Abstract
Background: Platelet indices are potentially useful markers for the early diagnosis of thromboembolic diseases. An increase in both mean platelet volume (MPV) and platelet distribution width (PDW) due to platelet activation, resulting from platelet swelling and pseudopodia formation was hypothesized.
Methods: Platelet indices (MPV and PDW) in three groups of persons, using impedance and optical technology were measured. The first group consisted of patients with established platelet activation and healthy control subjects. The second study group included pregnant women in different trimesters of pregnancy. The effect of storage time on MPV and PDW in blood samples of a third group of randomly chosen patients was also assessed.
Results: There was a significant increase in MPV (P<0.001) and PDW (P<0.001) in patients with confirmed platelet activation compared to healthy control subjects. Only PDW showed a significant increase from the first to the third trimester of pregnancy (P=0.009). Temporal changes of MPV and PDW over storage time revealed a significant increase in MPV (P<0.001), in contrast to a significant decrease in PDW (P<0.05).
Conclusion: MPV and PDW are simple platelet indices, which increase during platelet activation. PDW is a more specific marker of platelet activation, since it does not increase during simple platelet swelling.
Keywords: platelet distribution width, platelet activation
Thromboembolic diseases are among the major cause of mortality in developed countries. Early diagnosis of progressive activation of coagulation can help manage these diseases successfully. A significant list of reliable markers have been investigated recently, concerning activation of coagulation, such as prothrombin fragment 1+2, thrombin-antithrombin complex (TAT), and platelet activation, such as β-thromboglobulin (β-TG) or soluble platelet P-selectin1. However, laboratory measurement of these indices is laborious and expensive. Additionally, the above mentioned indices cannot be included in routine laboratory tests2–3.
Several investigators have used a series of platelet indices measured by hematology analyzers, given the fact that platelet activation causes morphologic changes of platelets. The mean platelet volume (MPV) is probably the most extensively studied platelet activation marker4–9. Recently, novel platelet indices such as mean platelet component (MPC) and platelet component distribution width (PCDW) have been investigated as prospective platelet activation markers10–11. However, not all hematology analyzers examine these indices.
The present effort for finding simple and widely used platelet activation indices focused on the fact that platelet activation causes morphologic changes of platelets, including both the spherical shape and pseudopodia formation. Platelets with increased number and size of pseudopodia differ in size, possibly affecting platelet distribution width (PDW).
The possibility whether platelet activation increases MPV and PDW as expected was examined. Moreover, the issue whether pseudopodia formation could cause specific changes, supporting the differential diagnosis between platelet activation and other causes of platelet swelling was assessed.
Materials and Methods
Analyses were performed in three study groups:
Ninty eight (98) healthy persons and 75 patients with established platelet activation (45 puerperas, 10 patients with phlebothrombosis before therapy with anticoagulants and 20 patients with myocardial infarction). Samples were analyzed by two hematology analyzers (CELL DYN 1700 and CELL DYN 3200, Abbott Diagnostics) two hours after blood sampling,
One hundred and three (103) pregnant women (41 in the first trimester of pregnancy, 32 in the second trimester and 30 in the third trimester). Samples were analyzed by the hematology analyzer GENS (Coulter) two hours after blood sampling,
Thirty five (35) randomly chosen patients. Blood samples were analyzed by the hematology analyzer GENS (Coulter) one, two, three and four hours after blood sampling.
All blood samples were collected in tubes with potassium ethylenediaminetetraacetate (EDTA) as an anticoagulant. The following hematological parameters were studied in all blood samples: platelet count (PLT), mean platelet volume (MPV) and platelet distribution width (PDW). Impedance resistance was used for the measurement of platelet indices in all blood samples (analyzers CELL DYNN 1700 and GENS). Blood samples in group (a) were further analyzed by optical method (CELL DYNN 3200).
Data were analysed using SPSS 12 software for Windows. Normality of continuous data was determined by Kolmogorov-Smirnov or Shapiro-Wilk normality test. All continuous data following normal distribution are presented as mean (SD, standard deviation). Independent- samples were examined with students' t-test, One- Way ANOVA, and paired-samples t-test were used for comparisons in group (a), (b) and (c), respectively. A two tailed P value <0.05 was considered statistically significant for all comparisons.
