Abstract
Objective:
To explore factors throughout the lifespan that influence cognition in midlife to late life.
Methods:
We conducted a retrospective birth cohort study of 2,062 individuals born during 1921-1954 in Beijing, China. In 2003-2005, birth records were abstracted, and participants then 50-82 years old received standardized examinations for health, cognition, and socio-environmental measures. Using cumulative logit models, we assessed adjusted relative effects of prenatal, early life, and adult factors on mid- to late-life cognition.
Results:
Most prenatal factors were associated with mid- to late-life cognition in the unadjusted models. However, when childhood and adult factors were sequentially added to the models, the impact of prenatal factors showed successive attenuation in effect size, and became insignificant. In contrast, early life factors remained significantly associated with mid- to late-life cognition even after full life-course adjustments. Specifically, those whose fathers had laborer vs professional occupations (odds ratio [OR]Laborer 1.74; 95% confidence interval [CI]: 1.25-2.42) had poorer cognitive outcomes, while individuals who drank milk daily in childhood (OR 0.65; 95% CI: 0.54-0.80), had more years of education (OR10-12 years 0.60; 95% CI: 0.45-0.81; OR13+ yrs 0.29; 95% CI: 0.23-0.38), and were taller adults (ORheight ≥ SD 0.65; 95% CI: 0.49-0.86) had better cognition. The high prenatal risk infants had similar patterns with a trend toward a stronger association between cognition and socioenvironmental factors.
Conclusion:
Mid- to late-life cognition is influenced by factors over the entire lifespan with the greatest impact coming from early life exposures. Nutrition, education, social, and family environment in early life may have a long-term impact on cognition in developing countries.
GLOSSARY
- CI
= confidence interval;
- HDL
= high-density lipoprotein;
- LDL
= low-density lipoprotein;
- OR
= odds ratio;
- PUMCH
= Peking Union Medical College Hospital;
- WAIS-R
= Wechsler Adult Intelligence Scale-Revised;
- WISC-R
= Wechsler Intelligence Scale for Children-Revised.
Numerous epidemiologic studies have reported that negative conditions in utero are associated with poorer adult life outcomes, including chronic disease and lower cognitive function.1–12 However, several recent studies have shown that early life postnatal exposures may have greater bearing on adult health compared with prenatal factors.4–7 Larger birthweight, the most widely researched birth size measure, was associated with better cognitive function from infancy through the third decade of life in geographically defined populations of several countries.3,7–12 However, the impact of birthweight has not been shown to persist after age 40.8 Birth head circumference has shown a similar pattern to birthweight, such that associations observed in young cohorts were not replicated in an older British sample, aged 48-74.13 Research with follow-up through mid- to late life has been sparse, but the few studies done suggest the association between birthweight and cognition may diminish as individuals reach middle age.8,13 However, these studies had varying adjustments for prenatal and postnatal factors and limited growth measures. We investigated the combined effect of birth size, early life factors, and adult factors on mid- to late-life cognition in a large, retrospective birth cohort study in Beijing, China.
METHODS
Study design and population.
We conducted a birth cohort study on individuals born during 1921-1954 at Peking Union Medical College Hospital (PUMCH) in Beijing, China. Established in 1921 by the Rockefeller Foundation, PUMCH has operated continuously under an American medical model except for 5 years of closure for World War II (1942-1947). The hospital serves a wide socioeconomic spectrum but higher on-average than the Chinese general population.14 During the periods 1921-1941 and 1948-1954, a total of 11,694 Chinese neonates were born in PUMCH. Using actuarial census projections, we estimated there would be about 6,570 survivors at the time of the planned assessment. Based on a pilot study in 1995-1996, we estimated that about 2,990 individuals would be successfully traced and that about 86% of these individuals would agree to participate in the study. In 2003-2005, we actually traced 2,503 subjects through community registries and media announcements. Among traced subjects, 418 did not participate due to refusal (n = 78), being deceased (n = 175), or residing outside Beijing (n = 165). Individuals could not be traced for multiple reasons. First, community registries, such as the Beijing population registry, delete individuals when they die or move from the area. Second, some individuals traced using the community registry were not the individuals identified in the medical records. Based on our pilot study and the great migration due to China’s domestic policy during the lifetime of this cohort, the loss to follow-up is likely due primarily to mortality and migration to other parts of the country or to other countries, with a smaller number due to birth record errors. Of the 2,085 individuals who were traced and agreed to participate, 2,062 had complete cognitive examinations, and 1,956 had no missing data and were used in the analyses.
