D. santomea and D. yakuba provide an example of reinforcement for a postmating-prezygotic trait in an organism that has internal fertilization. This work shows that reinforcement of barriers other than sexual and other forms of premating isolation is possible.
Abstract
Reinforcement, a process by which natural selection increases reproductive isolation between populations, has been suggested to be an important force in the formation of new species. However, all existing cases of reinforcement involve an increase in mate discrimination between species. Here, I report the first case of reinforcement of postmating prezygotic isolation (i.e., barriers that act after mating but before fertilization) in animals. On the slopes of the African island of São Tomé, Drosophila yakuba and its endemic sister species D. santomea hybridize within a well-demarcated hybrid zone. I find that D. yakuba females from within this zone, but not from outside it, show an increase in gametic isolation from males of D. santomea, an apparent result of natural selection acting to reduce maladaptive hybridization between species. To determine whether such a barrier could evolve under laboratory conditions, I exposed D. yakuba lines derived from allopatric populations to experimental sympatry with D. santomea, and found that both behavioral and gametic isolation become stronger after only four generations. Reinforcement thus appears to be the best explanation for the heightened gametic isolation seen in sympatry. This appears to be the first example in animals in which natural selection has promoted the evolution of stronger interspecific genetic barriers that act after mating but before fertilization. This suggests that many other genetic barriers between species have been increased by natural selection but have been overlooked because they are difficult to study.
Author Summary
What stops newly formed species from interbreeding? Answering this question is fundamental to our understanding of speciation. One mechanism is that where such would-be species meet, the barriers against interbreeding are reinforced by natural selection (e.g., leading to greater mate discrimination). On the slopes of the African island of São Tomé, Drosophila yakuba and its sister species D. santomea hybridize within a well-demarcated hybrid zone. I found that D. yakuba females from within this zone, but not from outside it, show an increase in gametic isolation from males of D. santomea, such that before fertilization, the females deplete sperm from D. santomea males faster than from conspecific males. Consequently, there are fewer progeny produced from interspecific matings. By experimentally evolving the populations, I also show that such postmating isolation can rapidly evolve. Natural selection, therefore, has promoted the evolution of stronger interspecific genetic barriers that act after mating but before fertilization. D. santomea and D. yakuba, then, appear to represent an example of reinforcement for a postmating-prezygotic trait in an organism that has internal fertilization. This work shows that reinforcement of barriers other than sexual and other forms of premating isolation is possible. This also suggests that there are many “cryptic” barriers to gene flow that might be increased by natural selection in areas where species overlap and hybridize.
Introduction
The evolutionary process of “reinforcement,” often suggested as an important component of speciation, involves the strengthening by natural selection of prezygotic isolation between closely related taxa in response to maladaptive hybridization [1]–[4]. Reinforcement has often been inferred from a pattern of “reproductive character displacement,” in which individuals of different species are more behaviorally isolated if they come from the area where two species overlap (sympatric) than from areas outside each other's range (allopatric; [1]–[8]). Reinforcing selection, however, need not be limited to increasing premating isolation: other reproductive barriers that act after mating, such as gametic isolation, can also be reinforced [9]–[14]. Lorch and Servedio [15], for example, proposed that a species preference for fertilizing the gametes of conspecific versus heterospecific individuals could evolve through a reinforcement-like process, depending on the nature of selection against heterospecific matings. Here, I report the first, to my knowledge, apparent case of reinforcement in the wild of a genetic barrier—reduced production of hybrid eggs—that acts after mating but before fertilization; and I also demonstrate that the evolution of this form of gametic isolation can occur in the laboratory.
I looked for evidence of reinforcement in postmating-prezygotic isolating mechanisms in two African species of Drosophila in the melanogaster subgroup: D. yakuba and D. santomea. D. yakuba is widespread throughout sub-Saharan Africa and has extended its range to neighboring islands, including the Gulf of Guinea islands in the eastern Atlantic Ocean [16]. D. santomea, the closest relative of D. yakuba, is endemic to São Tomé, a small (860 km2) volcanic island 255 km west of Gabón. Molecular data show that D. yakuba and D. santomea diverged about 400,000 y ago [17],[18]. On the extinct volcano of Pico de São Tomé, D. yakuba occurs at elevations below 1,450 m, and is also common in the lowlands, villages, and plantations. In contrast, D. santomea occupies the mist forests at elevations between 1,153 and 1,800 m [16]–[19]. These species are unique within Drosophila in showing a well-demarcated hybrid zone.
Previous studies uncovered at least 11 distinct reproductive barriers that act over the entire life cycle, ranging from habitat isolation to hybrid dysfunction, although no single barrier completely impedes gene flow [20]–[24]. Five known barriers are of the postmating-prezygotic form [22],[24], including both competitive (conspecific sperm precedence [CSP]) and noncompetitive mechanisms (lower production of eggs after heterospecific matings). The yakuba–santomea species pair is ideal for studying reinforcement because it meets the requirements that 1) mating and introgression occur between the species in nature (as observed in the hybrid zone between yakuba and santomea) and 2) that hybridization be costly (all male hybrids are sterile). Previous studies of these species have failed to find evidence of reinforcement in premating barriers [20], but there was no search for reinforcement in postmating-prezygotic barriers.
Here, I report that reinforcement for a form of postmating-prezygotic isolation—gametic isolation—has apparently evolved in natural populations of D. yakuba sympatric with the sister species D. santomea. I demonstrate a clear fitness advantage for those individuals who have increased gametic isolation, and this advantage apparently leads to a remarkably rapid evolution of gametic isolation in laboratory populations. This appears to be the first known example in animals of the evolutionary increase of interspecific genetic barriers that act after mating but before fertilization.
Materials and Methods
Gametic Isolation of Naturally Collected Lines
Figure 1 and Table S1 describe the collection sites of the stocks used in this study. I used isofemale lines to study the among-line component of variation in gametic isolation. (The among-population variation was not evaluated because it is not possible to sample multiple populations from the area of sympatry.) Females of each line from each species were mated to both conspecific and heterospecific males to estimate egg number produced by each type of cross. I collected virgin males and females under CO2 anesthesia and kept them for 3 d in single-sex groups of 20 flies. On day 4, I aspirated flies into fresh food-containing vials, with one female and one male per vial. All copulations were watched to ensure that they were not abnormally short. To prevent females from remating, males were removed from a vial by aspiration after mating. After 1 h, I ended the observations and discarded females who did not mate. Each mated female was allowed to oviposit for 24 h in a vial, after which I counted the total number of eggs laid and transferred the female to a fresh vial. The counting was repeated daily for 10 d. In interspecific crosses, the reduced number of offspring constitutes a noncompetitive form of gametic reproductive isolation, as each female carries sperm from only one male [22],[24]. Twelve females were scored for each cross.
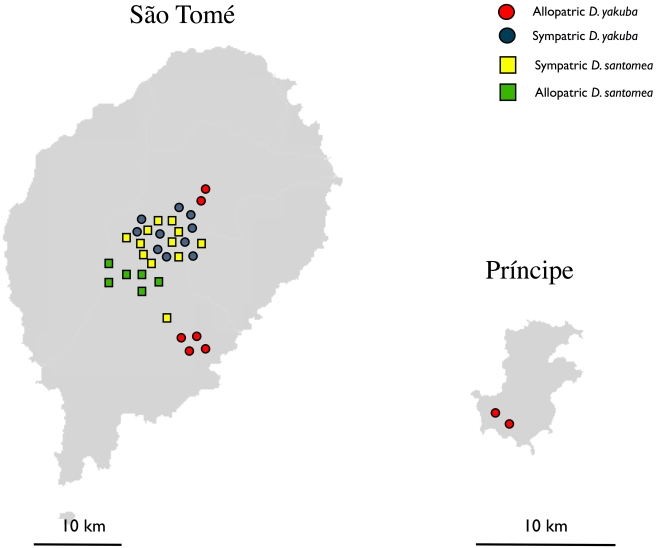
Figure 1. Map depicting the D. yakuba and D. santomea collection sites in the São Tomé and Príncipe islands.
