Abstract
Background and aim: Inherent property of the motoneurons of the peripheral nervous system is their ability to recover, at least in part, upon injury. To this end different factors are expressed and are thought to play important role in the regeneration processes. These factors are diverse, and range from transcription factors and chemokines, to molecules of the extracellular matrix. Transforming growth factor beta (TGF-β) is a protein with diverse actions controlling cell growth and proliferation. In the extracellular matrix it is found bound to decorin a proteoglycan involved in cell adhesion and cell signaling. In the present study we investigate the expression of TGF-β and decorin at different time points, in the regenerating sciatic nerve of a seven day old rat, having suffered nerve crush injury, over a period of one month.
Materials and methods: To achieve this, we evoked injury to male Wistar rats by exposing and applying pressure to the sciatic nerve using watchmaker's forceps. After that at 12h, 24h, 48h, 72h, one week, and one month intervals we investigated the gene expression of decorin using RT-PCR, and followed the expression of TGF-β molecule by immunohistochemistry in frozen sections of the L4-L5 region of the rat spinal cord.
Results: We report that both decorin mRNA and TGF- protein exhibit a concerted, biphasic expression after 12 hours and one month having the animal suffered the nerve crush.
Discussion: Our data reveal a biphasic modulation of TGF-β protein and decorin mRNA expression at lumbar segment of the spinal cord of animals having suffered unilateral sciatic nerve crush. We postulate that their concerted expression both at an early and a late phase after the nerve injury is of importance and can be part of a repair or neuroprotective mechanism as yet unclarified.
Keywords: TGF-beta, decorin, nerve crush, sciatic nerve, neurodegeneration
Peripheral nerve injury elicits a complex response pertaining to both nerve degeneration and repair through mechanisms that are not fully understood yet. In neonatal rats the survival and development of motoneurons is dependent upon the functional interaction with their target muscles. Peripheral nerve injury during this period causeds degeneration and loss of motoneurons1,2. In contrast to the central nervous system, mature neurons of the peripheral nervous system are able to survive and regenerate after injury. To this end different molecules such as cytokines and neurotrophins as well as molecules of the extracellular matrix are expressed and are thought to be part of a repair mechanism or act against nerve repair and axon regeneration3–7. The processes seem to be well coordinated since both the receptors for these molecules as well as their corresponding intracellular effectors' expression is modulated4,8,9. A central role to regulate the repair process was assigned to members of the TGF-β superfamily including TGF-β, activin and BMP which are all expressed by injured motoneurons4,10–12. In mammals TGF-β can be found in three isoforms TGF-β1, TGF-β2, and TGF-β3 which are highly conserved among species13. TGF-β is a pleiotropic cytokine synthesized in a latent form which upon activation exerts its functions as a homodimer through three classes of receptors βRI, TβRII and TβRIII found in most cell types14. Receptor activation is achieved when TGF-β binds to TβRII which then forms a heterodimer with TβRI which in turn is phosphorylated by the constitutively active kinase domain of TβRII. Thus, the molecular switch is set to "ON" and downstream signaling is initiated. The activated TβRI then interacts with the Smad proteins which in turn are classified into three classes depending on their structure and function. R-Smads are directly phosphorylated and activated by the activated TβRI, co-Smad (common partner) is Smad-4 and I-Smads exert inhibitory function competing with R-Smads for the activated TβRI receptor. Smads are then transferred to the nucleus were they interact with other signalling proteins to modulate the transcription of certain target genes15–17. In the extracellular matrix, TGF- β can be bound to biglycan, decorin, fibromodulin and betaglycan the later being a synonym for TRβIII. This association of TGF-β with the proteins of the extracellular matrix and specifically with decorin attenuates its function by generating an extracellular reservoir for the factor, or enhances its activity by helping to present TGF- β to its receptors or even activates alternative TGF-β signal transduction pathways by TGF-β signalling through lipoprotein-receptor related protein (LRP-1)18–21. Specifically the interaction of TGF-β with decorin is of interest as decorin is a proteoglycan involved in cell adhesion and cell signaling. Decorin belongs to a superfamily of small leucine-rich proteoglycans (SLRPs) and it carries a single glucosaminoglycan chain attached to its aminoterminus and its core protein consists of 12x24-aminoacid leucinerich repeats. Furthermore, decorin is expressed in most tissues and in the brain its expression is developmentally regulated22,23. It binds to the insulin-like growth factor-I, and interacts with TNF-α24,25. Furthermore, by binding to the EGF receptor decorin activates the mitogen activated protein kinase (MAP kinase) signaling pathways leading to p21 induction, and ultimately to cell cycle arrest26. Previous studies have decorin implicated in suppression of tumor cell-mediated angiogenesis27, attributed to it a functional role in adult brain injury and repair28 and demonstrated that it promotes axonal growth across rat spinal cord injuries7.
