Summary
A binomial outcome is a count s of the number of successes out of the total number of independent trials n = s + f, where f is a count of the failures. The n are random variables not fixed by design in many studies. Joint modeling of (s, f) can provide additional insight into the science and into the probability π of success that cannot be directly incorporated by the logistic regression model. Observations where n = 0 are excluded from the binomial analysis yet may be important to understanding how π is influenced by covariates. Correlation between s and f may exist and be of direct interest. We propose Bayesian multivariate Poisson models for the bivariate response (s, f), correlated through random effects. We extend our models to the analysis of longitudinal and multivariate longitudinal binomial outcomes. Our methodology was motivated by two disparate examples, one from teratology and one from an HIV tertiary intervention study.
Keywords: Logistic model, Longitudinal data, Multivariate discrete data, Poisson model, Random effects
1. Introduction
A logistic regression analysis with binomial outcome si for observation i conditions on the total number of trials ni = si + fi, where fi is the number of failures. In many studies, ni are not fixed by design but are random. In teratology studies on mice, researchers are interested in the proportion of fetuses with a given deformity (Paul, 1982; Machado et al., 2001). In public health studies on HIV transmission prevention, the proportion of protected sex acts, that is, sex acts using contraceptives, out of the number of total sex acts is a measure of the sexual risk behavior (Posner et al., 2001; Rotheram-Borus et al., 2001; Pinkerton, Chesson, and Layde, 2002; Foss et al., 2003; Rotheram-Borus et al., 2004). The total number of fetuses and sex acts, ni, are random and not under the control of the investigator.
Choosing to model only successes or failures out of the total is no longer appropriate with random ni. Teratology researchers expect the proportion of diabetic mice fetuses with a given deformity to increase as maternal mouse blood glucose levels increase. Analyzing the binomial outcome does not tell us if the increasing fraction of deformities is due to more deformities or fewer well-formed fetuses or a combination thereof. The effect of glucose levels on the number of viable fetuses may be uncertain, is also of interest, and is relevant to the proportion of viable fetuses.
A high ratio of protected sex acts out of the total number of sex acts si/ni and low number of unprotected sex acts fi are both successful outcomes. High ratios si/ni and low fi might be expected to be correlated across the population. A person with ni = 0 might, at a later observation, be anticipated to have high si/ni, should they have ni > 0 at that time. For example, low numbers of sex acts of any kind may be correlated with high fractions of protected acts. Both no sex acts and high fractions of protected acts are successes in HIV transmission prevention studies and the presence of no acts may indicate that a person is likely to have a high probability of protected acts, should any acts occur. This correlation is not included in the logistic regression of si successes out of fi + si trials, and the fi are redundant in the logistic regression.
Several analytic techniques have been proposed to account for the random ni. A logistic regression may include ni as a covariate but does not allow si and fi to be simultaneously modeled. Data points where ni = 0 are still excluded, for example, observations are excluded for people who did not have sex. The multinomial-Poisson model (Terza and Wilson, 1990) models ni ∼ Poisson (λi) and si ∣ ni ∼ binomial (ni, πi). Successes si ∼ Poisson (λiπi) and failures fi ∼ Poisson {λi(1 − πi)} are modeled as independent given covariates. In our two examples, this independence seems a priori implausible. In the multinomial-Poisson model, observations with ni = 0 contribute to the Poisson likelihood, but not to the binomial likelihood.
We recommend correlated bivariate Poisson models (Kocherlakota and Kocherlakota, 1992; Johnson, Kotz, and Balakrishnan, 1997) for the bivariate response (si, fi). We use random effects to model correlation between si and fi. The bivariate Poisson model offers a number of advantages over analytic techniques that do not model si and fi directly. The bivariate Poisson model may be easier to implement in standard statistical software packages than the multinomial-Poisson model; no transformation is needed to produce parameter estimates for outcomes si and fi. The relationship between si and fi is of interest and can be modeled. For models implemented in a Bayesian framework, regression coefficient and random effect priors are easier to formulate because they are on the same scale for both count responses, unlike for the multinomial-Poisson model.
The bivariate Poisson model is easily extended to longitudinal multivariate binomial responses that are prevalent in public health studies on HIV transmission prevention. For example, the proportion of protected sex acts and proportion of sex partners to whom HIV-positive status is disclosed may be assessed (Rotheram-Borus et al., 2001). A negative correlation between protection and disclosure may be hypothesized; people may feel less of an obligation to disclose their HIV-positive status if protection is used. Models for multivariate binomial responses (Lefkopoulou, Moore, and Ryan, 1989; McCullagh and Nelder, 1989) and longitudinal bivariate discrete responses (Rochon, 1996) capture correlation between proportions but do not model correlation between successes and failures within or between binomial responses. We will show in our example on HIV transmission risk data that the correlation of successes and failures provides useful information.