Lines collected in the African continent are not shown. Additional information about the collection sites can be found in Table S1.
Rate of Sperm Depletion (or Death)
One way to measure the efficiency of sperm storage or survival is to estimate the proportion of eggs laid every day that hatch, following the decline in this statistic over time [24]. To this end, I used six D. yakuba lines (three allopatric and three sympatric) and three D. santomea lines, measuring the decline of egg hatchability for all the possible D. yakuba × D. santomea crosses. For each cross, I produced 100 inseminated females, divided into five subgroups of 20 females. Each subgroup was transferred without anesthesia to colored medium. Eggs were collected every 24 h, and the hatchability of each batch was measured daily for 10 d.
Heterogeneity in hatchability among crosses was analyzed by fitting a minimal random linear mixed model (LMM) [25] to the hatchability of eggs laid each day. I analyzed five main effects—geographic origin of female (sympatric vs. allopatric populations), geographic origin of male, female line nested within geographic origin, male line nested within geographic origin, and days after mating—as well as all interactions between these factors. The effect due to differences between groups of females was considered random. I analyzed the data following the maximum-likelihood model simplification approach of Crawley [26],[27], in which the full model containing all factors and interactions was fitted and then simplified by a series of stepwise deletions, starting with the highest-order interaction and progressing to lower-order interaction terms and then to main effects. The critical probabilities for retaining factors and determining whether effects or interactions were significant were 5% for main effects, 1% for two-way interactions, and 0.5% for three-way interactions [28]. To assess whether slopes (i.e., the rate at which hatchability decays along time) differ between matings, I formulated two models that differed in the assumptions about these slopes and compared the models using a likelihood ratio test (LRT). Model 1 was a full factorial analysis (i.e., different slopes and intercepts for each of the possible crosses), whereas Model 2 assumed different intercepts but identical slopes (rate of decline of fecundity). Finally, to determine whether different treatments produced differences in initial hatchability (as a proxy for the amount of sperm transferred during heterospecific matings), I analyzed hatchability data from the first day using a one-way ANOVA, with hatchability as the response and two fixed effects (origin of the female, and female line nested within origin).
Selective Advantage
To study whether an initial interspecific mating had any effects on the fertility of D. yakuba females after a second conspecific mating, I scored the egg production of heterospecifically mated females. After 4 d, interspecifically mated females were remated to D. yakuba males (from the same line than the female), and I scored the number of eggs laid during the subsequent 10 d. Eggs were counted every 24 h over the entire 14-d period. This analysis used 12 lines (six allopatric and six sympatric), with 25 individuals scored per line.
I analyzed differences in overall fecundity between the different crosses by fitting a nested ANOVA to the total number of eggs laid per female (the sum of heterospecific and conspecific eggs), with geographic origin of the female and female line (nested within origin) as fixed effects and variation among females within line as a random effect. To determine whether the proportion of conspecific eggs (relative to the total number of eggs) differed between sympatric and allopatric lines, I followed the same procedure used to analyze total fertility, but fitted the model to the number of conspecific eggs laid (i.e., eggs laid after the second mating).
Experimental Sympatry
To test whether natural selection on gametic isolation could have been responsible for the observed reproductive character displacement in natural populations, I kept seven populations of D. yakuba, originally derived from allopatric populations (Table S2), in experimental sympatry with D. santomea for ten generations, following the design of Koopman [29] and Higgie et al. [30]. These conditions were created by maintaining four bottles per population, with each bottle containing 50 D. yakuba females, 50 D. yakuba males, 50 D. santomea females, and 50 D. santomea males. Since D. yakuba always outcompetes D. santomea under these conditions [24], I added D. santomea females and males to the experimental sympatry bottles each generation to maintain a constant ratio of the two species. To set up each successive generation, I collected 50 flies of each sex of D. yakuba (easily identifiable by pigmentation) as virgins from the experimental bottles and transferred them to a new bottle. To reconstitute the sympatry conditions, 50 D. santomea flies of each sex (collected as virgins from stock bottles) were added to the bottle. This procedure was followed for ten generations. Control populations of D. yakuba were maintained for each population (four replicates) at the same density (100 flies per bottle) but without adding D. santomea. The maintenance conditions and population size of D. yakuba were the same between experimental sympatry bottles and control groups. The strength of sexual and gametic isolation was measured every two generations using methods described previously [22],[31],[32].
Finally, I set up an internal control to make sure that elimination of hybrids was complete, i.e., there was no gene flow between the two species in the experimental bottles. Taking into account the complete sterility of F1 hybrid males, who lack motile sperm [17],[21], I collected D. yakuba females from each experimental sympatry bottle and mated them to D. santomea males to produce 100 F1 heterospecific males (♀ D. yakuba × ♂ D. santomea) every other generation. These F1 males were scored for sperm motility. The idea behind this test was that if motile sperm were seen, it meant that there had been gene flow between species (i.e., not all the hybrids were killed when setting up a new generation), and the bottle was discarded.
The results from this experiment were analyzed using a paired t-test to compare the values of gametic and sexual isolation (transformed with arcsine) between experimental populations that were exposed to D. santomea and the unexposed control populations.
Results
Gametic Isolation Is Stronger in Sympatric Than in Allopatric D. yakuba Females
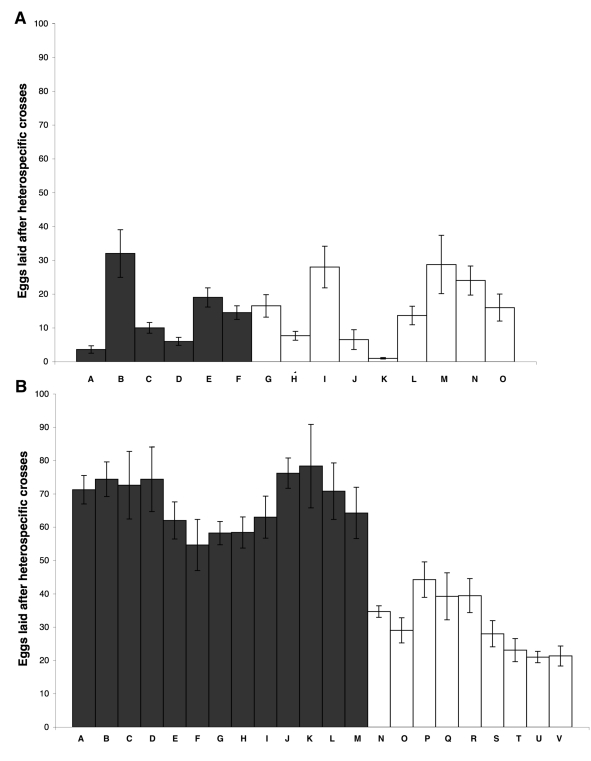
The test for reinforcement of gametic isolation involved mating D. yakuba and D. santomea females from either sympatric or allopatric populations to males of the other species and scoring the number of eggs produced by a single female—an index of the strength of noncompetitive gametic isolation—during the first 10 d after each mating (Figure 2). In D. santomea, I detected no heterogeneity in egg production when females were mated to D. yakuba males (gametic isolation) among lines (LMM, F 1,13 = 1.644, p = 0.222, Figure 2A). In contrast, D. yakuba females from sympatric lines yield significantly fewer progeny than those from allopatric females when both were mated to D. santomea males, even when allopatric females were derived from populations close to the hybrid zone on São Tomé. D. yakuba females, therefore, show the pattern predicted by reinforcement of gametic isolation (LMM, F 1,20 = 42.56, p<0.0001, Figure 2B). This suggests that in D. yakuba, increased gametic isolation has evolved as a response to the sympatric presence of the sister species. The results with synthetic lines (genetically heterogeneous strains of each species created by combining virgin males and females from several isofemale lines from the same location) were similar (unpublished data).