In this study we monitored the expression of TGF-β protein and decorin mRNA in the lumbar region of the spinal cord of seven day old rats after unilateral injury of the sciatic nerve over a period up to one month.
Methods
Nerve crush
Wistar rats were obtained from the Laboratory of Physiology (Medical School, Aristotle University of Thessaloniki, Greece). We complied with the guidelines for animal use established by the American Physiological Society approved by the local ethical committee in accordance with EEC Council Directive 86/609. The day of birth was counted as P0 (zero). Sciatic nerve crush was performed on the left side at the 7th postnatal day (P7) and successful axonotomy was certified postoperatively by clinical tests with the right side serving as control as described previously29,30. Only animals in which successful axonotomy was verified were included in our study. For each time point we divided the animals into two groups, control and nerve crush. Each group consisted of at least 7 animals and the TGF-β and decorin expression was determined at 12 hours, 24 hours, 48 hours, 72 hours, 1 week and 1 month after axonotomy.
Cardiac Perfusion
Control and operated animals, were deeply anaesthetized and underwent the procedure of cardiac perfusion with a fixative containing paraformaldehyde 4% in phosphate buffered saline (PBS). The spinal cord was then removed, post-fixed for 4 hours in the same fixative and finally stored in 30% sucrose at -40℃.
Immunochemistry
The L4-L5 region of the rat spinal cord was identified with the aid of a stereoscope. For identification, the control dorsal horn (right side) was marked with a fine micropin. Serial frozen sections of 4µm were cut with a HM 505 E Microm cryostat, collected on superfrost plus glass slides (Fisher Scientific, Pittsburgh, PA, USA) and stored at -70℃.
For immuno-staining, the sections were microwave fixed with 2% paraformaldehyde31 incubated for 10 minutes in dual endogenous enzyme block solution, for 3 hours with the primary antibody (diluted to 1:400) according to the vendors protocol. The antibody was a monoclonal anti-TGF-β1 antibody with possible actions for the other isoforms of TGF-β (NCL-TGFB, Novocastra Laboratories Ltd Newcastle upon Tyne NE12 8EW, U.K.). The sections were then processed using a horseradish peroxidase (HRP) conjugated secondary antibody for 30 min at room temperature and developed using diamino benzidine (DAB) as a substrate.