Notation for cross-sectional and longitudinal multivariate binomial data with random n is introduced in Section 2. We consider several covariance models for the random effects of the successes and failures in Section 3 and discuss model selection using Bayes factors in Section 4. The logistic model priors and posteriors induced by the Poisson model are discussed in Section 5. The Poisson models are applied to univariate binomial data from a teratology study and longitudinal bivariate binomial data from an HIV transmission risk study in Section 6. Discussion follows in Section 7.
2. Model Notation
A random-intercept logistic model (Anderson and Aitkin, 1985; Beitler and Landis, 1985; Wong and Mason, 1985; Zeger and Karim, 1991; Agresti, 2002) with observations on experimental units i = 1, …, N, taken at time point j, with longitudinal K-variate binomial data sijk ∼ Binomial (nijk, πijk), k = 1, …, K, with probability πijk of success has linear predictor
| (1) |
where γk is a vector of regression coefficients for the k th response and covariate vector xij is assumed the same for all responses k for simplicity and random effect vector δi = (δi1, …, δiK)′ ∼ NK (0, τ). The model and notation applies to cross-sectional data by dropping subscript j. We first consider univariate longitudinal binomial data, K = 1 dropping the subscript k. We jointly model zij1 = sij and zij2 = fij, our bivariate response, with a bivariate longitudinal Poisson model
| (2a) |
with means λijh = E(zijh), h = 1, 2. For longitudinal multivariate binomial data, zijh corresponds to successes for h = 2k − 1 and failures for h = 2k from the k th binomial observation, h = 1, …, H = 2 × K. Using a log link, the linear predictors are
| (2b) |
where again for simplicity the covariate vector xij is assumed the same for all responses h and αh is a vector of coefficients for xij for the hth outcome. We use random effects βih to induce correlation between zij1 and zij2. For H ≥ 2, the vector βi of random effects for each person βi = (βi1, …, βiH)′ ∼ NH(0, Σ) with elements βih where Σ is an H × H covariance matrix.
3. Covariance Models
We discuss covariance models for H-variate cross-sectional Poisson random effects. The most general covariance model has Σuns = (Σab), a, b = 1, …, H, to be the unstructured covariance matrix. Define and . Special cases of Σuns correspond to interesting models.
For H = 2, we consider five covariance matrices Σq, q = 1, …, 5, nested in Σuns. Uncorrelated random effects with ρ12 = 0, result in diagonal Σ1. The random effects account for overdispersion but do not induce correlation between zij1 and zij2. Covariance matrices Σ2 and Σ3 are special cases of Σuns and Σ1, respectively, with diagonal variances equal, . In some data sets the success and failure counts may share one random effect, βi1 = ±βi2 and singular Σ4 (+) or Σ5 (−) result. For longitudinal models we consider multivariate random-intercept models. Chen et al. (2003) discusses additional covariance models.
4. Comparing Covariance Models
We fit multivariate Poisson models to binomial data in a Bayesian framework and employ Bayesian model checking techniques to compare different covariance model fits to the data. Bayes factors (Jeffreys, 1961; Kass and Raftery, 1995) are used to test random effect covariance models.
We employ a Bayes factor B10 in favor of model M1 against M0 (Bernardo and Smith, 1994, p. 390; Raftery, 1995, p. 165) to compare models M1 and M0. Let ω be a parameter or set of parameters from M1 fixed at ω0 in M0 and let θ be the remaining model parameters. We assume prior densities under M1 and M0 satisfy p(θ ∣ M0) = p(θ ∣ ω = ω0, M1) and calculate B10 using the Savage–Dickey density ratio (Dickey, 1971; Verdinelli and Wasserman, 1995)
| (3) |
where p(ω) and p(ω ∣ Y) are the prior and posterior densities of ω under M1 evaluated at ω = ω0 and Y is the observed data. Nonnested covariance models M1 and M2 are also compared using Bayes factors. If model M2 can be compared to M0 by B20 then M2 can be compared to M1 by a Bayes factor because B21 = B20/B10. Kernel density estimation as implemented in S-PLUS 6.0 (Venables and Ripley, 1999) was used to estimate p(ω) and p(ω ∣ x).
For example, to test M0 : ρ12 = 0, draws are taken from the prior and posterior densities of the most general covariance matrix Σuns. Then βuns,1 is calculated as the ratio of the prior density over the posterior density of correlation ρ12 estimated at ρ12 = 0 by kernel density estimate for both the prior and the posterior.
5. The Induced Logistic Model
5.1 Model Parameters
Although we model the counts, we maintain interest in the binomial probabilities πik. The logistic model parameters are functions of the Poisson model parameters. For K-variate binomial data, πik = λi(2k−1)/{λi(2k−1) + λi2k}. Substituting for λih using equation (2b) gives
| (4) |
The logistic coefficient vector is γk = α2k−1 − α2k and random effect is δik = βi(2k−1) − βi2k with binomial random effect variance for K = 1 under Σuns. For covariance matrix Σq, q = 1, 2, …, 5, variance τ is , , , 0, and , respectively.