Figure 2. Noncompetitive gametic isolation between D. yakuba and D. santomea.
(A) D. santomea sympatric females produced the same amount of progeny as allopatric lines after being mated to D. yakuba males. Bars A–F (dark grey): allopatric lines; bars G–O (white bars): sympatric lines. (B) Reproductive character displacement in D. yakuba. Females derived from flies sympatric to D. santomea yield fewer progeny than do allopatric females after being mated with D. santomea, suggesting a higher level of gametic isolation in sympatric females. Bars A–M (dark grey): allopatric lines; bars N–V (white): sympatric lines. Each bar represents the mean (SE) number of eggs from independent heterospecific single matings of D. yakuba and D. santomea females. The list of crosses can be found in Tables S2 and S3.
To determine whether the reduced number of hybrid eggs laid by D. yakuba females from sympatric populations was caused by a female trait, a male trait or the interaction of both, I randomly selected six D. yakuba lines (three allopatric and three sympatric) and six D. santomea lines, mated the D. yakuba females to D. santomea males in all the possible combinations, and performed the egg-counting protocol described above. The data were analyzed with a LMM with four fixed effects: female origin, female line (nested within female origin), and male line (nested within male origin), as well as all interactions between these factors. The minimal linear model for this design showed that there is a high degree of heterogeneity (F 35,396 = 18.08, p<10–15) in the number of eggs produced. The results indicate that the among-line heterogeneity is explained by origin of the female (whether a population was allopatric or sympatric to D. santomea in the field, LMM, F 1,4 = 124.818, p = 0.0004). The male origin effect was not significant, suggesting that the genotype of the male does not have an effect on female fertility (F 1,4 = 1.822, p = 0.2484). More important, the interaction between female origin and male origin was not significant (F 16,396 = 1.86, p = 0.023), demonstrating that the heterogeneity in fecundity (and therefore, the observed reproductive character displacement) is a characteristic that depends primarily on the genotype of the female, regardless of the genotype of the D. santomea male involved in the heterospecific cross. This kind of reinforcement is expected to be due to changes in females, because they suffer more than do males from interspecific mating [2].
Sperm Depletion Rate
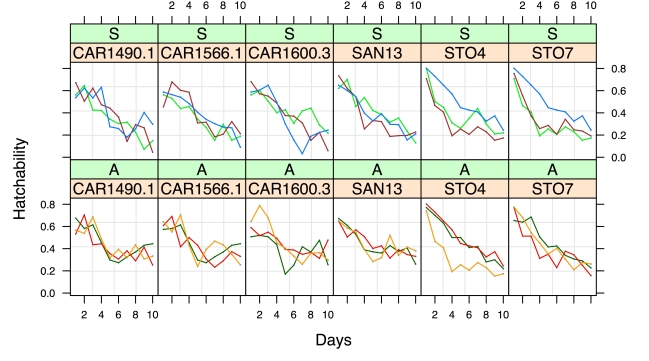
I estimated how long a female could retain and use viable sperm when she was mated to a heterospecific versus a conspecific male. The aim of this test was to determine whether the rate at which a D. yakuba female lost D. santomea sperm—either by depletion or sperm death—differed between allopatric and sympatric D. yakuba lines. Figure 3 shows that heterospecific sperm loss (the most likely cause of noncompetitive gametic isolation) is more pronounced in sympatric than in allopatric lines. This conclusion rests on two results of this analysis. First, the initial hatchability of eggs did not differ between allopatric and sympatric lines (LMM; female origin: F1,4 = 0.585, p = 0.4869). There was no heterogeneity between the intercepts of these crosses, suggesting no substantive difference in number of sperm transferred. Moreover, the decline in egg hatchability over time (slope) was significantly heterogeneous (Model 1 vs. Model 2: LRT = 12.086, p = 5×10−4). This shows that interspecific sperm stored after crosses involving sympatric lines was either retained for a shorter time or became inviable more quickly than in crosses involving allopatric lines. The more rapid loss (or death) of heterospecific sperm in sympatric females is consistent with the observation that sympatric females produce fewer progeny after heterospecific crosses compared to allopatric females. Apart from noncompetitive gametic isolation, no other reproductive barrier shows the signature of reinforcement (Figures S1 and S2, Tables S4, S5, S6, and S7).
Figure 3. Sperm retention in allopatric and sympatric females of D. yakuba.
A LMM was used to test for differences in sperm depletion/death over time between allopatric (A panels) and sympatric (S panels) lines of D. yakuba. The D. yakuba lines used in this experiment were SJ2 (brown), Cameroon 115 (blue), Anton 2 Principe (red), SA3 (light green), OBAT1200.15 (orange), and BAR1000.2 (dark green). I used six different D. santomea lines (tags highlighted in yellow) to make sure that the observed patterns were not line specific. Heterogeneity in slopes was detected between populations and was determined to be higher (i.e., faster sperm depletion/death) in sympatric than in allopatric lines.
Selective Advantage
Although the evolution of behavioral isolation is clearly advantageous in a hybrid zone when hybrids are semisterile or partly inviable, the benefits of increasing postmating-prezygotic isolation are not so obvious [2],[12],[13]. One possibility is that eliminating heterospecific sperm more quickly allows a female to remate with males of her own species, increasing her chances of passing her genes to the next generation. In such a case, alleles fostering quicker elimination of heterospecific sperm could be selectively advantageous. To check this possibility, I measured the reproductive capacity of D. yakuba females from both allopatric and sympatric populations that had been initially mated to heterospecific males. Four days after this first mating, these females were remated to conspecific males, and I counted the number of eggs produced every day for the next 10 d.