Results
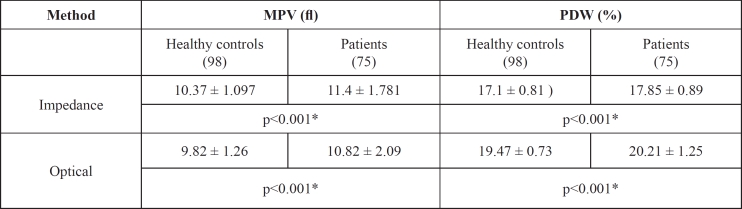
Mean values of MPV and PDW were significantly higher in patients with asserted platelet activation compared to healthy persons in group (a), measured both by impedance and optical technology (Table 1). Platelet counts did not show any changes. Comparison of platelet indices between healthy controls and patients in study group (a) showed a significant increase of MPV and PDW in patients compared to healthy controls measured by impedance and optical method (p<0.001).
Table 1. Platelet indices of the first study subgroups [group (a)].
MPV: Mean platelet volume (fl); PDW: Platelet distribution width (%)
Results expressed as mean ± SD (Standard Deviation)
* P<0.05
P-values estimated by independent-samples t test
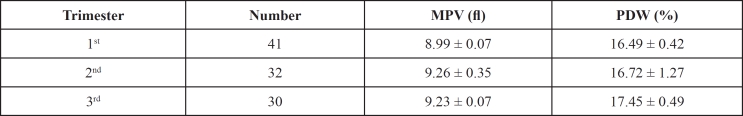
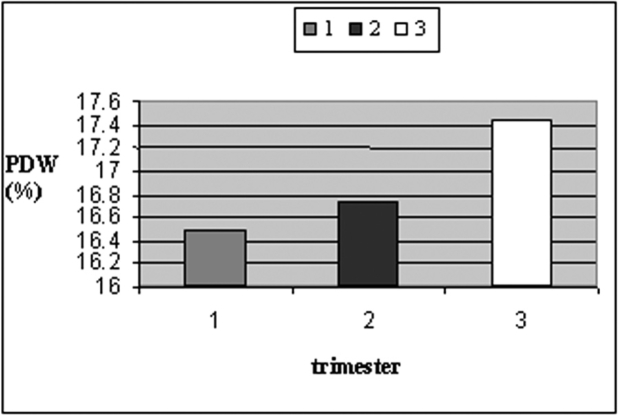
Mean values of PDW and MPV were higher in pregnant women in late pregnancy compared to those in early pregnancy [study group (b)] (Table 2, Figures 1 and 2). Only PDW values were significantly higher in women during the third trimester of pregnancy compared to those of the first trimester (One-Way ANOVA, P=0.009).
Table 2. Platelet indices among women during different trimesters of pregnancy of group (b).
MPV: Mean platelet volume (fl); PDW: Platelet distribution width (%)
Results expressed as mean ± SD (Standard Deviation)
Figure 1. Mean values of MPV during different trimesters of pregnancy.
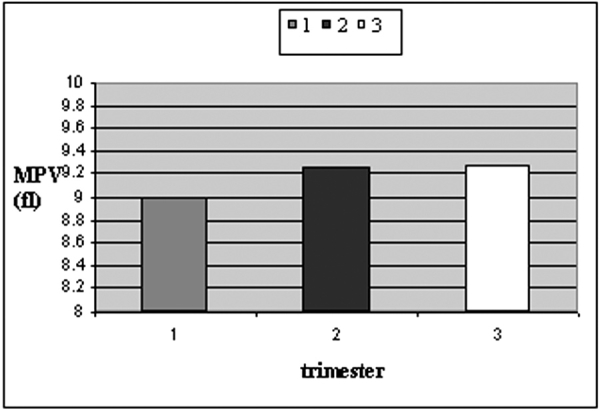
Figure 2. Mean values of PDW during different trimesters of pregnancy.
