Abstract
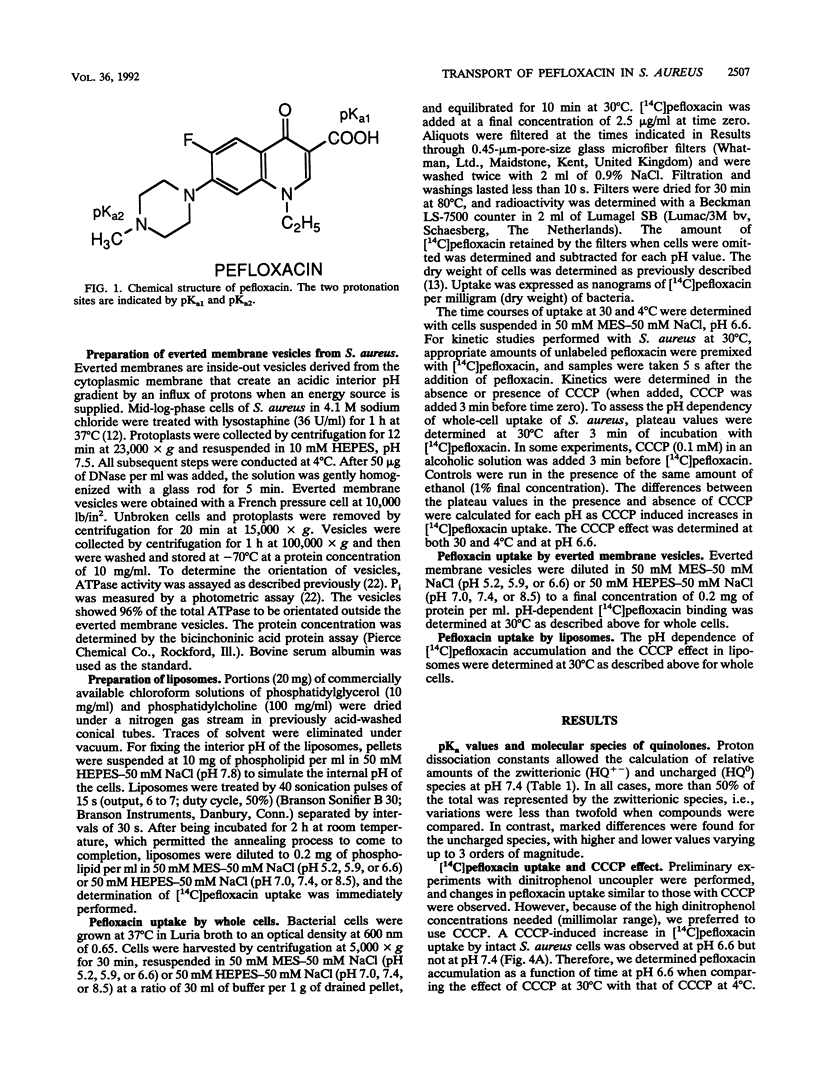
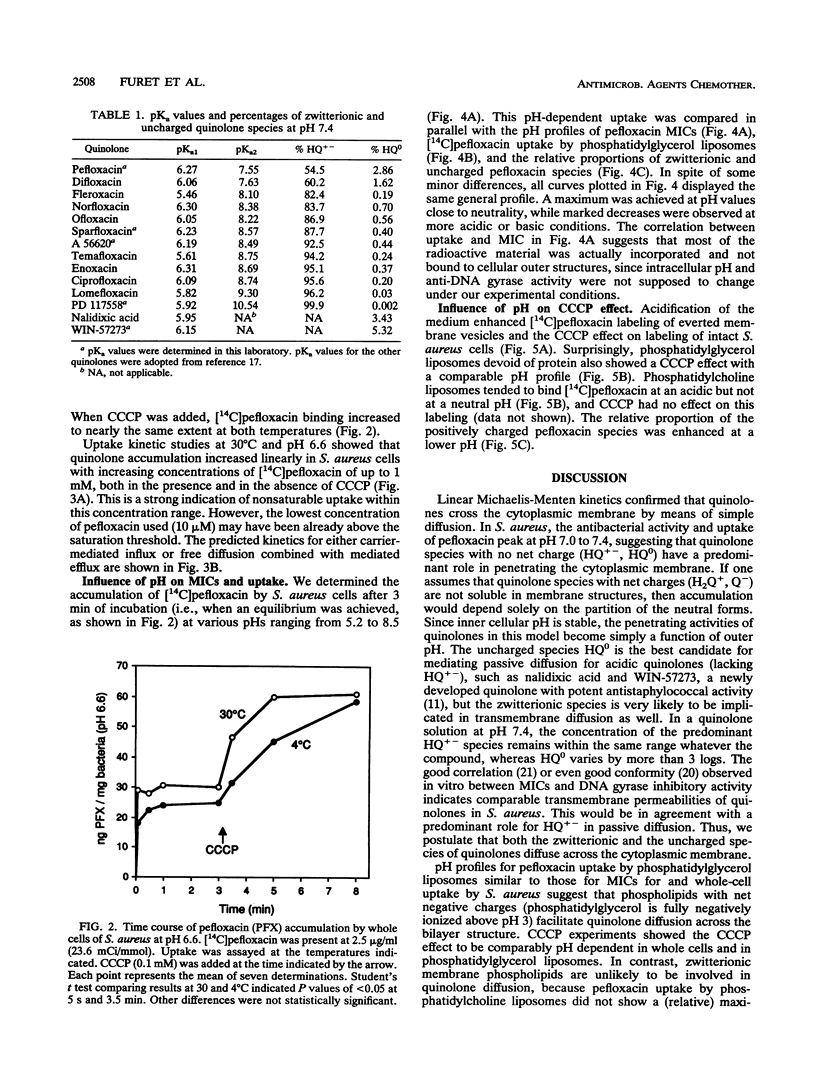
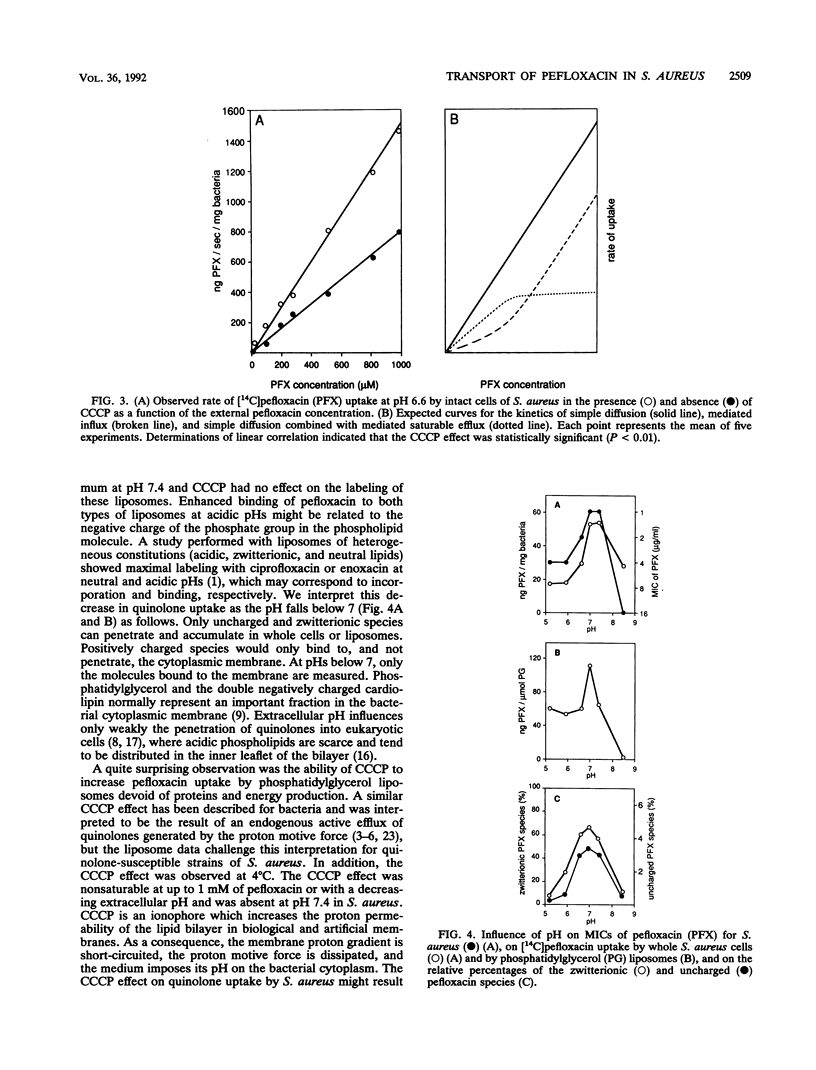
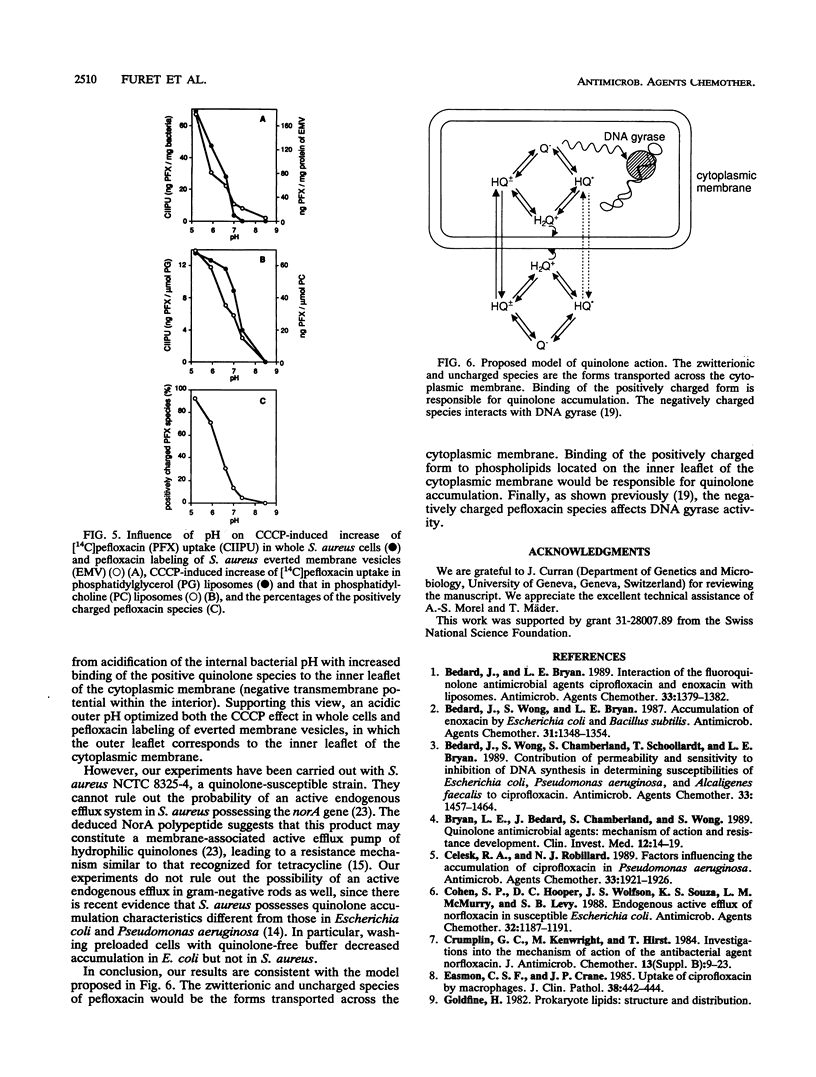
Binding to phospholipids, uptake by simple diffusion, and an energy-dependent, carrier-mediated efflux are thought to characterize interactions between fluoroquinolones and bacterial cytoplasmic membranes. Here, we have found that an endogenous active efflux is unlikely in quinolone-susceptible Staphylococcus aureus. The protonophore, carbonyl cyanide m-chlorophenylhydrazone (CCCP), increased pefloxacin uptake in different membrane systems under conditions which excluded carrier-mediated transport, i.e., in bacterial cells at 4 degrees C and in protein-free phosphatidylglycerol liposomes. When plotted as a function of outer pH, the CCCP effect, both in S. aureus cells and in phosphatidylglycerol liposomes, correlated with pefloxacin labeling of everted S. aureus membrane vesicles, with all three profiles showing maximal effect at an acidic pH. So the CCCP effect may result not from inhibition of the proton motive force, as previously thought, but rather from acidification of the intramembrane space by the protonophore, leading to enhanced binding of the positive pefloxacin species to the inner leaflet of the bilayer. Moreover, antistaphylococcal potency and uptake profiles of pefloxacin in S. aureus and phosphatidylglycerol liposomes, assayed as a function of outer pH, peaked at a neutral pH. These observations suggest that zwitterionic and positive quinolone species