SUMMARY
To safeguard from “permissive” NK cell reactivity towards target cells, activation by receptors such as NKG2D and 2B4 includes a requirement for synergistic coactivation. How synergy occurs is not known. Synergistic phosphorylation of PLC-γ2, Ca2+ mobilization, and degranulation triggered by NKG2D and 2B4 coengagement were blocked by Vav1 knockdown, but enhanced by knockdown of the ubiquitin ligase c-Cbl. c-Cbl inhibits Vav1-dependent signals, as c-Cbl knockdown did not rescue the Vav1 defect. Moreover, c-Cbl knockdown and Vav1 overexpression each circumvented the requirement for synergy, as NKG2D or 2B4 alone became sufficient for activation. Thus, synergy does not require strict complementation but, rather, enhanced Vav1 signals to overcome inhibition by c-Cbl. Inhibition of cytotoxicity by CD94-NKG2A binding to HLA-E on target cells was dominant over synergistic activation, even after c-Cbl knockdown. Therefore, NK cell activation by synergizing receptors is regulated at the level of Vav1 by a hierarchy of inhibitory mechanisms.
INTRODUCTION
Signaling pathways for the activation of immune cells often involve cooperation between different receptors, such as T cell receptor (TCR) and CD28 (Acuto and Michel, 2003), B cell receptor (BCR) and CD19 (Cherukuri et al., 2001; Fearon and Carroll, 2000), and chemokine receptors and integrins (Kinashi, 2005; Shamri et al., 2005). To ensure protective immunity without the induction of inflammatory and autoimmune diseases, activation signals are under the control of inhibitory receptors, such as CTLA-4 and PD-1 in T cells, and CD22 and FcγRIIb in B cells (Long, 2008). In contrast to the activation of T and B cells, which is dominated by antigen-specific receptors selected to not react with ‘self’, activation of natural killer (NK) cells relies on the integration of signals from ‘co-activation’ receptors, which bind endogenous, ‘self’ ligands on target cells (Bryceson et al., 2006a; Lanier, 2005). Thus, NK cell cytotoxicity towards target cells is tempered by a requirement for combined signals from multiple activating receptors (Bryceson et al., 2006a; Carlsten et al., 2007; Lanier et al., 1997; Pende et al., 2001). In addition, inhibitory receptors, including receptors specific for MHC class I and receptors that bind non-MHC molecules on target cells, antagonize signals from activating receptors, thereby also providing protection of healthy cells from lysis by NK cells (Long, 2008). In addition, engagement of inhibitory receptors on NK cells is required for the acquisition and/or maintenance of NK cell responsiveness (Anfossi et al., 2006; Fernandez et al., 2005; Johansson et al., 1997; Kim et al., 2005).
Signaling pathways triggered by individual NK cell receptors have been characterized to some extent (Billadeau et al., 2003; Bloch-Queyrat et al., 2005; Bonnema et al., 1994; Bryceson et al., 2006a; Moretta et al., 2001; Vivier et al., 2004), but one of the key unresolved questions is how signals delivered by several different receptors on NK cells are integrated in order to result in appropriate functional responses (Di Santo, 2008). A difficulty in the study of NK cell responses is the multiplicity of receptor–ligand interactions between NK cells and target cells, including ligands, and perhaps receptor–ligand pairs, that have not been identified yet (Bryceson and Long, 2008; Tassi et al., 2006). Recently, however, evidence was provided that primary, resting NK cells isolated from human blood, do not respond to single activating receptors but require co-engagement of specific pairs of activating receptors (Bryceson et al., 2009; Bryceson et al., 2006b). Understanding the basis for such synergy should provide insight into the regulation of NK cell cytotoxicity and cytokine production.
Among the pairwise combinations of receptors that synergize to activate resting NK cells are NKG2D (CD314) and 2B4 (CD244), and 2B4 and DNAM-1 (CD226). The combination of NKG2D and DNAM-1 did not synergize. This kind of information has been useful in determining the sensitivity of primary tumor cells to lysis by primary NK cells (Carlsten et al., 2007). NKG2D associates with DAP10, a small signaling subunit that recruits either PI3K or Grb2-Vav1 through its phosphorylated tyrosine (Billadeau et al., 2003; Graham et al., 2006; Upshaw et al., 2006). 2B4 carries several tyrosines in its cytoplasmic tail, which recruit the small adapter SAP bound to the tyrosine kinase Fyn (Chen et al., 2004). The signaling properties of DNAM-1 are still largely unknown. The natural ligands of NKG2D are MICA and MICB, and the small family of ULBP molecules (Bauer et al., 1999; Cosman et al., 2001). The expression of NKG2D ligands is inducible by stress, such as DNA damage (Gasser et al., 2005). The ligand of 2B4, CD48, is broadly expressed on hematopoietic cells.
Synergistic NK cell activation by two co-activation receptors could be due to a strict complementation, whereby enhanced signaling by either one of the receptors would not overcome the lack of signals from the other. Alternatively, synergistic signals may be required to overcome a high threshold for activation. In the latter hypothesis, enhanced signaling by each receptor may suffice to activate NK cell responses. In this study, we sought to test these hypotheses and to identify signaling molecules required for NKG2D and 2B4 synergy. Signaling during synergistic activation was studied not only by receptor ligation with Abs, but also in the context of defined and physiological receptor-ligand interactions, which was achieved by using Drosophila S2 cells as target cells. S2 cells that express ULBP1, CD48, or both were used. Primary, resting NK cells, or the cell line NKL after a period of rest, were used to assess the direct contribution of individual receptors to NK cell activation in the absence of exogenous cytokines. The results revealed that the guanine exchange factor Vav1 and the E3 ubiquitin ligase c-Cbl act coordinately to control NKG2D and 2B4 synergy, and uncovered a previously unappreciated role of c-Cbl as a gatekeeper for NK cell activation.
RESULTS
NKG2D and 2B4 Synergy Induces Degranulation but not Polarization
Drosophila Schneider line 2 (S2) cells with stable expression of ULBP1, CD48, and both ULBP1 and CD48 were generated (Figure S1A) in order to trigger NKG2D and 2B4 synergy in the context of natural ligands at the surface of a cell. Primary, resting NK cells isolated from human blood were incubated with transfected S2 cells for 2 hr and degranulation measured by the cell surface appearance of CD107a (LAMP-1). As shown with S2 cells with inducible expression of ULBP1 and CD48 (Bryceson et al., 2009), each ligand alone induced little degranulation, whereas ULBP1 and CD48 co-expression resulted in degranulation by ~13% of NK cells (Figure S1B), which was comparable to NK cell degranulation in response to antibody-dependent cellular cytotoxicity (ADCC) (Figure S1B). To test whether synergy between NKG2D and 2B4 induced polarization of cytolytic granules, as well as degranulation, perforin polarization was examined in resting NK cells stimulated by transfected S2 cells. NK-target cell conjugates were scored for granule polarization by confocal microscopy, using reconstructed 3-dimensional images. ICAM-1 expression on S2 cells induced polarization of perforin-containing granules in ~70% of NK cells (Figure S1C), as reported (Bryceson et al., 2005). In contrast, expression of ULBP1, CD48, or ULBP1 and CD48 on S2 cells triggered little or no granule polarization beyond the background from the random polarization observed with untransfected S2 cells (Figure S1C). Therefore, signals from NKG2D, 2B4, and the combination of NKG2D and 2B4, which is sufficient to induce degranulation, did not induce polarization of perforin-containing granules in resting NK cells. Granules ‘pre-docked’ at the plasma membrane in resting NK cells provide a source of cytolytic granules ready to fuse for degranulation in the absence of polarization of the bulk of cytolytic granules (Liu et al., 2009). The uncoupling of signals for polarization and for degranulation, a property of NK cells (Bryceson et al., 2005) that is not shared with T cells (Anikeeva et al., 2005), provided us with a unique tool to study the signaling pathways underlying synergistic activation of degranulation.
Synergistic Activation of PLC-γ2 by NKG2D and 2B4 Co-engagement
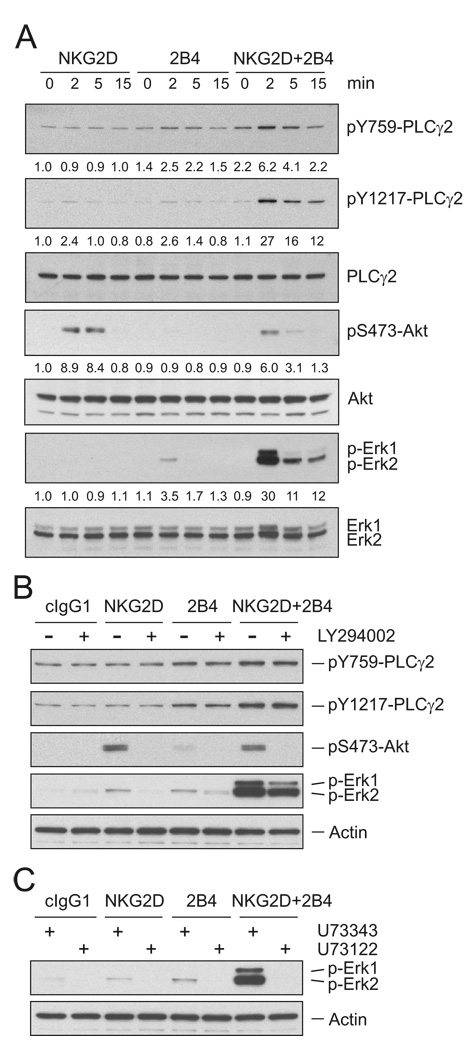
Signaling molecules reportedly associated with degranulation in NK cells include phospholipase C (PLC)-γ, phosphatidylinositol-3-kinase (PI3K), and the mitogen-associated protein kinase (MAPK) Erk (Bryceson et al., 2006a; Caraux et al., 2006; Chen et al., 2006; Jiang et al., 2000; Tassi et al., 2007). Their role in the synergy required to induce degranulation was therefore investigated. NKG2D and 2B4 on resting NK cells were stimulated, either individually or together, by preincubation with specific mAbs followed by cross-linking with goat F(ab′)2 antimouse IgG. Whereas stimulation of either NKG2D or 2B4 alone induced little phosphorylation of PLC-γ2 and Erk, co-engagement of NKG2D and 2B4 led to a synergistic increase in their phosphorylation, which was evident after 2 min and decreased thereafter (Figure 1A). In contrast, phosphorylation of Akt, which is a downstream effector of PI3K activation, was detected by cross-linking NKG2D, as expected (Sutherland et al., 2002), but not 2B4, and was not increased by co-engagement of NKG2D and 2B4 (Figure 1A). Therefore, synergy is not simply due to enhanced signaling by NKG2D. The weak phosphorylation of PLC-γ2 and of Erk, and the lack of Akt phosphorylation after 2B4 crosslinking could be due to a failure of 2B4 to signal under our conditions. However, phosphorylation of 2B4 and recruitment of the adapter SAP was clearly observed after 2B4 crosslinking (Figure S2A). 2B4 phosphorylation and binding of SAP were not enhanced by co-crosslinking NKG2D with 2B4 (Figure S2A). This result implies that synergy between NKG2D and 2B4 is not at the level of 2B4 phosphorylation. Although phosphorylation of PLC-γ2, Akt, and Erk upon stimulation with NKG2D and 2B4 varied somewhat among resting NK cells from different donors, reproducible and consistent results were obtained for the synergistic phosphorylation of PLC-γ2 and Erk, and for NKG2D-mediated phosphorylation of Akt. Thus, co-engagement of NKG2D and 2B4 induces signals for synergistic activation of PLC-γ2 and Erk.