mRNA extraction and RT-PCR analysis
Twelve, 24, 48, 72 hours, 1 week and 1 month after the nerve injury, the animals were sacrificed, and the region of the spinal cord (L4 – L5) was excised. Total RNA was extracted with the RNeasy Mini RNA extraction kit from Qiagen (Qiagen Greece BioAnalytica S.A. Athens). Two µg of total RNA were subjected to reverse transcription using 200 units of MMLV-RT (Promega Greece SB Biotechnology Suppliers S.A. Athens) in 100 l reaction volume, under standard conditions. Five µl of the reverse transcription reaction mixture were subjected to PCR amplification in 25 µl reaction volume, including primers for the amplification of the beta-actin mRNA as internal control for gene expression as described by others32,33. Amplification started with an initial step of 5 min denaturation at 94℃ followed by 30 cycles, each cycle consisted of denaturation at 94℃ for 30 sec annealing at 55℃ for 1 min and extension at 72℃ for 1 min. Finally, the PCR products were extended for 10 min at 72℃. Five µl of each PCR reaction mixture were analyzed in a 1.7% agarose gel. Visualization of DNA bands was achieved with UV illumination of ethidium bromide-stained gels. The intensity of the DNA bands was measured using the software from Kodak Digital Science 1DTM.
Primers
Primers were designed to be specific for both rat and human mRNA. For this purpose decorin mRNA sequnces for R. rattus (Z12298), R.norvegicus (X59859) and Homo sapiens (BC005322) were aligned using the Clustal W algorithm and the computing facilities of EBI http:// www.ebi.ac.uk/. The primers then were designed out of regions of complete homology. For beta-actin sence 5' ACACTGTGCCCATCTACGAGG 3' and antisence 5' AGGGGCCGGACTCGTCATACT 3' providing a pcr amplification product of 625 bp and for decorin sence 5' ATGATTGTCATAGAACTGGGC 3' and antisence 5' ATTGTTGTTATGAAGGTAGAC 3' with an amplicon of 385 bp.
Statistical Analysis
Non parametric data was analyzed using the Kruskal Wallis test and ANOVA. When significant, post hoc analysis Dunett test was used. The data is presented as mean ± SE of n ≥ 6 in each group. Results were considered significant with a probability z (p)<0.01 marked with(*).
Results
Animals successfully axonotomized lost reactive movements concerning the plantar- and the dorsi-flexion reflexes and the ability of normal movement of the left hindlimb when suspended by their tails. Impaired movement was evident up to two weeks after the nerve crush when gradual recovery was observed. By the end of one month the animals adopted an apparent normal walking pattern and reactive movements concerning the plantarand the dorsi-flexion reflexes were restored. The ability of animals for normal movement of the left hindlimb when suspended by their tails was also restored albeit diminished in strength and velocity. Our data suggests that peripheral nerve injury to a 7 day old newborn rat can be repaired and nerve regeneration and reinervation of the muscles involved is achieved resulting in apparently normal movement.
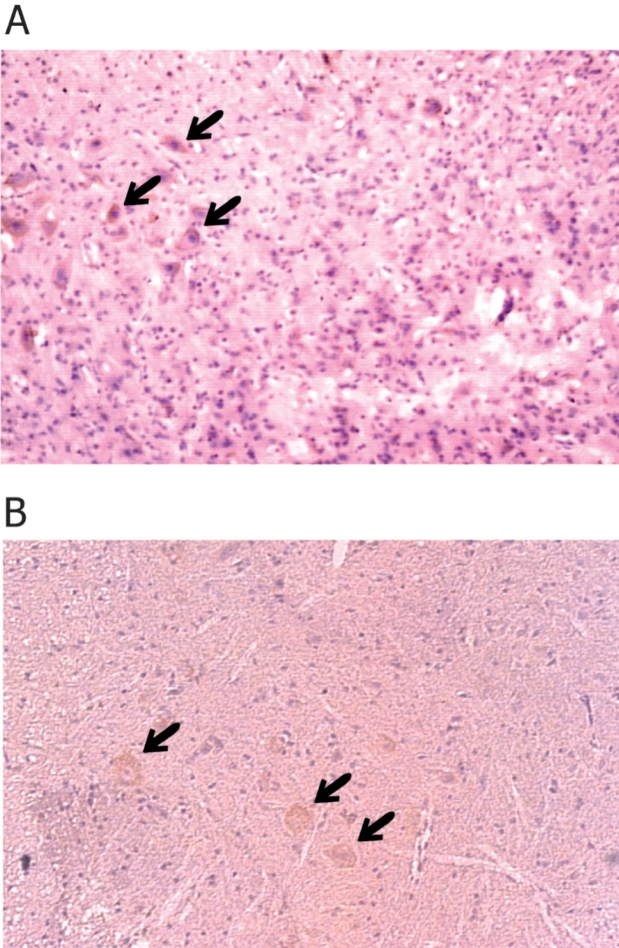
In our study the expression of TGF-β isoforms was assessed by immuno-staining of lumbar sections of spinal cords of axonotomized and control animals. We detected a strong immuno-reactivity for the TGF-β isoforms specific for the motoneurons of operated animals 12 hours and 1 month after the nerve crush (Figure 1, panel A and B).