are responsible for diffusion through and binding to the cytoplasmic membrane, respectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedard J., Bryan L. E. Interaction of the fluoroquinolone antimicrobial agents ciprofloxacin and enoxacin with liposomes. Antimicrob Agents Chemother. 1989 Aug;33(8):1379–1382. doi: 10.1128/aac.33.8.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard J., Chamberland S., Wong S., Schollaardt T., Bryan L. E. Contribution of permeability and sensitivity to inhibition of DNA synthesis in determining susceptibilities of Escherichia coli, Pseudomonas aeruginosa, and Alcaligenes faecalis to ciprofloxacin. Antimicrob Agents Chemother. 1989 Sep;33(9):1457–1464. doi: 10.1128/aac.33.9.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard J., Wong S., Bryan L. E. Accumulation of enoxacin by Escherichia coli and Bacillus subtilis. Antimicrob Agents Chemother. 1987 Sep;31(9):1348–1354. doi: 10.1128/aac.31.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Bedard J., Wong S., Chamberland S. Quinolone antimicrobial agents: mechanism of action and resistance development. Clin Invest Med. 1989 Feb;12(1):14–19. [PubMed] [Google Scholar]
- Celesk R. A., Robillard N. J. Factors influencing the accumulation of ciprofloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Nov;33(11):1921–1926. doi: 10.1128/aac.33.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., Hooper D. C., Wolfson J. S., Souza K. S., McMurry L. M., Levy S. B. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1187–1191. doi: 10.1128/aac.32.