI-FCS and 1mM hydroxyurea; the ribonucleotide reductase transfected KB cell clone, KB-M2D, was maintained in 10% HIFCS, with 300 μg/mL G418 and 0.1 mM hydroxyurea; both of these KB cell line variants were obtained from Dr Yun Yen, City of Hope Comprehensive Cancer Center [30]. The cisplatin-resistant human ovarian cancer cell line A2780DDP was maintained in RPMI medium containing 10% HIFCS and 50 U/ml Penstrep (Gibco, NY), as previously reported [31].
RNA isolation and reverse transcription
RNA was isolated from tissues after disruption with a Polytron homogenizer using RNA-zol B (TEL-TEST Inc., Friendswood, TX) according to the manufacturer's instructions. Genomic DNA contamination was removed with DNase I treatment (Ambion, Austin, TX). RNA from cell lines was isolated with RNAqueous-4 PCR kit (Ambion). RNA quality was tested on a 1% agarose gel (SeaKem, FMC, Rockland, ME) and RNA concentration and A260/A280 ratio were measured by UV spectrophotometry.
cDNA was prepared from 0.3–4 μg RNA using MMLV reverse transcriptase enzyme and random hexamers as primers (Invitrogen, Carlsbad, CA). Synthesis of cDNA was performed in a 20 μL volume for 45 min at 42°C, followed by 5 min denaturation at 75°C. The reaction was boosted by addition of 1000 units MMLV reverse transcriptase enzyme and repeating the 42°C-step for another 60 min. The enzyme was inactivated for 5 min at 95°C and the cDNA was stored at −20°C until use. Quantification of gene expression was carried out from the cDNA samples by real-time RT-PCR.
Real-time RT-PCR
Gene expression analysis was performed by quantitative real-time RT-PCR using 18S ribosomal RNA (rRNA) to normalize target gene expression for each sample. Primers and probes were designed following the manufacturer's guidelines (Primer Express software, Applied Biosystems, Foster City, CA); genomic DNA amplification was prevented by designing the primers around exon-intron splicing sites. Primer and probe sequences are listed in Table I.
Table I.
Primer sequences used for the evaluation of NOX isoforms and accessory genes.
| Gene | Sequence | GenBank no. |
|---|---|---|
| hNox1 | 5′-CCACTGTAGGCGCCCTAAGTT-3′ 5′-ATGACCGGTGCAAGGATCC-3′ 5′-FAM-AGGGCATCCCCCTGAGTCTTGGAA-TAMRA-3′ |
AF127763 |
| hNox2 | 5′-GCCCAAAGGTGTCCAAGCT-3′ 5′-TCCCCAACGATGCGGATAT-3′ 5′-FAM-TTACACTGACATCCGCCCCTGAGGA-TAMRA-3′ |
NM_000397 |
| hNox3 | 5′-CCTTCTGTAGAGACCGCTATGCA-3′ 5′-GACCACAGGGCCTAAAATCCA-3′ 5′-FAM-CCCAATGCCCCGTGCCTCAA-TAMRA-3′ |
AF190122 |
| hNox4 | 5′-GACTTTACAGGTATATCCGGAGCAA-3′ 5′-TGCAGATACACTGGGACAATGTAGA-3′ 5′-FAM-CCATCATTTCGGTCATAAGTCATCCCTCA-TAMRA-3′ |
AF261943 |
| hNox5 | 5′-CAGGCACCAGAAAAGAAAGCAT-3′ 5′-TGTTGATCCAGATAAAGTCCACCTT-3′ 5′-FAM-TTGCCCCAGCTGCCAGCACTC-TAMRA-3′ |
AF325189 |
| Duox1 | ABI TaqMan Gene Expression Assay: Hs00213694_m1 | NM_017434 |
| Duox2 | ABI TaqMan Gene Expression Assay: Hs00204187_m1 | NM_014080 |
| Gpx1 | ABI TaqMan Gene Expression Assay: Hs00829989_gH | NM_000581 |
| hNoxO1 | 5′-TGCAGATCAAGAGGCTCCAA-3′ 5′-TTCTTGAGCTGCCTGAATTCG-3′ 5′-FAM-TTGCCTTCTCTGTGCGCTGGTCAGA-TAMRA-3′ |
AF539796 |
| hNoxA1 | 5′-CCACGCTGCCATCGACTAC-3′ 5′-ACTGTGCCGACGCCACAT-3′ 5′-FAM-CCTGCGGTTCAAGCTGCAAGCC-TAMRA-3′ |
AY255769 |
| p22 phox (CYBA) | 5′-ACCGCCGTGGTGAAGCT-3′ 5′-ACCGAGAGCAGGAGATGCA-3′ 5′-FAM-TTCGGGCCCTTTACCAGGAATTACTATGTTC-TAMRA-3′ |
NM_000101 |
