Abstract
Traditionally, vaccines directly target a pathogen or microbial toxin. Lyme disease, caused by Borrelia burgdorferi, is a tick-borne illness for which a human vaccine is not currently available. B. burgdorferi binds a tick salivary protein, Salp15, during transmission from the vector, and this interaction facilitates infection of mice. We now show that Salp15-antiserum significantly protected mice from B. burgdorferi infection. Salp15-antiserum also markedly enhanced the protective capacity of antibodies against B. burgdorferi antigens, such as OspA or OspC. Mice actively immunized with Salp15 were also significantly protected from tick-borne Borrelia. In vitro assays showed that Salp15-antiserum increased the clearance of Salp15-coated B. burgdorferi by phagocytes, suggesting a mechanism of action. Vaccination with a vector molecule that a microbe requires for infection of the mammalian host suggests a new strategy for the prevention of Lyme disease, and this paradigm may be applicable to numerous arthropod-borne pathogens of medical importance.
Keywords: Lyme disease, Ixodes ticks, vaccine, Salp15, antibody
Introduction
Ixodes scapularis ticks can transmit diverse infectious agents, including Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti (de la Fuente et al., 2008). Lyme disease, caused by B. burgdorferi sensu lato, is the most common tick-borne illness in the United States and selected regions of Eurasia (Fikrig and Narasimhan, 2006). While taking a blood meal, ticks attach to their vertebrate host for several days and introduce saliva, together with pathogens, into the skin. Tick saliva contains a wide range of physiologically active molecules that are critical for effective attachment and engorgement (Hovius et al., 2008a).
Human vaccines against Lyme disease are not currently available (Nardelli et al., 2009). Several B. burgdorferi outer surface proteins (Osps) that elicit protective responses, including OspA, OspB and OspC among others, have been identified (Earnhart et al., 2007; Feng et al., 1998; Fikrig et al., 1997; Steere et al., 1998). OspA has been extensively studied, proven to elicit varying degrees of immunity in diverse animal models of Lyme borreliosis, and demonstrated 79% efficacy in phase III human trials -- resulting in an FDA-approved vaccine that was available from 1998 until 2002 (Abbott, 2006). To date, no other spirochetal antigen has been tested in phase III clinical trials (Earnhart et al., 2007; Feng et al., 1998; Fikrig et al., 1997).
Infection with B. burgdorferi, and potentially other tick-borne pathogens, can also theoretically be prevented by interfering with the ability of ticks to feed on a mammalian host (de la Fuente et al., 2007b). Repeated exposure of guinea pigs to ticks results in acquired resistance of the animals to subsequent tick bites, so-called “tick-immunity”, and can influence tick-transmitted B. burgdorferi infection (Narasimhan et al., 2007a). Recently, immunization of guinea pigs with a tick salivary antigen, sialostatin L2, diminished the capacity of I. scapularis nymphs to feed, thereby identifying one of the antigens that contributes to a protective response (Kotsyfakis et al., 2008). Vaccines based on tick gut antigens have also proven partially successful in combating Boophilus tick engorgement on cattle (Kocan et al., 2007).
We now propose a new type of vaccine -- targeting an arthropod protein that a pathogen requires for effective transmission to the mammalian host (Ramamoorthi et al., 2005). The presence of B. burgdorferi within I. scapularis up-regulates the expression of Salp15, a tick salivary protein. Salp15 is normally used by I. scapularis to inhibit the activation of CD4+ T cells (Anguita et al., 2002), among other functions, presumably to enhance successful engorgement on the host. Remarkably, B. burgdorferi coats itself with Salp15, via a direct interaction with OspC, and this facilitates spirochete survival in the mammalian host (Ramamoorthi et al., 2005). We now determine whether immunization with a tick protein that a pathogen requires for effective transmission to mice can be used to prevent infection in the mammalian host, using the Lyme disease agent and Salp15 as the paradigm.
Results
Salp15-antiserum thwarts infection with Salp15-coated B. burgdorferi
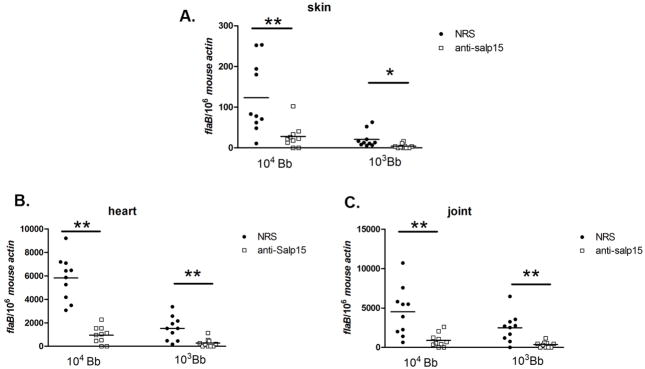
As Salp15 binds to the surface of B. burgdorferi, we determined whether Salp15 antibodies could protect mice from infection with Salp15-coated spirochetes. Groups of 10 mice were administered 200 μl of Salp15 antiserum, or control antiserum. One day later, the animals were challenged with an intradermal inoculation of 103 or 104 Salp15-coated B. burgdorferi, increasing doses that readily cause infection. At 1 week, the B. burgdorferi burden in the skin was markedly decreased in mice administered Salp15 antiserum, compared with controls (Figure 1, A) and 50% of the mice were fully protected from infection when challenged with 103 Salp15-coated B. burgdorferi. At 3 weeks, when spirochetes have disseminated to diverse organs, the pathogen burden in the heart and joints was also significantly lower in mice given Salp15-antiserum (Figure 1, B and C) and 40% of the mice were fully protected from infection with a dose of 103 Salp15-coated spirochetes. These studies, with an artificial challenge model, prompted us to examine the influence of Salp15-antiserum on naturally tick-transmitted B. burgdorferi infection.
Fig 1. Salp15-antiserum diminishes murine infection with Salp15-coated B. burgdorferi.
The B. burgdorferi load in different tissues was determined by Q-PCR, measuring the spirochete flaB gene copies and normalized using the murine β-actin gene. Spirochete burden in (A) skin at day 7, (B) heart and (C) joints at day 21. Horizontal lines represent the mean values. (* p < 0.05 and ** p < 0.01). A representative experiment of 3 performed is depicted.
