Abstract
C1q, the first component of the classical complement pathway, is also a pattern recognition receptor involved in the recognition and clearance of apoptotic cells. C1q deficiency in humans leads to development of lupus-like autoimmune disease, and it has been speculated that impaired clearance of apoptotic cells may contribute to disease development. Since phagocytes initiate specific and appropriate immune responses as a result of initial ligand-receptor interactions, regulation of gene expression by C1q may also contribute to the sculpting of an immune response to the ingested “self-Ags.” In this study, the role of C1q in apoptotic cell clearance and subsequent modulation of cytokine release by phagocytes was assessed including donor matched human monocytes, monocyte-derived macrophages (HMDMs), and dendritic cells (DCs). First, C1q binding is much greater to late compared with early apoptotic cells. Second, C1q binding to apoptotic cells significantly enhanced the levels of ingestion by monocytes but had no effect on HMDM and DC uptake. Third, in the presence of serum, C1q bound to apoptotic cells, activated the complement pathway, leading to C3b deposition, and enhancement of uptake of apoptotic cells by monocytes, HMDMs, and DCs. Finally, although C1q, either immobilized on a plate or bound to apoptotic cells, modulates the LPS-induced cytokine levels released by human monocytes, HMDMs, and DCs toward a more limited immune response, both the degree and direction of modulation differed significantly depending on the differentiation state of the phagocyte, providing further evidence of the integration of these cell- and environment-specific signals in determining appropriate immune responses.
Apoptosis is a form of programmed cell death, which is vital for developmental processes, maintaining tissue homeostasis, and maintenance of full functional capacity of the immune system. Billions of cells in the human body die by apoptosis every day, and under normal, healthy conditions, these cells are rapidly cleared by phagocytes, such as macrophages, without inducing inflammation. The failure of rapid clearance of these dying cells, however, can result in the disintegration of the dying cell(s) and the release of immunogenic or potentially toxic components into the surrounding tissue, ultimately evoking a proinflammatory response, a situation that can lead to the breakdown of the state of tolerance and development of autoimmunity (1). In addition, it is becoming evident that the tissue microenvironment and recognition mechanisms involved in the uptake of apoptotic cells help shape the immunological outcome (reviewed in Ref. 2). It is clear that the clearance of apoptotic cells must be a highly regulated innate immune activity to avoid initiation of a pathologic immune response.
The complement system is a powerful effector mechanism of the innate immune system. It is composed of proteins found both in circulation and in extracellular fluid. When activated, a series of proteolytic and protein-protein interactions occur, resulting in the opsonization of invading pathogens, initiation of a local inflammatory response through the recruitment of leukocytes to the site of infection or injury, and lysis of the pathogen (3, 4). Previous work has shown that certain collagen family members, such as the complement component C1q and the collectins mannose binding lectin (MBL) and pulmonary surfactant protein A (SPA), can act as a type of “costimulatory” molecule to enhance the phagocytosis of suboptimally opsonized targets (reviewed in Ref. 5). In addition, we showed that the interaction of C1q or MBL with human monocytes results in the modulation of cytokine production upon TLR stimulation (6).
It is generally accepted that phagocytic cells can respond to different immune stimuli (from pathogenic invasion or from injury) to initiate a specific program of events and/or signals that contribute to and direct an appropriate class of immune response, while also limiting tissue damage and/or the generation of immune responses to self-Ags (7). Rapid and efficient removal of apoptotic material by phagocytic cells likely plays an important role in controlling the immune response to Ags derived from apoptotic cells (8). It is well documented that C1q plays an important role in the clearance of apoptotic cells in vivo (9, 10), and in vitro it has been shown that C1q can enhance the uptake of apoptotic cells (11-13) and reviewed in Trouw et al. (14). C1q binds to apoptotic cells and cellular debris through its globular heads (15, 16), recognizing apoptotic cell-associated molecular patterns (ACAMPs)4 (17), many of which are yet unidentified, although phosphatidylserine and DNA are candidates (18, 19). Several studies support a role for the complement component C1q in influencing the uptake of apoptotic bodies and/or the subsequent cytokine profile of various phagocytic cells in a context dependent manner (6, 20, 21). However, these processes are not well understood, and there remains some controversy as to 1) the relative importance of the role of C1q, 2) the stage of the apoptotic cell (early vs late) at which C1q binding is most pronounced and influential in directing subsequent events, and 3) the influence of the differentiation state of the phagocyte being engaged. Therefore, we conducted systematic studies to examine the magnitude and quality of the C1q-mediated effects on these processes as a function of both the differentiation state of the phagocyte and the stage of apoptosis of the target cell. Our data demonstrate that C1q, in the absence of other complement components, can enhance the basal level of apoptotic cell clearance by monocytes but is not sufficient to enhance in vitro-generated human macrophage and immature dendritic cell (iDC) ingestion of early or late apoptotic cells. Furthermore, incubation with serum results in C1q-dependent C3 deposition on both early and late apoptotic cells and enhances apoptotic cell uptake by all phagocytes, although serum factors other than these complement proteins also contribute to the enhancement of uptake by monocytes. Finally, C1q evokes an altered class of immune response according to the differentiation state of the phagocyte. A better understanding of the parameters influencing these processes will be beneficial when designing therapeutic interventions to 1) promote host defense and/or vaccine design, 2) control inflammation, and 3) suppress autoimmunity in a wide range of pathophysiologic processes.
Materials and Methods
Media and reagents
RPMI 1640, penicillin/streptomycin, DMEM, trypsin-EDTA, and CFSE were purchased from Invitrogen. HL-1 medium (serum free), Xvivo-15 media, l-glutamine, and versene were purchased from BioWhittaker and defined FCS from HyClone. GM-CSF was obtained from Berlex and human recombinant IL-4 from PeproTech. The human serum albumin (HSA) used for the elutriation buffer was obtained from Talecris Biotherapeutics. Etoposide and LPS (from Escherichia coli 0127:B8; catalog no. L-3129) were obtained from Sigma-Aldrich. Factor P and Factor D were purchased from CompTech.
Isolation of C1q and C1q-depleted serum
C1q was isolated from plasma-derived normal human serum (NHS) by ion-exchange chromatography, followed by size-exclusion chromatography according to the method of Tenner et al. (22) and modified as described previously (23). The C1q preparations used were fully active as determined by hemolytic titration and homogeneous as assessed by SDS-PAGE. Protein concentration was determined using an extinction coefficient (E1%) at 280 nm of 6.82 for C1q (24). During the purification of C1q, serum depleted of C1q (C1qD) was collected after passage of plasma-derived serum over the ion-exchange resin and stored at −70°C until use. Calcium and magnesium were added to C1qD and plasma-derived serum to 3 mM Ca2+/10 mM Mg2+ (final concentration) immediately before use.
Cell isolation and culture
The human Jurkat T cell clone A3 (Jurkat) was obtained from American Type Culture Collection. Jurkat cells were maintained in RPMI 1640 supplemented with 10% FCS, 100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, and 10 mM HEPES (pH 7.4) (complete media) at 5% CO2. Apoptosis was induced by the addition of 40 μM etoposide to Jurkat cells cultured at 0.5 × 106/ml in complete media to obtain a predominantly Annexin V+/propidium iodide (PI)− (early apoptotic) phenotype, or by culturing Jurkat cells without serum, at 1 × 106/ml in Xvivo-15 media/1% penicillin/streptomycin, inducing a predominantly Annexin V+/PI+ (late apoptotic) phenotype. Measurement of early/late apoptosis was performed for every experiment by flow cytometry using the Apoptosis Detection Kit (Biovision) according to the manufacturer's instruction.
Human peripheral blood monocytes were isolated by counterflow elutriation using a modification of the technique of Lionetti et al. (25) as described previously (26). All blood samples were collected in accordance with the guidelines and approval of the University of California Irvine (UCI) Institutional Review Board. Blood units were collected into CPDA1 at the UCI Institute for Clinical and Translational Science. Greater than 90% of the cells in each preparation were monocytes according to size analysis on a Coulter Channelyzer.
Human monocyte-derived macrophages (HMDMs) were generated from monocytes by culture for 7 days in Xvivo-15 media supplemented with 10% heat-inactivated human AB+ serum, 1% l-glutamine, and 1% penicillin/streptomycin with 50% volume exchange with fresh media every 3–5 days. Human monocyte-derived iDCs were generated from monocyte cultures supplemented with GM-CSF and IL-4 as described previously (27). Briefly, isolated monocytes were cultured for 6–8 days in RPMI 1640 medium, which had been supplemented with 10% FCS, 1% penicillin/ streptomycin, 2 mM l-glutamine, 1% nonessential amino acids (Gibco), 1 mM sodium pyruvate, and 50 μM 2-ME. GM-CSF (200 U/ml) and IL-4 (200 U/ml) were added on day 0. Every 2–3 days, 50% of the culture media was exchanged with fresh media containing cytokines. Human monocyte-derived mature DCs (mDCs) were generated by treatment of iDCs with 1 μg/ml LPS for 24 h. HMDMs, iDCs, and mDCs were harvested from culture dishes/flasks using versene.