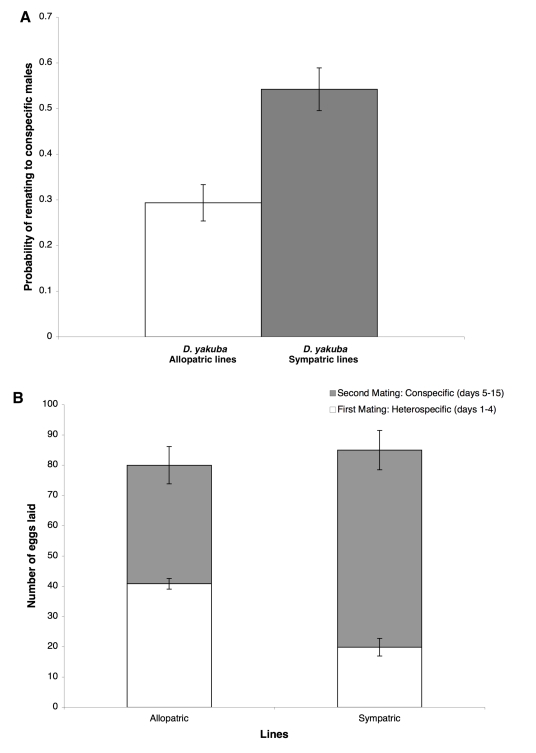
Data from this experiment give two kinds of support for the idea that natural selection might have increased the gametic isolation of sympatric D. yakuba females in nature. First, D. yakuba females from sympatric populations remated more quickly to conspecific males than did sympatric females (LMM on arcsine of the remating probability, F 1,12 = 13.4295, p = 0.0032; Figure 4A). Given the CSP that acts in D. yakuba (in double conspecific/heterospecific matings, regardless of mating order, conspecific sperm are used in fertilization much more often than heterospecific sperm, [22]), this faster mating would markedly reduce the proportion of hybrid progeny produced, decreasing the cost of maladaptive hybridization. Second, D. yakuba sympatric females mated to a conspecific male for a second time produced more conspecific progeny than did allopatric females (Figure 4B). Since the total number of eggs produced did not differ between allopatric and sympatric D. yakuba females after two matings (LMM, F 1,8 = 0.0031, p = 0.957), the stronger gametic isolation of sympatric females reduces the production of hybrid progeny and increases the number of (more fit) conspecific progeny that they can produce (LMM on number of eggs laid after conspecific mating: F 1,8 = 9.726, p = 0.0143). Taken together, these results show that increased gametic isolation can provide a fitness advantage to D. yakuba females who are sympatric with D. santomea.
Figure 4. Selective advantages of enhanced gametic isolation.
(A) Propensity of D. yakuba females to remate with a conspecific in a second, no-choice mating 4 d after an initial mating to a heterospecific male. Grey: sympatric females; white: allopatric females. (B) Mean (SE) number of eggs per D. yakuba female (from either sympatric or allopatric lines) sired by first (heterospecific, white) and second (conspecific, grey) male.
Experimental Sympatry
To establish whether natural selection would increase gametic isolation in the laboratory when species were given the opportunity to hybridize, I exposed seven distinct allopatric lines of D. yakuba (each collected in different years and geographic localities) to experimental sympatry with D. santomea for ten generations. If maladaptive hybridization promotes the evolution of postmating-prezygotic isolation, and there is genetic variance for the character, we might be able to observe such isolation evolving in the experimental sympatry lines. It is important to note, however, that in this study, hybrids are rendered completely inviable, whereas a few viable hybrids have been found in the wild. Although these species do mate in the wild, female hybrids have never been found, male hybrids are completely sterile, and most hybrids have been F1 individuals, with only 4% of them being from backcrosses [19].
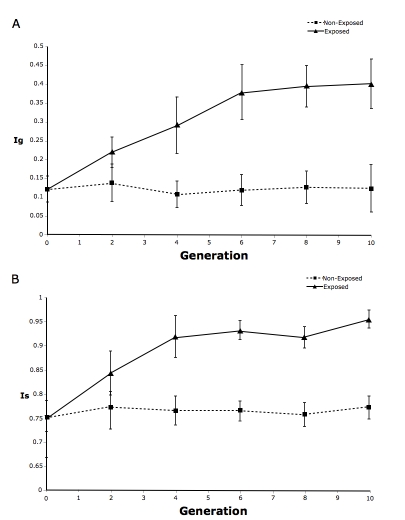
D. yakuba females exposed to experimental sympatry evolved substantial gametic isolation within four generations, whereas unexposed D. yakuba populations showed no change in isolation over time (Figure 5A). This difference was highly significant (paired t-test: t 5 = 4.32, p = 0.0076). I also observed a substantial increase in sexual isolation between D. yakuba females and D. santomea males in sympatric, but not in the unexposed control populations (paired t-test: t 5 = 4.85, p = 0.0047; Figure 5B). This is surprising in view of the lack of evidence for reinforcement of sexual isolation of these species in nature [20]. None of the other isolating barriers examined (copulation latency and duration) changed over time (Figures S3 and S4).
Figure 5. Effects of experimental sympatry on sexual (A) and gametic (B) isolation in D. yakuba.
The strength of gametic and sexual isolation was calculated according to the indexes proposed by Chang (I g; [22]), and Coyne and Orr (I s; [32]), respectively. Means and standard errors reflect the average of the seven lines (four replicates per line). D. yakuba lines, which in nature are allopatric to D. santomea, showed significant reproductive character displacement (triangles) when exposed to D. santomea, whereas unexposed lines (squares) experienced no change in their degree of isolation.
Although the experimental-sympatry study demonstrates the evolution of reproductive character displacement rather than reinforcement per se, for several reasons, these results increase the likelihood that increased gametic isolation in sympatry did result from reinforcement: i) gametic isolation is a heritable trait and responds to selection, ii) increased gametic isolation similar to that seen in nature is caused by the presence of D. santomea, and iii) the genetic variability required for sexual and gametic isolation to evolve is present in allopatric populations. Additionally, since D. santomea were added to the experimental sympatry bottles each generation, I did not examine the possibility of reinforcement of gametic (or sexual isolation) in that species.
Tests of Alternative Explanations
Increased reproductive isolation in sympatry can be generated through a variety of processes. Although reinforcement is the most commonly invoked explanation for reproductive character displacement, other processes—such as ecological character displacement and differential extinction or differential fusion— can generate the same pattern [2],[33]–[36]. Two results, however, suggest that ecological character displacement is an unlikely explanation for the observed pattern. First, to control for this possibility, I included several allopatric lines of D. yakuba collected from higher elevations off of São Tomé (e.g., Mount Cameroon, Pico Basile, and Nairobi, Table S1), which thus lived at elevations similar to the D. yakuba lines derived from the hybrid zone (Table S1). The aim of this test was to examine the possibility that the observed reproductive character displacement was a byproduct of adaptation to high elevation alone (allopatric lines collected at high elevations are represented by bars K–M, Figure 2B). These allopatric, high-elevation lines of D. yakuba did not, however, show elevated gametic isolation. Moreover, the results from the experimental sympatry experiment show that reproductive character displacement occurs if D. yakuba is exposed to D. santomea and when there is strong selection against the hybrids, even when the “ecology” is that of a food-filled milk bottle in the laboratory.
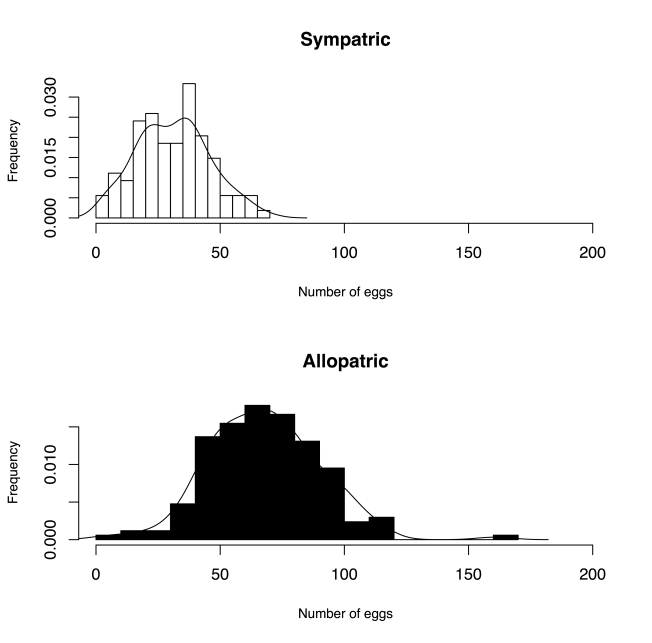
The second possibility is that the observed range of gametic isolation reflects the results of a deme-sorting process involving differential extinction (or differential fusion) of populations based upon levels of reproductive isolation. Under this scenario, only those populations that have a high, pre-existing level of reproductive isolation will be able to colonize and persist in a region where a potentially interbreeding sister species is present. This hypothesis yields a clear prediction: if biased extinction is the driving force behind the observed differences in levels of reproductive isolation, then non-allopatric populations should show a distribution of reproductive-isolation values lying within the range of phenotypic values seen in allopatric populations [31],[32]. The data suggest that this is not a likely explanation for the elevated gametic isolation seen in sympatric D. yakuba lines: values of gametic isolation in sympatric lines are not a subset of that of the distribution of values of allopatric individuals (Figure 6, Figure S5, ANOVA on pooled individual values with resampling of cells: F 1,310 = 341.93, p<1×10−4).