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumplin G. C., Kenwright M., Hirst T. Investigations into the mechanism of action of the antibacterial agent norfloxacin. J Antimicrob Chemother. 1984 May;13 (Suppl B):9–23. doi: 10.1093/jac/13.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- Easmon C. S., Crane J. P. Uptake of ciprofloxacin by macrophages. J Clin Pathol. 1985 Apr;38(4):442–444. doi: 10.1136/jcp.38.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Ng E. Y., McHugh G. L., Swartz M. N. Mechanisms of quinolone resistance in Escherichia coli: characterization of nfxB and cfxB, two mutant resistance loci decreasing norfloxacin accumulation. Antimicrob Agents Chemother. 1989 Mar;33(3):283–290. doi: 10.1128/aac.33.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. In vitro evaluation of WIN 57273, a new broad-spectrum fluoroquinolone. Antimicrob Agents Chemother. 1990 Feb;34(2):306–313. doi: 10.1128/aac.34.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. 2. The coupling of reduced phenazine methosulfate to the concentrative uptake of beta-galactosides and amino acids. J Biol Chem. 1971 Oct 10;246(19):5857–5861. [PubMed] [Google Scholar]
- Lucain C., Regamey P., Bellido F., Pechére J. C. Resistance emerging after pefloxacin therapy of experimental Enterobacter cloacae peritonitis. Antimicrob Agents Chemother. 1989 Jun;33(6):937–943. doi: 10.1128/aac.33.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey C., Bertasso A., Pace J., Georgopapadakou N. H. Quinolone accumulation in Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Antimicrob Agents Chemother. 