Evaluation of cell phenotypes was performed by standard flow cytometry on a FACSCalibur (BD Biosciences), using fluorochrome-conjugated mAbs against cell surface markers CD3, CD11b, CD11c, CD16, CD40, CD54, CD56, CD80, CD83, CD86, CD14, DC-SIGN, and MHC class II (MHC II). The appropriate fluorochrome-conjugated isotype control Abs were used as negative controls. Fluorochrome-conjugated Abs were obtained from Caltag Laboratories, with the following exceptions: anti-MHC II and anti-DC-SIGN (Biolegend); anti-CD3 and anti-CD14 (eBioscience); and anti-CD80 (BD Biosciences). Flow cytometry data were analyzed with FlowJo software (Tree Star).
LPS-stimulated cytokine assay
For cytokine analysis, cells were cultured in HL-1 medium supplemented with 1% l-alanyl-l-glutamine. Four-well LabTek chambers (Nunc) were coated with C1q or HSA control proteins (8 μg/ml) in coating buffer (0.1 M carbonate (pH 9.6)) and incubated at 37°C for 2 h. After washing chambers twice with PBS, phagocytic cells resuspended in HL-1 (0.5 ml of 106/ml) were added to chambers and cultured for 30 min at 37°C in 5% CO2 air. Where indicated, LPS (10–100 ng/ml) was added directly to the cells. Supernatants were harvested for cytokine analysis 18 h after the addition of LPS, centrifuged to remove cellular debris, and stored at −70°C until assayed for IL-1β, IL-6, IL-8/CXCL-8, IL-10, TNF-α, IL-12p70, MIP-1α/CCL3, and MCP-1/CCL2 using a multiplex cytokine kit (Linco/Millipore or Invitrogen) according to the manufacturer's protocol. The microbeads and reporter molecule were read on the Luminex100 version 1.7 (Luminex). Cytokine concentrations from the mean fluorescence values obtained were calculated from standard curves of each cytokine tested by using Miraibio Master Plex QT software (Miraibio).
C1q/C3-binding assay
Live or apoptotic Jurkat cells were washed twice in PBS before being resuspended at 5 × 106/ml in PBS/1% OVA. Purified human C1q was added to a final concentration of 0–300 μg/ml as indicated, or NHS, C1qD, or C1qD reconstituted with 75 μg/ml purified C1q (approximate serum concentration) (C1qD+C1q) was added to a final concentration of 10% in each sample volume. Binding reactions were incubated for 30 min at 37°C, after which cells were washed twice with FACS buffer (HBSS++/0.2% sodium azide/0.2% BSA). Binding of C1q or C3 was detected using mAbs to C1q or C3d obtained from Quidel, with secondary detection using fluorochrome-conjugated secondary Abs (Jackson ImmunoResearch Laboratories). The appropriate isotype control Abs were used as negative controls. Samples were analyzed by standard flow cytometry on a FACSCalibur.
Uptake of apoptotic cells
Jurkat cells were labeled with CFSE (Molecular Probes) as follows: cells were washed twice and resuspended at 2 × 107 cells/ml in HBSS++ and incubated with 5 μM CFSE at 37°C for 30 min. Remaining CFSE was quenched with RPMI 1640-10% FCS, and cells were washed twice in HBSS++ before induction of apoptosis. Live or apoptotic Jurkat cells were added to monocyte, HMDM, or DC cultures, which were at 106/ml in phagocytosis buffer (RPMI 1640, 25 mM HEPES, and 5 mM MgCl2) in LabTek chamber slides. Preliminary experiments demonstrated optimal uptake at a ratio of 3:1 (Jurkat:monocyte/HMDM) or 1:1 (Jurkat:DC). Amounts of purified human C1q were added to wells at 15 or 75 μg/ml final concentration or apoptotic Jurkats preincubated with C1q and washed before addition of targets to the phagocytes as indicated in the figures. Alternatively, phagocytosis buffer (no serum control), NHS, C1qD, or C1qD reconstituted with purified human C1q (C1qD+C1q) was then added to chambers at a final volume of 10%. Cells in LabTek chambers were centrifuged at 700 rpm for 3 min and incubated at 37°C, 5% CO2 for 30 min (HMDMs) or 60 min (monocytes and iDCs), as determined to be optimal in preliminary kinetic studies. After incubation, cells were harvested from the chamber by incubation with 0.25% trypsin/EDTA at 37°C for 3 min. The trypsin reaction was stopped with 0.5 ml of medium containing 10% FCS. Cells were pelleted by centrifugation at 1000 rpm for 10 min, washed with 1 ml of FACS buffer, and resuspended in 100 μl of FACS buffer for staining. DCs were stained with anti-human MHC II-PE or mouse IgG1-PE isotype control and monocytes/HMDMs with anti-human CD11b-PE or mouse IgG1-PE isotype control for 1 h on ice. After washing with FACS buffer, cell-associated fluorescence was measured using a FACSCalibur (BD Biosciences) and analyzed by using FlowJo software (Tree Star).
For cytokine analysis after the uptake of apoptotic cells, cells were treated essentially as for the uptake assays described above, with the following exceptions. Unlabeled live or apoptotic Jurkat cells were incubated with 0 or 300 μg/ml C1q for 30 min at 37°C in PBS/1% OVA. Cells were washed to remove unbound C1q, before incubation with phagocytic cells as described above. After ingestion, cells were washed twice with PBS, and phagocytosis buffer was replaced with HL-1 medium supplemented with 1% l-alanyl l-glutamine. Where indicated, LPS (30 ng/ml) was added directly to the cells. Supernatants were harvested for cytokine analysis 18 h after the addition of LPS, centrifuged to remove cellular debris, and stored at −70°C until analysis. Cytokine levels were subsequently measured by Luminex multiplex cytokine analysis.
C1q ELISA
Immulon 2HB plates (Thermo) were coated with affinity purified goat anti-human C1q F(ab)′2 (5 μg/ml) overnight at room temperature (28). Remaining sites were blocked with PBS containing 1% milk for 2 h at 37°C. Supernatants or purified C1q (100 μl) were incubated for 2 h at room temperature. Wells were washed with PBS/0.01% Tween, before addition of 100 μl of 1H11 monoclonal anti-C1q (0.5 μg/ml) (29) and incubation for 90 min at room temperature. Wells were washed, and HRP-conjugated secondary Ab (100 μl at 1/2000 dilution; Jackson Immunoresearch Laboratories) was added for 30 min at room temperature. The ELISA was developed by the addition of substrate OPD (Sigma-Aldrich). The concentration of C1q in culture supernatants was calculated using a standard curve generated with purified C1q from 0 to 50 ng/ml.
Results
Immobilized C1q differentially modulates LPS-induced cytokines released by human monocytes, macrophages, and DCs
Monocyte cytokine/chemokine expression in response to LPS is modified by surface bound C1q. Because monocytes are the precursors for macrophages and DCs in vivo, the influence of the differentiation state of the phagocyte on the response to C1q was examined. Freshly elutriated human monocytes were used to prepare monocyte-derived macrophages (HMDMs), iDCs, and LPS-activated mDCs. Characterization of the resulting populations by assessing cell surface markers shown in Table I provided evidence of generation of the expected cell types.
Table I.
Phenotype of monocytes, macrophages, and dendritic cellsa
| Relative Expression Level of Phenotypic Markers |
||||
|---|---|---|---|---|
| Monocytes | Macrophages | Immature DC | Mature DC | |
| MHC II | ++ | ++/+++ | +++ | +++ |
| CD3 | − | − | − | − |
| CD11c | + | + | + | + |
| CD14 | ++ | + | +/− | − |
| CD40 | + | + | + | ++ |
| CD54 | + | + | + | ++ |
| CD80 | − | + | + | +/++ |
| CD83 | − | − | − | + |
| CD86 | + | + | + | ++ |
| DC-SIGN | − | − | + | + |
Human monocytes, macrophages, and DCs were stained with fluorophore-conjugated Abs to the listed cell surface Ags and assessed by flow cytometry. The relative expression level for each marker is given and, in all cases, was consistent with the literature.