Figure 1. Synergistic Phosphorylation of PLC-γ2 by NKG2D and 2B4 Coengagement.
(A) Freshly isolated, resting NK cells were preincubated with mAbs specific for NKG2D and/or 2B4 on ice for 30 min and stimulated by cross-linking with secondary goat F(ab′)2 anti-mouse IgG at 37°C for the indicated time. Lysates were resolved on a 4 to 12% SDS-PAGE, transferred to a polyvinylidene difluoride (PVDF) membrane, and immunoblotted with Abs to phospho-PLC-γ2 at tyrosine 759 (pY759) or at tyrosine 1217 (pY1217), phospho-Akt at serine 473 (pS473) or phospho-Erk1 and 2. After stripping, the blots were re-probed with Abs to PLC-γ2, Akt, and Erk1 and Erk2. The normalized intensities of the phosphorylated PLC-γ2, Akt, and Erk1 and 2 relative to their total forms were quantified with ImageJ software and are presented for each condition.
(B) Resting NK cells were preincubated with isotype control mAb or mAbs specific for NKG2D and/or 2B4 in the absence or presence of the PI3K inhibitor LY294002 at 20 µM for 30 min. After receptor cross-linking for 2 min in the presence of the inhibitor, cells were lysed and the lysates were immunoblotted using Abs to phospho-PLC-γ2 at tyrosine 759 (pY759) or at tyrosine 1217 (pY1217), phospho-Akt at serine 473 (pS473) or phospho-Erk1 and 2. The blot was stripped and re-probed for actin to monitor sample loading.
(C) Resting NK cells were preincubated with isotype control mAb or mAbs specific for NKG2D and/or 2B4 in the presence of a PLC-γ inhibitor (U73122; 5 µM) or its inactive analog (U73343; 5 µM) for 30 min. After receptor cross-linking for 2 min in the presence of inhibitors, cells were lysed and the lysates were immunoblotted with Abs to phospho-Erk1 and 2. The blot was stripped and re-probed for actin. Data are representative of at least four independent experiments.
We next determined whether PI3K signaling could modulate the synergistic activation of PLC-γ2 and Erk by NKG2D and 2B4 in resting NK cells. Although synergistic degranulation induced by S2 cells expressing ULBP1 and CD48 was blocked by inhibition of both PLC-γ (U73122) and PI3K (LY294002 and wortmannin) (data not shown), PI3K is dispensable for transmission of synergistic signals, as it was not required for the phosphorylation of PLC-γ2, and only partially for Erk activation (Figure 1B). Supporting this, synergistic increase in Ca2+ mobilization, which is required for degranulation and typically depends on PLC-γ activation, was insensitive to PI3K inhibitors (data not shown). Moreover, the PLC-γ inhibitor U73122, but not its inactive analog U73343, completely abrogated synergistic Erk phosphorylation (Figure 1C). These data suggest that PLC-γ, rather than PI3K, is a critical upstream regulator of Erk activation. Taken together, our results suggest an important role of PLC-γ but not PI3K in synergistic signaling by NKG2D and 2B4.
As the supply of freshly isolated NK cells is limited, and as in vitro expansion of NK cells in the presence of cytokines changes their signaling properties, we screened human NK cell lines for a suitable surrogate of resting NK cells that could be used to study molecular signals underlying synergy. Among the cell lines NK92, NKL, NK3.3, and YTS, only NKL cells reproduced the synergistic increase in phosphorylation of PLC-γ2 and Erk, Ca2+ mobilization, and cytotoxic activity when stimulated by NKG2D and 2B4 co-engagement (Figure S3). NKL cells acquired these properties after 24 hr of IL-2 deprivation in a low serum concentration. Therefore, NKL cells were chosen to study NKG2D and 2B4 synergy.
Vav1 Is Required for NKG2D and 2B4 Synergy
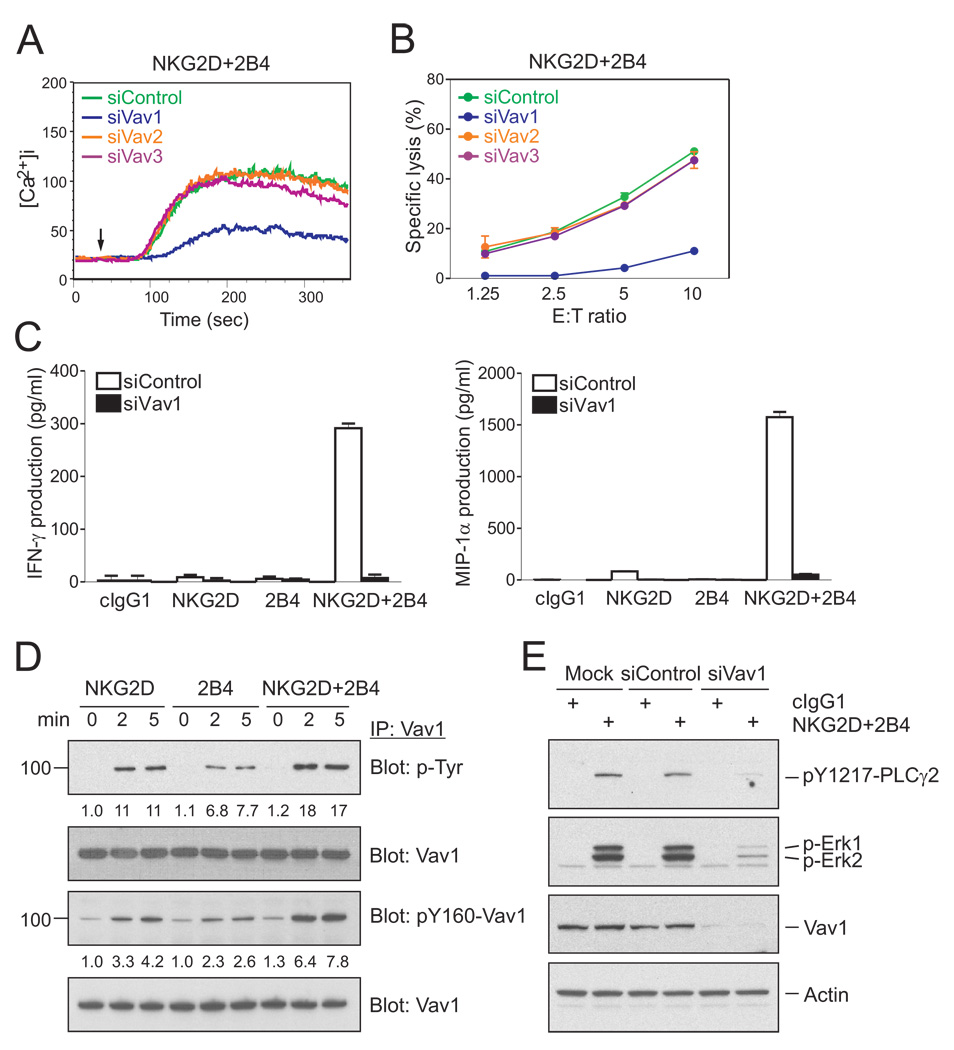
NKG2D can signal through either PI3K or Grb2-Vav1 (Upshaw et al., 2006). The PI3K-independent signaling by NKG2D during synergy suggested that NKG2D may signal through recruitment of Vav1 bound to the adapter Grb2. Vav proteins, which include Vav1, Vav2, and Vav3, have a central role in the regulation of actin cytoskeleton dynamics and lymphocyte-receptor signaling (Tybulewicz, 2005). Furthermore, Vav1 is a preferred substrate of the tyrosine phosphatase SHP-1 during inhibition of NK cell cytotoxicity by MHC class I-specific inhibitory receptors, suggesting that Vav1 may be a central regulator of NK cell cytotoxicity (Peterson and Long, 2008; Stebbins et al., 2003). We therefore tested if Vav proteins were required for NK cell effector function when triggered by NKG2D and 2B4 synergy. After knockdown by small interfering RNA (siRNA) in rested NKL cells (Figure S4A), the synergistic increase in Ca2+ mobilization and cytotoxicity were markedly diminished by knockdown of Vav1, but not Vav2 or Vav3 (Figure 2A and B). Synergistic signals for IFN-γ and MIP-1α release by NKL cells stimulated with beads coated with mAbs to NKG2D and 2B4 were also abrogated by Vav1 knockdown (Figure 2C). The complete dependence on Vav1 is more striking than the phenotype of Vav1−/− mouse NK cells, which retained partial cytotoxic activity towards sensitive target cells (Chan et al., 2001; Colucci et al., 2001) and is also consistent with the selective use of Vav1, but not Vav2 or Vav3, by the NKG2D-DAP10 signaling pathway in NK cells (Cella et al., 2004).
Figure 2. Vav1 Is Required for NKG2D and 2B4 Synergy.
(A) NKL cells were transfected with 300 pmoles of control siRNA or siRNA specific for Vav1, Vav2, or Vav3. After 24 hr, the cells were rested in 5% FBS-containing medium in the absence of IL-2 for another 24 hr, loaded with Fluo-4 at 30°C for 30 min, resuspended in HBSS containing 1% FBS, and preincubated with mAbs for NKG2D and 2B4. Cells were pre-warmed at 37°C and analyzed by flow cytometry. After 30 s, goat F(ab′)2 anti-mouse IgG was added to cross-link the receptors. Changes in fluorescence are shown as a function of time.