Salp15-antiserum enhances the protective capacity of OspA antibody in a model of tick-transmitted B. burgdorferi infection
Immunization with selected B. burgdorferi Osps can provide substantial, but not necessarily complete, protection against spirochete infection (de Silva et al., 1999). Our previous study in mice showed that a titer of at least 6.0 μg of OspA monoclonal antibody (mAb) C3.78/ml blood was required to protect animals from tick-transmitted B. burgdorferi (de Silva et al., 1999). We therefore determined whether Salp15-antiserum would enhance the protective capacity of this OspA mAb.
Groups of 5 mice were passively immunized with either a low dose of OspA mAb (4 μg/ml of murine blood), 4 μg/ml of OspA mAb plus 100 μl of Salp15-antiserum, a high dose of OspA mAb (25 μg/ml of mouse blood), 200 μl of Salp15-antiserum or normal rabbit serum (control), respectively. One day later, 10 B. burgdorferi-infected ticks were placed on each mouse and the pathogen burden in the murine skin, heart and joints was determined at 3 weeks. First, Salp15-antiserum alone provided significant protection in the tick-transmission model. Only 55% of the mice administered Salp15-antiserum had B. burgdorferi infection compared with 100% of the control animals (Figure 2A, Table 1). The B. burgdorferi load was also markedly reduced in the immunized mice, compared with controls and the incidence and severity of arthritis in animals administered Salp15-antiserum were decreased (Figure 2A, B and C, Table 1). Salp15-antiserum did not influence tick feeding (the tick engorgement weight was 4.0±0.2 mg, control versus 4.0±0.2 mg, anti-Salp15), suggesting that this protection was not due to an alteration in the ability of ticks to take a blood meal. The effect of Salp15 antibody was specific for B. burgdorferi, as experiments with A. phagocytophilum-infected ticks showed that Salp15 antiserum did not protect mice from challenge with this pathogen (Supplementary figure 1).
Fig 2. Salp15 antiserum enhances the protective capacity of OspA antibody.
(A) Spirochete burden in murine skin, heart and joints at day 21 post tick feeding. (B) Arthritis index of the murine joints at day 21 post tick feeding. (Horizontal lines represent the mean values of the spots. Results are pooled from at least three independent experiments.) (C) Histopathology of tarsal joints from mice infected with B. burgdorferi treated with different antibodies. Representative hematoxylin and eosin stained sections from naive control (no infection) (a., score of 0), normal rabbit serum control (b., score of 2.5), low dose of OspA Ab+Salp15Ab (c., score of 0.5), low dose of OspA Ab (d., score of 2.5), high dose of OspA Ab (e., score of 0), and Salp15 Ab (f., score of 1). Tarsal joints from mice treated with a low dose of OspA Ab +Salp15Ab (c.) and high dose of OspA Ab (e.) had the lowest average inflammation scores. (Scale bars = 1000 μm). Arrows shows the leukocytes infiltration and exudation of fibrin and leukocytes into the joint lumen. (D) Spirochete burden in ticks at day 7 post tick feeding. (Values represent the mean+ s.e.m of three independent experiments.) (E) Spirochete burden in mouse skin at day 3 post tick feeding. (F) Spirochete burden in skin at day 7 post tick feeding. Horizontal lines represent the mean values of the spots. Results are pooled from three independent experiments. * p < 0.05 and ** p < 0.01, when compare with the normal rabbit serum control group.
Table 1.
Salp15-antiserum enhances the protective capacity of OspA antibody in a model of tick-transmitted B. burgdorferi infection.
| Immunogen | Challenge (no. of borrelia infected nymphs) | No. of tissue specimens with flaB PCR-positive/no. of total mice analyzed | flab copy (normalized by mouse β-actin) (Mean±S.E.M.)a | No. of Arthritis-positive/no. of total mice analyzed | Arthritis Index (Mean±S.E.M.)b | ||||
|---|---|---|---|---|---|---|---|---|---|
| skin | heart | joint | skin | heart | joint | ||||
| Normal rabbit serum | 10 | 19/20 | 20/20 | 20/20 | 79.5± 14.9 | 1123± 142.5 | 961.2 ± 146.9 | 10/10 | 2.0 ± 1.0 |
| Low dose of OspA Ab + Salp15 Ab | 10 | 4/20 | 5/20 | 5/20 | 3.6± 1.7** | 41.4± 17.3 ** | 64.9± 33.0** | 2/10 | 0.5 ± 0.5 ** |
| Low dose of OspA Ab | 10 | 15/20 | 17/20 | 18/20 | 47.1± 10.0 | 690.0± 133.8 | 586.1 ± 147.3 | 9/10 | 1.75± 1.0 |
| High dose of OspA Ab | 10 | 0/20 | 1/20 | 1/20 | 0.0± 0.0 ** | 1.0± 1.0 ** | 1.1± 1.1** | 1/10 | 0.25± 0.25** |
| Salp15 Ab | 10 | 11/20 | 11/20 | 11/20 | 27.3± 7.1 * | 332.5± 93.7* | 290.4 ± 80.4* | 6/10 | 1.0± 1.0* |
Results are means + s.e.m. from three quantitative PCR experiments.
p < 0.05 and
p < 0.01, when compare with normal rabbit serum control group.
The knee and tibiotarsal joints were scored for arthritis severity on a scale of 0 (negative) to 3 (severe) in a blinded fashion.
p < 0.05 and
p < 0.01, when compare with normal rabbit serum control group.
Salp15-antiserum also enhanced the protective capacity of a low dose of OspA antibody. Only 25% of the mice treated with Salp15/OspA-antiserum were infected compared with 90% of the animals treated with a low dose of OspA antibody alone or 100% of the control mice. The spirochete burden in the combined antibody immunized group was significantly lower than other experimental, or control, groups. The degree of protection was comparable to the group administered the high dose of OspA mAb alone (Figure 2A, Table 1). In addition, only 20% of animals given Salp15/OspA-antiserum had arthritis compared with 100% of the control mice, and the degree of inflammation was very mild compared with control animals, further demonstrating the ability of antibodies against this tick protein to influence the course of murine B. burgdorferi infection (Figure 2, B and C, Table 1).
As expected, the OspA mAb reduced the B. burgdorferi burden in I. scapularis after the ticks digested the blood meal, and this effect was most prominent in the high-dose group. In contrast, Salp15 antiserum had no effect on the spirochete burden in ticks, either when given alone, or in conjunction with low-dose OspA antibody, further demonstrating that these antibodies predominantly influence B. burgdorferi in the mammalian host (Figure 2D).