Figure 1. Immunohistochemical staining for TGF-β of spinal cord lumbar sections of animals having undergone sciatic nerve crush. Panel A 12 hours after the nerve crush and panel B one month after the nerve crush. Arrows indicate motoneurons expressing TGF-beta stained with anti-TGF-β antibody.
No immuno-reactivity for TGF-β was detected in any of the other groups or in the control animals. In the sections where motoneurons were positively stained for TGF-β, staining was observed both ipsilatelral and contralateral to the sciatic nerve crush.
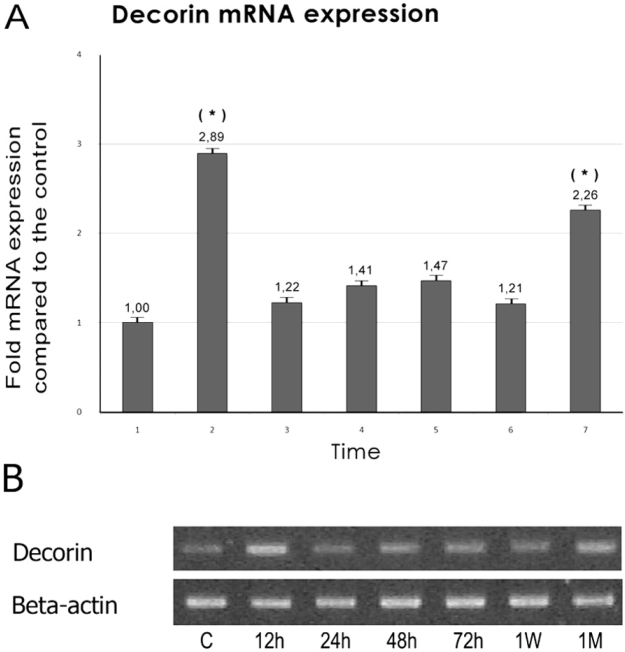
Decorin mRNA was expressed at the L4 - L5 region of the rat spinal cord of the 7 day old rat albeit to a much lower level compared to the expression of the beta-actin gene (Table 1).
Table 1. Relative intensities of ethidium bromide stained PCR bands.
Twelve hours after the unilateral sciatic nerve crush decorin mRNA levels increased significantly about threefold compared to those of the control animals (p < 0.01). At later time points decorin mRNA expression returned to normal levels with small variations of no statistical significance. However one month after the nerve crush, the levels of decorin mRNA expression were again significantly elevated, (p < 0.01), about 2.5–fold compared to those of the control animals (Figure 2).
Figure 2. Expression of Decorin mRNA was monitored from 12 hours after the sciatic nerve crush and over a period of one month. (A) The relative mRNA signal intensity at each time point is compared with that of the control. Bars represent means ± SE. (*) Significant difference compared with the control, (p < 0.01) (B). Agerose gel electrophoresis of RT-PCR products for decorin and beta-avtin mRNA from injured animals at different time points after sciatic nerve crush.