For K = 2, Σuns is a 4 × 4 matrix and τ = (τab) is a 2 × 2 matrix with elements a, b = 1, 2, where , , and τ12 = τ21 = σ13 + σ24 − σ14 − σ23. For K ≥ 2 and other covariance models, covariance matrix properties may be utilized to determine τ.
5.2 Priors
We discuss priors for the H-variate Poisson model and the induced priors from the logistic regression coefficients. Priors for p-vector coefficient αh and random effect vector βi are set to be Np(μh, ηI) and NH(0, Σuns), respectively, where I is an identity matrix. The prior density for Σuns is inverse-Wishart, IW (S, v),
where tr (A) is the trace of a square matrix A, Γ is the gamma function, S = (Sab), a, b = 1, …, k, is a known symmetric, positive definite k × k scale matrix and degrees of freedom parameter v. We set S to be diagonal, implying a priori the random effects are equally likely to be positively or negatively correlated. Model Σ1 has prior with independent random effects and results in independent prior densities for ∼ inverse-gamma(1.5, S11/2) and ∼ inverse-gamma(1.5, S22/2).
In the logistic model, the induced priors for γk and δi are Np(μ2k−1 − μk/2, 2ηI) and NK (0, τ), respectively. We choose invertible H × H matrix C so τ is the upper left K × K submatrix of
| (5) |
By properties of the IW distribution τ is also distributed inverse-Wishart (Press, 1982, p. 118). For H = 2,
| (6) |
where c22 ≠ −1 is an arbitrary real number. Then
| (7) |
where V = S11 + S22. For H = 4, C = (r1, r2, r3, r4)′ with row vectors r1 = (1, −1, 0, 0), r2 = (0, 0, 1, −1), and arbitrary row vectors r3 and r4, linearly independent of r1 and r2. Then
| (8) |
where W = (Wab), a, b = 1, 2, has elements W11 = S11 + S22, W22 = S33 + S44, and W12 = W21 = 0.
6. Examples
We consider teratology data containing a cross-sectional univariate binomial outcome and data from the Healthy Living Project (HLP), a public health study on HIV transmission behaviors containing a longitudinal bivariate binomial outcome. Multivariate Poisson models with random effects covariance matrix Σuns are fit to the data. We analyze data sets containing all observations, referred to as complete data sets, and data sets excluding observations whose success and failure counts sum to zero, referred to as nonzero subsets, for closer comparison with logistic models.
Analyses are conducted in WinBUGS version 1.41 software (Spiegelhalter, Thomas, and Best, 2000) and based on a 103 iteration burn-in followed by 106 iterations. Inspection of time series and lagged autocorrelation plots showed the number of iterations to be satisfactory. Posterior means (M) and standard deviations (SDs) of the parameters are reported in Tables 1 and 2.
Table 1. Posterior means (M) and SDs from bivariate Poisson and univariate logistic models fit to teratology data. Reference splotch allele group has neither parent with splotch allele.
| Bivariate Poisson model | Logistic model | |||||||
|---|---|---|---|---|---|---|---|---|
| Complete data | Nonzero subset | Induced | Estimated | |||||
| Part of model | M | SD | M | SD | M | SD | M | SD |
| With exencephaly | ||||||||
| Intercept | −1.57 | 0.89 | −2.15 | 0.96 | −4.69 | 1.08 | −5.20 | 1.21 |
| Blood glucose level | 0.0012 | 0.0011 | 0.0022 | 0.0012 | 0.0041 | 0.0014 | 0.0046 | 0.0016 |
| Splotch allele group | ||||||||
| Father | 0.33 | 0.43 | 0.41 | 0.43 | 0.39 | 0.48 | 0.53 | 0.52 |
| Mother | 0.56 | 0.51 | 0.53 | 0.50 | 0.73 | 0.58 | 0.82 | 0.62 |
| Without exencephaly | ||||||||
| Intercept | 2.78 | 0.46 | 2.53 | 0.45 | ||||
| Glucose level | −0.0024 | 0.00064 | −0.0019 | 0.00063 | ||||
| Splotch allele group | ||||||||
| Father | −0.027 | 0.21 | 0.013 | 0.21 | ||||
| Mother | −0.21 | 0.29 | −0.20 | 0.28 | ||||
| Random effect variances | ||||||||
| With exencephaly | 0.64 | 0.31 | 0.53 | 0.25 | 0.81 | 0.34 | 0.87 | 0.48 |
| Without exencephaly | 0.24 | 0.082 | 0.20 | 0.063 | ||||
| Correlation | 0.040 | 0.25 | −0.12 | 0.24 | ||||
Table 2. Posterior means (M) and SDs of parameters from longitudinal bivariate Poisson and univariate logistic models fit to HLP data. Models adjust for age at baseline and risk group.