(B) Redirected lysis of P815 cells by rested NKL cells transfected with the indicated siRNAs at the indicated effector to target cell ratios (E:T). Cytotoxicity against P815 cells preincubated with mAbs specific for NKG2D and 2B4 was determined after 2 hr with the Europium assay. Error bars represent the SD.
(C) Cytokine release assays with rested NKL cells after transfection with control siRNA or Vav1-specific siRNA and stimulation with beads coated with isotype control mAb (cIgG1) or mAbs specific for NKG2D and/or 2B4. After incubation for 16 hr, IFN-γ and MIP-1α in the supernatants were measured by ELISA. Values represent mean ± SD.
(D) Rested NKL cells were treated as in Figure 1A to stimulate NKG2D and/or 2B4 at 37°C for the indicated time. Lysates were immunoprecipitated with Ab to Vav1, immunoblotted with anti-p-Tyr mAb or anti-pY160-Vav1 Ab, and re-probed for Vav1. The normalized intensities of the phosphorylated Vav1 at tyrosines or at tyrosine 160 relative to total Vav1 are presented.
(E) Rested NKL cells that were mock-transfected or transfected with control siRNA or Vav1-specific siRNA were stimulated with isotype control mAb (cIgG1) or mAbs specific for NKG2D and 2B4. After receptor cross-linking for 2 min, lysates were immunoblotted for pY1217-PLC-γ2, p-Erk1 and 2, or Vav1. After stripping, the blots were re-probed for actin. Data are representative of at least four independent experiments.
Activation of the guanine exchange factor (GEF) activity of Vav1 is accompanied by Vav1 tyrosine phosphorylation. This phosphorylation includes tyrosine 160, which is one of three regulatory tyrosines for the GEF activity (Bustelo, 2001). NKG2D and 2B4 each induced Vav1 phosphorylation independently in rested NKL cells (Figure 2D). Similar results of Vav1 phosphorylation were obtained with primary resting NK cells (Figure S2B). Vav1 phosphorylation induced by NKG2D and 2B4 synergy was equivalent to the sum of the phosphorylation induced by each receptor. Therefore, synergistic activation of NK cells is not due to a complementarity of NKG2D and 2B4 signals for Vav1 phosphorylation. The enhanced Vav1 phosphorylation obtained during NKG2D and 2B4 co-engagement may be required to overcome a threshold for activation.
The role of Vav1 on downstream signaling, such as phosphorylation of PLC-γ2 and Erk, during NKG2D and 2B4 synergy was tested by Vav1 knockdown in NKL cells. Phosphorylation of PLC-γ2 and of Erk was almost eliminated (Figure 2E), in agreement with the control of PLC-γ and Erk activation by Vav1 in T cells (Reynolds et al., 2004; Reynolds et al., 2002).
To test whether Vav1 was required for synergistic activation of NK cells by a different combination of co-activation receptors, primary, resting NK cells and NKL cells were examined after co-engagement of receptor DNAM-1 with 2B4 and with NKG2D. DNAM-1 synergizes with 2B4 but not with NKG2D for Ca2+ mobilization and cytotoxicity (Bryceson et al., 2006b). Crosslinking DNAM-1 alone induced some phosphorylation of Vav1 at tyrosine 160, but little or no phosphorylation of PLC-γ2 and of Erk (Figure S5). However, the DNAM-1 synergy with 2B4 was almost equivalent to that of NKG2D and 2B4 (Figure S5). The synergistic increase in Ca2+ mobilization and cytotoxicity induced by 2B4 and DNAM-1 synergy was markedly impaired by Vav1 knockdown (Figure S5), underscoring the pivotal role of Vav1 in synergistic activation through different combinations of receptors.
Synergistic Activation Is Susceptible to Inhibition by CD94-NKG2A
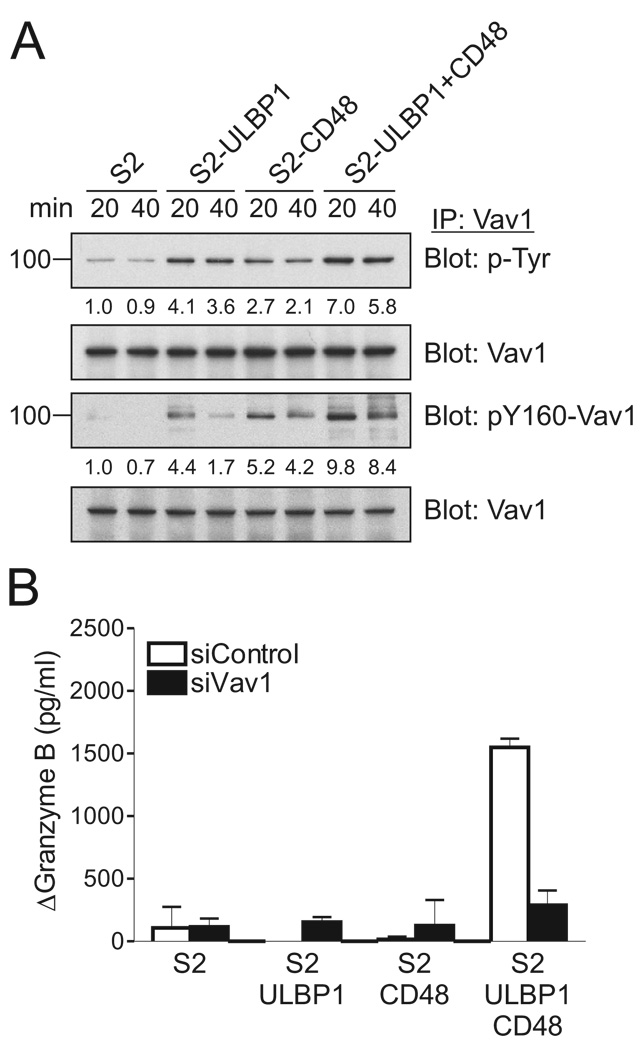
To test synergistic activation in the context of receptor-ligand interactions, NKL cells were mixed with S2 cells expressing ULBP1 and CD48, either alone or in combination. As shown previously, NK cell degranulation does not require co-engagement of the integrin LFA-1 with activation receptors (Bryceson et al., 2009; Bryceson et al., 2005). Tyrosine phosphorylation of Vav1, including that of tyrosine 160, was induced by mixing with S2-ULBP1 and S2-CD48 cells (Figure 3A). As seen after crosslinking receptors with Abs (Figure 2), mixing with S2 cells expressing both ligands (S2-ULBP1+CD48) resulted in greater phosphorylation of Vav1, corresponding to the sum of the phosphorylation induced by single receptor engagement (Figure 3A). In contrast, Granzyme B release depended on the synergistic combination of both ligands for NKG2D and 2B4 (Figure 3B). Granzyme B release triggered by S2-ULBP1+CD48 cells was greatly reduced after Vav1 knockdown (Figure 3B). These results, obtained in the context of receptors interacting with their physiological ligands, confirm that Vav1 is essential for activation of NK cell effector function through NKG2D and 2B4 synergy.
Figure 3. Coactivation by NKG2D and 2B4 Ligands Is Vav1-Dependent.
(A) Rested NKL cells were mixed with S2, S2-ULBP1, S2-CD48, or S2-ULBP1+CD48 insect cells, as indicated. After incubation for 20 or 40 min, Vav1 was immunoprecipitated from lysates and immunoblotted for p-Tyr and pY160-Vav1. Blots were re-probed for Vav1. The normalized intensities of the phosphorylated Vav1 at tyrosines or at tyrosine 160 relative to total Vav1 are presented.
(B) Granzyme B release assay with rested NKL cells that were transfected with control siRNA or Vav1-specific siRNA and incubated with S2 cells or S2 cells expressing ULBP1 and/or CD48, as indicated. After incubation for 2 hr, granzyme B in the supernatants was measured by ELISA. The increase of granzyme B after incubation with target cells relative to granzyme B upon incubation of NKL cells alone (ΔGranzyme B) is presented. Values represent mean ± SD. Data are representative of at least three independent experiments.
As Vav1 is a primary target for dephosphorylation during inhibition through MHC class I-specific inhibitory receptors (Peterson and Long, 2008; Stebbins et al., 2003), one would expect Vav1-dependent synergistic signaling by NKG2D and 2B4 to be susceptible to inhibition. Indeed, degranulation by resting NK cells induced by the ligands ULBP1 and CD48 was inhibited by co-expression of HLA-E (Bryceson et al., 2009). To test the effect of inhibition on Vav1 phosphorylation, NKL cells, which express the inhibitory receptor CD94-NKG2A, were incubated with transfected S2 cells expressing ULBP1, CD48, and HLA-E (Figure S6). Co-expression of peptide-loaded HLA-E inhibited Vav1 phosphorylation and Granzyme B release (Figure S6). Thus, inhibitory receptor signaling through CD94-NKG2A overrides Vav1-dependent synergy.
c-Cbl Inhibits Vav1-Dependent Activation Signals
To gain further insight into how PLC-γ2 activation may be achieved by NKG2D and 2B4 synergy, PLC-γ2 was immunoprecipitated from lysates of rested NKL cells stimulated by NKG2D and 2B4 co-crosslinking, and immunoblotted for phosphotyrosine. As before (Figure 1), synergistic tyrosine phosphorylation of PLC-γ2 was observed 2 min after stimulation (Figure S7). After a longer exposure, tyrosine-phosphorylated bands in the ~95 to ~120 kDa range were detected (Figure S7). The ~95 kDa tyrosine-phosphorylated band comigrated with Vav1 and was also detected by immunoblotting for pY160-Vav1 (Figure S7). pY160-Vav1 was also associated with PLC-γ2 after stimulation with NKG2D alone. A tyrosine-phosphorylated band at ~120 kDa that associated with PLC-γ2 comigrated with c-Cbl after synergistic activation by NKG2D and 2B4 (data not shown). We therefore tested the possible involvement of Cbl proteins in regulating signals delivered through PLC-γ2 and Vav1.