| p47 phox (NCFI) | 5′-GCTGGTGGGTCATCAGGAA-3′ 5′-GCCCCGACTTTTGCAGGTA-3′ 5′-FAM-ACGACGTCACAGGCTACTTTCCGTCCA-TAMRA-3′ |
NM_000265 |
| p67 phox (NCF2) | 5′-CCCTGCAACTACCTTGAACCA-3′ 5′-GGACTGCGGAGAGCTTTCC-3′ 5′-FAM-TTGAGCTGCGGATCCACCCTCAG-TAMRA-3′ |
NM_000433 |
| 18S rRNA | 5′-AACGAGACTCTGGCATGCTAACTA-3′ 5′-CGCCACTTGTCCCTCTAAGAA-3′ 5′-TET-TACGCGACCCCCGAGCGGT-TAMRA-3′ |
M10098 |
PCR reactions were performed in a 20 μL final volume adding 1 μL cDNA from each sample, using TaqMan Universal PCR mix (Applied Biosystems, Foster City, CA). For each target gene, the probe concentration was 0.3 μmol/L and the primer concentrations for the detection of Nox1 to 4, NoxO1 and NoxA1 genes were 0.4 μmol/L; 0.3 μmol/L was used for Nox5; 1 μL of the 20x primer and probe mix (ABI TaqMan Gene Expression Assays) was employed for Duox1, 2 and GPx1. PCR amplifications were performed on 384-well plates using the default cycling conditions and fluorescence was detected by the ABI 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA).
For the absolute calibration curve of the target genes and internal control gene (18S rRNA), serial dilutions of the plasmids (107 to 1 copy range) containing the gene insert were used. Nox1 and Nox5 plasmids were kindly provided by Dr B. Banfi and Dr K.-H. Krause (Geneva, Switzerland); gp91–1 (Nox2), gp91–2 (Nox1) and gp91–3 (Nox3) plasmids were a generous gift from Dr H. Kikuchi (Sendai, Japan); and the Nox4 plasmid was provided by Dr T. Leto (NIH). Plasmids containing Duox1 and Duox2 were a generous gift from Dr Corrine Dupuy (Chatenay-Malabry, France). Our laboratory cloned the 18S rRNA gene into the pCRII TA cloning vector (Invitrogen). Relative gene expression was determined as the ratio of the gene of interest to the internal reference gene expression based on standard curves. For the cell line studies, the data represent means of triplicate determinations of two and usually three separate experiments; the results of these experiment varied by <10%. For experiments with human tumour and adjacent normal tissues, the number of separate, independent studies for each of the patient samples was dependent upon the amount of tissue available. Colon, breast, liver, testis and lung studies were performed twice, in triplicate, for every patient's tissues; other tumour/normal pairs were examined once, in triplicate, for each patient sample.
Statistical evaluation
Differences in the expression levels of NOX family genes found in tumours were compared to mRNA expression levels in adjacent, pathologically-confirmed non-malignant surgical specimens using the Mann-Whitney test. A value of p <0.05 was considered significant.
Results
Expression of NOX isoforms and accessory genes in human tumour cell lines
Expression levels of NOX genes 1–5 and Duox1 and 2, relative to18S rRNA expression in 47 human cancer cell lines and normal human leukocytes, are shown in Table II. Expression was arbitrarily graded as low (NOX copy number/18S rRNA ratio <500×10−8), intermediate (ratio>500 but less than 2000×10−8) or high (ratio>2000×10−8). NOX genes with expression ratios>500×10−8 were routinely visible by Northern analysis using≥40 μg total RNA.
Table II.
Expression of Nox1 to 5, Duox1 and Duox2 relative to 18S rRNA expression (× 10−8) in human tumour cell lines.
