Abstract
Wnt/β-catenin signaling plays key roles in tooth development, but how this pathway intersects with the complex interplay of signaling factors regulating dental morphogenesis has been unclear. We demonstrate that Wnt/β-catenin signaling is active at multiple stages of tooth development. Mutation of β-catenin to a constitutively active form in oral epithelium causes formation of large, misshapen tooth buds and ectopic teeth, and expanded expression of signaling molecules important for tooth development. Conversely, expression of key morphogenetic regulators including Bmp4, Msx1 and Msx2 is down-regulated in embryos expressing the secreted Wnt inhibitor Dkk1 which blocks signaling in epithelial and underlying mesenchymal cells. Similar phenotypes are observed in embryos lacking epithelial β-catenin, demonstrating a requirement for Wnt signaling within the epithelium. Inducible Dkk1 expression after the bud stage causes formation of blunted molar cusps, down-regulation of the enamel knot marker p21, and loss of restricted ectodin expression, revealing requirements for Wnt activity in maintaining secondary enamel knots. These data place Wnt/β-catenin signaling upstream of key morphogenetic signaling pathways at multiple stages of tooth development and indicate that tight regulation of this pathway is essential both for patterning tooth development in the dental lamina, and for controlling the shape of individual teeth.
Keywords: tooth, mouse, embryo, Wnt, molar, incisor, β-catenin, dental development
Introduction
Delineating the mechanisms by which oral epithelial cells adopt and maintain dental fates is critical for understanding developmental tooth syndromes and for designing strategies for the regeneration or repair of teeth and enamel. A key question is how the coordinated actions of broadly used signaling pathways result in the formation of a specific organ, in this case the tooth.
An early initiating signal for tooth development arises in the oral ectoderm, causing thickening of the dental lamina and its down-growth into the mesenchyme to form a tooth bud (Lumsden, 1988). The specification of tooth and intervening regions may be regulated by a competition between Fibroblast growth factor (FGF) 8, expressed in pre-tooth epithelium, and Bone morphogenic protein (BMP) 4, expressed in intervening epithelium (Neubuser et al., 1997; St Amand et al., 2000). These factors regulate restricted expression of the homeobox transcription factor Pitx2 that is required for tooth development beyond the bud stage (Lin et al., 1999). The Sonic hedgehog (Shh) pathway genes Gli2 and Gli3, and the homeobox genes Msx1 and Msx2 are important for invagination of the dental lamina to form tooth buds (Bei and Maas, 1998; Hardcastle et al., 1998). However, the mechanisms controlling assignment of tooth fate are incompletely understood.
At the late bud stage, the tooth bud begins to fold at its base in response to mesenchymal signals (Mina and Kollar, 1987). The enamel knot, a non-proliferating, transient epithelial structure, appears at the cap stage and is thought to regulate tooth shape (Vaahtokari et al., 1996). These processes fail to occur in mice lacking the transcription factors LEF1, PITX2, MSX1 and PAX9, which are targets of the intercellular Wnt, BMP and FGF pathways (Bei and Maas, 1998; Kratochwil et al., 1996; Lin et al., 1999; Peters et al., 1998). Subsequent folding morphogenesis (the bell stage) results in the formation of multiple cusps and requires the TNF family member ectodysplasin (EDA), which signals via its receptor EDAR to activate the NF-κB pathway (Courtney et al., 2005; Jernvall et al., 1994; Ohazama et al., 2004; Pispa et al., 1999; Schmidt-Ullrich et al., 2006). Dental papilla cells adjacent to the epithelium differentiate into odontoblasts and begin to secrete dentin (Thesleff and Hurmerinta, 1981), while the epithelial cells differentiate into outer enamel epithelium, stellate reticulum, stratum intermedium, and inner enamel epithelium, lying adjacent to the papilla. The inner enamel epithelium differentiates into preameloblasts and then enamel-secreting ameloblasts (Thesleff and Hurmerinta, 1981).
Wnts form a large family of secreted ligands that activate several receptor-mediated pathways (Logan and Nusse, 2004). In the Wnt/β-catenin pathway, binding of Wnt ligands to Frizzled (FZ) receptors and LDL receptor related protein (LRP) family co-receptors causes β-catenin accumulation, nuclear translocation, and transcriptional activation by complexes of β-catenin and LEF/TCF transcription factor family members (Logan and Nusse, 2004). The Wnt/β-catenin pathway is specifically inactivated by endogenous secreted inhibitors of the Dickkopf (DKK) family, which bind to LRP and to high-affinity receptors of the Kremen family, causing rapid internalization of Kremen-Dkk-LRP complexes and removal of LRP from the plasma membrane (Mao et al., 2002).
Activation of Wnt/β-catenin signaling initiates the de novo formation of ectodermal appendages related to teeth, including hair follicles, feather buds, mammary placodes and taste papillae (Chu et al., 2004; Gat et al., 1998; Hogan, 1999; Liu et al., 2007; Noramly et al., 1999; Thesleff et al., 1995). Conversely, initiation of hair follicle, mammary and taste papilla placode development requires Wnt/β-catenin signaling (Andl et al., 2002; Chu et al., 2004; Liu et al., 2007).