| Bivariate Poisson model | Univariate Binomial model | |||||||
|---|---|---|---|---|---|---|---|---|
| Complete data | Nonzero subset | Induced | Estimated | |||||
| Part of model | M | SD | M | SD | M | SD | M | SD |
| Protected acts | ||||||||
| Base | 1.82 | 0.28 | 2.16 | 0.20 | 0.43 | 0.26 | −0.023 | 0.36 |
| Base * Intv | 0.31 | 0.12 | 0.45 | 0.091 | 0.66 | 0.12 | 0.87 | 0.15 |
| Follow | 1.69 | 0.29 | 2.22 | 0.21 | 0.62 | 0.098 | 0.73 | 0.36 |
| Follow * Wks | −0.11 | 0.014 | −0.13 | 0.014 | −0.024 | 0.018 | −0.15 | 0.026 |
| Follow * Wks2 | 0.0073 | 0.0097 | 0.0097 | 0.0010 | 0.0043 | 0.0013 | 0.014 | 0.0019 |
| Follow * Intv | 0.033 | 0.14 | 0.27 | 0.12 | 0.52 | 0.16 | 0.053 | 0.20 |
| Follow * Intv * Wks | 0.011 | 0.027 | −0.048 | 0.029 | 0.13 | 0.036 | 0.23 | 0.052 |
| Follow * Intv * Wks2 | 0.00046 | 0.0020 | 0.0057 | 0.0021 | −0.0083 | 0.0027 | −0.018 | 0.0039 |
| Unprotected acts | ||||||||
| Base | 2.54 | 0.25 | 1.74 | 0.21 | ||||
| Base * Intv | 0.37 | 0.098 | −0.21 | 0.077 | ||||
| Follow | 2.22 | 0.25 | 1.60 | 0.21 | ||||
| Follow * Wks | −0.061 | 0.010 | −0.11 | 0.011 | ||||
| Follow * Wks2 | 0.00040 | 0.00076 | 0.0054 | 0.00081 | ||||
| Follow * Intv | 0.064 | 0.11 | −0.25 | 0.10 | ||||
| Follow * Intv * Wks | −0.069 | 0.020 | −0.18 | 0.022 | ||||
| Follow * Intv * Wks2 | 0.0033 | 0.0015 | 0.014 | 0.0016 | ||||
| Random effect variances | ||||||||
| Protected acts | 3.38 | 0.21 | 2.74 | 0.17 | 5.03 | 0.29 | 6.28 | 0.41 |
| Unprotected acts | 2.25 | 0.12 | 1.86 | 0.11 | ||||
| Correlation | 0.13 | 0.037 | −0.095 | 0.038 | ||||
We plot posterior densities for probability πx given covariate vector x as approximated by the S-PLUS 6.0 kernel density estimator. Comparisons are made between πx estimated from logistic regression models and indirectly estimated from bivariate Poisson regression models as discussed in Section 5. We use a = 1, …, A draws and γ(a) from the joint posterior of the parameter estimates, with A = 1000; every hundredth iteration out of the total 106 iterations was used. The δ(a) are sampled from density N(0, τ(a)). The estimates are
| (9) |
6.1 Teratology Data
The teratology data was originally reported on in Machado et al. (2001). It was collected to measure the effects of diabetes in 68 pregnant female C57BL/6J mutant mice on malformations in their litters. Nonzero subset analyses exclude six females with no live births. The binomial outcome is the fraction of live births with exencephaly. We split the binomial ni into a bivariate count outcome, the number of viable fetuses with and without exencephaly from each litter. Diabetes was chemically induced in the female parents prior to pregnancy and assessed by blood glucose levels in milligrams per deciliter. Some parental mice contained a splotch allele thought to confer added susceptibility to malformations in their offspring. Covariates from the original analysis were used: an intercept; maternal blood glucose level (Glucose level); and a three-category splotch allele indicator, categorized as whether the father had the allele (Father), the mother had the allele (Mother), or neither parent had the allele. The experimental design excluded both parents having the allele. If both parents had the allele a fetus could not form. The linear predictor is parameterized as
| (10) |
6.1.1 Data priors
Priors on α1 and α2 are set to be N4 (0, 10I), proper but basically uninformative. The prior on Σ is IW(S, v), with degrees of freedom, v = 4 and H = 2. We specify a diagonal scale matrix S so the expectation E(Σ) = (v − H − 1)−1 S = S is a reasonable estimate of the random effect variances.