| Cell line | Nox1 | Nox2 | Nox3 | Nox4 | Nox5 | Duox1 | Duox2 | |
|---|---|---|---|---|---|---|---|---|
| Colorectal | LS180 | 6 050 | 107 | 8 | 0 | 0 | 1 | 0 |
| Caco2 | 19 381 | 553 | 5 | 93 | 124 | 1 | 66 | |
| LS174T | 14 904 | 1 637 | 12 | 4 | 2 | 117 | 13 | |
| HT-29 | 15 429 | 1 167 | 8 | 9 | 1 | 15 | 1 | |
| Prostate | PC-3 | 8 | 11 | 8 | 0 | 18 873 | 0 | 0 |
| LNCaP | 276 | 323 | 7 | 7 | 667 | 9 | 5 | |
| DU 145 | 22 | 477 | 10 | 5 | 93 | 496 | 137 | |
| Breast | MCF-10A | 8 | 16 | 4 | 0 | 229 | 0 | 0 |
| MCF-7 | 13 | 16 | 4 | 0 | 14 | 0 | 0 | |
| MCF-7/ADR | 10 | 151 | 9 | 27 | 41 | 1 | 3 | |
| MR-0 | 11 | 167 | 39 | 44 | 62 | 0 | 1 | |
| MR-30 | 10 | 303 | 39 | 58 | 124 | 0 | 1 | |
| JD-MCF-7/ADR | 140 | 668 | 29 | 132 | 83 | 6 | 12 | |
| BT474 | 8 | 2 | 7 | 0 | 1 | 2 | 0 | |
| ZR-75 | 9 | 53 | 5 | 3 | 4 264 | 0 | 0 | |
| MB-468 | 9 | 5 | 5 | 0 | 20 | 2 | 0 | |
| COH-BR6 | 12 | 653 | 11 | 0 | 678 | 1 | 4 | |
| Haematopoietic | K562 | 14 | 778 | 6 | 0 | 1 | 1 | 1 |
| CEM | 9 | 2 | 4 | 0 | 0 | 0 | 0 | |
| Jurkat | 11 | 5 | 4 | 0 | 1 | 0 | 0 | |
| Molt-4 | 11 | 11 | 6 | 0 | 0 | 0 | 0 | |
| HL-60 | 0 | 839 | 0 | 1 | 31 | 0 | 0 | |
| h leukocytes | 4 | 54 948 | 2 | 0 | 4 | 6 | 14 | |
| Ovarian | OVCAR-3 | 22 | 9 | 10 | 27 | 2 | 31 | 8 |
| A2780 | 12 | 6 | 13 | 6505 | 0 | 0 | 2 | |
| A2780/DDP | 8 | 16 | 7 | 8 | 0 | 0 | 0 | |
| Skov-3 | 19 | 945 | 9 | 600 | 5 | 0 | 2 | |
| Melanoma | SK-MEL 5 | 0 | 685 | 0 | 457 | 2 086 | 0 | 0 |
| SK-MEL 28 | 0 | 584 | 1 | 52 | 92 | 0 | 0 | |
| HMCB | 0 | 649 | 2 | 44 | 103 | 29 | 7 | |
| A2058 | 0 | 645 | 9 | 1489 | 300 | 0 | 0 | |
| HTB-65 | 0 | 84 | 0 | 0 | 0 | 0 | 137 | |
| Lung | HTB177 | 7 | 3 | 0 | 1 | 9 | 1 | 0 |
| HTB178 | 20 | 11 | 0 | 0 | 4 | 248 | 97 | |
| A549 | 0 | 25 | 0 | 0 | 56 | 762 | 0 | |
| A431 | 17 | 4 | 3 | 0 | 3 | 2281 | 621 | |
| Head and neck | KB | 18 | 247 | 7 | 0 | 17 | 0 | 0 |
| KB-HUR | 9 | 84 | 5 | 105 | 45 | 2 | 0 | |
| KB-M2D | 8 | 97 | 4 | 8 | 74 | 0 | 0 | |
| Hepatic | Hep G2 | 5 | 0 | 8 | 22 | 0 | 2 | 21 |
| Hep G2/C3A | 9 | 391 | 25 | 35 | 0 | 1 | 2 | |
| Hep 3B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Testis | HS1.Tes | 8 | 2 | 1 | 108 | 0 | 5 | 0 |
| Cates-1B | 53 | 26 | 4 | 8 | 4 | 0 | 1 | |
| NTERA-2 | 96 | 49 | 7 | 43 | 15 | 33 | 20 | |
| Brain | U 251 | 8 | 116 | 0 | 0 | 43 | 6 | 5 |
| Embryonic kidney | HEK293 | 10 | 58 | 6 | 128 | 10 | 2 | 3 |
| Ewing's sarcoma | TC-71 | 131 | 652 | 5 | 17 | 8 | 2 | 7 |
High-level Nox1 mRNA expression was observed, as expected [17,32], in colorectal cancer cell lines; this observation was confirmed by Northern blot analysis (data not shown). Three of the colorectal lines expressed Nox2 at an intermediate level, while expression of Nox4 and 5 and Duox1 and 2 was low or undetectable in all four colorectal lines. As required for functional Nox1 activity, and as shown in Table III, colorectal cancer cells express intermediate-to-high levels of the critical accessory genes NoxO1 and NoxA1, allowing these cells to transduce signals from a variety of growth factors, such as EGF, into intracellular reactive oxygen species [33-35]. None of the other cell lines tested expressed high levels of Nox1; expression was at either low or undetectable levels.