1992 Aug;36(8):1601–1605. doi: 10.1128/aac.36.8.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Pascual A., Garcia I., Perea E. J. Fluorometric measurement of ofloxacin uptake by human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1989 May;33(5):653–656. doi: 10.1128/aac.33.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. L., Mitscher L. A., Sharma P. N., O'Donnell T. J., Chu D. W., Cooper C. S., Rosen T., Pernet A. G. Mechanism of inhibition of DNA gyrase by quinolone antibacterials: a cooperative drug--DNA binding model. Biochemistry. 1989 May 2;28(9):3886–3894. doi: 10.1021/bi00435a039. [DOI] [PubMed] [Google Scholar]
- Takahata M., Nishino T. DNA gyrase of Staphylococcus aureus and inhibitory effect of quinolones on its activity. Antimicrob Agents Chemother. 1988 Aug;32(8):1192–1195. doi: 10.1128/aac.32.8.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Sato K., Kimura Y., Hayakawa I., Osada Y., Nishino T. Inhibition by quinolones of DNA gyrase from Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Jul;35(7):1489–1491. doi: 10.1128/aac.35.7.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura S., Ubukata K., Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990 Dec;172(12):6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Thienen G., Postma P. W. Coupling between energy conservation and active transport of serine in Escherichia coli. Biochim Biophys Acta. 1973 Oct 25;323(3):429–440. doi: 10.1016/0005-2736(73)90188-0. [DOI] [PubMed] [Google Scholar]