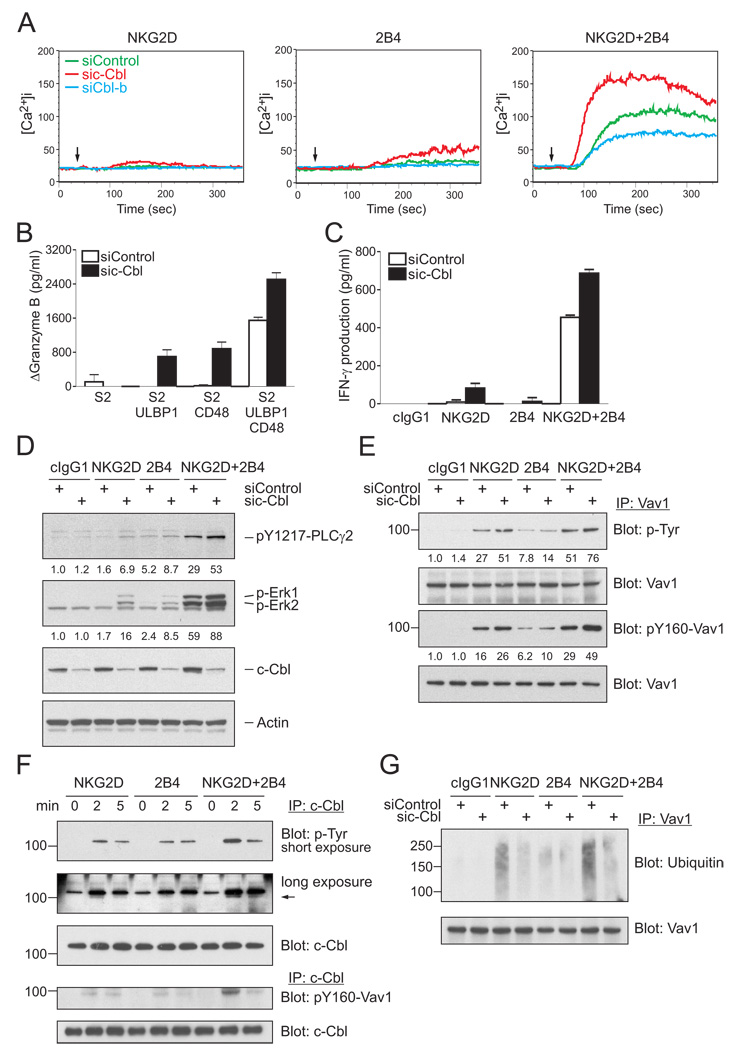
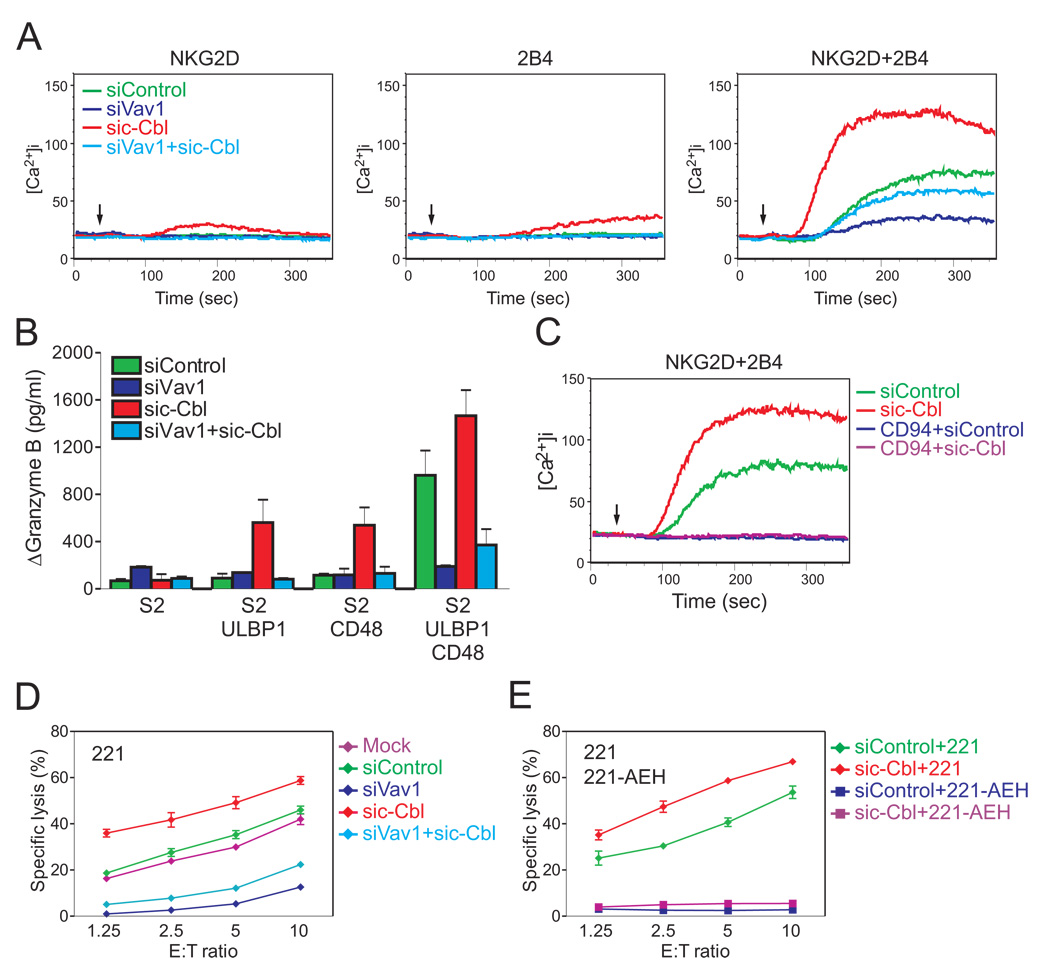
The E3 ubiquitin ligase proteins c-Cbl and Cbl-b are negative regulators of signaling by lymphocyte receptors (Huang and Gu, 2008; Thien and Langdon, 2005). By promoting ubiquitylation, Cbl proteins control the degradation or the trafficking of signaling components. We therefore tested the potential role of Cbl in signaling by NKG2D and 2B4, using siRNA-mediated knockdown of c-Cbl and Cbl-b (Figure S4). The Ca2+ mobilization induced by NKG2D and 2B4 synergy in NKL cells was clearly enhanced by knockdown of c-Cbl (Figure 4A). Knockdown of Cbl-b caused a reduction in the magnitude of Ca2+ mobilization. Furthermore, c-Cbl knockdown rendered otherwise unresponsive NKL cells responsive to the engagement of either NKG2D or 2B4 alone (Figure 4A). Although Ca2+ mobilization induced by NKG2D or by 2B4 after c-Cbl knockdown was low, robust degranulation, as measured by Granzyme B release, was detected after mixing NKL cells with S2 cells expressing either ULBP1 or CD48 (Figure 4B). The Granzyme B release induced by NKG2D and 2B4 synergy was further enhanced after c-Cbl knockdown (Figure 4B). c-Cbl knockdown also enhanced IFN-γ production in response to NKG2D and 2B4 synergy, and promoted IFN-γ secretion in response to NKG2D alone (Figure 4C). The bypass of the requirement for synergy observed after c-Cbl knockdown shows that c-Cbl serves as a gatekeeper and imposes a requirement for synergistic activation signals.
Figure 4. c-Cbl Limits NK Cell Activation and Imposes a Requirement for Synergy.
(A) Calcium flux in rested NKL cells transfected with either control siRNA or siRNA specific for c-Cbl or Cbl-b and stimulated with NKG2D and 2B4 as in Figure 2A.
(B) Granzyme B released by rested NKL cells transfected with control siRNA or c-Cbl-specific siRNA and incubated with S2 cells or S2 cells expressing ULBP1, CD48, or both, as in Figure 3B. Values (ΔGranzyme B) represent mean ± SD.
(C) IFN-γ released by rested NKL cells transfected with control siRNA or c-Cbl-specific siRNA and stimulated with beads coated with isotype control mAb (cIgG1) or mAbs to NKG2D and 2B4 as in Figure 2C. Values represent mean ± SD.
(D) Rested NKL cells transfected with control siRNA or c-Cbl-specific siRNA were stimulated with isotype control mAb (cIgG1) or mAbs to NKG2D and 2B4. After receptor cross-linking for 2 min, lysates were immunoblotted for pY1217-PLC-γ2, p-Erk1 and 2, and c-Cbl. Blots were re-probed for actin. The normalized intensities of the phosphorylated PLC-γ2 and Erk1 and 2 relative to actin are presented.
(E) Rested NKL cells were treated as in Figure 1A to stimulate NKG2D and 2B4 at 37°C for the indicated time. c-Cbl was immunoprecipitated from lysates and probed with a mAb to p-Tyr (top) and an Ab to pY160-Vav1 (bottom). The arrow points to a distinct tyrosine-phosphorylated protein associated with c-Cbl, which was visible after a long exposure. The blots were stripped and re-probed for c-Cbl.
(F and G) Rested NKL cells transfected with control siRNA or c-Cbl-specific siRNA were stimulated with isotype control mAb (cIgG1) or mAbs to NKG2D, 2B4, or both. After receptor cross-linking for 2 min, Vav1 was immunoprecipitated from lysates and probed with Abs to p-Tyr or pY160-Vav1 (F), or an Ab to ubiquitin (G). Blots were re-probed for Vav1. The normalized intensities of the phosphorylated Vav1 at tyrosines or at tyrosine 160 relative to total Vav1 are presented. Data are representative of at least four independent experiments.
c-Cbl knockdown enhanced the phosphorylation of PLC-γ2 and Erk observed during NKG2D and 2B4 synergy (Figure 4D). Although phosphorylation of PLC-γ2 and Erk after stimulation by NKG2D and by 2B4 alone was augmented by c-Cbl knockdown, it remained lower than the phosphorylation obtained during synergistic activation. Similarly, phosphorylation of Vav1 was enhanced by c-Cbl knockdown (Figure 4E). Although Vav1 phosphorylation induced by NKG2D after c-Cbl knockdown was equivalent to that induced by NKG2D and 2B4 synergy, the Vav1 phosphorylation induced by 2B4 after c-Cbl knockdown was not (Figure 4E). Therefore, degranulation by NKL cells is not controlled simply by a threshold of either Vav1 or PLC-γ2 phosphorylation.