Table III.
Expression of NOX accessory genes NoxO1, NoxA1, p67phox, p47phox and p22phox relative to 18S rRNA expression (× 10−8) in human tumour cell lines.
| Cell line | NoxO1 | NoxA1 | p67phox | p47phox | p22phox | |
|---|---|---|---|---|---|---|
| Colorectal | LS180 | 7595 | 8633 | 1257 | 4 | 53 674 |
| Caco2 | 361 | 2986 | 67 | 17 | 58 414 | |
| LS174T | 579 | 893 | 1908 | 2 | 17 712 | |
| HT-29 | 2198 | 464 | 10 | 12 | 61 203 | |
| Prostate | PC-3 | 318 | 5883 | 8315 | 14 | 42 |
| LNCaP | 640 | 279 | 17 | 9 | 14 | |
| DU 145 | 3131 | 296 | 197 | 665 | 28 962 | |
| Breast | MCF-7 | 89 | 1540 | 18 | 4 | 160 210 |
| BT474 | 29 | 1026 | 0 | 0 | 8 208 | |
| ZR-75 | 99 | 2350 | 4754 | 6 | 147 908 | |
| MB-468 | 39 | 192 | 9 | 0 | 58 258 | |
| Haematopoietic | K562 | 745 | 44 | 13 | 16 | 243 259 |
| HL-60 | 13 | 288 | 12 | 16 | 7 988 | |
| Ovarian | OVCAR-3 | 11 | 35 | 10 | 0 | 8 528 |
| Skov-3 | 455 | 554 | 1005 | 8 | 34 235 | |
| Melanoma | SK-MEL 5 | 27 | 70 | 0 | 1 | 4 133 |
| A2058 | 51 | 102 | 1 | 2 | 11 555 | |
| Hepatic | Hep G2 | 63 | 946 | 16 | 1 | 22 991 |
| Hep G2/C3a | 288 | 280 | 610 | 3 | 21 273 | |
| Embryonic Kidney | HEK293 | 141 | 10 | 13 | 6 | 125 963 |
| Ewing's Sarcoma | TC-71 | 744 | 7451 | 550 | 15 | 32 443 |
The three prostate carcinoma cell lines examined each expressed low or undetectable levels of Nox2, Nox 4 and Duox1 and 2; but two of the lines, PC-3 and LNCaP, expressed high and intermediate-levels of Nox5, respectively. High level Nox5 expression was also measured in one of 10 breast cell lines, ZR-75, and in one of five melanoma cell lines, SK-MEL 5; intermediate levels of Nox5 were found in COH-BR6 breast cancer cells. The four doxorubicin-resistant derivatives of the MCF-7 breast adenocarcinoma cell line, MCF-7/ADR, MR-0, MR-30 and JD-MCF-7/ADR, demonstrated no notable variations in NOX gene expression when compared to the parental cells.
Of the leukaemic lines tested, only K562 erythroleukaemia cells and HL-60 promyelocytic leukaemia cells expressed Nox2 at intermediate levels. For comparison, high levels of Nox2 expression levels were present in human polymorphonuclear leukocytes (Table II).
Nox4 expression was intermediate-to-high in two of the four tested ovarian cancer cell lines; notably, high-level acquired resistance to cisplatin in A2780/DDP cells was associated with a marked decrease in the expression level of Nox4. A2058 human melanoma cells also had an intermediate level of expression Nox4.
Of the four lung cancer cell lines tested, A431 cells expressed high levels of Duox1 and intermediate levels of Duox2 and A549 cells had intermediate levels of Duox1. All other genes tested in lung cancer cell lines were low or undetectable. NOX gene expression was low or undetectable in all hepatocellular, head and neck and testis cancer cell lines examined. Nox3 expression was essentially absent in all tumour cell lines tested.
Expression levels of the NOX accessory genes NoxO1, NoxA1, p67phox, p47phox and p22phox were also measured in a sub-set of cell lines in addition to those of colorectal origin (Table III). Expression of p22phox was high in every cell line tested, with the exception of PC-3 and LNCaP prostate carcinoma cells. All of the lines with the exception of DU 145 cells had low or undetectable levels of p47phox. PC-3 cells expressed high levels of NoxA1 and p67phox but low-levels of NoxO1, while LNCaP and DU 145 prostate cells expressed intermediate-to-high levels of NoxO1, respectively, but low-levels of NoxA1 and p67phox. Three of four breast lines expressed intermediate-to-high levels of NoxA1 with low level NoxO1, only ZR-75 cells also expressed a high level of p67phox. Intermediate p67phox expression was measured in one of two ovarian (Skov-3) and one of two hepatic (Hep G2/C3A) cell lines.