Since Salp15 facilitates spirochete survival during the early phase of transmission, we postulated that Salp15-antiserum may influence B. burgdorferi levels at this stage. A detailed analysis of the spirochete burden at the initial tick bite site showed that the pathogen load in the localized skin specimen was most markedly decreased in the combined antibody immunized group (Figure 2, E and F). At day 3, only 40% of mice given Salp15 antiserum alone and 10% of mice given Salp15/OspA antiserum were PCR- positive compared with 100% of the control mice. At day 7, 60% of mice with Salp15 antiserum alone and 30% of mice with Salp15/OspA antiserum were PCR- positive compared with 100% of the control animals, and those infected had markedly reduced levels of B. burgdorferi compared with controls (Figure 2, E and F). These data suggest that Salp15-antiserum interfered with spirochete survival at the early stage of infection.
Salp15-antiserum improves the protective capacity of B. burgdorferi OspC-antiserum
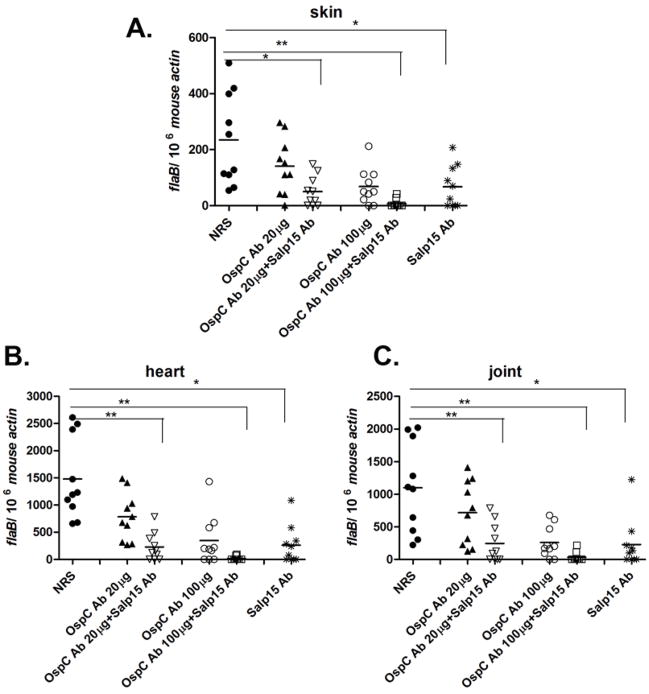
In order to assess whether Salp15-antiserum could also enhance the protective capacity of another B. burgdorferi antigen, we used OspC antibody in our tick-transmission studies. As Salp15 binds to OspC during B. burgdorferi transmission, and protects B. burgdorferi from antibody-mediated killing (Ramamoorthi et al., 2005), we postulated that Salp15-antiserum may alter the protective effect of OspC-antiserum. As previously reported, OspC antibody partially protected mice against tick-transmitted B. burgdorferi infection (Probert et al., 1997). We administered both OspC- (two different doses) and Salp15-antiserum to mice before tick challenge, and demonstrated that Salp15-antiserum enhanced the protective capacity of OspC antibody (Figure 3). The spirochete burden in the group administered both antiserum was significantly lower than other experimental or control groups, both at an early, in the skin at day 7, (Figure 3A), and at a later, in heart and joints at 21 days, stage of infection (Figure 3B and C). Only 2 of 10 mice treated with Salp15/high dose of OspC antiserum were infected, compared with 8 of 10 mice given a high dose of OspC antiserum alone, or 10 of 10 control mice given normal rabbit serum.
Fig 3. Salp15 antibody enhances the protective capacity of OspC antibody.
(A) Spirochete burden in murine skin at day 7 post tick feeding. Spirochete burden in the heart (B) and joints (C) at day 21 post tick feeding. Horizontal lines represent the mean values of the spots. * p < 0.05 and ** p < 0.01, when compare with the normal rabbit serum control group. Results are pooled from three independent experiments.
Borrelia transmission is impaired in mice actively immunized with Salp15
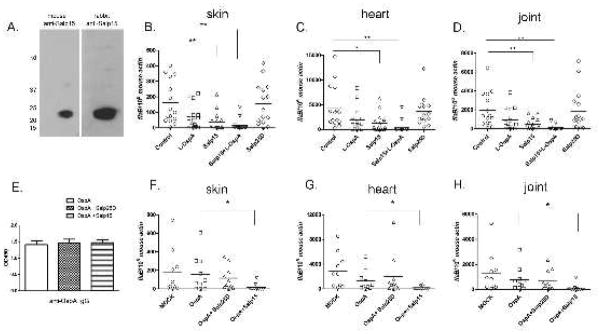
Our passive immunization study showed that Salp15 antiserum protects mice from tick-transmitted B. burgdorferi infection. We therefore tested whether mice actively immunized with Salp15 were also less susceptible to B. burgdorferi infection. Group of 5 mice were immunized with recombinant Salp15, Salp25D - another protein in tick saliva (Narasimhan et al., 2007b), or adjuvant alone (mock control). Additionally, 1 day prior to tick challenge, groups of 5 Salp15-immunized mice and 5 mock mice received a low dose of OspA mAb (~ 4 μg/ml mouse blood). First, mice generated antibodies against Salp15 following active immunization (Figure 4A); Second, after tick challenge, Salp15-immunized mice were partially protected against B. burgdorferi infection. At 3 weeks, 40% of Salp15 immunized mice were fully protected (based on absence of a detectable flaB signal in Q-PCR), while 95–100% of the control animals were infected (Figure 4, B, C and D). The mean B. burgdorferi load was also markedly reduced in Salp15 immunized mice which are positive, compared with controls. Salp15-immunized mice receiving a low-dose of OspA mAb shows the best protection rates (80% of mice were not infected with B. burgdorferi) (Figure 4, B, C and D). Arthritis was not visually observed in the mice actively immunized with Salp15 and then challenged with B. burgdorferi (data not shown). These data demonstrate that active immunization of mice with Salp15 prevents tick transmitted B. burgdorferi infection.
Fig 4. Salp15 active immunization provide mice protection against Borrelia infection.