Standard protocol approvals, registrations, and patient consents.
We received approval from the PUMCH Ethics Committee on human experimentation. Written informed consent was obtained from all study participants.
Measurements.
All participants were examined at PUMCH from September 2003 to March 2005, when they were ages 50 to 82, and were followed up for missing data until March 2006. The PUMCH team of neurologists, physicians, nurses, and trained technicians performed all assessments. The cognitive tests were administered by neurologists. The battery was comprised of the Fuld Object Memory Evaluation (0-20 points),15 Fuld Verbal Fluency, Wechsler Intelligence Scale for Children-Revised Block Design (0-62 points), and WAIS-R Digit Span (0-22 points).14,16
We abstracted birth records, including gender and information related to birth size (i.e., birthweight, birth height, head circumference, gestational age, placental weight), and early life family conditions (i.e., birth order, maternal age, father’s occupation); ponderal index was calculated as birthweight divided by birth length.3 Information on early life environment from childhood through early adulthood were obtained by questionnaire, including education and nutrition (i.e., daily childhood milk drinking, shortage of food during childhood, which was defined as being hungry due to lack of sufficient food for at least 6 months per year for at least 2 years during childhood). Measurement of height was obtained as an estimate of height in early adulthood.
Adult factors that were collected by in-person interviews were current alcohol consumption, current cigarette smoking, recreational activities ≥1/week after age 50 years (i.e., playing cards, traveling, and other activities), and physical activities ≥1/week after age 25 (i.e., running or swimming). To estimate the presence of selected health conditions, serum levels of triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and plasma concentrations of glucose (FPG) were assessed following a 12-hour overnight fast. Participants without a history of diabetes received a 75 g oral-glucose load, and after 2 hours, plasma glucose (PBG) was measured. Diagnosis of diabetes was made for FPG ≥126 mg/dL, PBG ≥200 mg/dL, or diabetic history or medication.2 Dyslipidemia was defined by the International Diabetes Federation definition.17 Medical records were reviewed to identify a history of stroke. In addition, all participants had a CT scan. Those with evidence of a previously undocumented stroke on CT using World Health Organization criteria18 were evaluated further with an MRI. Waist circumference and blood pressure measures followed standard protocols. Hypertension was defined as the presence of systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or a history of hypertension with medication.
Statistical analyses.
A cognitive global score was constructed by summing z-scores of the four cognitive tests. The summed z-scores were divided into 4 ordinal categories designating the 10th, 42nd, and 90th percentiles. Cronbach’s alpha was 0.66, indicating that the global score adequately summarized the individual test scores. Independent variables were selected a priori on the basis of postulated causal mechanisms and prior research, and were grouped as prenatal factors related to birth size, early life factors, and adulthood factors. Categories for each variable were formed using established cutpoints or gender-specific SD within the study population. The clinically normal/intermediate range was used as the reference group.
We compared cohort characteristics using χ2 tests for proportions and t tests for means. To assess prenatal and postnatal influences on mid- to late-life cognition, we constructed 5 successive classes of cumulative logit models19 that represent probabilities cumulated over the lower-ordered category values. Model I estimated unadjusted (crude) associations of individual factors with cognition. Model II adjusted for prenatal factors only. Model III included both prenatal and early life factors. Model IV examined the full life course (i.e., prenatal, early life, and adult periods). We also tested interactions between each prenatal factor with all other life-course variables, and 2-way interactions for age- and gender-specific effects for prenatal and early life factors by forward stepwise selection from the full life-course model, setting p < 0.05 as the level for retention (model V). To identify postnatal factors operating at the highest level of prenatal risk, we first estimated each participant’s risk of low cognition adjusted for prenatal factors alone, i.e., using a prenatal risk score based on coefficients from model II. Persons in the highest quintile of risk (n = 419) were then selected for further cumulative logit analysis.