Figure 6. Frequency distribution of individual levels of gametic isolation levels in D. yakuba.
The data correspond to the data shown in Figure 1 when pooled according to whether the line of each D. yakuba female was sympatric or allopatric.
Two further lines of evidence render differential fusion/extinction an unlikely explanation. First, differential fusion predicts that premating, postmating-prezygotic, and postzygotic isolation would be stronger in sympatry than allopatry [2],[31]. Tables S4, S5, S6, and S7 show that it is not the case: the only reproductive isolating barrier appears to be strengthened in sympatry is gametic isolation. Second, to explain the existence of substantial differences in gametic isolation before secondary contact, differential fusion/deme selection would require very low levels of gene flow between populations [2],[34]. Previous studies have shown that this is not the case for D. yakuba, which exhibits very little population structure [17].
All these considerations render alternative possibilities, such as ecological character displacement and differential extinction/fusion, unlikely. I suggest that reinforcement is the most likely cause of the reproductive character displacement observed in D. yakuba populations that are sympatric to D. santomea.
Discussion
Four conditions must be met before one can conclude that reinforcement is the cause of a pattern of reproductive character displacement between two species [33],[34]. First, gene flow, either current or recent, has to occur between them. Second, there must be, or have been, natural selection against maladaptive hybridization. Third, the trait causing reproductive isolation must be heritable and capable of responding to selection. Finally, one must rule out alternative explanations such as ecological character displacement. The work described above fulfills these requirements, suggesting that reinforcement for postmating prezygotic isolation has indeed evolved in populations of D. yakuba that are sympatric with D. santomea.
Some cases of reproductive character displacement of gametic isolation have been reported previously. Geyer and Palumbi [37] describe reproductive character displacement in the sequence of proteins involved in gametic interactions in sympatric populations of the sea urchin Echinometra oblonga. A similar example occurs in abalone and mussel species, which show a strong signature of positive selection in proteins involved in sperm–egg interaction, especially in sympatric species [38]–[42]. In all these cases, selection for local gamete coevolution (as a result of interactions between sympatric species) seems to be the driving force of speciation; however, the authors do not describe higher gametic isolation between sympatric than between allopatric populations of the same species pair, so it is possible that these patterns reflect processes other than reinforcement (e.g., differential fusion; [2],[34]).
D. santomea and D. yakuba, then, appear to represent the first example of reinforcement for a postmating-prezygotic trait in an organism that has internal fertilization. In this particular case, reinforcement operates when several reproductive barriers are already strong. Also, the major selection pressure seems to be direct—on the number of offspring produced—rather than indirect—on the fitness of hybrid offspring. The reason why only gametic isolation, and not sexual isolation, is reinforced in natural populations of D. yakuba remains an unanswered question, especially given that behavioral isolation mechanisms occurring earlier in the life history can more effectively reduce the costs of hybridization [2],[43],[44]. There are two explanations for why D. yakuba females show reinforced gametic isolation but no reinforced behavioral isolation. CSP reduces the cost of heterospecific matings for females, and thus reduces the likelihood reinforcement of sexual isolation [43]. It is possible that CSP reduces the likelihood of reinforcement of behavioral but not of gametic isolation; however, this seems unlikely given that CSP reduces the costs of hybridization as a whole, and its effects should reduce the likelihood of reinforcement of all mechanisms of reproductive isolation. A second possibility is that if behavioral isolation is not an effective isolating mechanism in nature, then gametic isolation can play a very prominent role on reproductive isolation, as occurs in free-spawning marine invertebrates. Again, previous inventories of reproductive isolation between D. yakuba and D. santomea and the low frequency of hybrids in nature (I psi = 0.54 for no choice experiments; [20],[21],[23],[45]) render this explanation as unlikely.
For sexual isolation, it has been predicted that reinforcement should be stronger in the rarer species, as rarity increases the probability of mating with the wrong species [2]–[7] and thus selection to avoid maladaptive hybridization stronger. Previous studies have demonstrated that in the hybrid zone D. yakuba is indeed rarer than D. santomea [19]. Although the reproductive mechanism that is reinforced in this case is not sexual but gametic isolation, our results do comply with this prediction.
Finally, I show that gametic isolation (and not only sexual isolation) can evolve under laboratory conditions—and can do so very quickly if natural selection is strong. These results, together with some previous examples [29],[30] in which artificial sympatry promoted the evolution of reproductive character displacement, demonstrate that prezygotic isolation (both premating and postmating-prezygotic) can evolve quickly given the strong selection regime and the presence of genetic variation. Whether reinforcement would evolve if gene flow was permitted and the selection regime was weaker is an unanswered question that I am currently investigating.
To date, the study of postmating-prezygotic barriers in speciation has focused largely on documenting their existence. The processes and mechanisms that generate such reproductive mechanisms are, understandably, less well understood than those that generate premating isolation [13],[46],[47]. Previous studies have shown that postmating-prezygotic characters can evolve rapidly and that such evolution can be the result of differences in the coevolutionary trajectory between males and females among populations or species [48],[49]. Postmating-prezygotic isolation can also evolve as a byproduct of ecological divergence and be heavily influenced by the ecology of a species [36],[48]–[51].
This work shows that reinforcement of barriers other than sexual and other forms of premating isolation is possible. This suggests that there are many “cryptic” barriers to gene flow that might be increased by natural selection in areas where species overlap and hybridize.
Supporting Information
Offspring production from double matings by D. yakuba females I. Mean (SE) number of offspring per D. yakuba female (from either sympatric or allopatric populations) sired by first (D. yakuba, red) and second (D. santomea STO.4, blue) male. The number of offspring produced during the first 4 d was subtracted from the total amount of produced progeny. The data were analyzed with a nested ANOVA in which the asin (progeny produced after the second mating/total progeny) was the response and line was nested within origin of the D. yakuba line (allopatric or sympatric). The results (Female origin: F 1,54 = 0.069, p = 0.794; Female line: F 4,54 = 1.188, p = 0.068) show no difference in the strength of CSP between sympatric and allopatric lines when D. yakuba is the first male.
(0.02 MB PDF)
Offspring production from double matings by D. yakuba females II. Mean (SE) number of offspring per D. yakuba female (from either sympatric or allopatric populations) sired by first (D. santomea STO.4, red) and second (D. yakuba, blue) male. The number of offspring produced during the first 4 d was subtracted from the total amount of produced progeny. The data were analyzed with a nested ANOVA in which the asin (progeny produced after the second mating/total progeny) was the response and line was nested within origin of the D. yakuba line (allopatric or sympatric). The results (Female origin: F 1,54 = 0.643; Female line: F 4,54 = 1.188, p = 0.327) show no difference in the strength of CSP between sympatric and allopatric lines when D. santomea is the first male.