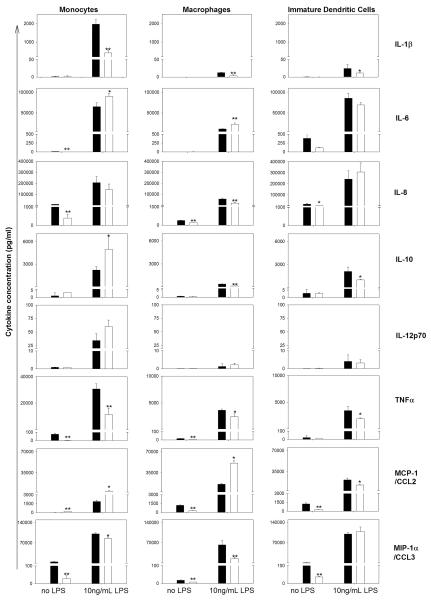
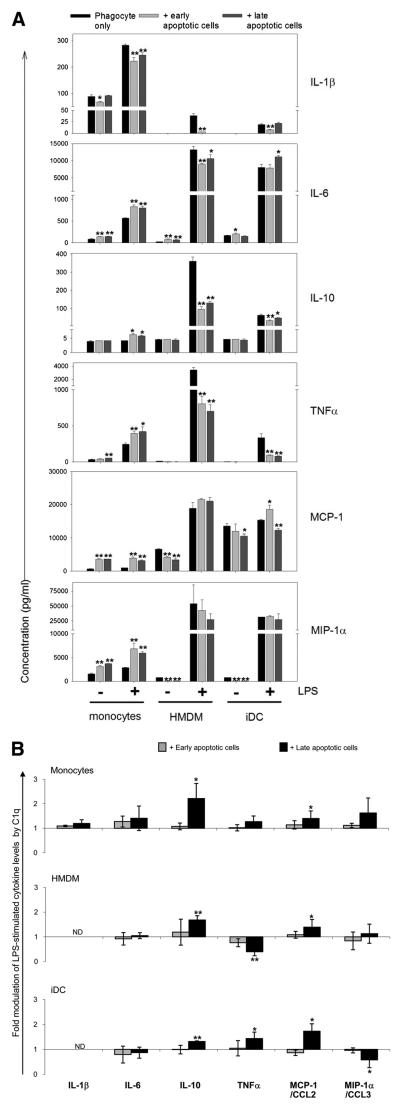
Multiplex cytokine analysis of supernatants from monocytes, HMDMs, and iDCs from the same donor is presented in Fig. 1, with similar results seen with cells obtained from different donors. Monocytes, HMDMs, and iDCs cultured in serum-free media for 18 h showed detectable levels of IL-1β, IL-6, IL-8/CXCL8, IL-10, IL-12p70, TNF-α, MCP-1/CCL2, and MIP-1α/CCL3 (Fig. 1). Although levels of IFN-γ were evaluated, IFN-γ production was not observed with or without stimulation (data not shown). Activation of these phagocytic cells with as little as 10 ng/ml LPS triggered a notable enhancement of the levels of all other cytokines tested. Both quantitative levels and C1q modulation of cytokines differed substantially with the differentiation state of the phagocyte (Fig. 1). In these studies, the Th1-associated proinflammatory cytokine IL-1β produced by LPS-stimulated monocytes was suppressed by C1q as shown previously (6) and was also significantly suppressed by C1q in LPS-activated HMDMs and iDCs, although absolute levels were low (<50 pg/ml). Similarly, secreted levels of another Th-1 associated proinflammatory cytokine, TNF-α, were significantly inhibited by C1q in both resting and LPS-activated monocytes, HMDMs, and iDCs. In contrast, although C1q significantly enhanced LPS-induced levels of IL-6 in monocytes and HMDMs, in iDCs there was a slight (though not statistically significant) trend toward suppression. Additionally, HMDMs and iDCs expression patterns for IL-10 and MCP-1/CCL2, two other cytokines that have been associated with Th2 polarization (30, 31), were not concordant with that in the monocyte. That is, MCP-1/CCL2 and IL-10 were enhanced by C1q in unstimulated and LPS-activated monocytes, consistent with our previous report (6), but were significantly reduced by C1q in LPS-activated iDCs (by 46%). Similar to iDCs, the LPS-activated levels of IL-10 in HMDMs were suppressed by C1q (by 29%); however, levels of MCP-1/CCL2 were significantly enhanced in HMDMs by C1q (320%), similar to that in monocytes (232%). Interestingly, in HMDMs not exposed to LPS stimulation, C1q suppressed secreted levels of MCP-1/ CCL2 (78%). Baseline levels of IL-8/CXCL8 and the chemokine MIP-1α/CCL3 were significantly down modulated by C1q in un-stimulated monocytes (by 96%), HMDMs (by 48%), and iDCs (by 75%) culture supernatants. LPS triggered high levels of IL-8/ CXCL8 and MIP-1α/CCL3 secretion by these phagocytes (>70 ng/ml), which were significantly down-regulated by C1q interaction with monocytes (by 16%) and HMDMs (by 76%), but not iDCs. Where seen, C1q modulations of cytokine levels were similar in phagocytic cells activated with 30 and 100 ng/ml LPS (data not shown) to those activated with 10 ng/ml LPS. Finally, because LPS was used to activate DCs to the mature phenotype, subsequent additional incubation of mDCs with LPS in the presence or absence of C1q did not induce detectable levels of any of the cytokines tested. Taken together, these data indicate that C1q modulation of chemokine and cytokine production, which contributes to the type of the subsequent elicited immune response, is dependent on the differentiation state of the cell but also influenced by other stimuli present in the microenvironment.
FIGURE 1.
Immobilized C1q differentially modulates LPS-induced cytokine release by human phagocytic cells according to their differentiation state. Cytokine levels were measured by Luminex multiplex analysis of the supernatant of phagocytic cells plated on LabTek chamber slides coated with 8 μg/ml C1q (□) or HSA control (■) and stimulated with 0 or 10 ng/ml LPS for 18 h. Data are mean values from a single experiment using donor-matched monocytes, HMDMs, and iDCs, performed in triplicate ± SD, and representative of data obtained in three experiments using different donors. *, p < 0.05; **, p < 0.005, Student's t test.
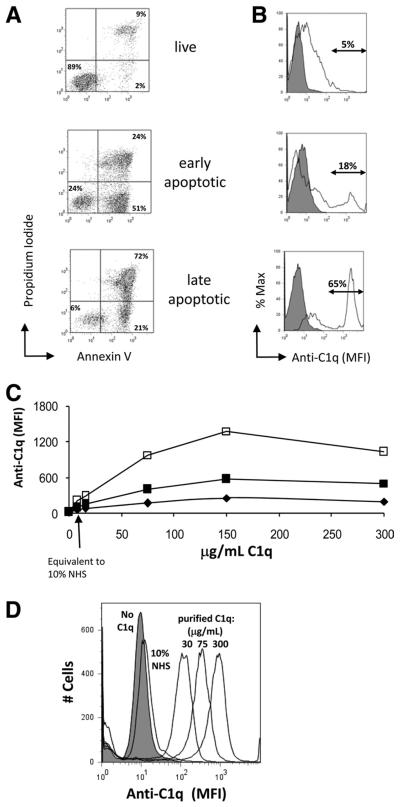
Differential and dose-dependent binding of C1q to early and late apoptotic cells
Control of cytokine expression may be influential in modulating or suppressing the immune response to self-Ags upon ingestion of apoptotic cells. C1q has previously been reported to bind a variety of apoptotic cells and cell blebs (11, 15, 16, 32). To determine the extent to which C1q could modulate the cytokine expression profile of the ingesting phagocyte, we first assessed whether the stage of apoptosis of the cell influenced C1q binding quantitatively. Jurkat cells were treated with etoposide to induce apoptosis in the presence or absence of serum to obtain cell populations enriched in early (Annexin V+PI−) or late (Annexin V+PI+) apoptotic cultures, respectively, as assessed by flow cytometric analysis of annexin V binding and PI staining. The resultant populations were 53% (±9%, n = 8) early apoptotic with <39% late apoptotic cells (range = 23-39%) or 74% (±10%, n = 8) late apoptotic cells (Fig. 2A). Purified C1q bound apoptotic but not live Jurkat cells in a dose-dependent manner (Fig. 2, B–D), reaching maximum binding at a C1q concentration of 150 μg/ml. Low levels of C1q binding were detected when late apoptotic Jurkats were incubated with 10% NHS (Fig. 2D), consistent with the lack of detectable binding of purified C1q at 7 μg/ml, because the concentration of C1q in serum is generally 70 μg/ml (33). Consistent with Nauta et al. (32), C1q bound late apoptotic cells to a greater extent than early apoptotic cells. C1q binding to early apoptotic cells is significantly lower than that of late apoptotic cells, which is particularly evident in the mixed population found in the late apoptotic cells in which the percentage of “low C1q” binding cells approaches that of the early apoptotic cells remaining in the population (Fig. 2, A and B). Conversely, analysis of the histogram binding data revealed that the percentage of cells that bound the higher amounts of C1q was similar to the percentage of cells that were Annexin V+/PI+ cells in each population, consistent with a preferential binding of C1q to late apoptotic cells (Annexin V+/PI+) (Fig. 2, A and B). To verify this, apoptotic cells incubated with 75 μg/ml C1q were double stained with Abs to C1q and Annexin V or PI. Cells that were positive for binding to C1q were also PI+ as well as Annexin V+ (data not shown), confirming the preferential stable binding of C1q to late apoptotic cells.
FIGURE 2.
C1q binds dose-dependently to apoptotic Jurkats but not to live cells. A, Characterization of apoptotic cells. Apoptosis of Jurkat cells was induced in complete media (early apoptotic) or Xvivo serum-free media (late apoptotic). Cells were stained with Annexin V and PI and analyzed by flow cytometry. Data are from one experiment, representative of at least 10. B, Live, early apoptotic, or late apoptotic Jurkat cells were incubated with 0 μg/ml (filled histogram) or 75 μg/ml (open histogram) C1q. C, Cultures of live (◆), early apoptotic (■), or late apoptotic (□) Jurkat cells were incubated with 0–300 μg/ml human C1q for 30 min at 37°C before being washed. C1q binding was measured by the MFI of fluorescently labeled secondary Ab detection of anti-human C1q using flow cytometry. D, A typical dose-response histogram of C1q binding to late apoptotic Jurkats when incubated with purified C1q or 10% NHS. Data (B–D) are from a single experiment, representative of three.