Expression of NOX isoforms and accessory genes in human tumours and adjacent normal tissues
Relative expression levels of NOX genes 1–5, Duox1 and 2 and NOX accessory genes were measured in surgically-resected tumours and, where available, adjacent non-malignant tissue from patients with solid tumour malignancies (Figure 1). A more detailed, disease-based representation of NOX isoform and accessory gene expression is presented in the Supplementary Appendix; estimates of the statistical differences between malignant and non-malignant tissues, where observed, are shown in Supplementary Table 1. Malignant cells from patients with chronic myelogenous leukaemia (CML; newly diagnosed or in relapse) were also examined.
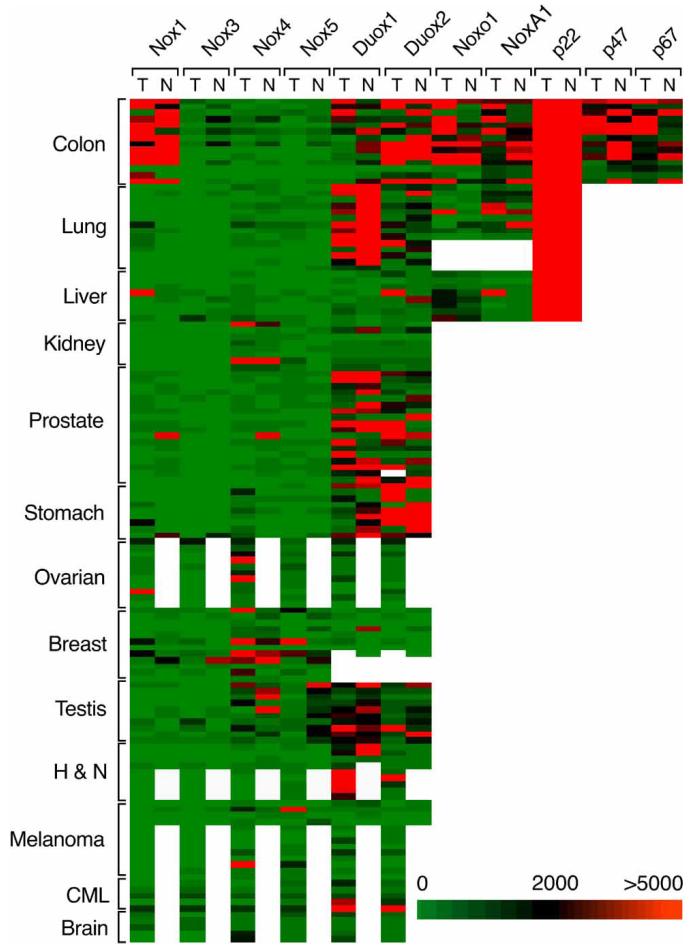
Figure 1.
mRNA expression of NOX isoforms and accessory genes in human tumours and adjacent non-malignant tissues relative to 18S rRNA levels. Of 237 clinical samples from 13 different malignancies, equal numbers of tumour and adjacent non-malignant tissue specimens were obtained from 17 patients with moderately- to poorly-differentiated prostate adenocarcinoma, 14 patients with moderately- to poorly-differentiated colorectal adenocarcinoma, 14 patients with moderately- to poorly-differentiated non-small cell lung cancer, 12 patients with moderately- to poorly-differentiated infiltrating ductal carcinoma of the breast, 10 patients with moderately- to poorly-differentiated testicular carcinoma, nine patients with moderately- to poorly-differentiated gastric adenocarcinoma, eight patients with moderately- to poorly-differentiated renal cell cancer and eight patients with primary hepatocellular cancer. Equal numbers of tumour and adjacent non-malignant surgical specimens were not always available for the following cancers: malignant melanoma (13 tumours, four adjacent non-malignant skin specimens); moderately- to poorly-differentiated ovarian adenocarcinoma (12 tumours, one non-malignant ovarian tissue sample); moderately- to poorly-differentiated squamous cell carcinoma of the head and neck (nine tumours, four adjacent non-malignant tissue samples); five samples of chronic myelogenous leukaemia in relapse; and five brain tumours of glioblastoma multiforme sub-type. The data shown in the figure represent the relative mRNA expression ratios for all of the clinical samples studied; the expression ratios are grouped by disease (y-axis), and NOX family or accessory gene expression (x-axis) subdivided by tumour (T) and normal (N) surgical samples.
Expression was arbitrarily graded as low (NOX gene copy number/18S rRNA ratio<500×10−8, intermediate (ratio>500 but less than 2000×10−8) or high (ratio>2000×10−), as outlined above for human tumour cell lines. Nox2 was excluded from our analysis (except for samples from patients with CML whose tumours consist of malignant leukocytes) because of the difficulty of differentiating expression of this NOX isoform in the tumour itself from that of infiltrating white blood cells.