(A). Salp15 probed with sera from mice or rabbits actively immunized with Salp15. (B) Spirochete burden in murine skin at day 7 post tick feeding. Spirochete burden in the heart (C) and joints (D) at day 21 post tick feeding (Horizontal lines represent the mean values). (E). Salp15 did not influence anti-OspA IgG levels at day 14 after immunization (Values represent the mean+ s.e.m of three independent experiments). (F) Spirochete burden in murine skin at day 7 post tick feeding. Spirochete burden in the heart (G) and joints (H) at day 21 post tick feeding. Horizontal lines represent the mean values. (* p < 0.05 and ** p < 0.01). Representative results from at least 3 independent experiments were shown.
To examine whether active immunization with Salp15 diminishes antibody production against a pathogen-specific antigen such as OspA, mice were immunized with OspA, OspA plus Salp15, or OspA plus Salp25D. Two weeks after immunization, mice were bled and the IgG titers of OspA were measured by ELISA. The OspA antibody titers were comparable among the 3 groups (Figure 4E). This suggests that Salp15 can be used administered in conjunction another antigen that elicits protective immunity, such as OspA, and will not influence the antibody production against pathogen-specific antigens.
An initial immunization with OspA, followed by several boosts is required to generate maximal protection against B. burgdorferi (Fikrig et al., 1990; Fikrig et al., 1992). We therefore determined whether active immunization with Salp15 could enhance the protective capacity of a single immunization with OspA. The results showed that 70% of the mice immunized with OspA plus Salp15 were protected; while only about 10–20% of the mice immunized with OspA alone, or OspA/Salp25D were PCR negative (Figure 4F,G and H). These data suggest that active immunization with Salp15 can be used to complement the protective effect of a B. burgdorferi specific-antigen.
Salp15-antiserum enhances the recognition and phagocytosis of spirochete by macrophages
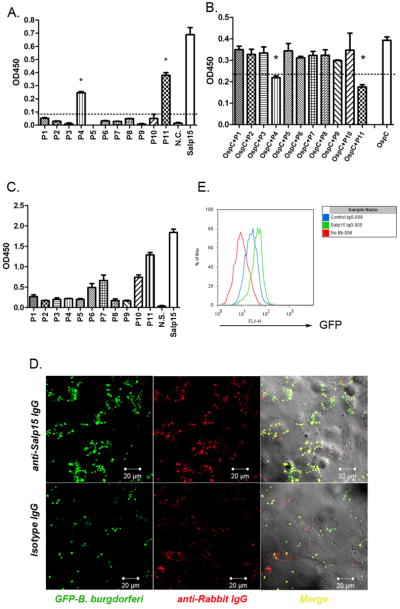
To assess the mechanism by which Salp15-antiserum controls B. burgdorferi infection, we examined the interaction between spirochetes, Salp15 and Salp15-antiserum. An in vitro binding assay using overlapping Salp15 peptides suggested that Salp15 peptides P11 (amino acids 95–114) and P4 (amino acids 31–50) are required for the Salp15-OspC interaction (Figure 5, A and B). As Salp15-antiserum recognize numerous epitopes on Salp15, including a dominant response to P11 (Figure 5C), the antibodies are likely to interact with Salp15 that has bound to OspC on the surface of B. burgdorferi, and thereby enhance clearance by phagocytes. We then assessed whether Salp15-antiserum augments the recognition and phagocytosis of Salp15-coated spirochetes by macrophages. Confocal microscopy showed that cells treated with Salp15 IgG could more readily identify and digest B. burgdorferi that express GFP (GFP-B. burgdorferi) (Eggers et al., 2002) than cells treated with control IgG (Figure 5D). The GFP-B. burgdorferi specifically co-localized with anti-Salp15 IgG (Figure 5D). FACS analysis using GFP-producing spirochetes showed that the Salp15 IgG-treated cells had a higher GFP intensity than control IgG-treated cells (Figure 5E) indicating that Salp15 IgG recognized the Salp15 that bound to OspC on the surface of B. burgdorferi, and these complexes were identified by host cells, and opsonized. To further confirm that the pathogens were intracellular, a detail immunostaining was performed. Cells were stained with OspA antibody prior to fixing and permeabilization, so that spirochetes outside the cells were detected as red. At the same time, the GFP signal represents spirochetes both inside and outside the cells (Supplementary figure 2).
Fig 5. Salp15 antibody enhances the phagocytosis of spirochetes by immune cells.
(A, B) Salp15 peptides bind to OspC. (C) Salp15 peptides bind Salp15 antibodies. (Results are means + s.e.m. of three independent experiments. * p<0.05.) (D) Confocal image of phagocytosis and spirochete-antibody co-localization (magnification, x 63). Rabbit IgG was stained with TRITC (red); GFP spirochetes were represented by green. Upper: Cells were treated with anti-Salp15 IgG; lower: Cells were treated with control rabbit IgG. (E) FACS analysis of the phagocytic efficiency of the cells. Histogram of GFP signal inside the cells. (red: no spirochetes; blue: control IgG treated cells; green: Salp15 IgG treated cells.) Representative results from at least 3 independent experiments were shown.
Salp15 antibodies facilitates phagocytosis of diverse Borrelia species by bone marrow-derived macrophages
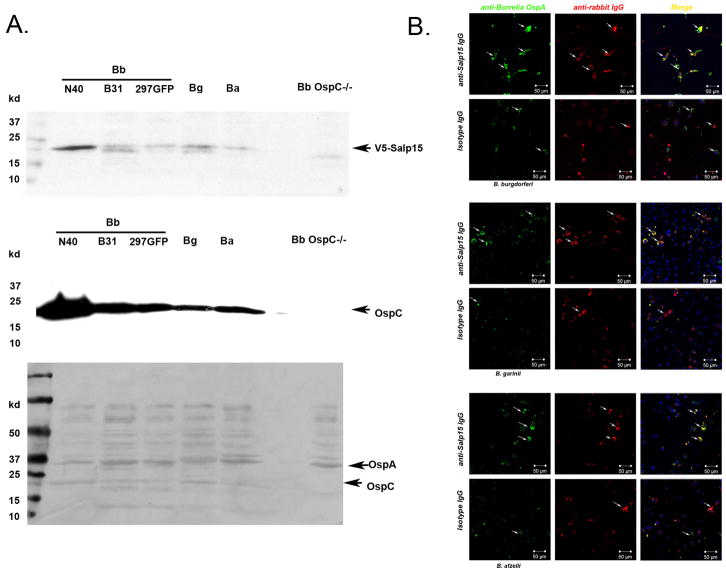
To examine whether Salp15-antiserum affords protection against other Borrelia species, we first whether Salp15 is able to bind to OspC from other Borrelia species. The overlay assay suggests that Salp15 not only binds to OspC from several Borrelia burgdorferi sensu stricto isolates (N40, B31, and 297), and also to OspC from Borrelia garinii and Borrelia afzelii – two species that contribute to Lyme borreliosis in Eurasia (Figure 6A). In an in vitro phagocytosis assay, we found that Salp15-antiserum enhanced the phagocytosis of different species of Salp15-coated Borrelia by bone marrow-derived macrophages (Figure 6B). These data suggest that Salp15 may be broadly effective as vaccine candidate against diverse Borrelia burgdorferi sensu lato species.