Regarding the indices among women during different trimesters of pregnancy, power analysis performed showed that for three levels of comparison, with an estimated mean for the MPV of 9 and a standard deviation of 0.16, with 30 participants per group, there is an 99,98% possibility to detect a difference of 0.25 among the three groups, if that difference exists. As regards PDW, for an estimated mean of 16.5 and a standard deviation of 0.73, with 30 participants per group, there is an 80.00% possibility to detect a difference of 0.55 among the three groups, if that difference exists.
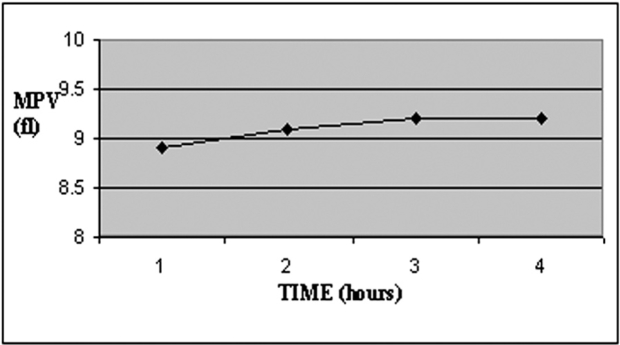
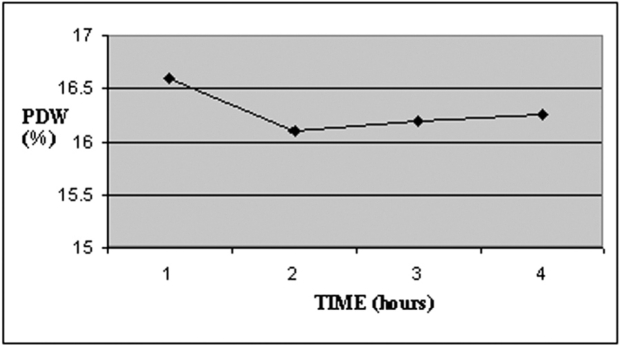
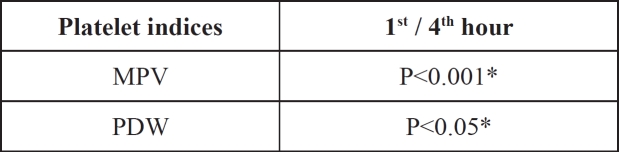
MPV increased during storage time in study group (c), while PDW decreased over time (Table 3, Figures 3 and 4). There was a significant increase of MPV in the fourth measurement compared to the first (P<0.001). On the contrary PDW showed a significant decrease (P<0.05), (Table 4).
Table 3. Mean values of MPV and PDW during sequential hourly measurements.
MPV: Mean platelet volume (fl); PDW: Platelet distribution width (%)
Results expressed as mean ± SD (Standard Deviation)
Figure 3. Temporal changes of MPV during blood storage.
Figure 4. Temporal changes of PDW during blood storage.
Table 4. Comparison of MPV and PDW values measured one and four hours after blood sampling.
MPV: Mean platelet volume (fl); PDW: Platelet distribution width (%)
* P<0.05
P-values estimated by paired t-test
Regarding the influence of time, power analysis performed showed that for four levels of comparison, during sequential hourly measurements with an estimated mean for the MPV of 9 and a standard deviation of 0.99, with 35 participants per group, there is an 83.7% possibility to detect a difference of 0.85 among the four measurements, if that difference exists. And as regards PDW, for a mean of 16 and a standard deviation of 0.44, with 35 participants per group, there is an 88.34% possibility to detect a difference of 0.4 among the four measurements, if that difference exists.
Discussion
MPV and PDW are easily measured platelet indices, which increase during platelet activation. In order to obtain a larger surface platelets change in shape during activation. Their shape changes from discoid to spherical. Pseudopodia are formed as well12. Hematology analyzers based on impedance technology measure platelet volume by the deformation of electrical field, which depends on the platelet vertical diameter. Analyzers with laser optical technology determine cell (platelet) volume according to the cross diameter of the cell (platelet)13. Therefore activated platelets seem larger independently of the principle of measurement.