Several sensitivity analyses to determine whether alternative assumptions or procedures affected results were conducted. Highly correlated factors were either dropped (e.g., body mass index related to serum levels of cholesterol and waist circumference) or combined into indices (i.e., ponderal index replaced birthweight and birth height), and only factors with phi coefficients <0.50 remained in the final models. Because large numbers of independent variables could change the statistical properties of estimated odds ratios (OR), we further reduced the number of adulthood factors, through an adjusted adult cumulative logit model, setting p < 0.05 as the significance level for retention. Thus, adult factors removed from the final adjusted full life-course model were cigarette smoking, dyslipidemia, physical activities, and hypertension, although the last 2 factors showed significant association with cognition in the unadjusted model.
RESULTS
Table 1 shows the characteristics at birth for the PUMCH’s source population of Chinese neonates (n = 11,694). Although participants (n = 2,062) differed on most birth factors compared to the nonenrolled source population (n = 9,632) or traced decedents (n = 175), they do not differ from traced survivors who did not complete examinations (n = 266). The distributions of the cognitive subscales and global scores and exposures in adulthood are summarized in table 2, and prenatal measures and early life conditions are summarized in table 3.
Table 1 Characteristics of the participants at birth by tracing and enrollment status in a Chinese 1921-1954 birth cohort
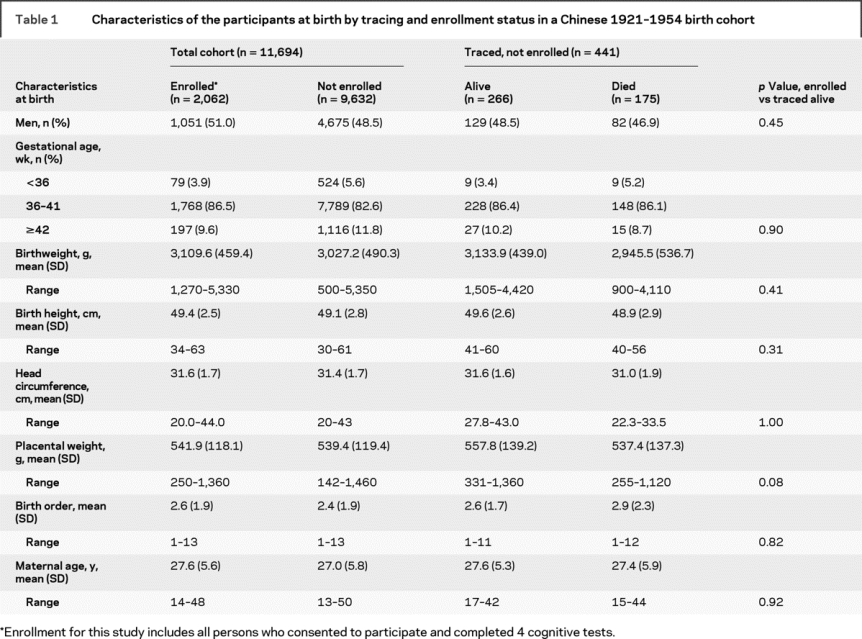
Table 2 Adult characteristics of the participants by midlife to late life cognitive function
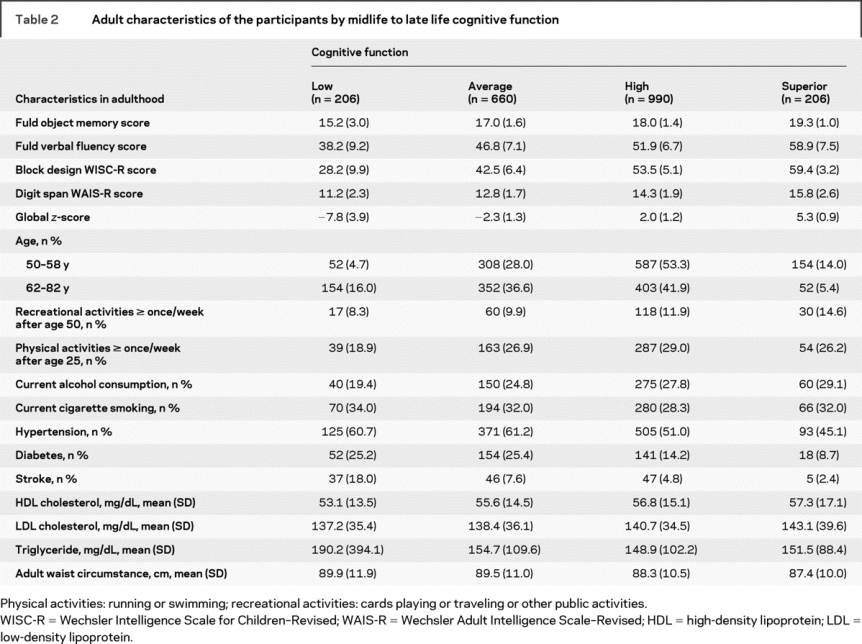
Table 3 Cumulative logit regression models for the association of lower cognition in midlife to late life with unadjusted factors (model I) and for prenatal combined with early life factors (model III)
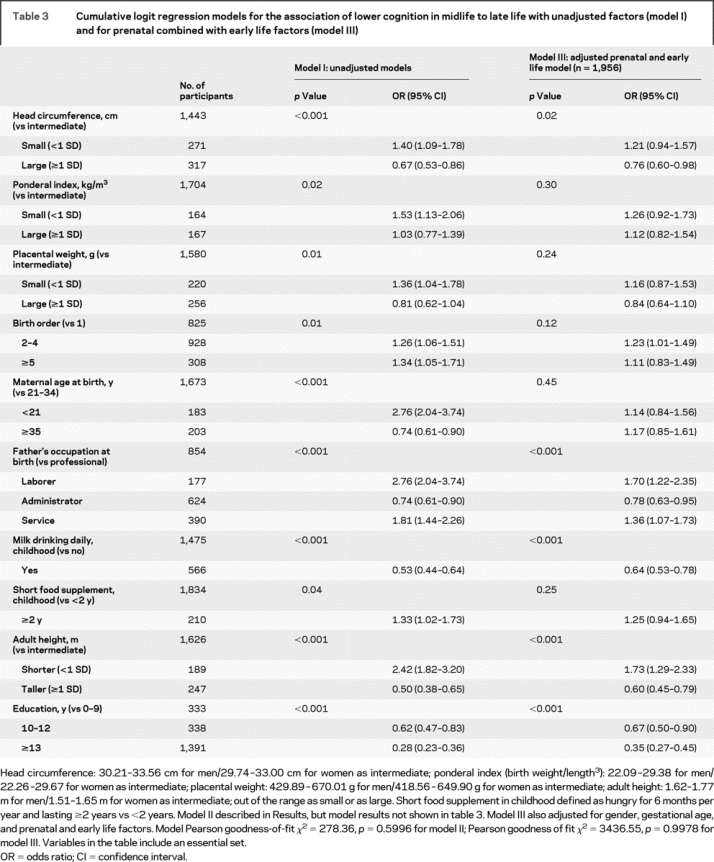
Prenatal effects.
Of the 4 measures at birth, head circumference, small ponderal index, and small placental weight showed significant crude associations with mid- to late-life cognition (table 3, model I). Gestational age was not associated with lower cognition in mid- to late life (<36 weeks’ gestation, OR = 1.18, 95% confidence interval [CI] = 0.77-1.80; ≥42 weeks, OR = 1.35, 95% CI = 1.03-1.78; overall p = 0.08). After adjusting for the 4 prenatal factors, the effects of small ponderal index (p = 0.18) and small placental weight (p = 0.12) were attenuated and became nonsignificant (model II not shown in table 3). When we controlled for prenatal and early life factors (table 3, model III) and then for full life-course variables (table 4, model IV), the effect size for large head circumference progressively diminished until it was no longer significant.