(0.02 MB PDF)
Effects of experimental sympatry on copulation latency in D. yakuba . Means and standard errors are based on the average of the seven lines (four replicates per line).
(0.02 MB PDF)
Effects of experimental sympatry on copulation duration in D. yakuba . Means and standard errors are based on the average of the seven lines (four replicates per line).
(0.02 MB PDF)
Frequency distributions of gametic isolation levels per D. yakuba line. The title of each graph shows the lines involved in the cross (♀ D. yakuba × ♂ D. santomea). Black distributions: allopatric lines; white distributions: sympatric lines. The data shown in this figure are the same data shown in Figure 1.
(0.04 MB PDF)
Isofemale lines of D. santomea and D. yakuba analyzed in this study.
(0.10 MB RTF)
Allopatric and sympatric crosses involving D. yakuba females. Cross corresponds to the letter shown in Figure 1B. S/A describes what is the geographical origin of the line (i.e., whether the lines involved in the cross are sympatric or allopatric).
(0.03 MB RTF)
Allopatric and sympatric crosses involving D. santomea females. Cross corresponds to the letter shown in Figure 1A. S/A describes what is the geographical origin of the line (i.e., whether the lines involved in the cross are sympatric or allopatric).
(0.03 MB RTF)
Degree of sexual isolation for allopatric (A) and sympatric (S) crosses. S/A describes what is the geographical origin of the line (i.e., whether the lines involved in the cross are sympatric or allopatric). N, the number of pairings observed for each mating type equaled 80 in all the cases. In the four mating columns, Y refers to D. yakuba, S to D. santomea, and the species of the female in each pairing is given first. Ipsi is the proposed statistic by Rolan-Alvarez to measure sexual isolation. SI(yak) and SI(san) are the degree of sexual isolation for D. yakuba females only and D. santomea females only, respectively. Allopatric (Al) and sympatric females (Sy) from both species showed no significant differences in sexual isolation in any of the three measurements (Ipsi: F 1,17 = 0.3899, p = 0.5406; SIyak: F 1,17 = 1.554, p = 0.2292; SIsan: F 1,17 = 0.1812, p = 0.6757).
(0.08 MB RTF)
Mean (SE) copulation latency in no-choice mating experiments involving D. yakuba females from sympatric and allopatric populations. N is equal to 12 for all crosses. The data were analyzed with a nested ANOVA in which copulation latency was the response. The fixed effects were female line, nested within origin of the D. yakuba line (allopatric or sympatric) and male line nested within male origin. Although the female and male line effects caused heterogeneity (Female line: F 16,277 = 10.176, p = 2.2×10−16; Male line: F 7,136 = 5.011, p = 1.22×10−5), there was no correlation between copulation latency and whether the populations were sympatric or allopatric (Female origin: F 1,99 = 3.633, p = 0.057; Male origin: F 1,17 = 0.6155, p = 0.432).
(0.10 MB RTF)
Mean (SE) copulation duration in no-choice mating experiments involving D. yakuba females from sympatric and allopatric populations. N is equal to 12 for all crosses. The data were analyzed with a nested ANOVA in which copulation duration was the response. The fixed effects were female line, nested within origin of the D. yakuba line (allopatric or sympatric) and male line nested within male origin. Although the female and male line effects caused heterogeneity (Female line: F 16,460 = 7.407, p = 2.2×10−6; Male line: F 1,230 = 4.352, p = 8.503×10−5), there was no correlation between copulation duration and whether the populations were sympatric or allopatric (Female origin: F 1,104 = 1.683, p = 0.195; Male origin: F 1,230 = 3.706, p = 0.054).
(0.11 MB RTF)
F1 ( D. yakuba × D. santomea ) larvae survival as a proxy for postzygotic isolation. Hybrid larvae from matings between females from allopatric or sympatric lines of D. yakuba and D. santomea were collected as first-instar larvae, and the number of recovered adults was scored. N equals 100 for all crosses. The data were analyzed with a nested ANOVA in which the asin (proportion of surviving larvae) was the response. The fixed effects were female line, nested within origin of the D. yakuba line (allopatric or sympatric) and male line nested within male origin. Female and male line effects caused heterogeneity (Female line: F 4,3589 = 65.39, p = 8.29×10−16; Male line: F 4,3589 = 53.011, p = 2.12×10−15), but there was no correlation between copulation latency and whether the populations were sympatric or allopatric (Female origin: F 1,3589 = 1.84, p = 0.1192; Male origin: F 1,3589 = 0.6155, p = 0.582).
(0.03 MB RTF)
Acknowledgments
I thank J. A. Coyne, M. A. F Noor, T. D. Price, J. T. Weir, R. Yukilevich, M. A. Sprigge, L. Quesada-Ocampo, N. Bloch for critical discussions and reading of the manuscript; and I. A. Butler and J. Gladstone for technical help. I also thank A. Llopart and P. Andolfatto for donating D. yakuba lines.
Abbreviations
- CSP
conspecific sperm precedence
- LMM
linear mixed model
Footnotes
The author has declared that no competing interests exist.
This work was funded by National Institutes of Health grant R01GM058260 to Jerry A. Coyne. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Servedio M. R, Noor M. A. The role of reinforcement in speciation: theory and data. Annu Rev Ecol Syst. 2003;34:339–364. [Google Scholar]
- 2.Coyne J. A, Orr H. A. Sunderland (Massachusetts): Sinauer Associates; 2004. Speciation.545 [Google Scholar]
- 3.Noor M. A. Speciation driven by natural selection in Drosophila. Nature. 1995;375:674–675. doi: 10.1038/375674a0. [DOI] [PubMed] [Google Scholar]
- 4.Dobzhansky T. G. Speciation as a stage in evolutionary divergence. Am Nat. 1940;74:312–321. [Google Scholar]
- 5.Gerhardt H. C. Reproductive character displacement of female mate choice in the grey treefrog, Hyla chrysoscelis. Anim Behav. 1994;47:959–969. [Google Scholar]
- 6.Rundle H. D, Schluter D. Reinforcement of stickleback mate preferences: sympatry breeds contempt. Evolution. 1998;52:200–208. doi: 10.1111/j.1558-5646.1998.tb05153.x. [DOI] [PubMed] [Google Scholar]
- 7.Haavie J, Borge T, Bures S, Garamszegi L. Z, Lampe H. M, et al. Flycatcher song in allopatry and sympatry–convergence, divergence and reinforcement. J Evol Biol. 2004;17:227–237. doi: 10.1111/j.1420-9101.2003.00682.x. [DOI] [PubMed] [Google Scholar]
- 8.Jaenike J, Dyer K. A, Cornish C, Minhas M. S. Asymmetrical reinforcement and Wolbachia infection in Drosophila. PLoS Biol. 2006;4:e325. doi: 10.1371/journal.pbio.0040325. doi: 10.1371/journal.pbio.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Servedio M. R. Beyond reinforcement: the evolution of premating isolation by direct selection on preferences and postmating, prezygotic incompatibilities. Evolution. 2001;55:1909–1920. doi: 10.1111/j.0014-3820.2001.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 10.Servedio M. R. The what and why of research on reinforcement. PLoS Biol. 2004;2:e420. doi: 10.1371/journal.pbio.0020420. doi: 10.1371/journal.pbio.0020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant V. The selective origin of incompatibility barriers in the plant Gilia. Am Nat. 1966;100:99–118. doi: 10.1073/pnas.54.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyne J. A. The evolutionary origin of hybrid inviability. Evolution. 1974;28:505–506. doi: 10.1111/j.1558-5646.1974.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 13.Howard D. J. Conspecific sperm and pollen precedence and speciation. Annu Rev Ecol Syst. 1999;30:109–132. [Google Scholar]