Several specific observations indicate that Wnt signaling plays key roles in tooth morphogenesis. Several Wnt genes are broadly expressed in oral and dental epithelium, while others are upregulated in developing teeth (Dassule and McMahon, 1998; Kratochwil et al., 2003; Sarkar and Sharpe, 1999). Loss of LEF1 causes arrested tooth development at the late bud stage, loss of expression of a direct LEF1/β-catenin target gene, Fgf4, and failure of survival of dental epithelial cells (Kratochwil et al., 2003; Sasaki et al., 2005). Other roles for Wnt signals in developing and postnatal teeth are likely masked in Lef1-null mice by functional redundancy of co-expressed Lef1, Tcf1, and possibly additional Tcf family members (Kratochwil et al., 2003; Oosterwegel et al., 1993). Consistent with additional Wnt functions, constitutive ectopic expression of Dkk1 in the oral epithelia of transgenic mouse embryos causes arrested tooth development at the lamina-early bud stage (Andl et al., 2002), and oral epithelium expressing constitutively active β-catenin develops multiple teeth following transplantation to a kidney capsule (Jarvinen et al., 2006).
Here we investigate the pattern of, and precise requirements for, Wnt pathway activation at multiple stages of tooth development, and use explant culture and in vivo loss and gain of function experiments to determine how Wnt signaling interacts with other factors important for tooth morphogenesis. Our results place Wnt upstream of other key signaling pathways at several stages of tooth development, and suggest the potential use of Wnt activation in strategies for tooth regeneration.
Materials and Methods
Generation of Mouse Lines and Genotyping
Wnt activity was monitored using TOPGAL (DasGupta and Fuchs, 1999) (Jackson Laboratories, Bar Harbor, ME, USA), BAT-gal (Maretto et al., 2003) and TCF/Lef-LacZ (Mohamed et al., 2005) Wnt reporter transgenic mice. For epidermal-specific activation of β-catenin signaling, Ctnnb1(Ex3)fl/+ mice (Harada et al., 1999) were crossed to K14-Cre line 43 transgenic mice (Andl et al., 2004). For epidermal-specific deletion of β-catenin, Ctnnb1f/lfl mice (Brault et al., 2001) (Jackson Laboratories) were crossed to K14-Cre line 43 transgenic mice (Andl et al., 2004). To assess the efficiency of K14-Cre mediated recombination in oral and dental epithelia, K14-Cre mice were crossed with the ROSA26R Cre reporter line (Soriano, 1999) (Jackson Laboratories). For Wnt pathway inhibition, K5-rtTA tetO-Dkk1 mice were generated and induced by feeding with chow formulated with 1g/kg doxycycline (BioServ, Laurel, MD, USA) as previously described (Chu et al., 2004). To monitor the efficiency of Wnt pathway activation and inhibition, K14-Cre Ctnnb1(Ex3)fl/+ mice and K14-Cre Ctnnb1f/lfl were crossed with TOPGAL mice and further crossed with K14-Cre mice; and K5-rtTA tetO-Dkk1 mice were crossed with TOPGAL mice. To monitor activation of NF-κB signaling, K5-rtTA tetO-Dkk1 mice were crossed with the NF-κB LacZ reporter transgenic mouse line (Ig)3xcona-lacZ (NFκB-GAL) (Schmidt-Ullrich et al., 1996). All experiments were performed with approved animal protocols according to the institutional guidelines established by the University of Pennsylvania IACUC committee.
Analysis of TOPGAL, BAT-gal and TCF/Lef-LacZ expression
Heads from E11.5 - E12.5 embryos were cryosectioned at 10-12μm followed by X-gal staining. Embryonic mandibles at E12.5 - E18.5 were fixed and whole mount stained with X-gal (Furth et al., 1994), photographed and/or paraffin-embedded, sectioned and counterstained with eosin.
Histology, Immunofluorescence, BrdU Incorporation, TUNEL Assays and In Situ Hybridization
Tissue preparation, histology, immunofluorescence with anti-β-catenin, BrdU assays, TUNEL staining and in situ hybridization with digoxygenin-labeled probes were as described previously (Andl et al., 2004; Andl et al., 2002; Chu et al., 2004). Sections were mounted with Vectashield containing DAPI (Vector Laboratories, Burlingame, CA, USA). Bmp4, Edar, Lef1, Shh, Wnt10b, Eda, Ctnnb1, Msx1, Msx2, p21 and Ectodin probes were as described previously (Andl et al., 2002; Kassai et al., 2005; Liu et al., 2007; Schmidt-Ullrich et al., 2006). The following PCR products each containing a T7 promoter were used as templates for sense and antisense probe synthesis: Pitx2, NM_011098, nt 972-1713; Pax9, NM_011041, nt 1431-1643 (Peters et al., 1998); Ambn, NM_009664.1, nt 248-957; Dspp: MN_010080.2, nt 385-1025 (Wang et al., 2004).