Tyrosine phosphorylation of c-Cbl was detected after stimulation for 2 min of rested NKL cells by either NKG2D, 2B4, or the synergistic NKG2D and 2B4 combination (Figure 4F). A c-Cbl-associated tyrosine-phosphorylated protein of ~95 kDa was also observed, as was pY160-Vav1 (Figure 4F). To test whether c-Cbl promoted ubiquitylation of Vav1, NKL cells were treated with c-Cbl siRNA and stimulated through NKG2D, 2B4, or both, and immunoprecipitates of Vav1 were immunoblotted for ubiquitin. c-Cbl-dependent ubiquitylation was clearly detected, even after synergistic activation (Figure 4G). Therefore, the overriding of c-Cbl-mediated inhibition during synergistic activation does not occur through protection of Vav1 from ubiquitylation. To obtain further evidence for Vav1 ubiquitylation, NKL cells were transfected with an HA-tagged ubiquitin, triggered through NKG2D and 2B4, and anti-HA immunoprecipitates were immunoblotted for pY160-Vav1. Several bands in the ~105 kDa to ~150 kDa range, which is larger than the ~95 kDa of Vav1, were detected (Figure S8A). Therefore, phosphorylated Vav1 is ubiquitylated during activation of NKL cells. The time course and the intensity of Vav1 ubiquitylation correlated closely to Vav1 phosphorylation (Figure S8B). Preclearing of c-Cbl removed a large fraction of both phosphorylated and ubiquitylated Vav1 (Figure S8C), suggesting that c-Cbl targets phospho-Vav1 for ubiquitylation. Finally, to test whether Vav1 ubiquitylation leads to its rapid degradation, the kinetics of Vav1 phosphorylation and of the Vav1-dependent Erk phosphorylation were monitored after inhibition of proteasome-mediated degradation with the inhibitor MG132. The decay of phosphorylated Vav1 and Erk was delayed only slightly during inhibition of proteasome (Figure S8D). Therefore, Vav1 is dephosphorylated more rapidly than it is degraded.
The enhanced NK cell response to receptor signaling after c-Cbl knockdown could be explained by the release from inhibition of a bypass activation pathway, independent of Vav1. Such a scenario occurs in the c-Cbl-mediated regulation of T cell development (Chiang et al., 2009; Chiang et al., 2004). The block of thymic positive selection in SLP76-deficient and SLP76-mutant mice was rescued in mice doubly deficient in SLP76 and c-Cbl. The role of c-Cbl in that case is to serve as gatekeeper to prevent a SLP76-independent pathway. To test whether a similar regulation by c-Cbl occurs in the case of Vav1-dependent NK cell activation, we performed double knockdown experiments with siRNA to Vav1 and c-Cbl (Figure S4). The defect in Ca2+ mobilization and Granzyme release due to Vav1 knockdown was not rescued by the combined c-Cbl knockdown (Figure 5A and B). The lack of rescue was observed after stimulation by NKG2D, 2B4, or both. Therefore, c-Cbl controls Vav1-dependent signals.
Figure 5. Signals Inhibited by c-Cbl Are Vav1-Dependent.
(A) Calcium flux in rested NKL cells transfected with either control siRNA or siRNAs specific for Vav1 and for c-Cbl. NKL cells were stimulated with Abs to NKG2D and 2B4 as in Figure 2A.
(B) Granzyme B released by rested NKL cells transfected with control siRNA or siRNAs specific for Vav1 and for c-Cbl and incubated with S2 cells or S2 cells expressing ULBP1, CD48, or both, as in Figure 3B. Values (ΔGranzyme B) represent mean ± SD.
(C) Calcium flux in rested NKL cells transfected with either control siRNA or siRNA specific for c-Cbl and stimulated with Abs to NKG2D and 2B4 combined with or without a mAb to CD94, as in Figure 2A.
(D) Lysis of 221 cells by rested NKL cells either mock-transfected or transfected with control siRNA or siRNAs specific for Vav1 and for c-Cbl.
(E) Lysis of 221 or 221-AEH cells by rested NKL cells transfected with control siRNA or siRNA specific for c-Cbl. Error bars represent the SD. Data are representative of at least three independent experiments.
Inhibition by CD94-NKG2A Is Independent of c-Cbl
Next, we asked whether the inhibition of NKG2D and 2B4 synergy by CD94-NKG2A, an inhibitory receptor specific for HLA-E, relied on the inhibitory contribution of c-Cbl. Ca2+ mobilization induced by NKG2D and 2B4 synergy was inhibited by CD94-NKG2A co-crosslinking, even after c-Cbl knockdown (Figure 5C). To validate these results in the physiological context of NK–target cell contacts, we tested the cytotoxic activity of rested NKL cells towards the HLA class I-negative human cell line 722.221 (referred to as 221) and 221 cells with cell surface expression of HLA-E (221-AEH). Lysis of 221 cells was enhanced after c-Cbl knockdown, and inhibited after knockdown of Vav1 (Figure 5D). As observed after synergistic activation of degranulation (Figure 5B), a very small amount of lysis was recovered after combined knockdown of c-Cbl and Vav1 (Figure 5D). As expected, 221-AEH cells were protected from lysis by NKL cells (Figure 5E). This HLA-E-dependent inhibition was dominant over the enhancement of activation through c-Cbl knockdown (Figure 5E). Therefore, inhibitory signaling through CD94-NKG2A binding to HLA-E is independent of an inhibitory contribution by c-Cbl.
Increased Vav1 Expression Overcomes Inhibition by c-Cbl
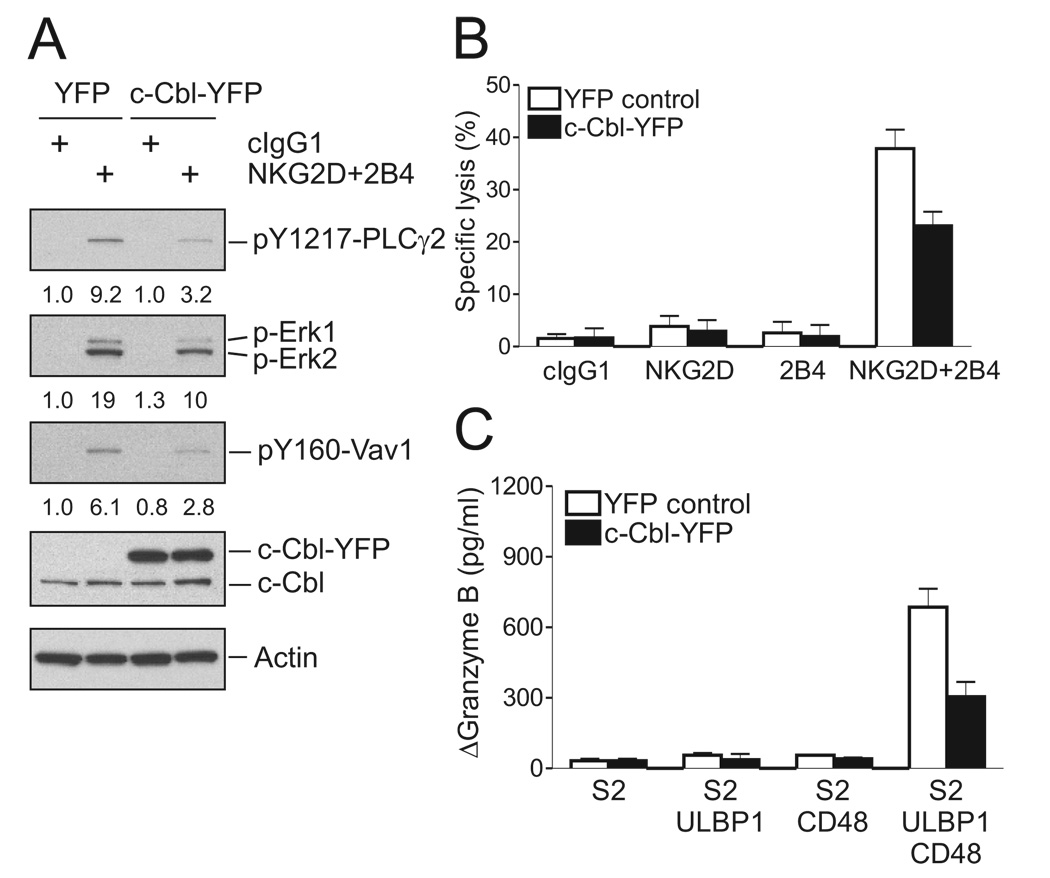
Despite Vav1 ubiquitylation, c-Cbl exerts only moderate control over Vav1 phosphorylation. Phosphorylation of Vav1 induced by crosslinking NKG2D, 2B4, or both was in each case augmented less than 2-fold after c-Cbl knockdown (Figure 4E). This raises the question of whether c-Cbl regulates Vav1-dependent signals through inhibition of another signaling component. In that case, synergistic activation could be due to a signaling pathway that is independent of the c-Cbl-sensitive component and, hence, resistant to inhibition by c-Cbl. Such a hypothesis predicts that c-Cbl overexpression would not inhibit synergistic activation signals. To test it, NKL cells were transfected with YFP-tagged c-Cbl and YFP-positive cells were isolated by flow cytometry. Expression of c-Cbl-YFP in those cells was higher than that of endogenous c-Cbl, and resulted in a 2 to 3-fold reduction in the phosphorylation of Vav1, PLC-γ2, and Erk after activation by NKG2D and 2B4 co-crosslinking (Figure 6A). A similar inhibition was obtained in functional readouts with c-Cbl-YFP expressing NKL cells, which had reduced cytotoxicity upon NKG2D and 2B4 co-engagement (Figure 6B), and reduced granzyme release after incubation with S2 cells expressing ULBP1 and CD48 (Figure 6C). Therefore, negative regulation by c-Cbl is, at least in part, at the level of Vav1-dependent synergistic phosphorylation of PLC-γ2, and is not targeting a protein that is outside of the Vav1-dependent synergistic activation pathway.
Figure 6. c-Cbl Overexpression Inhibits NKG2D and 2B4 Synergy.
NKL cells were transfected with 8 µg of YFP vector control or c-Cbl-YFP plasmid. After 24 hr, cells were sorted for YFP+ cells.
(A) Rested NKL cells transfected with the indicated plasmid were stimulated with isotype control mAb (cIgG1) or mAbs to NKG2D and 2B4. After receptor cross-linking for 2 min, lysates were immunoblotted for pY1217-PLC-γ2, p-Erk1 and 2, and pY160-Vav1. Blots were re-probed for c-Cbl and actin. The normalized intensities of the phosphorylated PLC-γ2, Erk1 and 2, and Vav1 at tyrosine 160 relative to actin are presented.
(B) Redirected lysis of P815 cells by rested NKL cells transfected with the indicated plasmid at an effector to target cell ratio of 10. NKL cells were stimulated with mAbs to NKG2D, 2B4, or both. Error bars represent the SD.
(C) Granzyme B released by rested NKL cells transfected with the indicated plasmid and incubated with S2 cells or S2 cells expressing ULBP1, CD48, or both, as in Figure 3B. Values (ΔGranzyme B) represent mean ± SD. Data are representative of at least three independent experiments.