- 14.Palumbi S. R. Speciation and the evolution of gamete recognition genes: pattern and process. Heredity. 2009;102:66–76. doi: 10.1038/hdy.2008.104. [DOI] [PubMed] [Google Scholar]
- 15.Lorch P. D, Servedio M. R. The evolution of conspecific gamete precedence and its effect on reinforcement. J Evol Biol. 2007;20:937–949. doi: 10.1111/j.1420-9101.2007.01306.x. [DOI] [PubMed] [Google Scholar]
- 16.Lachaise D, Harry M, Solignac M, Lemeunier F, Benassi V, et al. Evolutionary novelties in islands: Drosophila santomea, a new melanogaster sister species from São Tome. Proc Biol Sci. 2000;267:1487–1495. doi: 10.1098/rspb.2000.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llopart A, Lachaise D, Coyne J. A. Multilocus analysis of introgression between two sympatric sister species of Drosophila: Drosophila yakuba and D. santomea. Genetics. 2005;171:197–210. doi: 10.1534/genetics.104.033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llopart A, Elwyn S, Lachaise D, Coyne J. A. Genetics of a difference in pigmentation between Drosophila yakuba and Drosophila santomea. Evolution. 2002;56:2262–2277. doi: 10.1111/j.0014-3820.2002.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 19.Llopart A, Lachaise D, Coyne J. A. An anomalous hybrid zone in Drosophila. Evolution. 2005;59:2602–2607. [PubMed] [Google Scholar]
- 20.Coyne J. A, Kim S. Y, Chang A. S, Lachaise D, Elwyn S. Sexual isolation between two sibling species with overlapping ranges: Drosophila santomea and Drosophila yakuba. Evolution. 2002;56:2424–2434. doi: 10.1111/j.0014-3820.2002.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 21.Coyne J. A, Elwyn S, Kim S. Y, Llopart A. Genetic studies of two sister species in the Drosophila melanogaster subgroup, D. yakuba and D. santomea. Genet Res. 2004;84:11–26. doi: 10.1017/s0016672304007013. [DOI] [PubMed] [Google Scholar]
- 22.Chang A. S. Conspecific sperm precedence in sister species of Drosophila with overlapping ranges. Evolution. 2004;58:781–789. doi: 10.1111/j.0014-3820.2004.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 23.Matute D. R, Coyne J. A. Intrinsic reproductive isolation between two sister species of Drosophila. Evolution. E-pub ahead of print. 2009 doi: 10.1111/j.1558-5646.2009.00879.x. doi: 10.1111/j.1558-5646.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 24.Matute D. R, Novak C. J, Coyne J. A. Thermal adaptation and extrinsic reproductive isolation in two species of Drosophila. Evolution. 2009;63:583–594. doi: 10.1111/j.1558-5646.2008.00588.x. [DOI] [PubMed] [Google Scholar]
- 25.Pinheiro J, Bates D. New York (New York): Springer; 2000. Mixed-effects models in S and S-PLUS.528 [Google Scholar]
- 26.Crawley M. J. Oxford (United Kingdom): Blackwell Scientific Publications; 1993. GLIM for ecologists.379 [Google Scholar]
- 27.Crawley M. J. Chichester (United Kingdom): Wiley; 2002. Statistical computing: an introduction to data analysis using S-plus.761 [Google Scholar]
- 28.Wilson K, Hardy I. C. W. Statistical analysis of sex ratios: an introduction. In: Hardy I. C. W, editor. Sex ratios: concepts and research methods. Cambridge (United Kingdom): Cambridge University Press;; 2002. pp. 48–92. [Google Scholar]
- 29.Koopman K. F. Natural selection for reproductive isolation between Drosophila pseudoobscura and Drosophila persimilis. Evolution. 1950;4:135–148. [Google Scholar]
- 30.Higgie M, Chenoweth S, Blows M. W. Natural selection and the reinforcement of mate recognition. Science. 2000;290:519–520. doi: 10.1126/science.290.5491.519. [DOI] [PubMed] [Google Scholar]
- 31.Coyne J. A, Orr H. A. Patterns of speciation in Drosophila. Evolution. 1989;43:262–281. doi: 10.1111/j.1558-5646.1989.tb04233.x. [DOI] [PubMed] [Google Scholar]
- 32.Coyne J. A, Orr H. A. “Patterns of speciation in Drosophila” revisited. Evolution. 1997;51:295–202. doi: 10.1111/j.1558-5646.1997.tb02412.x. [DOI] [PubMed] [Google Scholar]
- 33.Howard D. J. Reinforcement: origin, dynamics, and fate of an evolutionary hypothesis. In: Harrison R. G, editor. Hybrid zones and the evolutionary process. Oxford (United Kingdom): Oxford University Press; 1993. pp. 46–69. [Google Scholar]
- 34.Noor M. A. Reinforcement and other consequences of sympatry. Heredity. 1999;83:503–508. doi: 10.1038/sj.hdy.6886320. [DOI] [PubMed] [Google Scholar]
- 35.Albert A, Schluter D. Reproductive character displacement of male stickleback mate preference: reinforcement or direct selection? Evolution. 2004;58:1099–1107. doi: 10.1111/j.0014-3820.2004.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 36.Nosil P, Crespi B, Sandoval C. P. Reproductive isolation driven by the combined effects of ecological adaptation and reinforcement. Proc Biol Sci. 2003;270:1911–1918. doi: 10.1098/rspb.2003.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geyer L. B, Palumbi S. R. Reproductive character displacement and the genetics of gamete recognition in tropical sea urchins. Evolution. 2003;57:1049–1060. doi: 10.1111/j.0014-3820.2003.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y. H, Vacquier V. D. The divergence of species-specific abalone sperm lysins is promoted by positive Darwinian selection. Biol Bull. 1992;182:97–104. doi: 10.2307/1542183. [DOI] [PubMed] [Google Scholar]
- 39.Lee Y. H, Ota T, Vacquier V. D. Positive selection is a general phenomenon in the evolution of abalone sperm lysin. Mol Biol Evol. 1995;12:231–238. doi: 10.1093/oxfordjournals.molbev.a040200. [DOI] [PubMed] [Google Scholar]
- 40.Wullschleger E. B, Wiehn J, Jokela J. Reproductive character displacement between the closely related freshwater snails Lymnaea peregra and L. ovata. Evol Ecol Res. 2002;4:247–257. [Google Scholar]
- 41.Springer S. A, Crespi B. J. Adaptive gamete-recognition divergence in a hybridizing Mytilus population. Evolution. 2007;61:772–783. doi: 10.1111/j.1558-5646.2007.00073.x. [DOI] [PubMed] [Google Scholar]
- 42.Slaughter C, McCartney M. A, Yund P. O. Comparison of gamete compatibility between two blue mussel species in sympatry and in allopatry. Biol Bull. 2008;214:57–66. doi: 10.2307/25066660. [DOI] [PubMed] [Google Scholar]
- 43.Marshall J. L, Arnold M. L, Howard D. J. Reinforcement: the road not taken. Trends Ecol Evol. 2002;17:558–563. [Google Scholar]
- 44.Nosil P, Crespi B. J. Ecological divergence promotes the evolution of cryptic reproductive isolation. Proc Biol Sci. 2006;273:991–997. doi: 10.1098/rspb.2005.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coyne J. A, Elwyn S, Rolán-Alvarez E. Impact of experimental design on Drosophila sexual isolation studies: direct effects and comparison to field hybridization data. Evolution. 2005;59:2588–2601. [PubMed] [Google Scholar]
- 46.Markow T. A. Female remating, operational sex ratio, and the arena of sexual selection in Drosophila. Evolution. 2002;56:1725–1734. doi: 10.1111/j.0014-3820.2002.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 47.Rosengrave P, Gemmel N. J, Metcalf V, McBride K, Montgomerie R. A mechanism for cryptic female choice in chinook salmon. Behav Ecol. 2008;19:1179–1185. [Google Scholar]