Nox1 expression was significantly higher in colon and stomach cancers compared with adjacent non-malignant tissues (p<0.044 and p<0.042, respectively). Individual patient examples of elevated Nox1 expression were also observed in patients with primary hepatocellular cancer.
Nox3 expression was low or undetectable in almost all of the samples tested.
Nox4 was expressed at intermediate-to-high levels in approximately half of the patients with ovarian cancer or brain tumours. Nox4 was significantly higher, although often in the intermediate range, for tumour samples from patients with hepatic and gastric cancers compared with adjacent normal tissue (p≤0.0379 and p≤0.0051, respectively), while Nox4 expression was high or intermediate in individual patients with melanoma. On the other hand, Nox4 levels were categorized as intermediate-to-high in both renal and breast cancer and in adjacent normal kidney and breast tissue.
Nox5 expression was high in selected patients with breast cancers and melanomas. Significantly less Nox5 expression was measured in the testis tumour samples than in the adjacent normal specimens (p≤0.00007).
Expression levels of the Duox genes have not previously been extensively evaluated in human tumours other than lung and thyroid carcinomas. We found high levels of Duox1 and 2 in colonic and gastric cancers and their adjacent non-malignant tissues, as well as in head and neck cancer and in CML. Duox1 and 2 were also highly expressed in both normal and malignant prostate, kidney and testicular tissues; however, Duox1 expression was significantly diminished in lung tumour samples compared with adjacent surgical specimens of normal tissue (p≤0.0159).
We also examined NOX accessory gene expression in some of our surgical samples. Levels of p22phox were constitutively high in both tumour and non-malignant tissues in the colon, lung and liver. NoxO1 and NoxA1 levels were significantly increased in colon cancer samples (p≤0.0036 and p≤0.05, respectively). To evaluate the possibility that the observed increase in Nox1 and NOX accessory gene expression in colon cancers compared with normal colonic mucosa was biased by the over-representation of epithelial cells in the tumour, we utilized the identical 14 matched samples studied in Figure 1 to measure the expression of the antioxidant gene glutathione peroxidase 1 (GPx1) in tumour and normal tissue. We found that GPx1 expression levels relative to 18S rRNA were not significantly different in colon cancers and their adjacent normal tissues, 2600±700 vs 1800±500 (×10−8; mean±SE), respectively, p>0.05.
Discussion
In this study, we have investigated the expression patterns of the seven members of the NOX gene family and several closely-related accessory genes, in a series of 47 human cancer cell lines, as well as normal human leukocytes and surgically-resected human cancers and adjacent non-malignant tissues from 13 different organs. Because the available literature examining the expression of NOX genes in patients with cancer is limited [32,36-40], our goal in this study was to evaluate the presence or absence of each member of the NOX gene family in the most common human tumours.
Our results confirm that expression of the NOX family of genes is highly organ-specific for both malignant and non-malignant tissue. Furthermore, four patterns of NOX gene expression in human tumours were observed: (1) high level expression in tumour that was significantly different than in non-malignant adjacent normal tissues; (2) high level NOX gene expression in tumour where adjacent non-malignant tissue was not available; (3) equivalent levels of expression in tumour and normal tissue; and (4) down-regulation of NOX family genes in tumour compared with non-malignant tissue.
In the gastrointestinal tract, expression of Nox1, as well as NoxA1 and NoxO1, is significantly increased in colon cancers compared with adjacent normal bowel mucosa (Figure 1 and Supplementary Appendix). Over-expression of Nox1 in human colon cancers has been viewed as controversial in the past [41]; however, we found by real-time RT-PCR that both Nox1 and its required accessory genes are present at significantly higher levels in colonic malignancies compared with surrounding normal mucosa. Consistent with a recently-published study [42], we also found that Nox1 expression is significantly higher in gastric cancers than in surrounding normal gastric mucosa. The mechanisms underlying the enhanced expression of Nox1 in human tumours remain to be fully elucidated.
In several tumour types that we examined, we found high levels of NOX gene expression (defined arbitrarily as NOX copy number/18S rRNA ratio>2000×10−8) in the primary malignancy, but, principally for anatomic or physiologic reasons, had either an insufficient number of normal samples or no normal tissues for comparison. Nox4 expression was high-to-intermediate in many of the ovarian carcinomas and brain tumours that we examined. These results support the observation of increased Nox4 levels in malignant gliomas that has recently been reported [43]. Duox expression levels are high in patients with squamous cancers of the head and neck (Duox1>Duox2). Although patients with active CML not unexpectedly demonstrate high levels of Nox2 (see Supplementary Appendix), the high-level expression of Duox1 and 2 in some of these patients was unanticipated.