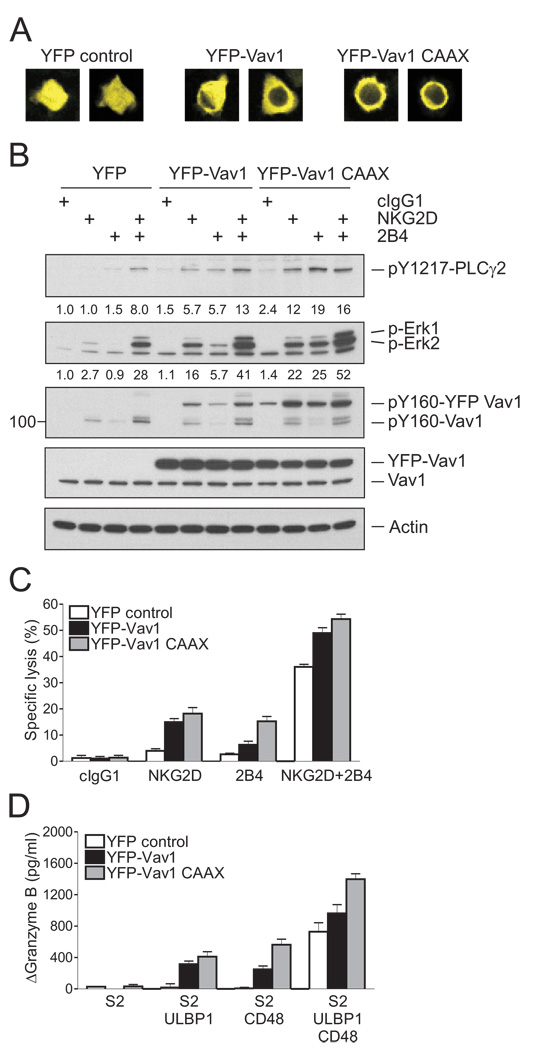
The negative regulation of NK cell responses by c-Cbl at the level of Vav1 during activation by a single receptor, such as NKG2D or 2B4, leads to the prediction that Vav1 overexpression may override c-Cbl-mediated inhibition and bypass the requirement for synergy. Conversely, if activation through a single receptor required complementation by a c-Cbl-sensitive signaling component outside of the Vav1-PLC-γ2 axis, Vav1 overexpression would not override c-Cbl-mediated inhibition. To test these hypotheses, NKL cells were transfected with YFP-tagged Vav1 and YFP-positive cells were isolated by flow cytometry. A membrane-targeted form of Vav1 carrying a CAAX palmitoylation motif at the C-terminus was also used.
As expected, YFP-Vav1 was dispersed throughout the cytosol, whereas YFP-Vav1-CAAX was distributed at the cell periphery (Figure 7A). Expression of YFP-tagged forms of Vav1 in those cells was higher than that of endogenous Vav1, and resulted in a drastic change of the PLC-γ2 and Erk phosphorylation induced by either NKG2D or 2B4 (Figure 7B). In the presence of YFP-Vav1, phosphorylation of PLC-γ2 tyrosine 1217 was no longer synergistic; NKG2D and 2B4 engagement each induced phosphorylation, and their co-engagement resulted in an additive amount of phosphorylation (Figure 7B). The impact of membrane-targeted YFP-Vav1 on PLC-γ2 phosphorylation was even greater; NKG2D and 2B4 crosslinking each induced a PLC-γ2 phosphorylation similar to that obtained by NKG2D and 2B4 co-engagement (Figure 7B). Erk phosphorylation was also greatly enhanced by membrane-targeted YFP-Vav1; NKG2D and 2B4 engagement each induced Erk phosphorylation, and their co-engagement resulted in an additive amount of phosphorylation (Figure 7B). In functional readouts for cytotoxicity and granzyme release, expression of YFP-Vav1 in NKL cells was sufficient to bypass the requirement for synergy (Figure 7C and 7D). Synergy-independent activation through 2B4 was very responsive to the membrane-targeted form of Vav1. We conclude that Vav1 overexpression can overcome c-Cbl-mediated inhibition and, therefore, that c-Cbl imposes a requirement for synergistic activation of NK cells through downregulation of Vav1-dependent signals.
Figure 7. Increased Vav1 Expression Overcomes Inhibition by c-Cbl.
NKL cells were transfected with 8 µg of YFP vector control, YFP-Vav1, or YFP-Vav1 CAAX plasmid. After 24 hr, cells were sorted for YFP+ cells.
(A) Representative confocal images of NKL cells transfected with the indicated plasmid.
(B) Rested NKL cells transfected with the indicated plasmid were stimulated with isotype control mAb (cIgG1) or mAbs to NKG2D, 2B4, or both. After receptor cross-linking for 2 min, lysates were immunoblotted for pY1217-PLC-γ2, p-Erk1 and 2, and pY160-Vav1. Blots were re-probed for Vav1 and actin. The normalized intensities of the phosphorylated PLC-γ2 and Erk1 and 2 relative to actin are presented.
(C) Redirected lysis of P815 cells by rested NKL cells transfected with the indicated plasmid at an effector to target cell ratio of 10. NKL cells were stimulated with mAbs to NKG2D, 2B4, or both. Error bars represent the SD.
(D) Granzyme B released by rested NKL cells transfected with the indicated plasmid and incubated with S2 cells or S2 cells expressing ULBP1, CD48, or both, as in Figure 3B. Values (ΔGranzyme B) represent mean ± SD. Data are representative of at least three independent experiments.
DISCUSSION
Cytotoxicity of NK cells towards target cells is governed by signals transmitted through multiple receptor-ligand interactions. Activation of primary NK cells, freshly isolated from human blood, is tightly regulated and requires signaling by combinations of synergistic receptors, which are not activating on their own (Bryceson et al., 2009; Bryceson et al., 2006a). The main question addressed here was how signals from complementary receptors synergize. Is the requirement for synergy due to complementation of distinct and necessary signals, or to a convergence of signals that have to override an activation threshold? Using co-activation by receptors NKG2D and 2B4 as a model, we have found that they synergize for PLC-γ activation by a pathway that is completely dependent on Vav1 and under negative regulation by the E3 ubiquitin ligase c-Cbl. Synergy is not achieved by signals that complement Vav1-dependent signals, nor by inhibition of c-Cbl-mediated ubiquitylation, but is required to overcome c-Cbl-mediated inhibition of Vav1-dependent signals. These results were obtained by stimulation of receptors either with Abs, or in the more physiological context of receptor-ligand interaction between NK cells and target cells.
The system used here for activation of resting NK cells did not involve signaling through ITAM- containing transmembrane adaptors, nor integrin-dependent signaling. ITAM-independent cytotoxicity has been demonstrated with mouse NK cells that lacked ZAP-70 and Syk kinases (Colucci et al., 2002). Synergistic signaling by NKG2D and 2B4 induced degranulation but not granule polarization, which is dependent on the β2 integrin LFA-1 (Bryceson et al., 2005). Therefore, our experimental approach avoided potential complications due to the contribution of Vav1 to “inside-out” signaling for LFA-1 (Ardouin et al., 2003; Krawczyk et al., 2002). Using Abs to crosslink receptors NKG2D and 2B4, or S2 cells expressing receptor ligands in the absence of LFA-1 ligands, we have shown that Vav1 has an indispensable role in synergistic signaling by NKG2D and 2B4 for PLC-γ2 activation, cytokine secretion, degranulation, and cytotoxicity by NK cells. Vav1 was also required for synergistic signaling by the combination of receptors 2B4 and DNAM-1.
Our results revealed that synergy does not occur by mutual amplification of NKG2D and 2B4 proximal signaling, but occurs downstream of 2B4 phosphorylation and recruitment of the adapter SAP, which were not enhanced by co-crosslinking with NKG2D. Conversely, PI3K-dependent phosphorylation of Akt induced by NKG2D crosslinking was not enhanced by co-crosslinking with 2B4. NKG2D signaling was sufficient to induce phosphorylation of Vav1 and its association with PLC-γ2. The C-terminal SH2 domain of PLC-γ can bind to a phosphorylated tyrosine in the regulatory, acidic domain of Vav1 (Miletic et al., 2006). 2B4 signaling was sufficient to induce a low amount of PLC-γ phosphorylation. Synergistic phosphorylation of PLC-γ by the co-engagement of NKG2D, which can recruit Vav1 through the adapter Grb2 (Graham et al., 2006; Upshaw et al., 2006), and 2B4 may be due to a combination of Vav1 recruitment and phosphorylation. In support of this hypothesis, a membrane-targeted form of Vav1 synergized with 2B4 signals to overcome the dependence on NKG2D signaling.
The Cbl proteins c-Cbl and Cbl-b are multifunctional adaptor molecules with ubiquitin ligase activity, which regulate signaling by diverse receptors (Duan et al., 2004; Huang and Gu, 2008; Liu, 2004; Swaminathan and Tsygankov, 2006; Thien and Langdon, 2005). Cbl-mediated ubiquitylation of receptors can result in internalization from the cell surface and delivery to lysosomal compartments. Ubiquitylation of cytosolic signaling molecules can lead to degradation by proteasomes. In our study, c-Cbl-dependent ubiquitylation of Vav1 was detected. However, the total amount of cellular Vav1 did not change appreciably under the different stimulation conditions, consistent with the fact that only a small fraction of total Vav1 was phosphorylated and ubiquitylated. Furthermore, as inhibition of proteasomal degradation had a very small effect on the half-life of phosphorylated Vav1, ubiquitylation may inhibit Vav1 signaling independently of degradation, as was shown for the inhibition of PI3K in primary T cells through Cbl-b-mediated ubiquitylation of p85 in the absence of degradation (Fang and Liu, 2001).