- 48.Knowles L. L, Markow T. A. Sexually antagonistic coevolution of a postmating prezygotic reproductive character in desert Drosophila. Proc Natl Acad Sci U S A. 2001;98:8692–8696. doi: 10.1073/pnas.151123998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knowles L. L, Hernandez B. B, Markow T. A. Exploring the consequences of postmating-prezygotic interactions between the sexes. Proc Biol Sci. 2004;271:S357–S359. doi: 10.1098/rsbl.2004.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calsbeek R, Bonneaud C. Postcopulatory fertilization bias as a form of cryptic sexual selection. Evolution. 2008;62:1137–1148. doi: 10.1111/j.1558-5646.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- 51.Knowles L. L, Hernandez B. B, Markow T. A. Non-antagonistic interactions between the sexes revealed by the ecological consequences of reproductive traits. J Evol Biol. 2004;18:156–161. doi: 10.1111/j.1420-9101.2004.00779.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Offspring production from double matings by D. yakuba females I. Mean (SE) number of offspring per D. yakuba female (from either sympatric or allopatric populations) sired by first (D. yakuba, red) and second (D. santomea STO.4, blue) male. The number of offspring produced during the first 4 d was subtracted from the total amount of produced progeny. The data were analyzed with a nested ANOVA in which the asin (progeny produced after the second mating/total progeny) was the response and line was nested within origin of the D. yakuba line (allopatric or sympatric). The results (Female origin: F 1,54 = 0.069, p = 0.794; Female line: F 4,54 = 1.188, p = 0.068) show no difference in the strength of CSP between sympatric and allopatric lines when D. yakuba is the first male.
(0.02 MB PDF)
Offspring production from double matings by D. yakuba females II. Mean (SE) number of offspring per D. yakuba female (from either sympatric or allopatric populations) sired by first (D. santomea STO.4, red) and second (D. yakuba, blue) male. The number of offspring produced during the first 4 d was subtracted from the total amount of produced progeny. The data were analyzed with a nested ANOVA in which the asin (progeny produced after the second mating/total progeny) was the response and line was nested within origin of the D. yakuba line (allopatric or sympatric). The results (Female origin: F 1,54 = 0.643; Female line: F 4,54 = 1.188, p = 0.327) show no difference in the strength of CSP between sympatric and allopatric lines when D. santomea is the first male.
(0.02 MB PDF)
Effects of experimental sympatry on copulation latency in D. yakuba . Means and standard errors are based on the average of the seven lines (four replicates per line).
(0.02 MB PDF)
Effects of experimental sympatry on copulation duration in D. yakuba . Means and standard errors are based on the average of the seven lines (four replicates per line).
(0.02 MB PDF)
Frequency distributions of gametic isolation levels per D. yakuba line. The title of each graph shows the lines involved in the cross (♀ D. yakuba × ♂ D. santomea). Black distributions: allopatric lines; white distributions: sympatric lines. The data shown in this figure are the same data shown in Figure 1.
(0.04 MB PDF)
Isofemale lines of D. santomea and D. yakuba analyzed in this study.
(0.10 MB RTF)
Allopatric and sympatric crosses involving D. yakuba females. Cross corresponds to the letter shown in Figure 1B. S/A describes what is the geographical origin of the line (i.e., whether the lines involved in the cross are sympatric or allopatric).
(0.03 MB RTF)
Allopatric and sympatric crosses involving D. santomea females. Cross corresponds to the letter shown in Figure 1A. S/A describes what is the geographical origin of the line (i.e., whether the lines involved in the cross are sympatric or allopatric).
(0.03 MB RTF)
Degree of sexual isolation for allopatric (A) and sympatric (S) crosses. S/A describes what is the geographical origin of the line (i.e., whether the lines involved in the cross are sympatric or allopatric). N, the number of pairings observed for each mating type equaled 80 in all the cases. In the four mating columns, Y refers to D. yakuba, S to D. santomea, and the species of the female in each pairing is given first. Ipsi is the proposed statistic by Rolan-Alvarez to measure sexual isolation. SI(yak) and SI(san) are the degree of sexual isolation for D. yakuba females only and D. santomea females only, respectively. Allopatric (Al) and sympatric females (Sy) from both species showed no significant differences in sexual isolation in any of the three measurements (Ipsi: F 1,17 = 0.3899, p = 0.5406; SIyak: F 1,17 = 1.554, p = 0.2292; SIsan: F 1,17 = 0.1812, p = 0.6757).
(0.08 MB RTF)
Mean (SE) copulation latency in no-choice mating experiments involving D. yakuba females from sympatric and allopatric populations. N is equal to 12 for all crosses. The data were analyzed with a nested ANOVA in which copulation latency was the response. The fixed effects were female line, nested within origin of the D. yakuba line (allopatric or sympatric) and male line nested within male origin. Although the female and male line effects caused heterogeneity (Female line: F 16,277 = 10.176, p = 2.2×10−16; Male line: F 7,136 = 5.011, p = 1.22×10−5), there was no correlation between copulation latency and whether the populations were sympatric or allopatric (Female origin: F 1,99 = 3.633, p = 0.057; Male origin: F 1,17 = 0.6155, p = 0.432).
(0.10 MB RTF)
Mean (SE) copulation duration in no-choice mating experiments involving D. yakuba females from sympatric and allopatric populations. N is equal to 12 for all crosses. The data were analyzed with a nested ANOVA in which copulation duration was the response. The fixed effects were female line, nested within origin of the D. yakuba line (allopatric or sympatric) and male line nested within male origin. Although the female and male line effects caused heterogeneity (Female line: F 16,460 = 7.407, p = 2.2×10−6; Male line: F 1,230 = 4.352, p = 8.503×10−5), there was no correlation between copulation duration and whether the populations were sympatric or allopatric (Female origin: F 1,104 = 1.683, p = 0.195; Male origin: F 1,230 = 3.706, p = 0.054).
(0.11 MB RTF)
F1 ( D. yakuba × D. santomea ) larvae survival as a proxy for postzygotic isolation. Hybrid larvae from matings between females from allopatric or sympatric lines of D. yakuba and D. santomea were collected as first-instar larvae, and the number of recovered adults was scored. N equals 100 for all crosses. The data were analyzed with a nested ANOVA in which the asin (proportion of surviving larvae) was the response. The fixed effects were female line, nested within origin of the D. yakuba line (allopatric or sympatric) and male line nested within male origin. Female and male line effects caused heterogeneity (Female line: F 4,3589 = 65.39, p = 8.29×10−16; Male line: F 4,3589 = 53.011, p = 2.12×10−15), but there was no correlation between copulation latency and whether the populations were sympatric or allopatric (Female origin: F 1,3589 = 1.84, p = 0.1192; Male origin: F 1,3589 = 0.6155, p = 0.582).
(0.03 MB RTF)