We also found that NOX gene expression was at a high level in both tumour and non-malignant tissues for Duox1 and 2 in the prostate and stomach and for Nox4 in the breast. At present, the physiologic function of these NOX family genes in the normal prostate, stomach or breast remains unclear. We did not observe the specific increase in Nox1 or Nox4 in prostate cancer that has been reported by others [36,38]. The surgical specimens from which our mRNA samples originated were flash frozen in the operating room in an attempt to maintain RNA integrity and were maintained in liquid nitrogen until use. However, it remains possible that the differences between our study and the reports of other investigators with respect to tissue-specific NOX gene expression may be related to differences in tissue handling or sample preparation.
The final pattern of NOX gene expression in tumours that we observed was that of significant down-regulation of expression in tumour vs adjacent non-malignant tissue. The epigenetic silencing of Duox1 and 2 expression in non-small cell lung cancers has recently been reported [39]. We also found that Duox1 expression in our lung cancer samples, as a group, was significantly diminished when compared with adjacent normal tissues (Figure 1 and Supplementary Table 1). We did not observe the same relationship for Duox2. For patients with testicular cancer, Nox5 expression was significantly decreased in tumour vs normal tissues. The mechanism for the down-regulation of NOX gene expression in testicular cancer is unknown.
We also evaluated NOX gene expression in a panel of 47 human tumour cells lines as well as mature polymorphonuclear leukocytes. It should be emphasized that each of these human tumour cell lines represents the outgrowth of a small, clonogenic population of cells derived from the malignant tissue of a single patient; and, thus, each cell line should not be viewed as necessarily being representative of either the disease or the tumour from which it originated [44]. It is not surprising, therefore, that the correlation of NOX gene expression in cell lines and tumours is modest. On the other hand, the human tumour cell lines shown in Tables II and III may provide important tools with which to study the biology of NOX expression. All four colon cancer cell lines that we investigated express Nox1 and its accessory genes at a high level and thus could potentially be useful in screening novel Nox1 inhibitors, for example. The A2780 ovarian carcinoma line and the A2058 melanoma may be helpful for the study of Nox4. SK-MEL 5 melanoma and PC-3 prostate cancer cells could assist in the investigation of Nox5 function. Knowledge of the expression levels of NOX accessory genes, as shown in Table III, should also further the investigation of the NOX gene family in tumour cell biology.
The variation in NOX gene and accessory factor expression in the prostate carcinoma cell lines we studied is intriguing and not related to androgen sensitivity, since PC-3 and DU 145 cells are both androgen-insensitive while LNCaP cells are androgen-sensitive. Our results do not confirm those of other investigators who have shown increasing amounts of Nox1 expression as prostate carcinoma cells progress from low to high tumourigenicity [38].
In conclusion, the studies presented herein demonstrate organ-specific patterns of NOX and NOX accessory gene expression in 13 primary human tumours. Although the comparison of NOX expression in tumour and adjacent normal tissue may be affected by the grade and stage of the tumours examined, the margin of normal tissue in the samples and the degree of tumour vascularization, this study of human tumour samples along with an examination of 47 different tumour cell lines provides an initial characterization of tumour-specific NOX gene expression. Further analysis of NOX and NOX accessory gene expression in primary tumour tissues awaits the development of a full panel of NOX-specific antibodies applicable to the immunohistochemical examination of tumour tissue microarrays.