Fig 6. Salp15 antibodies enhance phagocytosis of different Borrelia species.
(A) Overlay assay with Salp15 and Borrelia lysates. Upper panel: Borrelia lysates overlaid with Salp15 (with V5 tag), and detected by anti-V5 antibodies; Middle panel: Borrelia lysates probed with anti-OspC antibodies; Lower panel: coomassie staining control. Arrows show the position of OspA and OspC. (B) Confocal image of phagocytosis and spirochete-antibody co-localization (magnification, x 25). Rabbit IgG was stained with TRITC (red); spirochetes were represented by green (anti-OspA Alex-488). Nuclei were stained with TOPRO3 (blue). Arrows show the localization of rabbit IgG and spirochetes, and the phagocytosis of spirochetes.
Discussion
Vaccines generally target the pathogen directly using extracts of the organism or recombinant antigens as the immunogen: the influenza and hepatitis B vaccines are examples. A few vaccines, such as tetanus toxoid, are aimed at microbial toxins. Another tactic, applicable to arthropod-borne agents is immunization to alter vector engorgement or the inflammation at the feeding sites and thereby indirectly prevent an infection (Allen and Humphreys, 1979; de la Fuente et al., 2007a; Labuda et al., 2006; Narasimhan et al., 2007a). In this study, we propose a new approach for vaccine development: targeting an arthropod protein that a pathogen requires for effective transmission to the mammalian host. Interfering with this interaction may impede the ability of a microbe to colonize the vertebrate host, in a manner that is independent of any effect on vector engorgement, and may be used independently or in conjunction with traditional pathogen-based vaccines.
Salp15 is a tick protein that facilitates B. burgdorferi infection of the mammalian host (Ramamoorthi et al., 2005). The presence of B. burgdorferi within ticks causes a marked increase in the production of Salp15 by the I. scapularis salivary glands. As B. burgdorferi migrate through the tick, the spirochetes become coated with Salp15 via binding to OspC. Salp15-coated B. burgdorferi are protected from antibody-mediated killing (Ramamoorthi et al., 2005) and this increases the ability of B. burgdorferi to infect mice. These properties make it an attractive target to influence B. burgdorferi infection.
Mice receiving Salp15 antiserum by passive transfer were significantly protected from intradermal syringe challenge with Salp15-coated B. burgdorferi, or tick-transmitted spirochetes. A plausible reason that Salp15 antibody provided partial immunity against tick-borne B. burgdorferi is that there may be additional tick salivary proteins that interact with the spirochete in the tick, thereby affording additional protection. Salp15 antiserum did not influence the ability of ticks to normally engorge, or the transmission of another I. scapularis-borne pathogen, Anaplasma phagocytophilum, suggesting that this protection is unique to Borrelia. This is consistent with the observation that Salp15 specifically binds to Borrelia OspC, and this interaction is critical for spirochete infection. Salp15-antiserum also enhanced a traditional pathogen-based vaccine -- in this case, OspA. The OspA vaccine had 79% efficacy in phase III trials, and vaccine failure was strongly associated with low titers of OspA antibodies (Steere et al., 1998). Using a murine model, we delineated the amount of an OspA-specific mAb required to afford protection against tick-borne B. burgdorferi (de Silva et al., 1999). Our data now show that Salp15-antiserum clearly augments the protective capacity of the OspA mAb. In addition, in active immunization studies, Salp15 elicited protection, and also increased the protective effect of OspA immunization, when both antigens were used together -- these data suggest that although Salp15 was first identified as an immunosuppressive protein (Anguita et al., 2002), this may not limit its application as a vaccine. Nevertheless, a mutant Salp15, which has a truncated C-terminus and does not have immunosuppressive properties, can also be used to generate Salp15 antibodies (data not shown) eliminating any potential for immunosuppression. These data show that antibodies directed towards an arthropod protein that a pathogen uses to colonize the mammalian host have a role in immunity against a microbe, either individually or by complementing a pathogen-specific vaccine.
We extended our studies by assessing another B. burgdorferi antigen, OspC. OspC is induced on B. burgdorferi during tick engorgement and plays an important role in the establishment of mammalian infection. Xu et al. postulated that OspC is not essential, because other lipoproteins can compensated for OspC (Xu et al., 2008) based on needle-inoculation studies that are quite different from the natural mode of B. burgdorferi infection (transmitted by ticks, highly expressing OspC and surrounded by saliva). OspC antibodies have also been shown to be partially protective against B. burgdorferi infection in various animal models, but the heterogeneity of OspC among B. burgdorferi isolates makes it unlikely that a single OspC antigen can serve as a vaccine (Earnhart et al., 2007; Probert et al., 1997). We found that Salp15-antiserum increased the protective capacity of OspC antiserum, demonstrating that the complementary effect of Salp15 is not limited to OspA.
Our data suggest that Salp15 antibodies interact with Salp15 on the surface of B. burgdorferi, and thereby enhance clearance by phagocytes. The mechanisms for the action of Salp15 antibodies in vivo may involve opsonizing Salp15-bound B. burgdorferi to phagocytes, and/or displacing Salp15 from the surface of B. burgdorferi and unmasking the spirochetes for direct access by the host immune system. The co-localization of Salp15 antibodies on the surface of GFP-B. burgdorferi (Figure 5D) suggest that opsonization by the Abs may be the primary mechanism for enhancing the elimination of the spirochete.
A vaccine needs to be as broadly protective as possible. We then test whether Salp15 antibodies could also have effect on other Borrelia species. Our data suggest that Salp15 not only binds to different strain of Borrelia burgdorferi sensu stricto, but also binds to B. garinii, and B. afzelii. This is consisted with data showing that Salp15 homologs from tick I. ricinus can also binds to B. burgdorferi sensu stricto, B. garinii, and B. afzelii (Hovius et al., 2008b). Salp15 antibodies also enhanced the phagocytosis of B. garinii, and B. afzelii in bone marrow derived macrophages. These data suggest that Salp15 may have a role as a broad vaccine candidate against different Borrelia burgdorfeir sensu lato species.