The mean litter size for the C57BL/6J mouse is listed as 7 on the Laboratory Animal Science Center section of the Boston University Medical campus web site, http://www.bumc.bu.edu. We use this to develop a prior for the random effects variances. We start with a guess that half of the litter is malformed so our prior point estimate of the mean litter size of malformed and nonmalformed fetuses is 3.5. We take 0.001 and 7 as a lower and upper 0.005 and 0.995 probability bounds on the mean for an individual litter. We want the 0.005 and 0.995 percentiles of the random effects distribution on the log scale to give us end points of log(0.001) = −6.9 and log(7) = 1.17. Six SDs span 99% of a normal density. The SD of the random effects is approximately {1.9 − (−6.9)}/6 ≈ 1.5. The random effects variance is 1.52 ≈ 2 and we set S11 = S22 = 2.
Priors on γ and δi are then N4 (0, 20I) and N(0, τ), respectively, and the prior on τ is inverse-gamma(1.5, 1).
6.1.2 Results
Parameter estimates from random-intercept bivariate Poisson and univariate logistic models fit to the cross-sectional teratology data set are shown in Table 1. A higher proportion of malformed births with exencephaly is associated with increased blood glucose levels in the logistic model. The Poisson model shows the association with blood glucose is driven by a negative association between nonmalformed birth counts and increased glucose levels; an association with the number of malformed births is not seen. The association between the number of malformed births and blood glucose levels is close to being significant in the nonzero subset (M = 0.0022 and SD = 0.0012), showing that different conclusions might well be drawn from analyses on the complete data and on the nonzero subset.
For the nonzero subset, we estimate Bayes factors βuns,1, comparing covariance structures Σuns to Σ1, to be 0.47 and similarly βuns,2 = 0.60. The first Bayes factor favors independent random effects, ρ12 = 0, and βuns,2 favors equal random effect variances, .
6.2 HLP Data
We use data with n = 175 from the Choosing Life: Empowerment, Action, Results study (CLEAR; Rotheram-Borus et al., 2004) to create priors for models fit to HLP data (n = 936; Gore-Felton et al., 2005; Lightfoot et al., 2005). Nonzero subset analyses (n = 852) exclude observations for participants who were sexually inactive during the previous time period; 20%, 794 out of 3918, observations were excluded. The HLP study was modeled on the CLEAR study and bears many similarities. Both studies were designed to reduce HIV-transmission behaviors and improve the quality of life for persons living with HIV. Across studies, participants shared a similar socioeconomic status and were recruited from similar types of social service agencies in several metropolitan areas from 1999 to 2000 in the CLEAR study and from 2000 to 2002 in the HLP study. Sexual behavior, substance use, and quality-of-life measures were assessed at baseline, 3, 6, 9, and 15 months in the CLEAR study and at baseline and 5-month intervals up to 25 months in the HLP study. At baseline, participants in both studies were randomized to an immediate or delayed intervention condition.
There are two main differences across studies. The CLEAR sample is younger, ages 16 to 29 with a median age of 23, compared to the HLP sample, ages 19 to 67 with a median age of 40. Recruitment was based on different HIV-transmission behaviors. Current drug use and sexual activity were required to be recruited into the CLEAR and HLP studies, respectively. Study details may be found on the CHIPTS web site (http://chipts.ucla.edu/projects/chipts).
The bivariate binomial outcome, the proportion of sex partners to whom HIV-positive status is disclosed and the proportion of protected sex acts, is split into a multivariate Poisson outcome for the number of disclosed and undisclosed sex partners and number of protected and unprotected sex acts. We fit multivariate longitudinal Poisson random effects models. Relevant covariates based on analyses from prior studies include time, in units of 10 weeks (Wks); intervention arm (Intv), categorized as 1 = immediate or 0 = delayed; age at baseline (Age); and a four-category HIV risk indicator established by the Centers for Disease Control (CDC, 2001), categorized as men who are injecting drug users (IDU), non-IDU who are having sex with other men (MSM), non-IDU men not having sex with other men (HTM), and women.