Mandible Cultures and Bead Implantation Experiments
Mandibles dissected from E11.5 TOPGAL embryos were cultured in glutamine-supplemented DMEM/F12, 10% FBS on cell culture inserts (BD Biosciences, San Jose, CA, USA) in the presence or absence of 50 mM LiCl at 37°C, 5% CO2 for 24 hours, and stained with X-gal to assess reporter gene expression. Bead implantation and mandible culture experiments were performed according to previously described procedures (Chen et al., 1996; Vainio et al., 1993). For bead implantation, heparin acrylic beads (Sigma, St Louis, MO) were incubated with 100 μg/ml recombinant human BMP4 protein (R&D Systems, Minneapolis, MN USA) at 37°C for 30 minutes. Control beads were soaked with similar concentrations of BSA under the same conditions. Some BMP4 coated beads were subsequently washed in PBS and incubated with 20 μg/ml recombinant mouse DKK1 protein (R&D Systems, Minneapolis, MN USA) or similar concentrations of BSA at 37°C for 1 hour. Protein-soaked beads were stored at 4°C and used within 1 week. All explants were cultured on cell culture inserts (BD Biosciences, San Jose, CA, USA) in the presence of 50μg/ml doxycyline, in Dulbecco's minimal essential medium with 10% FCS at 37°C for 24 hours. After culture, explants were fixed and processed for whole-mount in situ hybridization.
Results
Wnt/β-catenin signaling is active at multiple stages of tooth morphogenesis
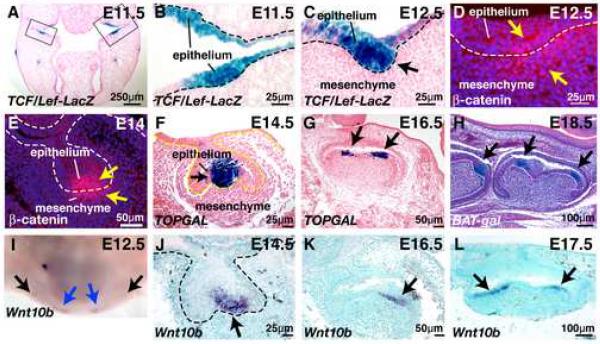
To determine the spatial and temporal pattern of WNT/β-catenin pathway activation during tooth development, we utilized mice carrying TCF/Lef-LacZ, TOPGAL or BAT-gal Wnt reporter transgenes that each contain multimerized consensus LEF/TCF binding sites upstream of a minimal promoter and lacZ coding sequences (DasGupta and Fuchs, 1999; Maretto et al., 2003; Mohamed et al., 2005). These three independent reporter transgenic lines displayed similar expression patterns at multiple stages of tooth development. In addition we utilized immunofluorescence for nuclear β-catenin (Andl et al., 2002) to identify cells with nuclear-localized β-catenin, an alternate readout for Wnt signaling activity. Reporter gene activity was apparent in the incisor and molar regions of the maxilla and mandible by embryonic day 11.5 (E11.5). X-gal staining of cryosectioned oral cavity revealed intense staining in developing dental epithelial placodes (Fig. 1A,B). At E12.5, reporter gene expression localized to invaginating tooth bud epithelium (Fig. 1C). Nuclear localized β-catenin was observed in epithelial and immediately underlying mesenchymal cells at this stage (Fig. 1D). At the cap stage, nuclear β-catenin and Wnt reporter gene expression localized to epithelial cells of the primary enamel knot (Fig. 1E,F). Nuclear β-catenin was also observed in a cluster of immediately underlying mesenchymal cells (Fig 1E). The slightly different expression patterns observed for Wnt reporter genes and nuclear β-catenin at the bud-cap stages could be due to relatively greater sensitivity of nuclear β-catenin detection in mesenchymal versus epithelial cells; under-reporting of mesenchymal Wnt signaling by the artificial reporter gene constructs; and/or absence in the mesenchyme of nuclear β-catenin co-factors required for activating transcription. At the early bell stage, Wnt reporter activity localized to the developing molar cusps (Fig. 1G), and by the late bell stage was present asymmetrically in the epithelial enamel knots of developing molar cusps (Fig. 1H). Notably, expression of Wnt10b localizes to dental placodes by E12.5 (Fig. 1I and data not shown), similar to, although slightly later than, the pattern of Wnt reporter gene activation. By the cap stage Wnt10b, like Wnt reporter gene expression, localizes to the primary enamel knot (Fig. 1J). Wnt10b expression subsequently localizes to the secondary enamel knots in molar cusp epithelium (Fig. 1K,L).
Fig. 1. Localization of Wnt reporter gene expression, β-catenin and Wnt10b expression in developing teeth.