Our results agree with the aforementioned data as well as with the international bibliography4–9,13–16. MPV was higher in subgroups of (a) study group, where activation of coagulation pathway was certain. However, there was no significant increase of MPV in women of late pregnancy compared to those of early pregnancy. Hourly measurements of MPV in random blood samples showed an increase of MPV over time, in accordance with other publications11,17. The temporal increase of MPV during storage of blood samples is usually attributed to platelet activation as it is combined with a decrease in platelet component concentration (MPC). However, platelet degranulation as well as simple platelet swelling can be the cause of MPC decrease11.
We also observed that PDW was higher in subgroups of (a) and (b) study groups with certain platelet activation. We ascribed the increase of PDW to platelet anisocytosis, which results from pseudopodia formation. In recent publications, Ihara et al as well as Khakendar et al made the same observation in patients with ischemic heart disease18,19.
Amin et al found that PDW increased in vaso-occlusive crisis in sickle cell disease. They concluded that megakaryote hyperplasia was responsible for PDW increase20. However, coagulation is activated in vaso-occlusive crisis, possibly causing this increase of PDW.
On the contrary, Beyan and coworkers concluded that platelet indices should not be used alone as direct indicators of platelet activation21. Their observation was derived from the lack of correlation of MPV and PDW with optical platelet aggregation responses in healthy volunteers. According to their results, large platelets did not show more intense activation in aggregometer. Indeed, large and variable in size platelets can be observed in several clinical conditions, such as hemorrhage and myeloproliferative disorders, without simultaneous activation of coagulation. Moreover, platelets shape and volume are variable, even in healthy persons. Thus, serial measurements of MPV and PDW would be useful, but impractical for the recognition of progressive platelet activation. Instead, simultaneous increase of MPV and PDW could reveal platelet activation, as depicted from our observations.
Finally, it is remarkable that in the present study a decrease in PDW over storage time in study group (c) was found. This observation can lead to the conclusion that there was no pseudopodia formation during storage of blood samples. Consequently, there was no platelet activation, except from single platelet distention and swelling. This is also supported by the fact that platelet count was not reduced over time because of aggregates formation, as expected if platelet activation took place. Van Cott and coworkers also observed a decreased effect of storage time on PDW22. However, they did not evaluate this result, as they focused on the other platelet indices.
In conclusion, MPV and especially PDW increase during platelet activation, as depicted by automated hematology analyzers. Even though our study sample was sufficient for revealing significant differences, smaller and minor differences might have been omitted. PDW seems to be a more specific indicator of platelet activation than MPV, since it was not elevated during single platelet distention caused by platelet swelling. The combined use of MPV and PDW could predict activation of coagulation more efficiently.
References
- 1.Ruf A, Patscheke H. Flow cytometric detection of activated platelets: comparison of determining shape change, fibrinogen binding, and P-selectin expression. Semin Thromb Hemost. 1995;21:146–151. doi: 10.1055/s-2007-1000389. [DOI] [PubMed] [Google Scholar]
- 2.Gurney D, Lip GY, Blann AD. A reliable plasma marker of platelet activation: does it exist? Am J Hematol. 2002;70:139–144. doi: 10.1002/ajh.10097. [DOI] [PubMed] [Google Scholar]
- 3.Kamath S, Blann AD, Lip GY. Platelet activation: assessment and quantification. Eur Heart J. 2001;22:1561–1571. doi: 10.1053/euhj.2000.2515. [DOI] [PubMed] [Google Scholar]
- 4.Coban E, Yazicioglu G, Avci A Berkant, Akcit F. The mean platelet volume in patients with essential and white coat hypertension. Platelets. 2005;16:435–438. doi: 10.1080/09537100500163572. [DOI] [PubMed] [Google Scholar]