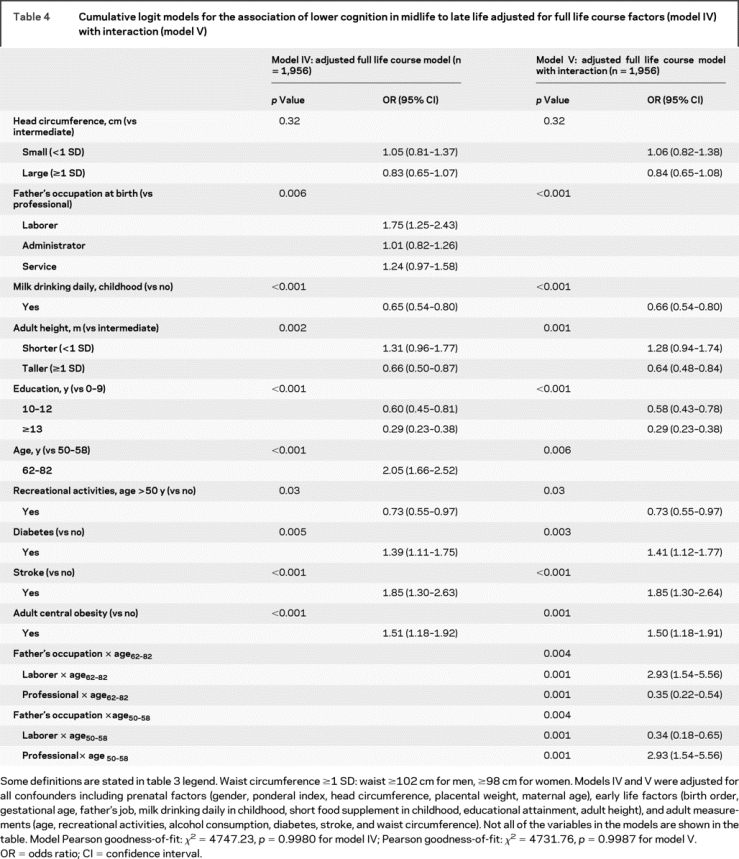
Table 4 Cumulative logit models for the association of lower cognition in midlife to late life adjusted for full life course factors (model IV) with interaction (model V)
Early life effects.
All postnatal early life measures showed significant crude associations with cognitive function in mid- to late-life (table 3, model I). In the model that included factors throughout the lifespan, all early life variables remained significant except for birth order, maternal age at birth, and short food supplement, and were independent of the various birth size measures or adult exposures (i.e., age, recreational activities, alcohol consumption, diabetes, stroke, and waist circumference) (table 4, model IV). Model IV in table 4 shows that poorer cognitive outcome was related to fathers being laborers vs being in professional occupations; higher cognitive scores were associated with daily childhood milk drinking, higher education, and taller adults. The only significant interaction was for age and father’s occupation, which showed that among the older participants (i.e., born prior to 1949, ages 62-82 years), cognition was lower when fathers were laborers compared to professionals, and reversed among younger participants (table 4, model V).
Adulthood effects.
Participation in recreational activities at least once a week after age 50 was associated with a lower risk of poor cognitive outcome, while the presence of diabetes, stroke, and adult central obesity was associated with a greater likelihood of low cognitive scores in middle to late adulthood (table 4, model IV). Because stroke impairs cognition, we reanalyzed the full life-course model excluding persons with stroke (n = 125), and the findings remained essentially unchanged (results not shown).
High prenatal risk subgroup.
Birth size factors did not have a noticeable influence on mid- to late-life cognition in the high prenatal risk subgroup (n = 419), although infants with small ponderal index comprised a larger portion of this subgroup compared to the overall group (32.9% vs 8.1%), as did those with small head circumference (59.4% vs 13.3%). On the other hand, we observed that compared with the reference group, better mid- to late-life cognition among the high prenatal risk individuals was associated with drinking milk daily during childhood (OR = 0.56, 95% CI = 0.36-0.88) and higher education (OReducation 10-12 years = 0.39, 95% CI = 0.20-0.76; OReducation ≥13 years = 0.24, 95% CI = 0.14-0.42). Lower cognition was associated with being shorter in adulthood (OR = 1.79, 95% CI = 1.02-3.15) and having a father with a nonprofessional occupation (ORLaborer = 2.29, 95% CI = 1.13-4.62; ORAdministrator = 2.41, 95% CI = 1.44-4.04; ORService = 1.77, 95% CI = 1.06-2.96). There was a trend toward early life factors having a stronger effect on mid- to late-life cognition in the high prenatal risk group compared with the full sample.