Tissues were cryosectioned and then X-gal stained to reveal sites of Wnt reporter expression (blue) (A-C); sectioned prior to immunofluorescence staining for β-catenin (red) (D-E); or whole mount X-gal stained prior to paraffin sectioning (F-H). (A) Transverse section of E11.5 TCF/Lef-LacZ head showing reporter gene expression in molar tooth placodes (bracketed) and adjacent oral epithelium. (B) Higher magnification view of the area bracketed on the right in (A) showing LacZ expression in epithelial cells of the first molar. (C) Transverse section of E12.5 TCF/Lef-LacZ maxilla showing LacZ expression in molar epithelial cells. (D) Transverse section of E12.5 maxilla showing nuclear and cytoplasmic localization of β-catenin in epithelial cells and immediately underlying mesenchymal cells of the first left molar. Nuclei are counterstained with DAPI and appear blue. (E) Transverse section of E14 maxilla showing nuclear localization of β-catenin in epithelial cells and immediately underlying mesenchymal cells of the first left molar. Nuclei are counterstained with DAPI and appear blue. (F) Frontal section of E14.5 TOPGAL mandible showing reporter gene expression in the enamel knot of a first lower molar. (G) Frontal section of E16.5 TOPGAL mandible showing reporter gene expression in developing molar cusps. (H) Sagittal section of E18.5 BAT-gal mandible showing reporter gene expression in molar cusp cells (arrows). (I) E12.5 mandible subjected to whole mount in situ hybridization with digoxygenin-labeled probe for Wnt10b. Note hybridization signals (purple) in incisor placodes (blue arrows) and in the developing molar placodes (black arrows). (J-L) In situ hybridization of frontally sectioned mandibles with digoxygenin-labeled probe for Wnt10b. (J) E14.5 first lower molar showing hybridization to the enamel knot. (K) E16.5 first lower molar showing hybridization to developing cusp epithelium. (L) E17.5 first lower molar showing expression in cusp epithelia. Sections in (A-C,F,G) were counterstained with eosin, section (H) with hematoxylin, and sections in (J-L) with methyl green. A dashed line indicates the epithelial-mesenchymal boundary in (B-F and J).
Stimulation of Wnt signaling can initiate tooth development and results in expanded expression of multiple signaling factors
To determine whether stimulation of Wnt/β-catenin signaling is capable of initiating tooth development we first examined the consequences of pathway stimulation ex vivo in cultured mandibles. TOPGAL transgenic mandibles were dissected at E11.5 and cultured for 24 hours under conditions permissive for inductive stages of tooth development (Jowett et al., 1993), in either the presence or absence of 50mM LiCl which inhibits GSK3-β, preventing targeting of cytoplasmic β-catenin for degradation (Hedgepeth et al., 1997). In LiCl-treated mandibles, the sizes of clusters of TOPGAL-expressing cells in the regions of the incisor rudiments were significantly expanded relative to controls (Supplementary Fig. S1, white arrows) and the appearance of TOPGAL-expressing cell clusters in the molar regions was markedly accelerated compared with controls (Supplementary Fig. S1, red arrows) (n=8 control and n=9 LiCl-treated mandibles). In approximately 50% of LiCl-treated mandibles, clusters of blue cells with the appearance of dental placodes were formed at ectopic locations, adjacent to the incisor and molar clusters (Supplementary Fig. S1, pink arrows). Ectopic TOPGAL-expressing cell clusters were not observed in control mandibles (Supplementary Fig. S1). These data suggest that LiCl treatment enhances Wnt/β-catenin pathway mediated transcription in a regionspecific fashion within the mandible, and may promote the formation of dental placodes.
To determine the consequences of Wnt pathway stimulation in vivo, we mutated β-catenin to a stabilized form in epithelial cells. Ctnnb1(Ex3)fl/+ mice, in which exon 3 of the endogenous β-catenin gene (Ctnnb1), encoding all phosphorylation target serine/threonine residues, is flanked by loxP sequences that are targets for Cre-mediated recombination (Harada et al., 1999), were mated to K14-Cre line 43 transgenic mice (Andl et al., 2004) expressing Cre recombinase under the control of a Keratin 14 promoter (Byrne et al., 1994). K14-Cre mediated recombination of a ROSA26R Cre reporter gene (Soriano, 1999) was observed broadly in oral epithelia by E12.5 (Supplementary Fig. S2).
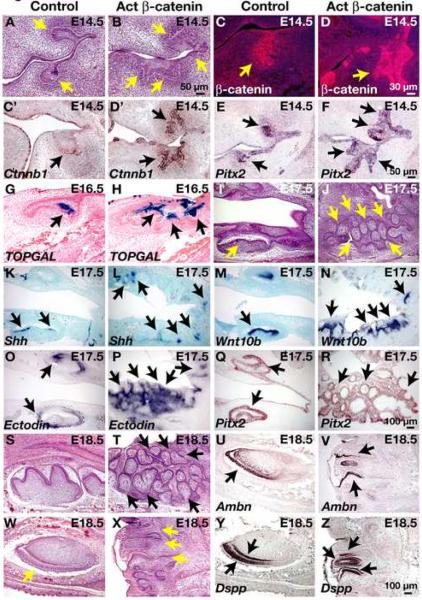
K14-Cre Ctnnb1(Ex3)fl/+ mice die at birth and exhibit multiple defects including open eyes and protruding tongues (Liu et al., 2007). Histological examination of the oral cavity at E14.5 revealed gross abnormalities in tooth development (Fig. 2A,B). Molar teeth developing in their normal locations were enlarged and exhibited multiple epithelial protrusions into the underlying mesenchyme. Smaller, ectopic invaginations were observed in adjacent epithelia. Similar phenotypes resulting from mutation of β-catenin to a stabilized form have also been described recently by another group (Jarvinen et al., 2006).
Fig. 2. Mutation of epithelial β-catenin to a stabilized form causes abnormal dental invaginations and ectopic tooth formation.