Discussion
Our data reveal a biphasic modulation of TGF-β protein and decorin mRNA expression at lumbar segment of the spinal cord of animals having suffered unilateral sciatic nerve crush. This modulation of expression occurs at two stages, an early one after 12 hours and a late one, 1 month after the axonotomy. Bearing in mind that these molecules are functionally related and both alone and together activate different signaling pathways we conclude that their concerted expression both at an early and a late phase after the nerve injury is of importance and can be part of a repair or neuroprotective mechanism not clarified yet.
It is well established that injury to the peripheral nerve triggers a complex, developmental stage specific, gene expression response, which regulates interplay of motor neuron death or survival. It has been reported that in spinal cord injuries inflammation is a key feature of this interplay, causing substantial secondary damage and thus undermining the processes of neuroregeneration34,35. In addition it has been reported that IL-10, a powerful anti-inflammatory cytokine, administered at the site of sciatic nerve crush reduced scar formation and permitted better regeneration of the damaged axons36. In this inflammatory response TGF- β is an important factor and it has been shown that inhibition of its expression by neutralizing antibodies at the site of sciatic nerve repair reduced scar formation but did not enhance nerve regeneration37. In contrast TGF-β inhibition by decorin over-expression in the rat brain induced severe inflammation and acute neuronal death37. Others reported that TGF-β is not a neurotrophic factor by itself rather it potentiated the action of neurotrophins either though interaction with other growth/differentiation factors38 or by activating other signaling pathways such as the MAP Kinases pathway (MAPK/ERK/p38) that also has been reported to be activated in peripheral nerve injury39,40.
On the other hand decorin has been shown to help reduce scar formation by inhibiting the expression of other chondroitin sulfate proteoglycans and promote axon growth by direct interaction with myelin in adult spinal cord injuries7,41. Our data is consistent with these reports and it would account for the late expression of decorin being involved in processes employed in remyelination. Additional implication of decorin in nerve repair comes from reports stating that the MAPK ERK pathway is activated in peripheral nerve after injury39,40.
Our findings are in agreement with the above reports and clearly demonstrat that TGF-β and decorin expression is modulated as part of the response to sciatic nerve injury. This modulation of expression is biphasic and includes an early and a late stage consistent with the inflammatory process during which both cellular and exudative components are mobilized. It is of interest that these molecules are expressed in a concerted manner after the sciatic nerve crush. Both of these molecules activate different branches of the same pathway (MAPK) making it an important target for further investigation. The fact that TGF-β and decorin are functionally related both in a negative and a positive way and are expressed simultaneously implies that their function depends on the microenvironment created at the site of the injury or that it is exerted through other unidentified mechanisms as yet. Recent reports revealed that TGF-β required decorin for signaling through the large endocytotic lipoprotein related receptor-118. It is of interest to elucidate the contribution of this pathway to nerve injury and repair and whether it is indeed activated upon of sciatic nerve injury. This would help identify the events following the injury and assign to both TGF-β and decorin specific places in the nerve repair processes.
Acknowledgments
The authors would like to thank I. Klagas for expert technical assistance and Dr. Ch. Pourzitaki for providing the statistical analysis of the data.