DISCUSSION
We found that early life and adulthood factors have a greater effect on cognitive function in 50- to 82-year-old individuals than do prenatal measures related to birth size. A few other studies have suggested that factors during infancy and early childhood are more important than growth in utero in determining cognitive function up until middle adult life.4–7 Our findings extend those of previous studies by assessing individuals into their 80s and also adjusting for multiple potentially influential factors throughout the lifespan. We conducted post hoc analyses using partial correlation coefficients to assess the interrelationship between adult factors (e.g., diabetes, stroke) and father’s occupation or childhood milk drinking patterns. The partial correlations were small, typically similar to the non-partial correlations, and not significant, thus providing additional support for the independent contribution of early childhood and adult factors on cognition.
Overall, father’s occupation was influential in mid- to late-life cognition, but we found the effect differed based on the individual’s age. Father’s occupation has a broad effect on the social environment in many cultures, including China’s. In our study, older and younger age groups reflected different cohort experiences pre- and post-1949, when the People’s Republic of China was founded. Older members of the cohort were born in the Republic of China, a period with pronounced social class differences in living conditions. In this older cohort, we found that participants whose fathers worked as laborers, compared with professionals, had significantly lower cognition in mid- to late life, a social class association that has been previously noted, but mainly for childhood cognitive outcomes.3,7,8 In comparison, among younger cohort members born after 1949, we found that those with professional class fathers had an enhanced risk of lower cognitive function. The events that happened in contemporary Chinese history could help us interpret the seemingly counterintuitive findings. When the radical revolution led by a party who claimed that they represented the interest of the labor class was introduced after 1949, the scope was broad enough to affect every individual’s life. One major goal of the revolution was not only to eliminate privileges, but also downgrade the role of selected social classes (e.g., intellectuals) and elevate living standards and social status for the labor classes. Our findings suggest that the impact of social class on cognition needs to be interpreted within a particular social and historical context.
Nutrition is thought to have its greatest effect on brain development during periods of rapid growth known as growth spurts,20 which occur at specific time points spanning the prenatal to adolescent periods in different areas of the brain. Some research has reported an association between breastfeeding as a marker of early childhood nutrition and childhood cognition, but little has been studied on the link between post-infancy childhood diet and mid- to late-life cognition.21–22 Our analyses demonstrated the association between daily milk drinking and cognition later in life, independent of other factors across the life course.
Even with adjustments for many other variables, we cannot rule out the possibility that daily milk drinking in childhood may be a proxy for childhood health and enriched environment. It may suggest that in populations with traditional rice-based diets and insufficient nutrition, as in Beijing from the 1920s to 1950s, milk may have been an important supplement providing essential nutrients that enhanced cognitive development. On the other hand, we found that childhood malnutrition due to a shortage of food in childhood did not appear to influence risk of lower cognition in mid- to late-life in the fully adjusted model. We further examined whether collinearity between childhood malnutrition and short adult stature explained the lack of an association between a shortage of food in childhood and lower mid- to late-life cognition, but the results did not support this explanation. Some studies have shown that early to middle adulthood smaller height is related to increased dementia prevalence, consistent with the protective effect we observed for mid- to late-life cognition.8,23 Our study suggests that early life nutrition may have a long-lasting effect across the lifespan.
Our findings showed a trend toward a stronger association between early life factors and cognition among the high prenatal risk group. With respect to preventing poor long-term cognitive outcomes, controlling risk factors especially beginning in early postnatal life may supersede and compensate for fetal vulnerabilities such as small birth size.
Strengths of this study include the relatively large sample with a comparatively long follow-up period compared to previous research on this topic. We also were able to assess the influence of numerous variables from different dimensions of social status, lifestyle factors, and health across developmental periods, including details of birth measures from medical records. In addition, our study provides a unique opportunity to examine the impact of a naturally occurring socioeconomic experiment. Specifically, the 8 decades that span the duration of the study correspond to great social and economic change within China. For example, the establishment of the People’s Republic of China in 1949 signified major changes in social classifications of Chinese citizens. In addition, prior to and following 1949, citizens experienced 2 widespread famines that likely led to malnutrition. Because participants were in their early life years between the 1920s and 1960s, this sample provides a unique setting for investigating the impact of major socio-historical events within China’s history on cognition in mid- to late-life.