Control littermate (A,C,C',E,G,I,K,M,O,Q,S,U,W,Y) and K14-Cre Ctnnb1(Ex3)fl/+ (Act β-catenin) mutant(B,D,D',F,H,J,L,N,P,R,T,V,X,Z) oral cavity from embryos at E14.5 (A-F), E16.5 (G,H), E17.5 (I-R) and E18.5 (S-Z) paraffin embedded and sectioned frontally (A-F) or sagitally (G-Z) and subjected to hematoxylin and eosin staining (A,B,I,J,S,T,W,X); immunofluorescence with anti-β-catenin (red) (C,D); X-gal staining (G,H) (blue); or in situ hybridization with digoxygenin-labeled probe for Ctnnb1 (C',D'), Pitx2 (E,F,Q,R), Shh (K,L), Wnt10b (M,N), ectodin (O,P), Ambn (U,V) or Dspp (Y,Z) (purple or brown signals). Sections in (G,H) were taken from control and mutant embryos that also carried the TOPGAL Wnt reporter gene and were counterstained with eosin (pink). Size bar in (B) applies to (A,B); size bar in (D) applies to (C,D); size bar in (R) applies to (C'-R); size bar in (Z) applies to (S-Z).
Strongly elevated levels of β-catenin protein were observed in the abnormal teeth and ectopic invaginations, compared with the levels in control littermate teeth (Fig. 2C,D). However, β-catenin protein levels were not uniformly elevated throughout the oral surface epithelium, suggesting either non-uniform activation of the K14 promoter at this stage, or the existence of negative and positive feedback signaling downstream of activated β-catenin that impacts on protein levels. Interestingly, Ctnnb1 mRNA was strongly upregulated in the abnormal teeth and ectopic invaginations, suggesting that stabilized β-catenin positively regulates its own expression in dental epithelial cells at the level of transcription or message stability (Fig. 2C',D').
Pitx2 expression was observed throughout the abnormal epithelial structures (Fig. 2E,F). As Pitx2 expression is characteristic of developing teeth (Mucchielli et al., 1997), but is not observed in other embryonic epithelial appendages such as hair follicle germs and mammary buds (EYC and SEM, unpublished data), this result identifies the abnormal structures as developing teeth.
Expanded Wnt/β-catenin pathway activity, assayed by TOPGAL expression, was observed in the abnormal and ectopic dental structures in mutant oral cavities (Fig. 2G,H). By E17.5, multiple small, ectopic teeth could be observed on histological analysis (Fig. 2I,J). These were positive for the dental epithelial marker Shh (Fig. 2K,L). Similarly, expanded and ectopic expression of the enamel knot marker Wnt10b was observed in the mutant teeth (Fig. 2M,N). In addition, broad expression of Lef1, Bmp4, Msx1 and Msx2 mRNAs was observed in the abnormal teeth and ectopic invaginations (Supplementary Fig. S3, and data not shown).
Broad expression of ectodin (also known as Wise), a Bmp and Wnt inhibitor critical for molar cusp patterning (Itasaki et al., 2003; Kassai et al., 2005; Laurikkala et al., 2003), was observed in the abnormal and ectopic mutant teeth (Fig. 2O,P) in a pattern inverse to that of TOPGAL and Wnt10b. This finding suggests that ectodin expression is suppressed in cells with high Wnt signaling activity but induced in adjacent cells by a secreted factor downstream of Wnt/β-catenin. Pitx2 expression was maintained in the mutant dental epithelium at E17.5 and E18.5 (Fig. 2Q,R and Supplementary Fig. S3).
By E18.5, dental differentiation, including the formation of ameloblasts and odontoblasts, could be observed in mutant oral cavities upon histological analysis (Fig. 2S,T,W,X), and was confirmed by in situ hybridization for the ameloblast differentiation marker ameloblastin (Ambn) and the preodontoblast/preameloblast differentiation marker dentin sialophosphoprotein (Dspp) (Fig. 2U,V,Y,Z and data not shown). Developing mutant molar teeth were irregular in shape, and lacked the normal regular pattern of developing cusps (Fig. 2S,T), consistent with the observed development of unicuspoid teeth in transplanted dental epithelium expressing constitutively activated β-catenin (Jarvinen et al., 2006). Serial sections through incisor tooth structures revealed them to be misoriented and abnormally shaped (Supplementary Fig. S3K). These results indicate that stimulation of β-catenin signaling specifically within epithelial cells can initiate tooth development and promotes a cascade of signaling events associated with this process. Our data further suggest that limitation of Wnt/β-catenin signaling activity is critical for establishing normal tooth patterning and shape.
WNT/β-catenin signaling is required within the epithelium for early stages of tooth development
To determine the requirements for WNT/β-catenin signaling in tooth development and expression of key regulatory factors, we utilized inducible bi-transgenic K5-rtTA tetO-Dkk1 mice, in which expression of Dkk1, a secreted Wnt antagonist capable of blocking the actions of Wnts that signal via LRP co-receptors and β-catenin (Zorn, 2001), can be induced by doxycycline in cells in which the Keratin 5 promoter is active including surface and oral ectoderm and tooth buds (Chu et al., 2004; Diamond et al., 2000). Ectopic Dkk1 expressed in surface epithelia blocks WNT/β-catenin pathway activity in the epithelium and in underlying mesenchymal cells (Chu et al., 2004).