These data demonstrate a new approach for vaccine development against arthropod-borne diseases – in this case targeting an I. scapularis protein that B. burgdorferi uses to enable infection of the vertebrate host. This strategy may prove effective, either by using the immune response to Salp15 exclusively, or as a complement to a vaccine based on a specific antigen on the spirochete. Currently vaccines against many life-threatening arthropod-borne diseases, including dengue, malaria and leishmaniasis, among others, are not highly efficacious or unavailable. Our data suggest that the extension of this paradigm, developed with the Lyme disease agent, may be applicable to other vector-borne infections of medical importance.
Materials and Methods
Spirochetes, ticks and mice
A low-passage clonal isolate of B. burgdorferi N40 that is infectious in mice was used throughout the study. B. burgdorferi B31 and GFP-B. burgdorferi isolate 297, which constitutively express green fluorescent protein(Eggers et al., 2002), were used in the in vitro assays. B. garinii, and B. afzelii were purchased from ATCC and cultured in our lab. B. burgdorferi N40-infected I. scapularis nymphs were obtained from The Connecticut Agricultural Experiment Station. Female C3H/HeJ (C3H) mice, 4 to 6 weeks of age, were obtained from the Jackson Laboratory.
Protection from needle inoculated B. burgdorferi infection
Recombinant Salp15 and rabbit Salp15 antiserum were made as described (Anguita et al., 2002). Groups of 10 mice were passively administered 200 μl of rabbit Salp15-antiserum while control mice were administered 200 μl of normal rabbit serum. Before challenge, 107 B. burgdorferi were first allowed to adhere to 30 μg of recombinant Salp15 for 1h at room temperature. B. burgdorferi were pelleted by centrifugation and free Salp15 was removed by washing the spirochetes twice in PBS. 24 h after the passive immunization, mice were challenged with an intradermal inoculation of 103 or 104 Salp15-coated B. burgdorferi. Murine skin samples were collected at one week post-infection; murine heart and joint samples were collected at 3 weeks post-infection. The murine β-actin gene was used to normalize the amount of DNA in these samples and flaB was used to quantify the levels of spirochetes in the tissue samples by quantitative PCR.
Protection from tick-transmitted infection
We previously showed that, in C3H mice, a minimum titer of 6.0 μg OspA IgG/ml blood was required to protect mice from tick-transmitted B. burgdorferi infection (de Silva et al., 1999). Groups of 5 mice were passively immunized with normal rabbit serum (as control), a low dose of OspA mAb (4 μg/ml mouse blood, little protection), a low dose of OspA mAb plus 100 μl of Salp15 antiserum, a high dose of OspA mAb (20 μg/ml mouse blood, full protection), or 200 μl of Salp15 antiserum, respectively. 24 h after immunization, 10 B. burgdorferi-infected nymphal ticks were placed on each mouse. The pathogen burden in the localized skin specimen at day 3 and 7 post tick repletion and in the murine skin, heart and joints at 3 weeks post-infection were determined by measuring flaB copies using quantitative PCR.
For A. phagocytophilum infection, 24 h after immunization, 3 A. phagocytophilum -infected nymphs were placed on each mouse. At day 3, 5, 7 and 12, mice were bled, and the Anaplasma burden in murine blood was determined by measuring Anaplasma16srRNA gene copies using quantitative PCR.
In the OspC studies, OspC rabbit antiserum was generated by immunizing a rabbit with recombinant OspC protein in Freund’s adjuvant. Groups of 5 mice were passively immunized with normal rabbit serum (as control), a low dose of OspC Ab (20 μg/mouse, a low degree of protection), a low dose of OspC Ab plus 100 μl of Salp15 antiserum, a high dose of OspC Ab (100 μg/mouse, a higher degree of protection), a high dose of OspC Ab plus 100 μl of Salp15 antiserum, or 200 μl of Salp15 antiserum, respectively. The tick challenge and pathogen burden analysis were performed using the same methods described above.
Active immunization study
In active immunization study, group of 5 mice were immunized by subcutaneously injecting 10 μg of purified recombinant Salp15, Salp25D (a control saliva protein) suspended in complete Freund’s adjuvant, or adjuvant alone (mock control). Recombinant Salp15 and Salp25D were made as described previously (Anguita et al., 2002; Narasimhan et al., 2007b). Mice were boosted with 5 μg of antigen suspended in incomplete Freund’s adjuvant every two weeks. Additionally, at one day before tick challenge, groups of 5 Salp15-immunized mice and 5 mock mice received a low dose of OspA mAb (~ 4 μg/ml mouse blood.) The tick challenge and pathogen burden analysis were performed using the same methods described above.
To test whether the immunosuppressive properties of Salp15 influenced antibody production against a prototypic pathogen-specific antigen (OspA), mice were immunized with 10 μg of OspA, OspA plus Salp15 (5 μg) and OspA plus Salp25D (5 μg) together with complete Freund’s adjuvant (CFA). Two weeks after immunization, mice were bled and the IgG titer of OspA was measured by ELISA. The tick challenge and pathogen burden analysis were performed using the same methods described above.
Q-PCR analysis
All quantitative PCR (Q-PCR) assays were performed with an iCycler (Bio-Rad Laboratories) using high-fidelity Platinum Taq DNA polymerase (Invitrogen), gene-specific primers, and TaqMan probes with a program consisting of an initial denaturing step of 3 min at 95°C and 50 amplification cycles with 30 s at 95°C followed by 1 min at 60°C. The forward and reverse primers and probe used for Q-PCR analysis of flaB were 5′-AGC TGA AGA GCT TGG AAT GC-3′, 5′-TTG GTT TGC TCC AAC ATG AA-3′, and 5′-TCC AAG ACG CTT GAG ACC CTG AAA-3′, respectively. The forward and reverse primers and probe used for Q-PCR analysis of mouse β-actin were 5′-AGA GGG AAA TCG TGC GTG AC-3′, 5′-CAA TAG TGA TGA CCT GGC CGT-3′, and 5′-CAC TGC CGC ATC CTC TTC CTC CC-3′, respectively. The forward and reverse primers and probe used for Q-PCR analysis of tick actin were 5′-GAT CAT GTT CGA GAC CTT CA-3′, 5′-CGA TAC CCG TGG TAC GA-3′, and 5′-CCA TCC AGG CCG TGC TCT C-3′, respectively. The forward and reverse primers and probe used for Q-PCR analysis of A. phagocytophilum 16srRNA gene is 5′-TCG AAC GGA TTA TTC TTT ATA GCT TG-3′, 5′-CCA TTT CTA GTG GCT ATC CCA TAC TAC-3′ and 5′-TCA CCC GTC TGC CAC TAA CTA T-3′, respectively.