References
- 1.Lawson SJ, Lowrie MB. The role of apoptosis and excitotoxicity in the death of spinal motoneurons and interneurons after neonatal nerve injury. Neuroscience. 1998;87:337–348. doi: 10.1016/s0306-4522(98)00120-1. [DOI] [PubMed] [Google Scholar]
- 2.Lowrie MB, Vrbova G. Dependence of postnatal motoneurones on their targets: review and hypothesis. Trends Neurosci. 1992;15:80–84. doi: 10.1016/0166-2236(92)90014-y. [DOI] [PubMed] [Google Scholar]
- 3.Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Snider WD, Zhou FQ, Zhong J, et al. Signaling the pathway to regeneration. Neuron. 2002;35:13–16. doi: 10.1016/s0896-6273(02)00762-6. [DOI] [PubMed] [Google Scholar]
- 5.Szpara ML, Vranizan K, Tai YC, et al. Analysis of gene expression during neurite outgrowth and regeneration. BMC Neurosci. 2007;8:100. doi: 10.1186/1471-2202-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hossain-Ibrahim MK, Rezajooi K, Stallcup WB, et al. Analysis of axonal regeneration in the central and peripheral nervous systems of the NG2-deficient mouse. BMC Neurosci. 2007;8 doi: 10.1186/1471-2202-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies JE, Tang X, Denning JW, et al. Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur J Neurosci. 2004;19:1226–1242. doi: 10.1111/j.1460-9568.2004.03184.x. [DOI] [PubMed] [Google Scholar]
- 8.Makwana M, Raivich G. Molecular mechanisms in successful peripheral regeneration. FEBS J. 2005;272:2628–2638. doi: 10.1111/j.1742-4658.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- 9.Okuyama N, Kiryu-Seo S, Kiyama H. Altered expression of Smad family members in injured motor neurons of rat. Brain Res. 2007;1132:36–41. doi: 10.1016/j.brainres.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, McLennan IS, Koishi K, et al. Transforming growth factor-beta 2 is anterogradely and retrogradely transported in motoneurons and up-regulated after nerve injury. Neuroscience. 2000;97:735–742. doi: 10.1016/s0306-4522(00)00084-1. [DOI] [PubMed] [Google Scholar]
- 11.DAntonio M, Droggiti A, Feltri ML, et al. TGFbeta type II receptor signaling controls Schwann cell death and proliferation in developing nerves. J Neurosci. 2006;26:8417–8427. doi: 10.1523/JNEUROSCI.1578-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buss A, Pech K, Kakulas BA, et al. TGF-beta1 and TGF-beta2 expression after traumatic human spinal cord injury. Spinal Cord. 2008;46:364–371. doi: 10.1038/sj.sc.3102148. [DOI] [PubMed] [Google Scholar]
- 13.Clark DA, Coker R. Transforming growth factor-beta (TGFbeta) Int J Biochem Cell Biol. 1998;30:293–298. doi: 10.1016/s1357-2725(97)00128-3. [DOI] [PubMed] [Google Scholar]
- 14.Brand T, Schneider MD. Transforming growth factor-beta signal transduction. Circ Res. 1996;78:173–179. doi: 10.1161/01.res.78.2.173. [DOI] [PubMed] [Google Scholar]
- 15.Massague J, Wotton D. Transcriptional control by the TGF-beta/ Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazawa K, Shinozaki M, Hara T, et al. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells. 2002;7:1191–1204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- 17.Wrana JL, Attisano L, Wieser R, et al. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 18.Cabello-Verrugio C, Brandan E. A novel modulatory mechanism of transforming growth factor-beta signaling through decorin and LRP-1. J Biol Chem. 2007;282:18842–18850. doi: 10.1074/jbc.M700243200. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi Y, Kodama Y, Matsumoto T. Bone matrix decorin binds transforming growth factor-beta and enhances its bioactivity. J Biol Chem. 1994;269:32634–32638. [PubMed] [Google Scholar]
- 20.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 21.Droguett R, Cabello-Verrugio C, Riquelme C, et al. Extracellular proteoglycans modify TGF-beta bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix Biol. 2006;25:332–341. doi: 10.1016/j.matbio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 23.Kappler J, Stichel CC, Gleichmann M, et al. Developmental regulation of decorin expression in postnatal rat brain. Brain Res. 1998;793:328–332. doi: 10.1016/s0006-8993(98)00260-1. [DOI] [PubMed] [Google Scholar]