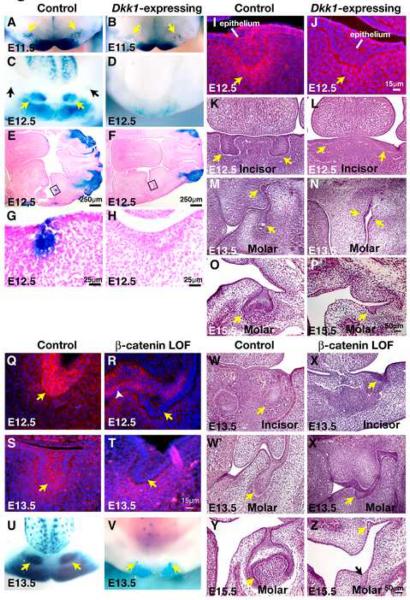
To determine the efficiency of Wnt inhibition resulting from ectopic Dkk1 expression in oral ectoderm, pregnant females carrying K5-rtTA tetO-Dkk TOPGAL and control TOPGAL littermate embryos were placed on oral doxycycline from the time of detection of a copulation plug and sacrificed at E11.5 or E12.5. Mandibles were dissected, subjected to whole mount X-gal staining, and sectioned. TOPGAL expression was reduced, but not entirely absent, in the dental region of induced K5-rtTA tetO-Dkk TOPGAL embryos at E11.5 (Fig. 3A,B). However, by E12.5, TOPGAL activity was absent from K5-rtTA tetO-Dkk TOPGAL dental lamina, indicating efficient Wnt inhibition by this stage (Fig. 3C-H). Immunofluorescence staining revealed that in controls, nuclear and cytoplasmic β-catenin localized to mesenchymal cells underlying the epithelium at E12.5 and was also observed in some dental epithelial cells (Fig. 3I). Nuclear and cytoplasmic localized β-catenin was not detected in Dkk1-expressing samples at E12.5, consistent with Wnt/β-catenin pathway inhibition (Fig. 3J). The small number of epithelial cells displaying nuclear and cytoplasmic localized β-catenin in control molars at E12.5 despite strong expression of epithelial TOPGAL at this stage could be due to lower sensitivity of nuclear β-catenin detection in epithelial cells.
Fig. 3. Ectopic expression of Dkk1 or epithelial deletion of β-catenin blocks Wnt pathway activity and tooth development.
(A-D) X-gal stained mandible whole mounts from E11.5 (A,B) and E12.5 (C,D) littermate control TOPGAL (A,C) and K5-rtTA tetO-Dkk1 TOPGAL (B,D) (Dkk1-expressing) embryos treated with doxycycline from E0.5. (E-H) Transverse sectioned X-gal stained heads from E12.5 control TOPGAL (E,G) and K5-rtTA tetO-Dkk1 TOPGAL (F,H) (Dkk1-expressing) embryos. Note absence of TOPGAL staining and arrest of molar tooth development in the Dkk1-expressing embryo (F,H). Panels G,H shower higher magnification photographs of the boxed regions in (E,F). (I,J) Frontally sectioned oral cavities from E12.5 control (I) and K5-rtTA tetO-Dkk1 (Dkk1-expressing) (J) embryos subjected to immunofluorescence for β-catenin (red). Sections are DAPI counterstained (blue). Note nuclear and cytoplasmic as well as membrane localization of β-catenin in dental mesenchymal and some epithelial cells in the control (I) and predominantly membrane localization in the Dkk1-expressing molar (J). (K-P) Frontally sectioned oral cavities from control (K,M,O) and littermate K5-rtTA tetO-Dkk1 (Dkk1-expressing) (L,N,P) embryos subjected to hematoxylin and eosin staining at E12.5 (K,L), E13.5 (M,N) and E15.5 (O,P). Size bar in (J) applies to (I,J); size bar in (P) applies to (K-P). (Q-T) Frontally sectioned oral cavities from E12.5 (Q, R) and E13.5 (S, T) control (Q,S) and K14-Cre Ctnnb1fl/fl (β-catenin loss of function (LOF)) (R, T) embryos subjected to immunofluorescence for β-catenin (red). Sections are DAPI counterstained (blue). Note mosaic depletion of β-catenin at E12.5 (R, yellow arrow and white arrowhead) and more efficient depletion at E13.5 (T). (U,V) X-gal stained mandible whole mounts from E13.5 littermate control TOPGAL (U) and K14-Cre Ctnnb1fl/fl TOPGAL (β-catenin null) (V) embryos. (W-Z) Frontally sectioned oral cavities from control (W,W',Y) and littermate β-catenin LOF mutant (X,X',Z) embryos subjected to hematoxylin and eosin staining at E13.5 (W-X') and E15.5 (Y,Z). Size bar in (T) applies to (Q-T); size bar in (Z) applies to (W-Z).
Histological examination at E12.5 and later stages revealed that molar tooth development was blocked at the lamina-early bud stage in Dkk1-expressing oral cavity (Fig. 3G,H,I,J,M-P), consistent with results from constitutive K14-Dkk1 transgenic mice (Andl et al., 2002), while incisor tooth development was blocked at the placode stage (Fig. 3K,L). Significant differences in proliferation were not observed in Dkk1-expressing molars compared with controls assayed at E12.5 (Supplementary Fig. S4A,B).