Visual inspection of the HLP data suggested a piecewise regression allowing different trajectories for each intervention arm from baseline to 5 months and from 5 months to 25 months. Indicators for baseline (Base) and follow-up (Follow = 1 − Base) allow the trajectories to be modeled. Quadratic terms for time are included to model an observed curvilinear trend from 5 to 25 months. The linear predictor is parameterized as
| (11) |
We plot estimated mean counts λ̂.jh for outcome h at time point j in Figure 1. Outcomes are modeled on the logarithmic scale and transformed back to the original scale prior to plotting. The transformed expectation is of course not the expectation on the transformed scale for nonlinear transformations. A parametric version of the smearing estimate (Duan, 1983) is used to calculate the mean on the transformed scale. We use a = 1, …, A draws and from the joint posterior of the parameter estimates and random effects, with A = 100; every ten-thousandth iteration out of the total 106 iterations was used. The estimate is
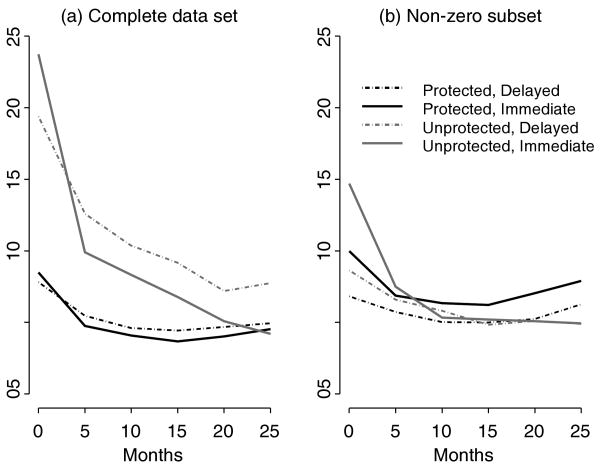
Figure 1.
Estimated mean outcomes over time by intervention condition from longitudinal bivariate Poisson model fit to HLP (a) complete data set and (b) nonzero subset.
| (12) |
6.2.1 Data priors
Prior distribution parameter values for HLP data analyses were obtained from the CLEAR data set. Separate random-intercept Poisson regression models were fit to each outcome in SAS software, version 8.2, using the GLIMMIX macro (Wolfinger and O'Connell, 1993; Littell et al., 1996) using a pseudolikelihood approach. For the hth outcome and pth coefficient a N(μhp, ωhp) prior was used with μhp and ωhp set to the coefficient and coefficient variance estimate from the CLEAR data analyses.
The prior on Σ was set to be IW (S, v), with v = 6. The diagonal scale matrix, S, was specified so the expectation E(Σ) = (v − H − 1)−1 S = S had diagonal variance elements corresponding to random effect variance estimates from the CLEAR data analysis.
Inferred priors on γk and δik are Np(Θk, Λk) and NK(0, τ), respectively, where Θk is a vector with elements Θkp = μ2k−1,p − μ2k,p and diagonal covariance matrix Λk has diagonal elements Λkpp = ω2k−1,pp + ω2k,pp. The prior on τ is inferred to be IW(S, v), where diagonal scale matrix S has elements S11 = 5.49 and S22 = 4.47, calculated from CLEAR random effect variance estimates of 0.99, 1.53, 0.74, and 1.50 for the number of partners disclosed to, partners not disclosed to, protected acts, and unprotected acts, respectively.
6.2.2 Analysis results
We fit random-intercept multivariate Poisson and bivariate logistic models to the bivariate binomial outcome for the fraction of protected sex acts and disclosure. Parameter estimates are similar to parameter estimates from random-intercept bivariate Poisson and univariate logistic models fit to each binomial outcome separately. This is not surprising considering the low correlation of −0.085, 95% probability interval = (−0.17, 0.0068), between random effects in the bivariate binomial model, indicating binomial outcomes are nearly independent. We present parameter estimates for one of the binomial outcomes, the fraction of protected sex acts, in Table 2.
The proportion of protected sex acts increases over time more rapidly at first in the immediate condition, compared to the delayed condition. In Figure 1, the Poisson model shows the initial increase in the logistic model to be driven by an initial decrease in the number of unprotected acts in the immediate condition, compared to the delayed condition. The number of protected acts follow a similar trajectory across intervention conditions; inclusion of the zero counts eliminates the level shift in trajectories between intervention conditions at follow-up time points. Inclusion of the zero counts also reverses the direction of the correlation between random effects.
For the nonzero subset, we estimate Bayes factors βuns,1 to be 1.23, favoring independent random effects, ρ12 = 0, and βuns,2 to be 105.96, favoring unequal random effect variances, .
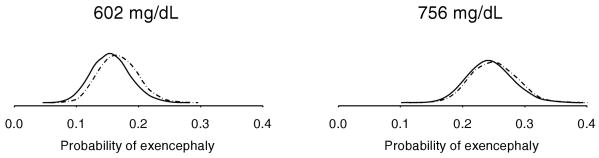
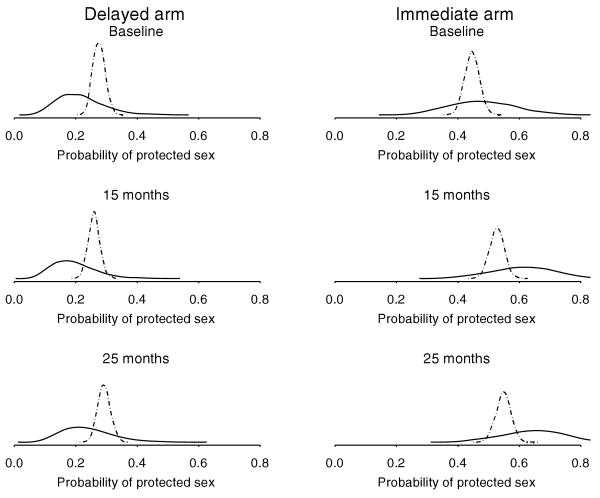
Approximate posterior densities for teratology and HLP outcome probabilities under the logistic and bivariate Poisson models are shown in Figures 2 and 3, respectively. The density shapes from the cross-sectional teratology data are quite similar, whereas the bivariate Poisson densities resulting from the longitudinal HLP data are much narrower compared to the logistic densities. In the HLP data, the bivariate Poisson model produces much smaller confidence intervals compared to the logistic model.