Limitations of our study are that we were not able to locate and assess all individuals born at PUMCH during 1921-1954. The participation rate for those who were traced and alive was 89% and among persons we traced, no differences in birth measures were observed between participants and living nonparticipants who had moved or refused. Even so, it is possible that our findings would not apply to individuals who survived to midlife, but died prior to this study or could not be located. Although our sample is not population-based, the PUMCH cohort encompasses a broad socioeconomic spectrum of China from indigent to wealthy, except for peasant farmers. Distributions of birth measurements were similar to the Chinese general population of the time and adult cognitive scores are comparable to contemporary Western normative samples. Consequently, our findings for prenatal factors may generalize, but first warrant replication in other populations. Another possible limitation of this study is the reliance on retrospective recall for some of the early life and midlife factors. Although this methodology is commonly used by studies that assess factors throughout the lifespan, it is subject to recall error and potential bias. One might predict if recall error was an issue that its effect would be similar for related information. For example, if recall error resulted in those with low cognitive scores being less likely to endorse daily milk drinking during childhood, then one might also expect this group to less frequently endorse food shortages during childhood. Our data do not show this pattern, suggesting that selective recall error does not explain our results.
Our findings suggest that cognition in mid- to late-life is impacted by the cumulative effect of nutrition, education, social, and family environment in early life and midlife. Prenatal measures reflecting birth size had little effect on cognition once factors throughout the lifespan were considered.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by Q. Xu (Statistician), T. Xu (Statistician), and D. Hu (Statistician).
ACKNOWLEDGMENT
The authors thank the Beijing Population Registry Office for its assistance in tracing members of the Peking Union Medical College Hospital birth cohort. The authors also thank Dr. James Schlesselman for helpful suggestions on this manuscript. Dr. Schlesselman is affiliated with the Department of Biostatistics, University of Pittsburgh, and is the Director, Biostatistics Facility, University of Pittsburgh Cancer Institute. Zeng Yi, PhD, as PI at Duke University, contributed to acquisition of funding for NIA Program Project 5P01AG17937-03, for the main birth cohort study. The authors also thank all the study participants for their time and support.
DISCLOSURE
Dr. Zhang reports no disclosures. Dr. Plassman receives research support from the NIH [U01 AG09740 (Site PI), R01 AG027010 (Co-investigator), R21 DE-016970 (Co-investigator), and R21 DE019518-01(Co-investigator)], the Veterans Administration, and the Alzheimer’s Association [IIRG 0891522 (PI)], and her spouse has received royalty payments from Abbott Laboratories for pulmonary/respiratory instruments. Dr. Xu reports no disclosures. Dr. Zahner has received honoraria from Pfizer China for 2 lectures on writing research grants applications. Dr. Wu serves on the Editorial Boards of Ageing International and the Journal of Applied Gerontology; receives research support from National Institutes of Health (NIH)/National Institute for Dental and Craniofacial Research [1 R21 DE019518-01 (PI) and 5R21DE016970 (PI)], NIH/Fogarty International Center, Chinese Dementia Care Research Center [Infrastructure, Training And Pilot Studies (Co-investigator)], and Centers for Disease Control and Prevention [Prevention Research to Promote and Protect Brain Health (Consultant)]. Dr. Gai reports no disclosures. Dr. Wen reports no disclosures. Dr. Chen reports no disclosures. Dr. Gao reports no disclosures. Dr. Hu reports no disclosures. Dr. Xiao reports no disclosures. Dr. Shen reports no disclosures. Dr. Liu reports no disclosures. Dr. Xu reports no disclosures.
DISCLAIMER
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Supplementary Material
Address correspondence and reprint requests to Prof. Zhen-Xin Zhang, Department of Neurology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, 1 Shuaifuyuan, Wanfujing, Beijing 100730, China wuzhangzhenxin@medmail.com.cn
Supported by NIH, National Institute on Aging Project 5P01AG17937-03; also funded in part by Chinese National Science and Technology Project 96-096-05-01 and Chinese Medical Board of New York, Inc., New York Grant 99-699.
Disclosure: Author disclosures and a disclaimer are provided at the end of the article.
Received December 16, 2008. Accepted in final form April 13, 2009.