Arthritis analysis
Bilateral hind limb joints (knees and tibiotarsi) were processed and stained with hematoxylin and eosin by HSRL Laboratories (Mount Jackson, VA) or the Section of Comparative Medicine, Yale School of Medicine. The knee sand tibiotarsai were scored for arthritis severity on a scale of 0 (negative) to 3 (severe) in a blinded fashion as described previously (Barthold et al., 1997; Barthold et al., 1992).
Microtiter binding assay
Chemically synthesized overlapping peptides of Salp15 (Anguita et al., 2002) were purchased from the Yale University KECK facility. The different peptides (0.5 μg) were coated overnight at 4°C at the indicated concentrations in 0.1 M sodium carbonate buffer (pH 9.5) in 96-well plates. The wells were blocked with 10% FCS/PBS, and incubated with recombinant OspC (0.5 μg), anti-OspC Ab and HRP-conjugated anti-rabbit IgG followed by microwell peroxidase substrate (Kirkegaard & Perry Laboratories). For Salp15 peptide and Salp15 antibody binding, the wells (coated with individual peptide) were probed with rabbit anti-Salp15 polyclonal antibodies followed by HRP-conjugated anti-Rabbit IgG and detected with peroxidase substrate. In a competition assay, wells were coated with 0.5 μg of Salp15 overnight, probed with mixture of OspC and individual peptide and detected by anti-OspC Ab and HRP-conjugated anti-rabbit IgG.
In vitro phagocytosis study
107 GFP-B. burgdorferi were first allowed to adhere to 30 μg of recombinant Salp15 for 1 h at room temperature. B. burgdorferi were pelleted by centrifugation and free Salp15 was removed by washing the spirochetes twice in PBS. Murine bone marrow-derived macrophages (BMDM) were recovered from femurs and differentiated as described previously (Shin et al., 2008). BMDMs were sub-cultured in 12 well plates for 16 h and then Salp15-coated GFP spirochetes were added into the wells. The cells were either treated with 30 μg of normal rabbit IgG or rabbit anti-Salp15 IgG, respectively. The anti-Salp15 IgG and isotype IgG were purified from sera by using Proten A/G argrose according to standard protocol. After incubation for 4 h, the cells were washed 3 times with PBS, and fixed in 1% paraformaldehyde (PFA). For confocal microscopy analysis, the cells were incubated with TRITC-conjugated anti-rabbit IgG and phagocytosis was detected by the colocalization of GFP-spirochetes and TRITC staining. For flow cytometric analysis, the cells were fixed in 1% PFA, scraped off the plate with a policeman and analyzed in a FACSCalibur™ (BD Biosciences).
For the phagocytosis assay with different Borrelia species, 107 of B. burgdorferi N40, B. garinii, or B. afzelii were coated with Salp15, and then incubated with BMDMs and different antibodies for 4 hrs. Cells were fixed and probed with anti-OspA antibody, and stained with Alex 488 labeled anti-mouse IgG (Invitrogen), and TRITC-labeled anti-rabbit IgG. Cells were then examined by confocal microscope.
Salp15 Overlay assay
To test whether Salp15 is also able to bind to OspC from other Borrelia species, an overlay assay was performed as described (Ramamoorthi et al., 2005). Extract from different Borrelia burgdorferi sensu lato species were loaded on the SDS-PAGE gel, and transferred to a PVDF membrane. The membrane was overlaid with recombinant Salp15 (with a V5 tag). After 3 washes by PBST, the membrane was probed by HRP- conjugated anti-V5 antibody and the signal was analyzed by enhanced luminol-based detection (ECL) method. Then the membrane was stripped and re-probed with OspC Ab followed by HRP-conjugated anti rabbit IgG, and analyzed by ECL.
Statistical analysis
The significance of the difference between the mean values of the groups was evaluated using the Student’s t test with StatView software (SAS Institute).
Supplementary Material
Supplementary figure 1. Salp15 antiserum did not influence Anaplasma phagocytophilum transmission. Anaplasma burden in murine blood at day 3, 5, 7 and 12 was measured by amplifying the Anaplasma 16srRNA gene in DNA from blood using Q-PCR and normalized using the murine β-actin gene. Horizontal lines represent the mean values. Representative results from at least 3 independent experiments were shown.
Supplementary figure 2. The cellular distribution of B. burgdorferi in the phagocytosis assay. The extracellular (EC) spirochetes were stained with TRITC (red). GFP signal represents spirochetes both inside and outside the cells. Upper: Cells were treated with anti-Salp15 IgG; lower: Cells were treated with control rabbit IgG. Nuclei were stained with TOPRO3 (blue). Representative results from at least 3 independent experiments were shown.