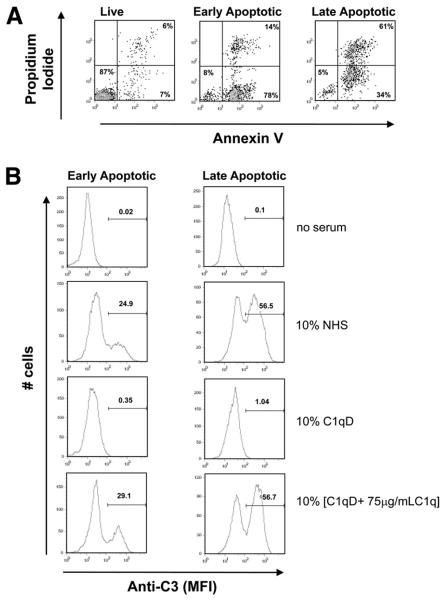
C3b is deposited on the surface of etoposide-induced apoptotic cells predominantly via classical complement pathway activation
C1q binding to apoptotic cells has previously been reported to activate complement (32). To confirm this in our system, C3b deposition on early and late apoptotic Jurkats was assessed by flow cytometry. Early or late apoptotic Jurkat cells, as assessed by measurement of Annexin V/PI staining by flow cytometry (Fig. 3A), were incubated with 10% C1q-depleted serum (C1qD) alone or 10% [C1q D + C1q], in which C1qD had been reconstituted with 75 μg/ml C1q to approximate serum concentration. Cells were also incubated with 10% NHS as a control. Interestingly, whereas little C1q binding was detectable on cells incubated with 10% serum, deposition of C3 was seen on both early and late apoptotic cells in 10% NHS. Because no C3 binding was seen in the absence of C1q (C1qD) (Fig. 3B) but could be completely restored upon reconstitution of C1q-depleted serum with serum levels of C1q (Fig. 3B), the activation of complement by apoptotic cells under these conditions is predominantly C1q dependent and thus is initiated by the classical pathway. The percentage of cells binding C3 in the early apoptotic cell “population” was 2-fold the percentage of PI-staining cells, suggesting that at least some portion of the early apoptotic (Annexin V+PI−) cells are activating complement. Although the amount of C3 bound to early and late cells was comparable (i.e., peak mean fluorescent intensity (MFI) = 332 and 316 for early and late apoptotic cells, respectively), the percentage of cells positive for C3 deposition is much greater in the late apoptotic cell populations, suggesting that complement activation is enhanced or accelerated on late apoptotic cells relative to early apoptotic cells (Fig. 3B).
FIGURE 3.
C3b deposition on apoptotic cells. Early and late apoptotic Jurkats, as assessed by Annexin V/PI staining (A), were incubated with no serum, 10% NHS, 10% C1q-depleted serum (C1qD) (generated from the same NHS), or 10% [C1qD + 75 μg/ml C1q] for 30 min at 37°C and subsequently washed and probed with anti-C3 as described in Materials and Methods. C3 deposition was assessed by flow cytometry. Data shown are a typical histogram depicting the MFI from a single experiment, representative of three (B).
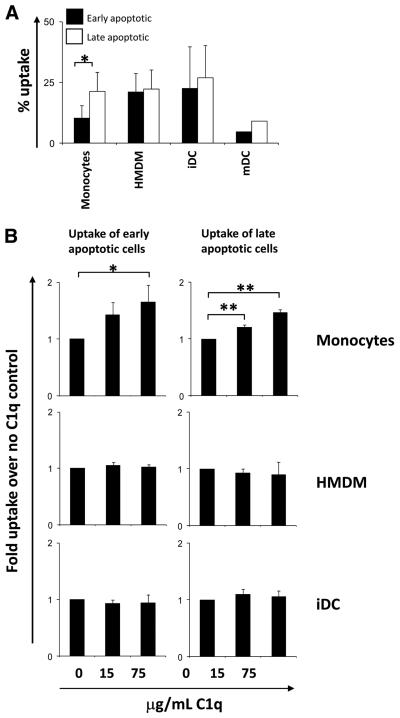
C1q enhances monocyte but not macrophage or DC uptake of apoptotic cells in the absence of other complement components
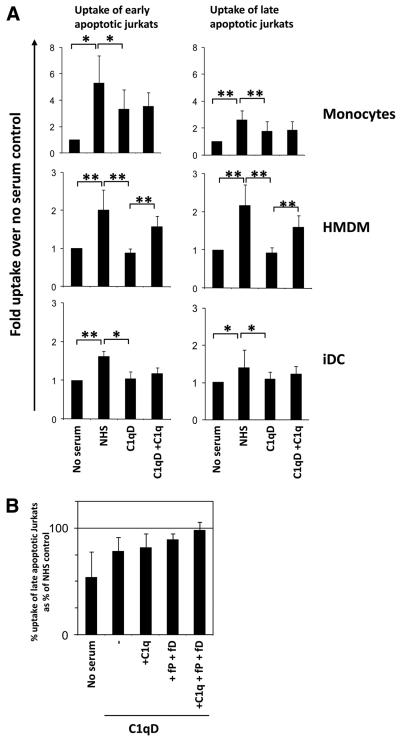
To assess specifically the effect of C1q acutely bound to the dying cell, apoptotic Jurkat cells in the presence or absence of 15 or 75 μg/ml C1q were incubated with monocytes, HMDMs, and iDCs as described above. The basal level of uptake (i.e., in the absence of C1q) of late apoptotic cells by monocytes was significantly greater, by ~2-fold, than that of early apoptotic cells (Fig. 4A). In contrast, although basal levels of uptake by iDCs and HMDMs were higher relative to monocytes, levels of uptake of early and late apoptotic cell populations by iDCs and HMDMs were not substantially different (Fig. 4A). Analysis of the fold enhancement of uptake by C1q showed a small but significant dose-dependent enhancement of the uptake of apoptotic cells by monocytes, but C1q had no effect on the uptake of apoptotic cells by HMDMs or iDCs (Fig. 4B). Although the basal level of uptake was ~2-fold higher for late apoptotic cells (Fig. 4A), the relative C1q enhancement of uptake of early apoptotic cells by monocytes was similar to the C1q enhancement of uptake of late apoptotic cells by this phagocyte population. The results did not differ when apoptotic cells were preopsonized for 30 min with similar amounts of C1q, washed, and added to the phagocytes (data not shown).
FIGURE 4.
Uptake of early and late apoptotic Jurkats by monocytes, HMDMs, and iDCs. Jurkat cells were labeled with CFSE and treated with 40 μM etoposide for 16 h to induce apoptosis in complete media (early apoptotic) or serum-free Xvivo (late apoptotic). Apoptotic cells were fed to phagocytes at a ratio of 3:1 (Jurkat: monocyte/HMDM) or 1:1 (Jurkat:DC) for 30 min (HMDMs) or 60 min (monocytes and iDCs) at 37°C and the basal percent uptake determined by flow cytometry (A). Data are the mean ± SD. *, p < 0.05, Student's t test (n = 3). B, Uptake of early and late apoptotic cells was assayed in the presence or absence of C1q as indicated. Uptake was expressed as fold enhancement of percent apoptotic cell uptake over control levels (no C1q). Data are the mean ± SD. *, p < 0.05; **, p < 0.005, ANOVA, (n > 3).
C1q contributes to the serum enhancement of apoptotic cell clearance by macrophages, monocytes, and DCs
Because multiple serum components have been implicated in the uptake of apoptotic cells, the relative contribution of C1q to serum-mediated enhancement of apoptotic cells was investigated. As expected, addition of NHS significantly enhanced the uptake of early and late apoptotic Jurkat cells by monocytes, HMDMs, and iDCs (Fig. 5) with ingestion of apoptotic cells confirmed by confocal microscopy (data not shown). This enhancement of ingestion was not seen with the addition of C1q-depleted serum (C1qD) to HMDM or iDC and was only partially seen with the addition of C1qD to monocytes, suggesting that C1q plays a role in the serum-mediated enhancement of apoptotic cell clearance by these phagocytes. Interestingly, although reconstitution of C1qD with serum levels of C1q (C1qD+C1q) was able to fully restore classical pathway activity as measured by hemolytic titer and C3d deposition on the apoptotic cells (data not shown and Fig. 3), addition of C1qD+C1q restored serum-mediated uptake of apoptotic cells by HMDMs completely but only slightly increased ingestion by monocytes or iDCs, implying involvement of a further serum factor in these cell populations, which may be lost during the depletion of C1q. C1qD serum used here is generated by ion-exchange chromatography under conditions in which only highly basic proteins such as C1q (isoelectric point > 9) are removed. Although levels of MBL, as measured by ELISA (34), were found to be unaffected by the process of C1q removal (data not shown), levels of similarly charged alternative pathway components, factor P/properdin and factor D in our C1q-depleted serum, were determined to be much lower than in matched NHS (1 and 7%, respectively) (P. Giclas, unpublished observation). Incubation of late apoptotic cells with 10% C1qD reconstituted with C1q, factor P, and factor D to 2× normal serum levels show similar levels of apoptotic cell uptake by monocytes to those seen with cells incubated in the presence of 10% NHS (to 97 ± 7%) (Fig. 5B), thus suggesting these molecules are candidates for the missing components. As expected, basal levels of apoptotic cell uptake by mDCs were substantially lower than iDCs (Fig. 4A) and not significantly enhanced by serum above basal levels (data not shown).