- 24.Schonherr E, Sunderkotter C, Iozzo RV, et al. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem. 2005;280:15767–15772. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- 25.Tufvesson E, Westergren-Thorsson G. Tumour necrosis factoralpha interacts with biglycan and decorin. FEBS Lett. 2002;530:124–128. doi: 10.1016/s0014-5793(02)03439-7. [DOI] [PubMed] [Google Scholar]
- 26.Moscatello DK, Santra M, Mann DM, et al. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest. 1998;101:406–412. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant DS, Yenisey C, Rose RW, et al. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 28.Stichel CC, Kappler J, Junghans U, et al. Differential expression of the small chondroitin/dermatan sulfate proteoglycans decorin and biglycan after injury of the adult rat brain. Brain Res. 1995;704:263–274. doi: 10.1016/0006-8993(95)01131-5. [DOI] [PubMed] [Google Scholar]
- 29.Gougoulias N, Kouvelas D, Albani M. Protective effect of PNQX on motor units and muscle property after sciatic nerve crush in neonatal rats. Pharmacol Res. 2007;55:370–377. doi: 10.1016/j.phrs.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Gougoulias N, Hatzisotiriou A, Kapoukranidou D, et al. Magnesium administration provokes motor unit survival, after sciatic nerve injury in neonatal rats. BMC Musculoskelet Disord. 2004;5:33. doi: 10.1186/1471-2474-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barsony J, Pike JW, DeLuca HF, et al. Immunocytology with microwave-fixed fibroblasts shows 1 alpha, 25-dihydroxyvitamin D3-dependent rapid and estrogen-dependent slow reorganization of vitamin D receptors. J Cell Biol. 1990;111:2385–2395. doi: 10.1083/jcb.111.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enomoto T, Fujita M, Cheng C, et al. Loss of expression and loss of heterozygosity in the DCC gene in neoplasms of the human female reproductive tract. Br J Cancer. 1995;71:462–467. doi: 10.1038/bjc.1995.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonson T, Mahlamaki EH, Karhu R, et al. Characterization of genomically amplified segments using PCR: optimizing relative-PCR for reliable and simple gene expression and gene copy analyses. Genes Chromosomes Cancer. 2000;29:192–199. [PubMed] [Google Scholar]
- 34.Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Blumbergs PC, Jones NR, et al. Early expression and cellular localization of proinflammatory cytokines interleukin- 1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine. 2004;29:966–971. doi: 10.1097/00007632-200405010-00004. [DOI] [PubMed] [Google Scholar]
- 36.Atkins S, Loescher AR, Boissonade FM, et al. Interleukin-10 reduces scarring and enhances regeneration at a site of sciatic nerve repair. J Peripher Nerv Syst. 2007;12:269–276. doi: 10.1111/j.1529-8027.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- 37.Boche D, Cunningham C, Docagne F, et al. TGFbeta1 regulates the inflammatory response during chronic neurodegeneration. Neurobiol Dis. 2006;22:638–650. doi: 10.1016/j.nbd.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Krieglstein K, Strelau J, Schober A, et al. TGF-beta and the regulation of neuron survival and death. J Physiol Paris. 2002;96:25–30. doi: 10.1016/s0928-4257(01)00077-8. [DOI] [PubMed] [Google Scholar]
- 39.Yongchaitrakul T, Pavasant P. Transforming growth factor-beta1 up-regulates the expression of nerve growth factor through mitogen-activated protein kinase signaling pathways in dental pulp cells. Eur J Oral Sci. 2007;115:57–63. doi: 10.1111/j.1600-0722.2007.00420.x. [DOI] [PubMed] [Google Scholar]
- 40.Agthong S, Kaewsema A, Tanomsridejchai N, et al. Activation of MAPK ERK in peripheral nerve after injury. BMC Neurosci. 2006;7:45. doi: 10.1186/1471-2202-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minor K, Tang X, Kahrilas G, et al. Decorin promotes robust axon growth on inhibitory CSPGs and myelin via a direct effect on neurons. Neurobiol Dis. 2008;32:88–95. doi: 10.1016/j.nbd.2008.06.009. [DOI] [PubMed] [Google Scholar]