Acknowledgments
This work was supported by grants from the NIH and Hatch Act funds. We thank Dr. Justin D. Radolf and Dr. Melissa Caimano for providing the GFP-B. burgdorferi, and Nandhini Ramamoorthi, Deborah Beck and Lindsay Rollend for assistance. Erol Fikrig is an Investigator with the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott A. Lyme disease: uphill struggle. Nature. 2006;439:524–525. doi: 10.1038/439524a. [DOI] [PubMed] [Google Scholar]
- Allen JR, Humphreys SJ. Immunisation of guinea pigs and cattle against ticks. Nature. 1979;280:491–493. doi: 10.1038/280491a0. [DOI] [PubMed] [Google Scholar]
- Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, Fikrig E. Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity. 2002;16:849–859. doi: 10.1016/s1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- Barthold SW, Feng S, Bockenstedt LK, Fikrig E, Feen K. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin Infect Dis. 1997;25(Suppl 1):S9–17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- Barthold SW, Sidman CL, Smith AL. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. The American journal of tropical medicine and hygiene. 1992;47:605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Almazan C, Canales M, Perez de la Lastra JM, Kocan KM, Willadsen P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Animal health research reviews/Conference of Research Workers in Animal Diseases. 2007a;8:23–28. doi: 10.1017/S1466252307001193. [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Kocan KM, Almazan C, Blouin EF. Targeting the tick-pathogen interface for novel control strategies. Front Biosci. 2008;13:6947–6956. doi: 10.2741/3201. [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Kocan KM, Blouin EF. Tick vaccines and the transmission of tick-borne pathogens. Veterinary research communications. 2007b;31(Suppl 1):85–90. doi: 10.1007/s11259-007-0069-5. [DOI] [PubMed] [Google Scholar]
- de Silva AM, Zeidner NS, Zhang Y, Dolan MC, Piesman J, Fikrig E. Influence of outer surface protein A antibody on Borrelia burgdorferi within feeding ticks. Infection and immunity. 1999;67:30–35. doi: 10.1128/iai.67.1.30-35.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Buckles EL, Marconi RT. Development of an OspC-based tetravalent, recombinant, chimeric vaccinogen that elicits bactericidal antibody against diverse Lyme disease spirochete strains. Vaccine. 2007;25:466–480. doi: 10.1016/j.vaccine.2006.07.052. [DOI] [PubMed] [Google Scholar]
- Eggers CH, Caimano MJ, Clawson ML, Miller WG, Samuels DS, Radolf JD. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the lyme disease spirochaete. Molecular microbiology. 2002;43:281–295. doi: 10.1046/j.1365-2958.2002.02758.x. [DOI] [PubMed] [Google Scholar]
- Feng S, Hodzic E, Stevenson B, Barthold SW. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infection and immunity. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Fikrig E, Barthold SW, Marcantonio N, Deponte K, Kantor FS, Flavell RA. Roles of OspA, OspB, and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infection and immunity. 1992;60:657–661. doi: 10.1128/iai.60.2.657-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E, Barthold SW, Sun W, Feng W, Telford SR, 3rd, Flavell RA. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- Fikrig E, Narasimhan S. Borrelia burgdorferi--traveling incognito? Microbes and infection/Institut Pasteur. 2006;8:1390–1399. doi: 10.1016/j.micinf.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Hovius JW, Levi M, Fikrig E. Salivating for knowledge: potential pharmacological agents in tick saliva. PLoS medicine. 2008a;5:e43. doi: 10.1371/journal.pmed.0050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius JW, Schuijt TJ, de Groot KA, Roelofs JJ, Oei GA, Marquart JA, de Beer R, van ‘t Veer C, van der Poll T, Ramamoorthi N, Fikrig E, van Dam AP. Preferential protection of Borrelia burgdorferi sensu stricto by a Salp15 homologue in Ixodes ricinus saliva. The Journal of infectious diseases. 2008b;198:1189–1197. doi: 10.1086/591917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan KM, de la Fuente J, Blouin EF. Targeting the tick/pathogen interface for developing new anaplasmosis vaccine strategies. Veterinary research communications. 2007;31(Suppl 1):91–96. doi: 10.1007/s11259-007-0070-z. [DOI] [PubMed] [Google Scholar]
- Kotsyfakis M, Anderson JM, Andersen JF, Calvo E, Francischetti IM, Mather TN, Valenzuela JG, Ribeiro JM. Cutting edge: Immunity against a “silent” salivary antigen of the Lyme vector Ixodes scapularis impairs its ability to feed. J Immunol. 2008;181:5209–5212. doi: 10.4049/jimmunol.181.8.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuda M, Trimnell AR, Lickova M, Kazimirova M, Davies GM, Lissina O, Hails RS, Nuttall PA. An antivector vaccine protects against a lethal vector-borne pathogen. PLoS pathogens. 2006;2:e27. doi: 10.1371/journal.ppat.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Deponte K, Marcantonio N, Liang X, Royce TE, Nelson KF, Booth CJ, Koski B, Anderson JF, Kantor F, Fikrig E. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS ONE. 2007a;2:e451. doi: 10.1371/journal.pone.0000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Sukumaran B, Bozdogan U, Thomas V, Liang X, DePonte K, Marcantonio N, Koski RA, Anderson JF, Kantor F, Fikrig E. A tick antioxidant facilitates the Lyme disease agent’s successful migration from the mammalian host to the arthropod vector. Cell host & microbe. 2007b;2:7–18. doi: 10.1016/j.chom.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli DT, Munson EL, Callister SM, Schell RF. Human Lyme disease vaccines: past and future concerns. Future microbiology. 2009;4:457–469. doi: 10.2217/fmb.09.17. [DOI] [PubMed] [Google Scholar]
- Probert WS, Crawford M, Cadiz RB, LeFebvre RB. Immunization with outer surface protein (Osp) A, but not OspC, provides cross-protection of mice challenged with North American isolates of Borrelia burgdorferi. The Journal of infectious diseases. 1997;175:400–405. doi: 10.1093/infdis/175.2.400. [DOI] [PubMed] [Google Scholar]
- Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, Koski RA, Fikrig E. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin OS, Isberg RR, Akira S, Uematsu S, Behera AK, Hu LT. Distinct roles for MyD88 and Toll-like receptors 2, 5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction. Infection and immunity. 2008;76:2341–2351. doi: 10.1128/IAI.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, Nowakowski J, Schmid CH, Laukamp S, Buscarino C, Krause DS. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. The New England journal of medicine. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- Xu Q, McShan K, Liang FT. Essential protective role attributed to the surface lipoproteins of Borrelia burgdorferi against innate defences. Molecular microbiology. 2008;69:15–29. doi: 10.1111/j.1365-2958.2008.06264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Salp15 antiserum did not influence Anaplasma phagocytophilum transmission. Anaplasma burden in murine blood at day 3, 5, 7 and 12 was measured by amplifying the Anaplasma 16srRNA gene in DNA from blood using Q-PCR and normalized using the murine β-actin gene. Horizontal lines represent the mean values. Representative results from at least 3 independent experiments were shown.
Supplementary figure 2. The cellular distribution of B. burgdorferi in the phagocytosis assay. The extracellular (EC) spirochetes were stained with TRITC (red). GFP signal represents spirochetes both inside and outside the cells. Upper: Cells were treated with anti-Salp15 IgG; lower: Cells were treated with control rabbit IgG. Nuclei were stained with TOPRO3 (blue). Representative results from at least 3 independent experiments were shown.