FIGURE 5.
Serum enhances uptake of early and late apoptotic cells. A, Early or late apoptotic Jurkat cells labeled with CFSE were incubated with phagocytes at a ratio of 3:1 (Jurkat:monocyte/HMDM) or 1:1 (Jurkat:DC) for 30 min (HMDMs) or 60 min (monocytes and iDCs) at 37°C in the absence or presence of 10% NHS, 10% C1q-depleted serum (C1qD) (generated from the same NHS), or 10% [C1qD + 140 μg/ml C1q] (C1qD+C1q). Uptake was assessed by flow cytometry and plotted as the fold uptake over no serum control. Data are the mean ± SD. *, p < 0.05; **, p < 0.005, ANOVA (n = 3, monocytes and iDCs; n = 5, HMDMs). B, Late apoptotic Jurkat cells (prelabeled with CFSE) were incubated in the absence or presence of 10% NHS, 10% C1qD, 10% [C1qD + 140 μg/ml C1q] (C1qD+C1q), 10% [C1qD + 10 μg/ml fP + 4 μg/ml fD] (C1qD +fP +fD), or 10% [C1qD + 140 μg/ml C1q + 10 μg/ml fP + 4 μg/ml fD] (C1qD +C1q +fP +fD) as indicated. Data are expressed as the mean percent uptake relative to that in the presence of 10% matched NHS of two experiments ± SD.
C1q modulates cytokine release during the uptake of apoptotic cells
It has previously been shown that apoptotic cells have immunomodulatory effects on cytokine production and intracellular signaling molecules by phagocytic cells (35-38). However, because many of these studies have investigated a single phagocytic cell type at a time, we chose to isolate monocytes and subsequently generate HMDMs and iDCs from the same donor and assess the influence of apoptotic cells on unstimulated and LPS-triggered cytokine expression in each of these populations. Levels of IL-10, IL-1β, IL-6, MCP-1/CCL2, MIP-1α/CCL3, and TNF-α were measured by Luminex analysis. LPS significantly enhanced the secreted levels of all cytokines tested. In general, apoptotic cells slightly diminished the levels of most cytokines and chemokines secreted by HMDMs and robustly suppressed IL-10 and TNF-α in HMDMs. Although levels of TNF-α secreted by iDCs were modest compared with macrophage levels, this cytokine was also significantly depressed by apoptotic cells. In contrast, where signifi-cant LPS-induced modulations were observed, the presence of apoptotic cells slightly enhanced the production of cytokines and chemokines by monocytes, with the notable exception of proinflammatory cytokine IL-1β, which was significantly suppressed in monocytes by apoptotic cells (Fig. 6A and Table II).
FIGURE 6.
C1q modulation of cytokines released from phagocytes during the uptake of apoptotic cells. A, Cytokine levels were measured by Luminex multiplex analysis of the supernatant of monocytes, HMDMs, or iDCs from the same donor, plated on LabTek chamber slides, and fed early or late apoptotic cells as described in Materials and Methods and stimulated with 0 or 30 ng/ml LPS for 18 h. Data are the average concentrations ± SD from measurements of triplicate wells from a single experiment, representative of three. B, Cytokine levels were measured in the supernatant of monocytes, HMDMs, or iDCs, which had been incubated with early or late apoptotic cells opsonized with 300 μg/ml C1q for 30 min and subsequently stimulated with 30 ng/ml LPS for 18 h. Results are expressed as fold difference in expression compared with control levels from LPS-stimulated phagocytes, which had ingested apoptotic cells in the absence of C1q. Data are plotted as average fold differences ± SD from three experiments. *, p < 0.05; **, p < 0.005, C1q-treated levels vs control levels in the absence of C1q, Student's t test.
Table II.
Summary of cytokine modulation by C1qa
| Summary of Cytokine Modulation by C1q |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| C1q immobilized/bound to plate |
C1q bound to late apoptotic cells |
||||||||
| Modulation of basal levels |
Modulation of LPS-induced levels |
Modulation of apoptotic cells only levels |
|||||||
| Monocytes | HMDMs | iDCs | Monocytes | HMDMs | iDCs | Monocytes | HMDMs | iDCs | |
| IL-1β | ND | ND | ND | ↓ | ↓ | ↓ | – | – | – |
| TNF-α | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | – | ↓ | ↑ |
| IL-8 | ↓ | ↓ | ↓ | ↓ | ↓ | – | ND | ND | ND |
| MIP-1α/CCL3 | ↓ | ↓ | ↓ | ↓ | ↓ | – | – | – | ↓ |
| MCP-1/CCL2 | ↑ | ↓ | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ |
| IL-6 | ↓ | ↑ | ↓ | ↑ | ↑ | ↓ | – | – | – |
| IL-10 | – | – | – | ↑ | ↓ | ↓ | ↑ | ↑ | ↑ |
Levels of cytokines secreted by human monocytes, macrophages (HMDMs), and iDCs were measured by Luminex multiplex analysis. Significant increases (↑,↑) and decreases (↓,↓) in cytokine levels triggered by C1q for the conditions indicated are shown. Differences that did not reach statistical significance are noted (–). Data are representative of at least three individual experiments per condition. Bold (↑,↓, –) indicates a response different from that in monocytes.
Next, we determined the effect of C1q bound to apoptotic cells on the LPS-induced cytokine responses by these phagocytes (Fig. 6B). The influence of apoptotic cells on LPS-induced levels of IL-1β or IL-6 was not significantly altered by the presence of C1q on those dying cells in any of the phagocytic cells tested (Fig. 6B). In contrast, secretion of IL-10 and MCP-1/CCL2 by all phagocytes was significantly enhanced by C1q opsonization of late apoptotic cells but did not reach statistical significance by opsonization of early apoptotic cells. C1q restored levels of IL-10 to 80% (± 4%) or 101% (± 40%) of the levels released by LPS-stimulated HMDMs and iDCs in the absence of apoptotic cells, respectively, whereas levels of MCP-1/ CCL2 released by HMDMs and iDCs were enhanced 1.3- to 1.6-fold by C1q above the LPS-stimulated levels, respectively. Interestingly, levels of TNF-α and MIP-1α/CCL3 were differentially modulated upon ingestion of C1q-opsonized late apoptotic cells, depending on the differentiation state of the phagocyte. Levels of TNF-α released by LPS-stimulated monocytes and iDCs, which had ingested late apoptotic cells, were enhanced 1.3- to 1.4-fold by the presence of C1q but were significantly reduced (by 60%) in HMDMs. In contrast, although the presence of C1q triggered an enhanced release of LPS-induced MIP-1α/CCL3 from monocytes ingesting late apoptotic cells, levels were unaffected in HMDM and significantly reduced by 40% in iDCs (Fig. 6B). Since we have confirmed that C1q binds late apoptotic cells to a greater extent than early apoptotic cells (Fig. 2), it is perhaps not surprising that ingestion of C1q-opsonized late apoptotic cells modulated the cytokines from monocytes, HMDMs, and iDCs to a greater extent than C1q-opsonized early apoptotic cells, although there was no difference in the direction of modulation between early or late apoptotic cells.
Secretion of C1q is regulated by the differentiation state of the phagocyte
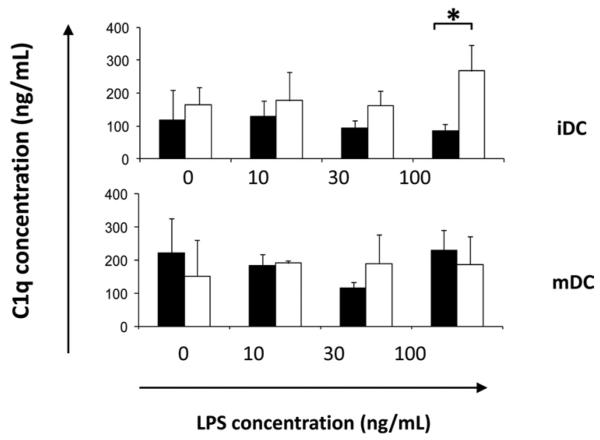
Studies have shown that cells of the monocyte/macrophage lineage can be the major source of C1q biosynthesis (39). To determine the levels of C1q secreted by monocytes, HMDMs, and DCs under our assay conditions, levels of C1q were measured by Ag capture ELISA in the supernatants of cells cultured on LabTek chamber slides coated with control protein (HSA) or C1q with or without stimulation with LPS for 18 h. In this assay, monocytes in HL-1 did not secrete detectable levels of C1q. C1q was detectable in the supernatant of culture-generated HMDMs only after 7–9 days of culture (data not shown), consistent with earlier observations (28). However, iDCs and mDCs did secrete detectable and similar levels of C1q (100–250 ng/ml) (Fig. 7). C1q expression was not significantly altered by the addition of LPS; however, C1q itself triggered enhanced levels of C1q released by iDCs at the highest level of LPS tested (100 ng/ml). Levels of C1q were undetectable in identical C1q-coated control wells that did not contain phagocytic cells (data not shown), suggesting that the detected C1q was not due to contaminating coating protein. These data are consistent with a previous report that iDCs secrete functionally active C1q at similar levels (112 ± 21 ng/ml) (40).