- 5.Nadar S, Blann AD, Lip GYetal. Platelet indexes in relation to target organ damage in high-risk hypertensive patients: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) J Am Coll Cardiol. 2004;44:415–422. doi: 10.1016/j.jacc.2004.03.067. [DOI] [PubMed] [Google Scholar]
- 6.Papanas N, Symeonidis G, Maltezos E, Mavridis G, Karavageli E, Vosnakidis T. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15:475–478. doi: 10.1080/0953710042000267707. [DOI] [PubMed] [Google Scholar]
- 7.Nadar SK, Lip GY, Blann AD. Platelet morphology, soluble P selectin and platelet P-selectin in acute ischaemic stroke. The West Birmingham Stroke Project. Thromb Haemost. 2004;92 doi: 10.1160/TH04-07-0433. [DOI] [PubMed] [Google Scholar]
- 8.Greisenegger S, Endler G, Hsieh K, Tentschert S, Mannhalter C, Lalouschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35:1688–1691. doi: 10.1161/01.STR.0000130512.81212.a2. [DOI] [PubMed] [Google Scholar]
- 9.Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;338:1409–1411. doi: 10.1016/0140-6736(91)92719-i. [DOI] [PubMed] [Google Scholar]
- 10.Boos CJ, Beevers GD, Lip GY. Assessment of platelet activation indices using the ADVIATM 120 amongst high-risk patients with hypertension. Ann Med. 2007;39(1):72–78. doi: 10.1080/07853890601040063. [DOI] [PubMed] [Google Scholar]
- 11.Van Cott EM, Fletcher SR, Kratz A. Effects of the blood-collection tube material and long-term storage on platelet activation parameters on the ADVIA 120/2120 hematology system. Lab Hematol. 2005;11(1):71–75. [PubMed] [Google Scholar]
- 12.Jagroop IA, Clatworthy I, Lewin J, Mikhailidis DP. Shape change in human platelets: measurement with a channelyzer and visualisation by electron microscopy. Platelets. 2000;11(1):28–32. doi: 10.1080/09537100075760. [DOI] [PubMed] [Google Scholar]
- 13.Park Y, Schoene N, Harris W. Mean platelet volume as an indicator of platelet activation: methodological issues. Platelets. 2002;13(5-6):301–306. doi: 10.1080/095371002220148332. [DOI] [PubMed] [Google Scholar]
- 14.Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost. 2003;9(3):177–190. doi: 10.1177/107602960300900301. [DOI] [PubMed] [Google Scholar]
- 15.Howarth S, Marshall LR, Barr Aletal. Platelet indices during normal pregnancy and preeclampsia. Br J Biomed Sci. 1999;56:20–22. [PubMed] [Google Scholar]
- 16.von DadelszenP, Magee LA, Devarakonda RM, et al. The prediction of adverse maternal outcomes in preeclampsia. J Obstet Gynaecol Can. 2004;26(10):871–879. doi: 10.1016/s1701-2163(16)30137-2. [DOI] [PubMed] [Google Scholar]
- 17.Furlanello T, Tasca S, Caldin M, et al. Artifactual changes in canine blood following storage, detected using the ADVIA 120 hematology analyzer. Vet Clin Pathol. 2006;35(1):42–46. doi: 10.1111/j.1939-165x.2006.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 18.Ihara A, Kawamoto T, Matsumoto K, Shouno S, Morimoto T, Noma Y. Relationship between hemostatic factors and the platelet index in patients with ischemic heart disease. Pathophysiol Haemost Thromb. 2006;35(5):388–391. doi: 10.1159/000097694. [DOI] [PubMed] [Google Scholar]
- 19.Khandekar MM, Khurana AS, Deshmukh SD, Kakrani AL, Katdare AD, Inamdar AK. Platelet volume indices in patients with coronary artery disease and acute myocardial infarction: an Indian scena. J Clin Pathol. 2006;59(2):146–149. doi: 10.1136/jcp.2004.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amin MA, Amin AP, Kulkarni HR. Platelet distribution width (PDW) is increased in vaso-occlusive crisis in sickle cell disease. Ann Hematol. 2004;83(6):331–335. doi: 10.1007/s00277-003-0833-8. [DOI] [PubMed] [Google Scholar]
- 21.Beyan C, Kaptan K, Ifran A. Platelet count, mean platelet volume, platelet distribution width, and plateletcrit do not correlate with optical platelet aggregation responses in healthy volunteers. J Thromb Thrombolysis. 2006;22(3):161–164. doi: 10.1007/s11239-006-9014-7. [DOI] [PubMed] [Google Scholar]
- 22.Van CottEM, Fletcher SR, Kratz A. Effects of the blood-collection tube material and long-term storage on platelet activation parameters on the ADVIA 120/2120 hematology system. Lab Hematol. 2005;11(1):71. [PubMed] [Google Scholar]