Supplementary Material
Acknowledgements
We wish to thank Dr Mel Simpson and Dr Yvonne A. Evrard, SAIC-Frederick, Inc., for editorial assistance in the preparation of this manuscript. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, Grant number CA33572. The content of this publication does not necessarily reflect the views or policies of the United States Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Abbreviations
- NOX
NADPH oxidase
- Duox
dual oxidase
- HI-FCS
heat-inactivated foetal calf serum
- H2O2
hydrogen peroxide
superoxide
- ROS
reactive oxygen species
- RT-PCR
reverse transcriptase-polymerase chain reaction
- VSMC
vascular smooth muscle cell
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Whorton AR, Hoidal JR. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol. 2002;282:C1212–C1224. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- 2.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 5.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 6.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 7.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A, Hirose K, Kiyosawa K, Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT//apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–3707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 9.Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 10.Kamiguti AS, Serrander L, Lin K, Harris RJ, Cawley JC, Allsup DJ, Slupsky JR, Krause KH, Zuzel M. Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J Immunol. 2005;175:8424–8430. doi: 10.4049/jimmunol.175.12.8424. [DOI] [PubMed] [Google Scholar]
- 11.Cheng G, Cao Z, Xu X, Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 12.Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis. 2004;57:28–29. [PubMed] [Google Scholar]
- 13.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 14.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 15.Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, Krause KH. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–142. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- 16.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 17.Perner A, Andresen L, Pedersen G, Rask-Madsen J. Superoxide production and expression of NAD(P)H oxidases by transformed and primary human colonic epithelial cells. Gut. 2003;52:231–236. doi: 10.1136/gut.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 19.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 20.Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 21.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 22.Donko A, Peterfi Z, Sum A, Leto T, Geiszt M. Dual oxidases. Philos Trans R Soc Lond B Biol Sci. 2005;360:2301–2308. doi: 10.1098/rstb.2005.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 24.Lambeth JD. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr Opin Hematol. 2002;9:11–17. doi: 10.1097/00062752-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 26.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 27.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 28.Chu FF, Esworthy RS, Akman S, Doroshow JH. Modulation of glutathione peroxidase expression by selenium: effect on human MCF-7 breast cancer cell transfectants expressing a cellular glutathione peroxidase cDNA and doxorubicin-resistant MCF-7 cells. Nucl Acids Res. 1990;18:1531–1539. doi: 10.1093/nar/18.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esworthy RS, Baker MA, Chu FF. Expression of selenium-dependent glutathione peroxidase in human breast tumor cell lines. Cancer Res. 1995;55:957–962. [PubMed] [Google Scholar]
- 30.Zhou BS, Hsu NY, Pan BC, Doroshow JH, Yen Y. Over-expression of ribonucleotide reductase in transfected human KB cells increases their resistance to hydroxyurea: M2 but not M1 is sufficient to increase resistance to hydroxyurea in transfected cells. Cancer Res. 1995;55:1328–1333. [PubMed] [Google Scholar]
- 31.Morgan RJ, Jr, Margolin K, Raschko J, Akman S, Leong L, Somlo G, Scanlon K, Ahn C, Carroll M, Doroshow JH. Phase I trial of carboplatin and infusional cyclosporin in advanced malignancy. J Clin Oncol. 1995;13:2238–2246. doi: 10.1200/JCO.1995.13.9.2238. [DOI] [PubMed] [Google Scholar]
- 32.Fukuyama M, Rokutan K, Sano T, Miyake H, Shimada M, Tashiro S. Overexpression of a novel superoxide-producing enzyme, NADPH oxidase 1, in adenoma and well differentiated adenocarcinoma of the human colon. Cancer Lett. 2005;221:97–104. doi: 10.1016/j.canlet.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 33.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 34.Cheng G, Cao Z, Xu X, Van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 35.Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ, Rhee SG, Bae YS. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol. 2004;24:4384–4394. doi: 10.1128/MCB.24.10.4384-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapointe J, Li C, Higgins JP, Van De RM, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, Ekman P, DeMarzo AM, Tibshirani R, Botstein D, Brown PO, Brooks JD, Pollack JR. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurent E, McCoy JW, III, Macina RA, Liu W, Cheng G, Robine S, Papkoff J, Lambeth JD. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int J Cancer. 2008;123:100–107. doi: 10.1002/ijc.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, Petros JA, Arnold RS. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62:200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 39.Luxen S, Belinsky SA, Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 2008;68:1037–1045. doi: 10.1158/0008-5472.CAN-07-5782. [DOI] [PubMed] [Google Scholar]
- 40.Shono T, Yokoyama N, Uesaka T, Kuroda J, Takeya R, Yamasaki T, Amano T, Mizoguchi M, Suzuki SO, Niiro H, Miyamoto K, Akashi K, Iwaki T, Sumimoto H, Sasaki T. Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int J Cancer. 2008;123:787–792. doi: 10.1002/ijc.23569. [DOI] [PubMed] [Google Scholar]
- 41.Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E, Herrmann F, Hadengue A, Krause KH. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol. 2005;207:164–176. doi: 10.1002/path.1824. [DOI] [PubMed] [Google Scholar]
- 42.Tominaga K, Kawahara T, Sano T, Toida K, Kuwano Y, Sasaki H, Kawai T, Teshima-Kondo S, Rokutan K. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radic Biol Med. 2007;43:1627–1638. doi: 10.1016/j.freeradbiomed.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 43.Shono T, Yokoyama N, Uesaka T, Kuroda J, Takeya R, Yamasaki T, Amano T, Mizoguchi M, Suzuki SO, Niiro H, Miyamoto K, Akashi K, Iwaki T, Sumimoto H, Sasaki T. Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int J Cancer. 2008;123:787–792. doi: 10.1002/ijc.23569. [DOI] [PubMed] [Google Scholar]
- 44.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.