Expression patterns of Wnt-reporter genes and β-catenin immunofluorescence (Fig. 1) suggested that Wnt/β-catenin signaling activity localizes prominently in dental epithelium at the dental lamina and bud stages. To determine whether Wnt/β-catenin signaling is required specifically within epithelial cells at these stages, we used Keratin 14 (K14) promoter-driven expression of Cre recombinase to delete the cell autonomous Wnt effector gene β-catenin in dental epithelium. We crossed K14-Cre line 43 mice (Andl et al., 2004) to mice carrying a conditional allele of β-catenin (Ctnnb1fl) that, when recombined, produces a nonfunctional allele (Brault et al., 2001). Immunofluorescence for β-catenin showed that in K14-Cre Ctnnb1fl/fl embryos, β-catenin was deleted in a mosaic fashion in the oral and dental epithelium at E12.5 (Fig. 3Q,R). By E13.5, β-catenin was almost completely absent from dental epithelium (Fig. 3S,T). TOPGAL activity was markedly reduced in the dental region of K14-Cre Ctnnb1fl/flTOPGAL compared with control TOPGAL embryos (Fig. 3U,V). Histological examination at E13.5 and later stages revealed that molar tooth development was blocked at the early bud stage in epithelial β-catenin depleted oral cavity (Fig. 3W',X',Y,Z), while incisor tooth development was blocked at the lamina-early bud stage (Fig. 3W,X). Significant differences in proliferation were not observed in epithelial β-catenin depleted molars compared with controls assayed at E13.5, consistent with the results of similar assays in Dkk1-expressing embryos (Supplementary Fig. S4C,D). These data indicate that Wnt/β-catenin signaling is required within dental epithelial cells for tooth development beyond the lamina-early bud stage.
Wnt/β-catenin signaling lies upstream of multiple secreted signals at early stages of tooth development
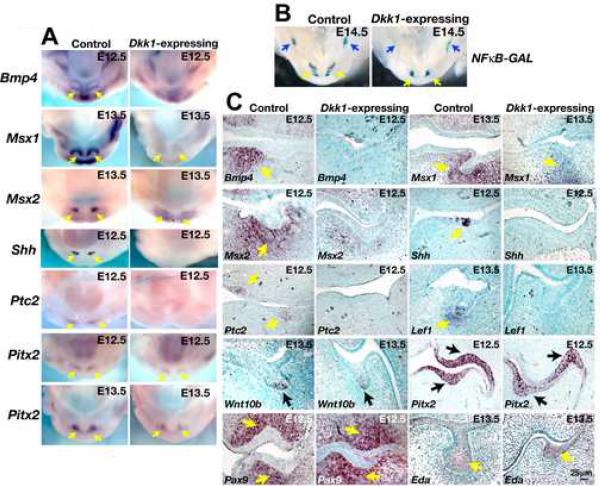
Bmp4 is expressed in the epithelium at the initiation of tooth development, and induces expression of the homeobox transcription factor genes Msx1 and Msx2 in the underlying mesenchyme. Lack of both Msx1 and Msx2 genes causes arrest at the dental lamina-early bud stage (Bei and Maas, 1998) indicating essential roles for these factors at early stages of tooth formation. Mesenchymal Msx1 is required for expression of Bmp4 in the mesenchyme (Chen et al., 1996). Bmp4 then signals back to the epithelium, inducing expression of p21 that is thought to cause cell cycle arrest and formation of the enamel knot (Jernvall et al., 1998). Consistent with this role for Bmp signaling in epithelial cells, deletion of epithelial Bmpr1a causes arrest of tooth development at the bud stage (Andl et al., 2004). To determine whether Wnt signaling is required for expression of Bmp4 or Msx genes, we carried out whole mount and section in situ hybridization for Bmp4, Msx1 and Msx2 on oral cavities from E12.5 embryos expressing Dkk1 and littermate controls. Bmp4 expression was absent and expression of Msx1 and Msx2 was significantly reduced in dental mesenchymal cells of Dkk1-expressing embryos at E12.5 (Fig. 4A,C). Shh signaling is required within the dental epithelium for regulation of tooth growth and shape (Dassule et al., 2000; Gritli-Linde et al., 2002). Shh expression was completely blocked by ectopic Dkk1 at E12.5 (Fig. 4A,C), indicating that Wnt signaling lies upstream of Shh expression. Expression of the Shh pathway gene Ptc2 was also absent from the dental region of Dkk1-expressing transgenics (Fig. 4A,C).
Fig. 4. Wnt inhibition blocks expression of multiple developmental regulators.
(A) Mandibles dissected from littermate control (left panels) and K5-rtTA tetO-Dkk1 (Dkk1-expressing) (right panels) embryos doxycycline treated from E0.5, sacrificed at E12.5 or E13.5, and subjected to in situ hybridization with digoxygenin-labeled probes for Bmp4, Msx1, Msx2, Shh, Ptc2, and Pitx2. Arrows indicate positive signals (purple). (B) X-gal stained mandibles dissected from E14.5 littermate control NFκB-GAL and K5-rtTA tetO-Dkk1 NFκ-GAL (Dkk1-expressing) embryos doxycycline treated from E0.5. Note that NFκ-GAL expression in the incisor (yellow arrows) and molar (blue arrows) regions persists in Dkk1-expressing mandibles. (C) Transverse sections of oral cavities from littermate control and K5-rtTA tetO-Dkk1 (Dkk1-expressing) embryos doxycycline treated from E0.5, sacrificed at the stages indicated, and subjected to in situ hybridization with digoxygenin-labeled probes for Bmp4, Msx1, Msx2, Shh, Ptc2, Lef1, Wnt10b, Pitx2, Pax9 and Eda. Arrows indicate positive signals (brown/purple). Sections are counterstained with methyl green.