FIGURE 7.
Cultured DCs secrete C1q. Secreted levels of C1q were measured by ELISA analysis of the supernatant of iDCs and mDCs plated on LabTek chamber slides coated with 8 μg/ml C1q (□) or HSA (■) control and stimulated with 0–100 ng/ml LPS for 18 h. Results are average concentration ± SD of three separate experiments. *, p < 0.05, ANOVA.
Discussion
The orderly uptake of apoptotic cells is important in a wide variety of physiologic processes to prevent the release of immunogenic and/or toxic components into the surrounding tissue, potentially evoking a proinflammatory response and leading to autoimmunity (8, 41). The physiologic clearance of apoptotic cells involves processes designed to prevent inflammation and autoimmunity while also limiting tissue toxicity. Indeed, this process has been postulated to induce active microenvironment suppression of inflammation (35). Although various groups have studied the interactions of C1q with apoptotic cells and the role C1q may be playing in the ingestion of these dying cells by phagocytes, they have used a variety of experimental designs and phagocytic cells (10, 11, 13, 15, 16, 18, 20, 32, 42-45). To our knowledge, this is the first study to perform a comparative analysis of how C1q modulates the response of multiple differentiation states of monocyte-derived phagocytes by using donor-matched monocytes, monocyte-derived macrophages, and DCs (Figs. 1 and 6). Our data demonstrate first that while C1q aids in the uptake of apoptotic cells, the contribution to the clearance of apoptotic cells differs with the phagocyte phenotype. Second, the influence of C1q on the program of cytokine production that is induced upon engagement of apoptotic cells varies with the differentiation state of the phagocytic cell but generally appears to generate mediators that could modulate either the Th class of immune response toward an enhanced Th2-biased immune profile or promote an “anti-inflammatory” state.
Schwaeble et al. (46) were able to detect C1q in the interdigitating cells of the spleen (mDCs in vivo), which is consistent with our findings (Fig. 7) and that of Cao et al. (47) that mDCs secrete detectable C1q. In this study, we further document the synthesis of C1q in differentiated HMDMs similar to reports by other groups (48). In the CNS, in addition to synthesis by microglia, it has been shown that C1q is synthesized by neurons in response to injury (reviewed in Ref. 49). Thus, local synthesis of C1q in the tissue microenvironment can be the source of C1q to promote enhanced ingestion of apoptotic cells, without the need for or before recruitment of plasma-derived complement.
Similar to other studies, our data demonstrate that C1q binds directly and stably to late apoptotic cells, much less to early apoptotic cells, but not to live cells (15, 16, 32). Although significant and substantial C1q-mediated enhancement of uptake by monocytes was seen, no such enhancement of uptake was seen by HMDMs or human immature DCs when purified C1q was bound to either early or late apoptotic cells, perhaps reflecting differences in the location and function of phagocytic monocytes. Monocytes undergo rapid mobilization into tissues upon injury, and enhanced clearance of apoptotic cells and cellular debris stimulated by C1q, which may be synthesized in these regions in the absence of other complement components, may play an important role in wound healing and tissue homeostasis. Indeed, in a recent study (50), reservoirs of splenic monocytes were shown to accumulate at sites of injury and participate in tissue repair and wound healing after myocardial infarction in mice.
The lack of influence of C1q alone on iDC ingestion differs from a previous report in which C1q did enhance uptake (20). The discrepancy between our results and previous reports of in vitro experiments could be due to the presence of C1q during the induction of apoptosis and/or to the difference in how the phagocyte populations were generated and thus to the differentiation and activation state of the phagocytic cell (20). We routinely use elutriation as a means to isolate monocytes, because this method does not activate monocytes and quickly removes contaminating platelets, whereas other studies have made use of monocyte isolation from PBMCs by their adherence to tissue culture plates. The adherence method for isolating monocytes from PBMCs results in a less pure population, with the presence of some lymphocytes as well as contaminating platelets, which may provide a source of other activating components and/or promote phagocytosis (51). Our studies with elutriated monocytes eliminate or minimize those confounding factors.
Because clearance of apoptotic cells and cellular debris is such a critical continuous process, it is not surprising that in in vivo settings, there would be multiple factors present that may directly or synergistically modulate uptake of apoptotic cells. The presence of serum during the course of apoptotic cell ingestion in our experiments resulted in C3 deposition on the apoptotic cells via activation of the classical complement pathway. Apoptotic cell up-take was enhanced compared with the absence of serum for all phagocytic cells tested, albeit to differing degrees, with the exception of mDCs. This is not surprising since the primary role of mDC is Ag presentation of already ingested material rather than continued phagocytosis. Because the addition of C1q reconstituted C3 deposition by C1qD (Fig. 3B), the fact that the level of uptake by monocytes and iDCs cannot be completely restored by the addition of C1q alone to C1qD (Fig. 5A) indicates that other serum factors have a substantial influence on these cell types. Indeed, monocyte incubation with C1qD reconstituted with C1q, factor P, and factor D shows similar levels of apoptotic cell uptake to those seen with cells incubated in the presence of 10% NHS (Fig. 5B), supporting a contribution of alternative complement pathway components and/or a direct effect of properdin/factor P on apoptotic cell uptake by these cells, as has been reported previously (51, 52). Opsonization with iC3b has previously been shown to enhance uptake of apoptotic cells by human but not murine iDCs (53, 54). However, the ratio of C3b to iC3b on the target may also be important in mediating uptake by monocytes and iDCs, because complement receptor 1 (CR1/CD35), the receptor for C3b on these cells, requires activation before monocytes and iDCs are able to phagocytose C3b-opsonized targets (Ref. 55; D. A. Fraser, unpublished observations). Although others have suggested a role for MBL in the clearance of apoptotic cells (56), MBL levels in our C1qD remained intact (data not shown), suggesting that the lectin pathway is not involved in the clearance of apoptotic Jurkat cells by HMDM and iDC.
There is an increasing awareness of the role of the differentiation state of the phagocyte and the sum of the signals present in the microenvironment in shaping immunological outcomes (reviewed in Ref. 2). With the exception of the Th2-associated anti-inflammatory cytokine IL-10, which was induced by immobilized C1q from human monocytes, as has previously been shown (6), all other cytokines tested were similarly regulated by immobilized C1q in monocytes and HMDMs, including a reduction in LPS-induced levels of proinflammatory (and Th1-associated) cytokines TNF-α, IL-1β, and MIP-1α, and enhancement of LPS-induced levels of the chemokine MCP-1/CCL2 (Fig. 1 and Table II). MCP-1/CCL2 is a potent chemokine, capable of attracting monocytes, T lymphocytes, and DCs (57, 58), and enhancing phagocytosis and clearance of cellular debris in vivo (59), although it alone does not appear to alter cytokine production in these cells (60), allowing the microenvironment (including target associated triggers) to further dictate responses. In contrast, the presence of immobilized C1q suppressed the LPS-induced iDC production of all the cytokines tested, with the exception of IL-8/CXCL8 and MIP-1α/CCL3 (Fig. 1 and Table II). This may reflect important functional distinctions between the differentiated phagocytes. Because DCs play a primary role in Ag presentation, upon danger signal stimulation, iDCs become activated and acquire the mature phenotype en route to secondary lymphoid tissue. Although it is known that activated DCs produce increased levels of many cytokines, C1q, in the absence of other signals on ingested targets, may dampen the LPS-induced cytokine production in DCs to prevent the activation and chemotactic response of immature nonactivated macrophages residing in the periphery. This is consistent with the concept that chemokines are involved in mobilization of iDCs to become activated and migrate to secondary lymphoid tissues, as described by Mantovani and colleagues (61). Finally, none of the cytokines assayed was produced by mDCs with our experimental design, which is concordant with the literature. IL-10 produced by activated DCs can also induce DC cytokine “exhaustion” (62) and may be important in switching DCs from a Th1- to a Th2-inducing mode (63).