c-Cbl is required for T cell development past the double-negative stage (Naramura et al., 1998). c-Cbl can inhibit TCR signals through ubiquitylation of the TCR ζ chain (Naramura et al., 1998; Wang et al., 2001) and Vav1 (Miura-Shimura et al., 2003), and by promoting internalization of LAT (Balagopalan et al., 2007). Work in mice with single and double deficiencies in c-Cbl and the adapter SLP76 showed that c-Cbl inhibits a bypass signaling pathway that is independent of SLP76, as a double SLP76 and c-Cbl deficiency restored development (Chiang et al., 2009; Chiang et al., 2004). In contrast, in our study with NK cells, c-Cbl knockdown did not rescue the Vav1 defect, implying that c-Cbl regulates a Vav1-dependent signaling pathway. Overexpression of c-Cbl and Vav1 led to further mechanistic insights into the synergy-dependent activation of NK cells. If c-Cbl were to regulate a Vav1-independent pathway, c-Cbl overexpression would not overcome Vav1-dependent synergistic activation signals. However, inhibition of synergistic activation by overexpression of c-Cbl provided independent evidence that c-Cbl regulates Vav1-dependent signaling. If c-Cbl were to control an obligate complement to Vav1 signals, Vav1 overexpression would not overcome inhibition by c-Cbl. However, Vav1 overexpression resulted in permissive NK cell degranulation after stimulation with S2 cells expressing either ULBP1 or CD48, and in phosphorylation of PLC-γ2 after stimulation with NKG2D or 2B4 alone. These results exclude the possibility of an obligate complement to Vav1 signals that is controlled by c-Cbl. Synergistic signals from NKG2D and 2B4 did not block c-Cbl phosphorylation or Vav1 ubiquitylation, but were required to overcome inhibition by c-Cbl. Whereas the mechanism by which c-Cbl regulates activation signals in NK cells is very different from that by which c-Cbl regulates signaling for T cell development, it exhibits similarities to inhibition by Cbl-b in naïve T cells, which has to be overcome by CD28 through activation of Vav1 (Acuto and Michel, 2003; Bachmaier et al., 2000; Chiang et al., 2000; Huang and Gu, 2008; Krawczyk et al., 2000). The assignment of specific functions to Cbl isoforms is therefore different in T cells and NK cells.
Inhibition of NK cell responses by ITIM-containing receptors occurs at a proximal step, upstream of Ca2+ mobilization and of actin polymerization-dependent processes (Long, 2008). The identification of Vav1 as a primary substrate for dephosphorylation by SHP-1 during inhibitory receptor engagement by MHC class I on target cells (Peterson and Long, 2008; Stebbins et al., 2003), and as an essential component in the NKG2D and 2B4 synergy, as shown here, provides an explanation for the dominance of inhibitory receptors over synergistic activation signals. Our data revealed a hierarchy of inhibitory signals to control NK cell activation: a Vav1-dependent synergy was required to overcome inhibition by c-Cbl but was dominantly inhibited by CD94-NKG2A, even when inhibition by c-Cbl had been lifted.
The expression of ligands for synergistic natural cytotoxicity receptors correlated with the sensitivity of primary tumor cells to killing by freshly isolated, primary NK cells (Carlsten et al., 2007). As the co-activation receptor NKG2D provides innate protection from spontaneous tumor cells (Guerra et al., 2008; Smyth et al., 2005), and downregulation of Cbl-b in mice results in spontaneous tumor rejection by CD8 T cells (Chiang et al., 2007; Loeser et al., 2007), our new understanding of the regulation of NK cell responses through c-Cbl, and the synergy-independent activation through individual receptors after c-Cbl downmodulation, may open ways to optimize NK cell effector functions.
EXPERIMENAL PROCEDURES
Cell Lines and Reagents
Human NK cells were isolated from peripheral blood by negative selection using the MACS NK isolation kit (Miltenyi) as described (Bryceson et al., 2006b). Resting NK cells were resuspended in IMDM (Invitrogen) supplemented with 10% human serum (Valley Biomedical) and used within 2 d of isolation. The human NK cell line NKL (gift of M. Robertson) was cultured in RPMI supplemented with 10% FBS, 1 mM sodium pyruvate, and 200 U/ml rIL-2. NKL cells were rested by culturing 5% FBS and without rIL-2 for 24 hr. YTS cells, NK92 cells, NK3.3 cells were obtained from G. Cohen, H. G. Klingemann, and J. Kornbluth, respectively. P815 cells (American Type Culture Collection) were maintained in IMDM supplemented with 10% FBS and 2mM L-glutamine. 721.221 cells (221; gift of J. Gumperz and P. Parham) and 221 cells transfected with an HLA-E carrying the HLA-A2 signal peptide (gift of D. Geraghty) were cultured as described (Lee et al., 1998). The transfection and maintenance of the Drosophila S2 cell line has been described (Barber et al., 2004). Expression of human ligands for NK cell receptor in S2 cells is described in the supplemental data. All chemicals were from Calbiochem unless indicated otherwise.
Abs
Abs were obtained from the following sources: Anti-NKG2D (149810; R&D Systems); Anti-CD244/2B4 (C1.7; Beckman Coulter); Anti-CD226/DNAM-1 (DX11), CD94 (HP-3D9), and actin (C4) (BD Biosciences); Isotype control mouse IgG1 (MOPC-21; Sigma); Anti-phosphotyrosine (4G10), c-Cbl (7G10), and HA (06–831) (Upstate); Anti-Vav1 (H211), PLC-γ2 (Q-20), PLC-γ2 (B-10), Cbl-b (G-1), Sap (1D12) and Ubiquitin (P4D1) (Santa Cruz); Anti-Vav1 (VAV-30), Vav2 (EP1067Y), and pY731-c-Cbl (EP973Y) (Abcam); Anti-pY160 Vav1 (Biosource); and Anti-Vav1 (2153), Vav3 (2398), pY759 PLC-γ2 (3874), pY1217 PLC-γ2 (3871), pS473 Akt (9271), Akt (9272), pErk (9101), and Erk (9102) (Cell Signaling). Goat F(ab′)2 anti-mouse IgG and PE-conjugated anti-mouse secondary Ab were from Jackson Immunoresearch. Anti-mouse and rabbit Abs conjugated to horseradish peroxidase (HRP) were from Santa Cruz. Anti-rat-HRP and streptavidin-HRP were from GE Healthcare.
Degranulation Assays
To measure degranulation of NK cells toward target S2 cells, NK cell surface CD107a expression was determined by flow cytometry and granzyme B release by ELISA (Cell Sciences) as described (Bryceson et al., 2005).
Ca2+ flux analysis, Cytotoxicity Assay and Cytokine Release Assay
Intracellular Ca2+ flux was measured by flow cytometry after labeling with 2 µM Fluo-4 AM (Invitrogen), as described (Bryceson et al., 2006b). NK cell cytotoxicity against P815 and 221 cells were determined by a Europium assay (Peterson and Long, 2008). IFN-γ (Pierce) and MIP-1α (R&D Systems) release after stimulation by beads coated with mAbs to NK receptors were determined by ELISA as described (Bryceson et al., 2006b).
RNA Interference
NKL cells were transfected with 300 pmoles of siRNAs using Amaxa Nucleofector II system. 2 × 106 cells were resuspended in 100 εl of Amaxa Kit V, mixed with siRNA and then immediately transfected using program O-017. For a total of 48 hr incubation at 37°C, cells were rested for the last 24 hr and then assayed as indicated. The following siRNA sequences were used: Vav1, CGUCGAGGUCAAGCACAUU; c-Cbl, CCUCUCUUCCAAGCACUGA; Cbl-b, GGACAGACGAAAUCUCACA. Prevalidated Vav2 and Vav3-specific siRNAs were purchased from Qiagen. The negative siRNA control was obtained from Dharmacon. Comparable results were obtained with ON-TARGETplus SMARTpool siRNA specific for Vav1 (Dharmacon).
DNA Constructs and Transfections
A human Vav1 cDNA (a gift of D. Billadeau, Mayo Clinic College of Medicine) was subcloned into pEYFP-C1 (Clontech) to tag YFP at the N-terminus of Vav1 (YFP-Vav1). To generate CAAX containing YFP-Vav1 construct (YFP-Vav1 CAAX), an oligonucleotide encoding the 12 C-terminal amino acids of RhoA was inserted by “loop-in” mutagenesis using QuikChange (Stratagene), such that this membrane-targeting sequence of RhoA was incorporated at the C-terminus of Vav1. All final constructs were verified by sequencing. The c-Cbl-YFP and HA-tagged ubiquitin plasmids were provided by Lawrence Samelson (National Cancer Institute) and Dirk Bohmann (University of Rochester), respectively. NKL cells (5 × 106 cells) were transfected with 5 to 8 µg of plasmid DNA by Amaxa Nucleofector II, solution V, and program O-017. Transfected cells were assayed 24 hr posttransfection after a period of rest.
Receptor Cross-Linking and Cell Mixing Experiments
For Ab-mediated cross-linking of NK receptors, NK cells were preincubated with 10 µg/ml isotype control mAb or mAbs specific for NK receptors for 30 min on ice. After washing with medium, NK cells were stimulated by cross-linking with 30 µg/ml goat anti-mouse F(ab′)2 Ab at 37°C for the indicated times. For cell mixing, NK cells and S2 target cells separately chilled on ice were mixed at an effector to target ratio of 1. Cells were incubated for 20 min on ice and then transferred to 37°C for the indicated times. Cells were moved to ice and then lysed for further analysis.