REFERENCES
- 1.Langley-Evans SC. Fetal programming of adult disease: an overview. In: Langley-Evans SC, ed. Fetal Nutrition and Adult Disease. Oxfordshire: CABI Publishing; 2004:1–16. [Google Scholar]
- 2.Xiao X, Zhang ZX, Cohen HJ, et al. Evidence of a relationship between infant birth weight and later diabetes and impaired glucose regulation in a Chinese population. Diabetes Care 2008;31:483–487. [DOI] [PubMed] [Google Scholar]
- 3.Shenkin SD, Starr JM, Pattie A, Rush MA, Whalley LJ, Deary IJ. Birth weight and cognitive function at age 11 years: the Scottish Mental Survey 1932. Arch Dis Child 2001;85:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gale CR, O’Callaghan FJ, Godfrey KM, Law CM, Martyn CN. Critical periods of brain growth and cognitive function in children. Brain 2004;127:321–329. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord 2006;20:63–72. [DOI] [PubMed] [Google Scholar]
- 7.Jefferis BJ, Power C, Hertzman C. Birth weight, childhood socioeconomic environment, and cognitive development in the 1958 British birth cohort study. BMJ 2002;325:305–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards M, Hardy R, Kuh D, Wadsworth ME. Birthweight, postnatal growth and cognitive function in a national UK birth cohort. Int J Epidemiol 2002;31:342–348. [PubMed] [Google Scholar]
- 9.Strauss RS. Adult functional outcome of those born small for gestational age: twenty-six-year follow-up of the 1970 British Birth Cohort. JAMA 2000;283:625–632. [DOI] [PubMed] [Google Scholar]
- 10.Sommerfelt K, Andersson HW, Sonnander K, et al. Cognitive development of term small for gestational age children at five years of age. Arch Dis Child 2000;83:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Birth weight and intellectual performance in late adolescence. Obstet Gynecol 1992;79:543–546. [PubMed] [Google Scholar]
- 12.Sørensen HT, Sabroe S, Olsen J, Rothman KJ, Gillman MW, Fischer P. Birth weight and cognitive function in young adult life: historical cohort study. BMJ 1997;315:401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martyn CN, Gale CR, Sayer AA, Fall C. Growth in utero and cognitive function in adult life: follow up study of people born between 1920 and 1943. BMJ 1996;312:1393–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang ZX, Zahner GE, Román GC, et al. Dementia subtypes in China: prevalence in Beijing, Xian, Shanghai, and Chengdu. Arch Neurol 2005;62:447–453. [DOI] [PubMed] [Google Scholar]
- 15.Zhao JH, Zhang ZX, Hong X, et al. Functions and limitations of neurological tests on diagnosis of dementia. Chin J Neurol 2002;35:333–335. [Google Scholar]
- 16.Fuld PA, Masur DM, Blau AD, Crystal H, Aronson MK. Object-memory evaluation for prospective detection of dementia in normal functioning elderly: predictive and normative data. J Clin Exp Neuropsychol 1990;12:520–528. [DOI] [PubMed] [Google Scholar]
- 17.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. Available at: http://www.idf.org/webdata/docs/Metabolic_syndrome_definition.pdf. Accessed September 2, 2005.
- 18.Thorvaldsen P, Asplund K, Kuulasmaa K, Rajakangas AM, Schroll M. Stroke incidence, case fatality, and mortality in the WHO MONICA project: World Health Organization Monitoring Trends and Determinants in Cardiovascular Disease. Stroke 1995;26:361–367. [DOI] [PubMed] [Google Scholar]
- 19.Ananth CV, Kleinbaum DG. Regression models for ordinal responses: a review of methods and applications. Int J Epidemiol 1997;26:1323–1333. [DOI] [PubMed] [Google Scholar]
- 20.Isaacs E, Oates J. Nutrition and cognition: assessing cognitive abilities in children and young people. Eur J Nutr 2008;47(suppl 3):4–24. [DOI] [PubMed] [Google Scholar]
- 21.Richards M, Hardy R, Wadsworth ME. Long-term effects of breast-feeding in a national birth cohort: educational attainment and midlife cognitive function. Public Health Nutr 2002;5:631–635. [DOI] [PubMed] [Google Scholar]
- 22.Isaacs EB, Gadian DG, Sabatini S, et al. The effect of early human diet on caudate volumes and IQ. Pediatr Res 2008;63:308–314. [DOI] [PubMed] [Google Scholar]
- 23.Abbott RD, White LR, Ross GW, et al. Height as a marker of childhood development and late-life cognitive function: the Honolulu-Asia Aging Study. Pediatrics 1998;102:602–609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