A scenario in which a regulated immune response is particularly warranted is in the clearance of apoptotic cells, as an immune response to self-Ags presented by phagocytosing professional APCs would lead to the dire consequence of autoimmunity. Thus, multiple pathways are induced to provide checkpoints and brakes on a potentially detrimental activation of immune responses. First, either due to an absence of other downstream complement components (e.g., C1q synthesis is known to be induced rapidly in response to injury in the CNS in the absence of other complement components) or to endogenous regulators of the complement cascade that limit activation in the absence of a danger signal, there is limited to no activation of the terminal complement cascade, thus preventing the lytic membrane attack complex formation and premature release of potentially inflammatory intracellular contents (14). Second, C1q opsonization of apoptotic cells alters the direction and extent of cytokine secretion after ingestion of these dying cells by phagocytes. In all phagocytes tested, C1q triggers cells to secrete chemokines (MCP-1/CCL2) resulting in the recruitment of more phagocytic cells for cleanup of debris, and in the absence of additional “danger” signals, C1q induces cytokines such as IL-10 that can suppress Th1-type responses. C1q also significantly suppressed the high levels of LPS-induced Th1-type cytokine TNF-α produced by HMDMs, which were over 10-fold higher in HMDMs than monocytes or iDCs (Fig. 6A). These high levels of LPS-induced TNF-α in HMDMs, while decreased by 80% by late apoptotic cells alone, were significantly further inhibited by C1q (to 5% of LPS-induced levels). These results are consistent with a role for C1q in the prevention of autoimmunity, maintaining an immunosuppressive microenvironment and/or promoting a Th2 environment during the clearance of apoptotic cells.
It has been previously demonstrated that C1q contributes inhibitory and/or competing signals to human monocytes by the generation of inhibitory NFκB complexes (p50p50) and enhancing CREB phosphorylation, which modulate cytokine production at the mRNA and protein levels (38). Further studies are required to dissect the molecular mechanisms of the action of C1q on directing cellular responses in HMDMs and iDCs to identify common and divergent signaling pathways in the different cell types. Indeed, recent studies have shown that activation of a common intracellular signaling molecule (cAMP) can differentially regulate inflammatory mediator production in response to LPS in distinct phagocytic cell types via alternative intracellular signaling molecules (64). Thus, pleiotropic intracellular signaling molecules generated in response to extracellular signals can modulate a number of diverse cellular functions according to the differentiation state of the cell and the nature of the extracellular signals it receives.
In summary, although it is important to stress that the presence of C1q alone does not necessarily avoid autoimmunity (65), these data demonstrate that C1q alone is sufficient to mediate enhanced apoptotic cell clearance by monocytes. We further show that C1q modulates phagocyte cytokine responses toward a more Th2-biased state and dampened Th1 response, and thus, this may be at least partially responsible for the autoimmune phenotypes of both human and mice genetically deficient in C1q. These responses appear to be fine-tuned according to the differentiation state of the phagocyte and other target-expressed signals received by the phagocyte. These findings clarify and extend the evidence for the importance of differentiation-specific myeloid cell responses in determining the nature and potency of the subsequent immune responses (64, 66) and distinctively provide further support for the role of C1q in immune homeostasis. Additional identification of the molecular basis/pathways for these cellular and environmental differences should provide novel therapeutic targets for host defense, vaccine design, and suppression of, or resolution of, inflammation and autoimmunity.
Acknowledgments
We thank Ozkan Yazan for technical assistance, as well as contributions from Hazel Rosete, Elysia Chin, and Lindsey Weiner. We are very grateful to Dr. Patsy Giclas, National Jewish Health (Denver, CO), for serum analysis of Factor P and Factor D and to the staff of the UCI Institute for Clinical and Translational Science for obtaining human blood for monocyte purification.
Footnotes
This work was supported by National Institutes of Health Grant AI41090 (to A.J.T.). The project described was also supported by Grant Number T32CA009054 from the National Cancer Institute (to A.K.L. and E.L.N.) and the Chao Family Comprehensive Cancer Center at University of California Irvine (to E.L.N.). Support for obtaining human blood products used in this study was provided in part by Public Health Service Research Grant M01RR00827.
Abbreviations used in this paper: ACAMP, apoptotic cell-associated molecular pattern; DC, dendritic cell; HMDM, human monocyte-derived macrophage; HSA, human serum albumin; iDC, immature DC; mDC, mature DC; MFI, mean fluorescent intensity; MHC II, MHC class II; NHS, normal human serum; PI, propidium iodide.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Hart SP, Dransfield I, Rossi AG. Phagocytosis of apoptotic cells. Methods. 2008;44:280–285. doi: 10.1016/j.ymeth.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Erwig LP, Henson PM. Immunological consequences of apoptotic cell phagocytosis. Am. J. Pathol. 2007;171:2–8. doi: 10.2353/ajpath.2007.070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walport MJ. Complement: second of two parts. N. Engl. J. Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 4.Walport MJ. Complement: first of two parts. N. Engl. J. Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 5.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol. Immunol. 2007;44:33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Fraser DA, Bohlson SS, Jasinskiene N, Rawal N, Palmarini G, Ruiz S, Rochford R, Tenner AJ. C1q and MBL, components of the innate immune system, influence monocyte cytokine expression. J. Leukocyte Biol. 2006;80:107–116. doi: 10.1189/jlb.1105683. [DOI] [PubMed] [Google Scholar]
- 7.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat. Immunol. 2004;5:971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 8.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 9.Botto M, Dell'agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 10.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J. Exp. Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 2001;194:781–796. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lillis AP, Greenlee MC, Mikhailenko I, Pizzo SV, Tenner AJ, Strickland DK, Bohlson SS. Murine low-density lipoprotein receptor-related protein 1 (LRP) is required for phagocytosis of targets bearing LRP ligands but is not required for C1q-triggered enhancement of phagocytosis. J. Immunol. 2008;181:364–373. doi: 10.4049/jimmunol.181.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J. Immunol. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 14.Trouw LA, Blom AM, Gasque P. Role of complement and complement regulators in the removal of apoptotic cells. Mol. Immunol. 2008;45:1199–1207. doi: 10.1016/j.molimm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J. Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- 16.Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J. Immunol. 2001;166:3231–3239. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- 17.Gregory CD. CD14-dependent clearance of apoptotic cells: relevance to the immune system. Curr. Opin. Immunol. 2000;12:27–34. doi: 10.1016/s0952-7915(99)00047-3. [DOI] [PubMed] [Google Scholar]
- 18.Paidassi H, Tacnet-Delorme P, Garlatti V, Darnault C, Ghebrehiwet B, Gaboriaud C, Arlaud GJ, Frachet P. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J. Immunol. 2008;180:2329–2338. doi: 10.4049/jimmunol.180.4.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paidassi H, Tacnet-Delorme P, Lunardi T, Arlaud GJ, Thielens NM, Frachet P. The lectin-like activity of human C1q and its implication in DNA and apoptotic cell recognition. FEBS Lett. 2008;582:3111–3116. doi: 10.1016/j.febslet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Nauta AJ, Castellano G, Xu W, Woltman AM, Borrias MC, Daha MR, van Kooten C, Roos A. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J. Immunol. 2004;173:3044–3050. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- 21.Yamada M, Oritani K, Kaisho T, Ishikawa J, Yoshida H, Takahashi I, Kawamoto S, Ishida N, Ujiie H, Masaie H, et al. Complement C1q regulates LPS-induced cytokine production in bone marrow-derived dendritic cells. Eur. J. Immunol. 2004;34:221–230. doi: 10.1002/eji.200324026. [DOI] [PubMed] [Google Scholar]
- 22.Tenner AJ, Lesavre PH, Cooper NR. Purification and radiolabeling of human C1q. J. Immunol. 1981;127:648–653. [PubMed] [Google Scholar]
- 23.Young KR, Jr., Ambrus JL, Jr., Malbran A, Fauci AS, Tenner AJ. Complement subcomponent C1q stimulates Ig production by human B lymphocytes. J. Immunol. 1991;146:3356–3364. [PubMed] [Google Scholar]
- 24.Reid KBM, Lowe DM, Porter RR. Isolation and Characterization of C1q, a Subcomponent of the first component of complement, from human and rabbit sera. Biochem. J. 1972;130:749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lionetti FJ, Hunt SM, Valera CR. Methods of Cell Separation. Plenum Publishing; New York: 1980. [Google Scholar]