SUPPLEMENTAL DATA
Supplemental Data include Supplemental Experimental Procedures and eight figures
Supplementary Material
ACKNOWLEDGMENTS
We thank M. March for useful discussions; G. Cohen, D. Geraghty, J. Gumperz, P. Parham, H. G. Klingemann, J. Kornbluth, and M. Robertson for cell lines; and L. Samelson and D. Bohman for plasmids. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL DATA
Supplemental Data include Supplemental Experimental Procedures and eight figures
REFERENCES
- Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:6437–6442. doi: 10.1073/pnas.0502467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardouin L, Bracke M, Mathiot A, Pagakis SN, Norton T, Hogg N, Tybulewicz VLJ. Vav1 transduces TCR signals required for LFA-1 function and cell polarization at the immunological synapse. Eur J Immunol. 2003;33:790–797. doi: 10.1002/eji.200323858. [DOI] [PubMed] [Google Scholar]
- Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- Balagopalan L, Barr VA, Sommers CL, Barda-Saad M, Goyal A, Isakowitz MS, Samelson LE. c-Cbl-mediated regulation of LAT-nucleated signaling complexes. Mol Cell Biol. 2007;27:8622–8636. doi: 10.1128/MCB.00467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173:3653–3659. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4:557–564. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- Bloch-Queyrat C, Fondaneche MC, Chen R, Yin L, Relouzat F, Veillette A, Fischer A, Latour S. Regulation of natural cytotoxicity by the adaptor SAP and the Src-related kinase Fyn. J Exp Med. 2005;202:181–192. doi: 10.1084/jem.20050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnema JD, Karnitz LM, Schoon RA, Abraham RT, Leibson PJ. Fc receptor stimulation of phosphatidylinositol 3-kinase in natural killer cells is associated with protein kinase C-independent granule release and cell-mediated cytotoxicity. J Exp Med. 1994;180:1427–1435. doi: 10.1084/jem.180.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114:2657–2666. doi: 10.1182/blood-2009-01-201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20:344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006a;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006b;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustelo XR. Vav proteins, adaptors and cell signaling. Oncogene. 2001;20:6372–6381. doi: 10.1038/sj.onc.1204780. [DOI] [PubMed] [Google Scholar]
- Caraux A, Kim N, Bell SE, Zompi S, Ranson T, Lesjean-Pottier S, Garcia-Ojeda ME, Turner M, Colucci F. Phospholipase C-gamma2 is essential for NK cell cytotoxicity and innate immunity to malignant and virally infected cells. Blood. 2006;107:994–1002. doi: 10.1182/blood-2005-06-2428. [DOI] [PubMed] [Google Scholar]
- Carlsten M, Bjorkstrom NK, Norell H, Bryceson Y, van Hall T, Baumann BC, Hanson M, Schedvins K, Kiessling R, Ljunggren HG, Malmberg KJ. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007;67:1317–1325. doi: 10.1158/0008-5472.CAN-06-2264. [DOI] [PubMed] [Google Scholar]
- Cella M, Fujikawa K, Tassi I, Kim S, Latinis K, Nishi S, Yokoyama W, Colonna M, Swat W. Differential requirements for Vav proteins in DAP10- and ITAM-mediated NK cell cytotoxicity. J Exp Med. 2004;200:817–823. doi: 10.1084/jem.20031847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Hanke T, Fischer KD. Vav-1 regulates NK T cell development and NK cell cytotoxicity. Eur J Immunol. 2001;31:2403–2410. doi: 10.1002/1521-4141(200108)31:8<2403::aid-immu2403>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Chen R, Relouzat F, Roncagalli R, Aoukaty A, Tan R, Latour S, Veillette A. Molecular dissection of 2B4 signaling: implications for signal transduction by SLAM-related receptors. Mol Cell Biol. 2004;24:5144–5156. doi: 10.1128/MCB.24.12.5144-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Allan DS, Krzewski K, Ge B, Kopcow H, Strominger JL. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci U S A. 2006;103:10346–10351. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherukuri A, Cheng PC, Sohn HW, Pierce SK. The CD19/CD21 complex functions to prolong B cell antigen receptor signaling from lipid rafts. Immunity. 2001;14:169–179. doi: 10.1016/s1074-7613(01)00098-x. [DOI] [PubMed] [Google Scholar]
- Chiang JY, Jang IK, Hodes R, Gu H. Ablation of Cbl-b provides protection against transplanted and spontaneous tumors. J Clin Invest. 2007;117:1029–1036. doi: 10.1172/JCI29472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YJ, Jordan MS, Horai R, Schwartzberg PL, Koretzky GA, Hodes RJ. Cbl enforces an SLP76-dependent signaling pathway for T cell differentiation. J Biol Chem. 2009;284:4429–4438. doi: 10.1074/jbc.M808679200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- Chiang YJ, Sommers CL, Jordan MS, Gu H, Samelson LE, Koretzky GA, Hodes RJ. Inactivation of c-Cbl reverses neonatal lethality and T cell developmental arrest of SLP-76-deficient mice. J Exp Med. 2004;200:25–34. doi: 10.1084/jem.20040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci F, Rosmaraki E, Bregenholt S, Samson SI, Di Bartolo V, Turner M, Vanes L, Tybulewicz V, Di Santo JP. Functional dichotomy in natural killer cell signaling: Vav1-dependent and -independent mechanisms. J Exp Med. 2001;193:1413–1424. doi: 10.1084/jem.193.12.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci F, Schweighoffer E, Tomasello E, Turner M, Ortaldo JR, Vivier E, Tybulewicz VL, Di Santo JP. Natural cytotoxicity uncoupled from the Syk and ZAP-70 intracellular kinases. Nat Immunol. 2002;3:288–294. doi: 10.1038/ni764. [DOI] [PubMed] [Google Scholar]
- Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- Di Santo JP. Natural killer cells: diversity in search of a niche. Nat Immunol. 2008;9:473–475. doi: 10.1038/ni.f.201. [DOI] [PubMed] [Google Scholar]
- Duan L, Reddi AL, Ghosh A, Dimri M, Band H. The Cbl family and other ubiquitin ligases: destructive forces in control of antigen receptor signaling. Immunity. 2004;21:7–17. doi: 10.1016/j.immuni.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Fang D, Liu YC. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DB, Cella M, Giurisato E, Fujikawa K, Miletic AV, Kloeppel T, Brim K, Takai T, Shaw AS, Colonna M, Swat W. Vav1 controls DAP10-mediated natural cytotoxicity by regulating actin and microtubule dynamics. J Immunol. 2006;177:2349–2355. doi: 10.4049/jimmunol.177.4.2349. [DOI] [PubMed] [Google Scholar]
- Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Gu H. Negative regulation of lymphocyte development and function by the Cbl family of proteins. Immunol Rev. 2008;224:229–238. doi: 10.1111/j.1600-065X.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Jiang K, Zhong B, Gilvary DL, Corliss BC, Hong-Geller E, Wei S, Djeu JY. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- Johansson MH, Bieberich C, Jay G, Karre K, Hoglund P. Natural killer cell tolerance in mice with mosaic expression of major histocompatibility complex class I transgene. J Exp Med. 1997;186:353–364. doi: 10.1084/jem.186.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- Krawczyk C, Bachmaier K, Sasaki T, Jones RG, Snapper SB, Bouchard D, Kozieradzki I, Ohashi PS, Alt FW, Penninger JM. Cbl-b is a negative regulator of receptor clustering and raft aggregation in T cells. Immunity. 2000;13:463–473. doi: 10.1016/s1074-7613(00)00046-7. [DOI] [PubMed] [Google Scholar]
- Krawczyk C, Oliveira-Dos-Santos A, Sasaki T, Griffiths E, Ohashi PS, Snapper S, Alt F, Penninger JM. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity. 2002;16:331–343. doi: 10.1016/s1074-7613(02)00291-1. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Corliss B, Phillips JH. Arousal and inhibition of human NK cells. Immunol Rev. 1997;155:145–154. doi: 10.1111/j.1600-065x.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- Lee N, Goodlett DR, Ishitani A, Marquardt H, Geraghty DE. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J Immunol. 1998;160:4951–4960. [PubMed] [Google Scholar]
- Liu D, Bryceson YT, Meckel T, Vasiliver-Shamis G, Dustin ML, Long EO. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 2009;31:99–109. doi: 10.1016/j.immuni.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC. Ubiquitin ligases and the immune response. Annu Rev Immunol. 2004;22:81–127. doi: 10.1146/annurev.immunol.22.012703.104813. [DOI] [PubMed] [Google Scholar]
- Loeser S, Loser K, Bijker MS, Rangachari M, van der Burg SH, Wada T, Beissert S, Melief CJ, Penninger JM. Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells. J Exp Med. 2007;204:879–891. doi: 10.1084/jem.20061699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletic AV, Sakata-Sogawa K, Hiroshima M, Hamann MJ, Gomez TS, Ota N, Kloeppel T, Kanagawa O, Tokunaga M, Billadeau DD, Swat W. Vav1 acidic region tyrosine 174 is required for the formation of T cell receptor-induced microclusters and is essential in T cell development and activation. J Biol Chem. 2006;281:38257–38265. doi: 10.1074/jbc.M608913200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura-Shimura Y, Duan L, Rao NL, Reddi AL, Shimura H, Rottapel R, Druker BJ, Tsygankov A, Band V, Band H. Cbl-mediated ubiquitinylation and negative regulation of Vav. J Biol Chem. 2003;278:38495–38504. doi: 10.1074/jbc.M305656200. [DOI] [PubMed] [Google Scholar]
- Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- Naramura M, Kole HK, Hu RJ, Gu H. Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc Natl Acad Sci U S A. 1998;95:15547–15552. doi: 10.1073/pnas.95.26.15547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S, Nanni M, Biassoni R, Bottino C, Moretta A, Moretta L. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31:1076–1086. doi: 10.1002/1521-4141(200104)31:4<1076::aid-immu1076>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Peterson ME, Long EO. Inhibitory receptor signaling via tyrosine phosphorylation of the adaptor Crk. Immunity. 2008;29:578–588. doi: 10.1016/j.immuni.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LF, de Bettignies C, Norton T, Beeser A, Chernoff J, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1. J Biol Chem. 2004;279:18239–18246. doi: 10.1074/jbc.M400257200. [DOI] [PubMed] [Google Scholar]
- Reynolds LF, Smyth LA, Norton T, Freshney N, Downward J, Kioussis D, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways. J Exp Med. 2002;195:1103–1114. doi: 10.1084/jem.20011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamri R, Grabovsky V, Gauguet JM, Feigelson S, Manevich E, Kolanus W, Robinson MK, Staunton DE, von Andrian UH, Alon R. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 2003;23:6291–6299. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland CL, Chalupny NJ, Schooley K, VandenBos T, Kubin M, Cosman D. UL16-binding proteins, novel MHC class I-related proteins, bind to NKG2D and activate multiple signaling pathways in primary NK cells. J Immunol. 2002;168:671–679. doi: 10.4049/jimmunol.168.2.671. [DOI] [PubMed] [Google Scholar]
- Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- Tassi I, Cella M, Gilfillan S, Turnbull I, Diacovo TG, Penninger JM, Colonna M. p110gamma and p110delta phosphoinositide 3-kinase signaling pathways synergize to control development and functions of murine NK cells. Immunity. 2007;27:214–227. doi: 10.1016/j.immuni.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Tassi I, Klesney-Tait J, Colonna M. Dissecting natural killer cell activation pathways through analysis of genetic mutations in human and mouse. Immunol Rev. 2006;214:92–105. doi: 10.1111/j.1600-065X.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- Thien CB, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem J. 2005;391:153–166. doi: 10.1042/BJ20050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opin Immunol. 2005;17:267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Upshaw JL, Arneson LN, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat Immunol. 2006;7:524–532. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- Wang HY, Altman Y, Fang D, Elly C, Dai Y, Shao Y, Liu YC. Cbl promotes ubiquitination of the T cell receptor zeta through an adaptor function of Zap-70. J Biol Chem. 2001;276:26004–26011. doi: 10.1074/jbc.M010738200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.