Figure 2.
Approximate marginal posterior density of π, the probability of exencephaly, from bivariate Poisson (- -) and logistic (—) regressions fit to teratology nonzero subset. Densities are shown for 25th and 75th percentiles of sample blood glucose levels.
Figure 3.
Approximate marginal posterior density of π.j, the probability of protected sex, from longitudinal bivariate Poisson (- -) and longitudinal logistic (—) regressions fit to HLP nonzero subset. Densities are shown for each intervention arm at three time points.
7. Discussion
Our analyses highlight the importance of capturing the complex nature of binomial data with random numbers of trials through the multivariate Poisson model. In both of our examples, the expansion of the binomial outcome to the bivariate Poisson model allowed us to examine the relationship between successes and failures and key covariates, giving a more detailed picture of the data. Biologists may be interested to examine whether teratogenic agents lead to different rates of malformed and normal births. Interventions targeting HIV-transmission behaviors may be very different, depending on whether the goal is to decrease unprotected acts or increase the proportion of protected acts.
More complex random effect models are possible. We could replace the random effects in equations (1) and (2b) with a more complicated random effects structure. For example, we could have a random intercept and slope instead of a random intercept, or more complex random effects. Alternatively, we could induce an autoregressive structure on the random effects over time, so that within-subject random effects were not constant over time (refer to Chen et al., 2003). Another possible covariance model for time-varying random effects is the product correlation model (Weiss, 2005, Chapter 13). For both the autoregressive and product correlation models, there would be additional Poisson variability in addition to the random effect variance.
Acknowledgments
The authors thank Michael Collins, Ph.D., for permission to use the teratology data set and Mary Jane Rotheram-Borus, Ph.D., for permission to use the CLEAR and HLP HIV transmission risk data sets in our analyses. The CLEAR data was collected with the support of NIDA grant DA07903 and the HLP data was collected with the support of NIMH grant U10MH057615. REW was partially supported by NIMH grant 1 R01 MH60213 and by the Center for HIV Identification, Prevention and Treatment Services (CHIPTS) within the Center for Community Health (P30MH-58107). We thank the referees and editors for helpful comments.
References
- Agresti A. Categorical Data Analysis. 2nd. New York: Wiley; 2002. [Google Scholar]
- Anderson DA, Aitkin M. Variance component models with binary response: Interviewer variability. (B).Journal of the Royal Statistical Society. 1985;47:203–210. [Google Scholar]
- Beitler PJ, Landis JR. A mixed-effects model for categorical data. Biometrics. 1985;41:991–1000. [PubMed] [Google Scholar]
- Bernardo JM, Smith AFM. Bayesian Theory. Chichester: Wiley; 1994. [Google Scholar]
- CDC. HIV/AIDS Surveillance Report 13. 2001. [Google Scholar]
- Chen MH, Ibrahim JG, Shao QM, Weiss RE. Prior elicitation for model selection and estimation in generalized linear mixed models. Journal of Statistical Planning and Inference. 2003;111:57–76. [Google Scholar]
- Dickey J. The weighted likelihood ratio, linear hypotheses on normal location parameters. The Annals of Statistics. 1971;42:204–223. [Google Scholar]
- Duan N. Smearing estimate: A nonparametric retransformation method. Journal of the American Statistical Association. 1983;78:605–610. [Google Scholar]
- Foss AM, Vickerman PT, Heise L, Watts CH. Shifts in condom use following microbicide introduction: Should we be concerned? AIDS. 2003;17:1227–1237. doi: 10.1097/00002030-200305230-00015. [DOI] [PubMed] [Google Scholar]
- Gore-Felton C, Rotheram-Borus MJ, Weinhardt LS, Kelly JA, Lightfoot M, Kirshenbaum SB, Johnson MO, Chesney MA, Catz SL, Ehrhardt AA, Remien RH, Morin SF, The NIMH Healthy Living Project Team The Healthy Living Project: An individually tailored, multidimensional intervention for HIV-infected persons. AIDS Education and Prevention. 2005;17 A:21–39. doi: 10.1521/aeap.17.2.21.58691. [DOI] [PubMed] [Google Scholar]
- Jeffreys H. Theory of Probability. 3rd. Oxford: Oxford University Press; 1961. [Google Scholar]