- 26.Bobak DA, Frank MM, Tenner AJ. Characterization of C1q receptor expression on human phagocytic cells: effects of PDBu and FMLP. J. Immunol. 1986;136:4604–4610. [PubMed] [Google Scholar]
- 27.Nelson EL, Strobl S, Subleski J, Prieto D, Kopp WC, Nelson PJ. Cycling of human dendritic cell effector phenotypes in response to TNF-α: modification of the current “maturation” paradigm and implications for in vivo immunoregulation. FASEB J. 1999;13:2021–2030. doi: 10.1096/fasebj.13.14.2021. [DOI] [PubMed] [Google Scholar]
- 28.Tenner AJ, Volkin DB. Complement subcomponent C1q secreted by cultured human monocytes has subunit structure identical with that of serum C1q. Biochem J. 1986;233:451–458. doi: 10.1042/bj2330451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilchherr E, Schumaker VN, Curtiss LK. Activation of C1 by monoclonal antibodies directed against C1q. Biochem. Biophys. Res. Commun. 1985;126:785–791. doi: 10.1016/0006-291x(85)90253-0. [DOI] [PubMed] [Google Scholar]
- 30.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of Th2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 31.Viallard JF, Pellegrin JL, Ranchin V, Schaeverbeke T, Dehais J, Longy-Boursier M, Ragnaud JM, Leng B, Moreau JF. Th1 (IL-2, interferon γ (IFN-γ)) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE) Clin. Exp. Immunol. 1999;115:189–195. doi: 10.1046/j.1365-2249.1999.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nauta AJ, Trouw LA, Daha MR, Tijsma O, Nieuwland R, Schwaeble WJ, Gingras AR, Mantovani A, Hack EC, Roos A. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur. J. Immunol. 2002;32:1726–1736. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 33.Ziccardi RJ, Cooper NR. Direct demonstration and quantitation of the first complement component in human serum. Science. 1978;199:1080–1082. doi: 10.1126/science.75568. [DOI] [PubMed] [Google Scholar]
- 34.Arora M, Munoz E, Tenner AJ. Identification of a site on mannan-binding lectin critical for enhancement of phagocytosis. J. Biol. Chem. 2001;276:43087–43094. doi: 10.1074/jbc.M105455200. [DOI] [PubMed] [Google Scholar]
- 35.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 37.Williams CA, Harry RA, McLeod JD. Apoptotic cells induce dendritic cell-mediated suppression via interferon γ-induced IDO. Immunology. 2008;124:89–101. doi: 10.1111/j.1365-2567.2007.02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fraser DA, Arora M, Bohlson SS, Lozano E, Tenner AJ. Generation of inhibitory NF-κB complexes and phosphorylated cAMP response element-binding protein correlates with the activity of complement protein C1q in human monocytes. J. Biol. Chem. 2007;282:7360–7367. doi: 10.1074/jbc.M605741200. [DOI] [PubMed] [Google Scholar]
- 39.Petry F, Botto M, Holtappels R, Walport MJ, Loos M. Reconstitution of the complement function in C1q-deficient (C1qa−/−) mice with wild-type bone marrow cells. J. Immunol. 2001;167:4033–4037. doi: 10.4049/jimmunol.167.7.4033. [DOI] [PubMed] [Google Scholar]
- 40.Vegh Z, Goyarts EC, Rozengarten K, Mazumder A, Ghebrehiwet B. Maturation-dependent expression of C1q binding proteins on the cell surface of human monocyte-derived dendritic cells. Int. Immunopharmacol. 2003;3:39–51. doi: 10.1016/s1567-5769(02)00211-4. [DOI] [PubMed] [Google Scholar]
- 41.Lleo A, Selmi C, Invernizzi P, Podda M, Gershwin ME. The consequences of apoptosis in autoimmunity. J. Autoimmun. 2008;31:257–262. doi: 10.1016/j.jaut.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortes-Hernandez J, Fossati-Jimack L, Carugati A, Potter PK, Walport MJ, Cook HT, Botto M. Murine glomerular mesangial cell uptake of apoptotic cells is inefficient and involves serum-mediated but complement-independent mechanisms. Clin. Exp. Immunol. 2002;130:459–466. doi: 10.1046/j.1365-2249.2002.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bottcher A, Gaipl US, Furnrohr BG, Herrmann M, Girkontaite I, Kalden JR, Voll RE. Involvement of phosphatidylserine, αvβ3, CD14, CD36, and complement C1q in the phagocytosis of primary necrotic lymphocytes by macrophages. Arthritis Rheum. 2006;54:927–938. doi: 10.1002/art.21660. [DOI] [PubMed] [Google Scholar]
- 44.Donnelly S, Roake W, Brown S, Young P, Naik H, Wordsworth P, Isenberg DA, Reid KB, Eggleton P. Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum. 2006;54:1543–1556. doi: 10.1002/art.21783. [DOI] [PubMed] [Google Scholar]
- 45.Castellano G, Woltman AM, Schlagwein N, Xu W, Schena FP, Daha MR, van Kooten C. Immune modulation of human dendritic cells by complement. Eur. J. Immunol. 2007;37:2803–2811. doi: 10.1002/eji.200636845. [DOI] [PubMed] [Google Scholar]
- 46.Schwaeble W, Schafer MK, Petry F, Fink T, Knebel D, Weihe E, Loos M. Follicular dendritic cells, interdigitating cells, and cells of the monocyte-macrophage lineage are the C1q-producing sources in the spleen: identification of specific cell types by in situ hybridization and immunohistochemical analysis. J. Immunol. 1995;155:4971–4978. [PubMed] [Google Scholar]
- 47.Cao W, Bobryshev YV, Lord RS, Oakley RE, Lee SH, Lu J. Dendritic cells in the arterial wall express C1q: potential significance in athero-genesis. Cardiovasc. Res. 2003;60:175–186. doi: 10.1016/s0008-6363(03)00345-6. [DOI] [PubMed] [Google Scholar]
- 48.Castellano G, Woltman AM, Nauta AJ, Roos A, Trouw LA, Seelen MA, Schena FP, Daha MR, van Kooten C. Maturation of dendritic cells abrogates C1q production in vivo and in vitro. Blood. 2004;103:3813–3820. doi: 10.1182/blood-2003-09-3046. [DOI] [PubMed] [Google Scholar]
- 49.Tenner AJ, Pisalyaput K. The complement system in the CNS: thinking again. In: Lane TE, Carson MJ, Bergmann C, Wyss-Coray T, editors. Central Nervous System Diseases and Inflammation. Springer; New York: 2008. pp. 153–174. [Google Scholar]
- 50.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kemper C, Mitchell LM, Zhang L, Hourcade DE. The complement protein properdin binds apoptotic T cells and promotes complement activation and phagocytosis. Proc. Natl. Acad. Sci. USA. 2008;105:9023–9028. doi: 10.1073/pnas.0801015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu W, Berger SP, Trouw LA, de Boer HC, Schlagwein N, Mutsaers C, Daha MR, van Kooten C. Properdin binds to late apoptotic and necrotic cells independently of C3b and regulates alternative pathway complement activation. J. Immunol. 2008;180:7613–7621. doi: 10.4049/jimmunol.180.11.7613. [DOI] [PubMed] [Google Scholar]
- 53.Verbovetski I, Bychkov H, Trahtemberg U, Shapira I, Hareuveni M, Ben Tal O, Kutikov I, Gill O, Mevorach D. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J. Exp. Med. 2002;196:1553–1561. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Behrens EM, Ning Y, Muvarak N, Zoltick PW, Flake AW, Gallucci S. Apoptotic cell-mediated immunoregulation of dendritic cells does not require iC3b opsonization. J. Immunol. 2008;181:3018–3026. doi: 10.4049/jimmunol.181.5.3018. [DOI] [PubMed] [Google Scholar]
- 55.Bobak DA, Frank MM, Tenner AJ. C1q acts synergistically with phorbol dibutyrate to activate CR1-mediated phagocytosis by human mononu-clear phagocytes. Eur. J. Immunol. 1988;18:2001–2007. doi: 10.1002/eji.1830181220. [DOI] [PubMed] [Google Scholar]
- 56.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Man-nose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J. Immunol. 2005;174:3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 57.Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J. Leukocyte Biol. 1996;60:365–371. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
- 58.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T lymphocyte chemoattractant. Proc. Natl. Acad. Sci. USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amano H, Morimoto K, Senba M, Wang H, Ishida Y, Kumatori A, Yoshimine H, Oishi K, Mukaida N, Nagatake T. Essential contribution of monocyte chemoattractant protein-1/C-C chemokine ligand-2 to resolution and repair processes in acute bacterial pneumonia. J. Immunol. 2004;172:398–409. doi: 10.4049/jimmunol.172.1.398. [DOI] [PubMed] [Google Scholar]
- 60.Gunn MD, Nelken NA, Liao X, Williams LT. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J. Immunol. 1997;158:376–383. [PubMed] [Google Scholar]
- 61.Sozzani S, Allavena P, D'Amico G, Luini W, Bianchi G, Kataura M, Imai T, Yoshie O, Bonecchi R, Mantovani A. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J. Immunol. 1998;161:1083–1086. [PubMed] [Google Scholar]
- 62.Kajino K, Nakamura I, Bamba H, Sawai T, Ogasawara K. Involvement of IL-10 in exhaustion of myeloid dendritic cells and rescue by CD40 stimulation. Immunology. 2007;120:28–37. doi: 10.1111/j.1365-2567.2006.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of Th1, Th2 and nonpolarized T cells. Nat. Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 64.Aronoff DM, Carstens JK, Chen GH, Toews GB, Peters-Golden M. Short communication: differences between macrophages and dendritic cells in the cyclic AMP-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J. Interferon Cytokine Res. 2006;26:827–833. doi: 10.1089/jir.2006.26.827. [DOI] [PubMed] [Google Scholar]
- 65.Bygrave AE, Rose KL, Cortes-Hernandez J, Warren J, Rigby RJ, Cook HT, Walport MJ, Vyse TJ, Botto M. Spontaneous auto-immunity in 129 and C57BL/6 mice—implications for autoimmunity described in gene-targeted mice. PLoS. Biol. 2004;2:E243. doi: 10.1371/journal.pbio.0020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu W, Roos A, Daha MR, van Kooten C. Dendritic cell and macrophage subsets in the handling of dying cells. Immunobiology. 2006;211:567–575. doi: 10.1016/j.imbio.2006.05.023. [DOI] [PubMed] [Google Scholar]