- Johnson N, Kotz S, Balakrishnan N. Discrete Multivariate Distributions. New York: Wiley; 1997. [Google Scholar]
- Kass RE, Raftery AE. Bayes factor and model uncertainty. Journal of the American Statistical Association. 1995;90:773–795. [Google Scholar]
- Kocherlakota S, Kocherlakota K. Bivariate Discrete Distributions. New York: Marcel Dekker; 1992. [Google Scholar]
- Lefkopoulou M, Moore D, Ryan L. The analysis of multiple correlated binary outcomes; applications to rodent teratology experiments. Journal of the American Statistical Association. 1989;84:810–815. [Google Scholar]
- Lightfoot M, Rogers T, Goldstein R, Rotheram-Borus MJ, May S, Kirshenbaum S, Weinhardt L, Zadoretzky C, Kittel L, Johnson M, Gore-Felton C, Morin SF. Predictors of substance use frequency and reductions in seriousness of use among persons living with HIV. Drug and Alcohol Dependency. 2005;77:129–138. doi: 10.1016/j.drugalcdep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, North Carolina: SAS Institute; 1996. [Google Scholar]
- Machado AF, Zimmerman EF, Hovland DN, Jr, Weiss RE, Collins MD. Diabetic embryopathy in C57BL/6J mice: Altered fetal sex ratio and impact of the splotch allele. Diabetes. 2001;50:1193–1199. doi: 10.2337/diabetes.50.5.1193. [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. 2nd. London: Chapman and Hall; 1989. [Google Scholar]
- Paul SR. Analysis of proportions of affected foetuses in teratological experiments. Biometrics. 1982;38:361–370. [PubMed] [Google Scholar]
- Pinkerton SD, Chesson HW, Layde PM. Utility of behavioral changes as markers of sexually transmitted disease risk reduction in sexually transmitted disease/HIV prevention trials. Journal of Acquired Immune Deficiency Syndromes. 2002;31:71–79. doi: 10.1097/00126334-200209010-00010. [DOI] [PubMed] [Google Scholar]
- Posner SF, Pulley L, Artz L, Cabral R, Macaluso M. Psychosocial factors associated with self-reported male condom use among women attending public health clinics. Sexually Transmitted Diseases. 2001;28:387–393. doi: 10.1097/00007435-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Press JS. Applied Multivariate Analysis: Using Bayesian and Frequentist Methods of Inference. Melbourne: Krieger Publishing Co.; 1982. [Google Scholar]
- Raftery AE. Hypothesis testing and model selection. In: Gilks WR, Richardson S, Spiegelhalter DJ, editors. Markov Chain Monte Carlo in Practice. London: Chapman and Hall/CRC; 1995. [Google Scholar]
- Rochon J. Analyzing bivariate repeated measures for discrete and continuous outcome variables. Biometrics. 1996;52:740–750. [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Lee MB, Murphy D, Futterman D, Duan N, Birnbaum J, Lightfoot M. Efficacy of a preventative intervention for youths living with HIV. American Journal of Public Health. 2001;91:400–405. doi: 10.2105/ajph.91.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Swendeman D, Comulada WS, Weiss RE, Lee M, Lightfoot M. Prevention for substance using HIV positive young people: Telephone and in-person delivery. Journal of Acquired Immune Deficiency Syndromes. 2004;37:S68–S77. doi: 10.1097/01.qai.0000140604.57478.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalter DJ, Thomas A, Best NG. WinBUGS Version 1.4 User Manual. MRC Biostatistics Unit; 2000. [Google Scholar]
- Terza JV, Wilson PW. Analyzing frequencies of several types of events: A mixed multinomial-poisson approach. The Review of Economics and Statistics. 1990;72:108–115. [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with SPLUS. New York: Springer; 1999. [Google Scholar]
- Verdinelli I, Wasserman L. Computing Bayes factors using a generalization of the Savage-Dickey density ratio. Journal of the American Statistical Association. 1995;90:614–618. [Google Scholar]
- Weiss RE. Modeling Longitudinal Data. New York: Springer-Verlag; 2005. [Google Scholar]
- Wolfinger R, O'Connell M. Generalized linear mixed models: A pseudo-likelihood approach. Journal of Statistical Computation and Simulation. 1993;48:233–243. [Google Scholar]
- Wong G, Mason W. The hierarchical logistic regression model for multilevel analysis. Journal of the American Statistical Association. 1985;80:513–524. [Google Scholar]
- Zeger SL, Karim MR. Generalized linear models with random effects: A Gibbs sampling approach. Journal of the American Statistical Association. 1991;86:79–86. [Google Scholar]