Lef1 expression is Wnt-regulated in several developmental contexts, and the Lef1 promoter can be controlled synergistically by PITX2 and β-catenin (Vadlamudi et al., 2005). In developing teeth, Lef1 expression can also be induced by ectopic Bmp4 (Kratochwil et al., 1996), but does not regulate its own expression (Kratochwil et al., 2003). We found that ectopic Dkk1 blocked expression of Lef1 in dental epithelial and mesenchymal cells (Fig. 4C). Consistent with loss of Lef1 expression, Fgf3, which has previously been shown to act in an FGF cascade downstream of epithelial Lef1 and Fgf4, was downregulated in Dkk1-expressing dental epithelium at E13.5 (data not shown). Interestingly, Wnt10b expression was down-regulated and more diffuse in Dkk1-expressing dental epithelium at E13.5 compared with littermate controls, but was not entirely absent (Fig. 4C). Expression of Wnt10b was lost from Dkk1-expressing dental epithelium by E14.5 (data not shown).
Although Pitx2 can be regulated by Wnt/β-catenin signaling in certain developmental contexts (Kioussi et al., 2002), we found that Pitx2 expression was maintained in Dkk1-expressing dental epithelia when expression of other regulatory molecules such as Bmp4, Msx genes and Shh was abrogated (Fig. 4A,C). Thus maintenance of Pitx2 expression does not require Wnt/β-catenin signaling in the dental lamina. We found that Dkk1 expression also had no effect on Pax9 expression (Fig. 4C), indicating that, like Pitx2, Pax9 is regulated independently of Wnt.
Eda can promote survival of dental placodes (Mustonen et al., 2004) and has been suggested as a direct Wnt target (Durmowicz et al., 2002). EDA signals through its receptor EDAR to activate NF-κB signaling. We used the NF-κB lac Z reporter transgenic mouse line (Ig)3xcona-lacZ (NFκBGAL) (Schmidt-Ullrich et al., 1996) to determine whether Wnt inhibition blocks signaling through NF-κB at early stages of tooth development. Pregnant females bearing K5-rtTA tetO-Dkk NFκB-GAL and control littermate embryos were doxycycline treated from the beginning of pregnancy. In X-gal stained littermate control mandibles, NFκB-GAL activity was clearly detectable in the incisor and molar regions at E14.5 (Fig. 4B). Similarly, despite much less extensive tooth development, clear X-gal staining was detectable in the comparable regions of K5-rtTA tetO-Dkk NFκB-GAL mandibles (Fig. 4B). Consistent with these data, Eda expression in dental epithelium was unaffected by ectopic Dkk1 at E12.5 and E13.5 (Fig. 4C and data not shown). Thus expression of Eda and NF-κB signaling in dental epithelial cells at early stages of tooth development is independent of Wnt/β-catenin activity.
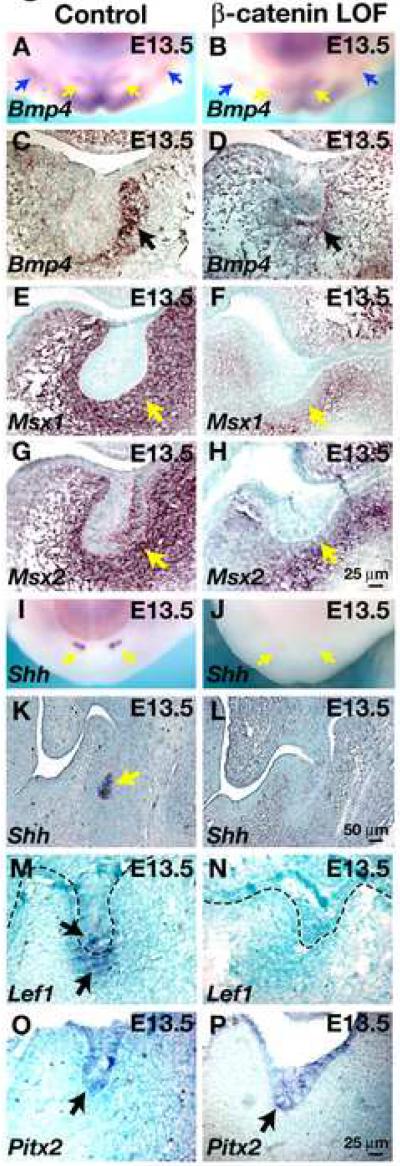
To determine the extent to which these alterations in gene expression reflect a requirement for Wnt signaling specifically within dental epithelial cells, we carried out whole mount and section in situ hybridization on oral tissues from K14-Cre Ctnnb1fl/fl and littermate control embryos with probes for Bmp4, Msx1, Msx2, Shh, Lef1, Eda and Pitx2 at E13.5, the earliest stage at which β-catenin is efficiently deleted in dental ectoderm. Expression of Pitx2 and Eda was unaffected by the loss of function β-catenin mutation, in line with data from Dkk1-expressing embryos (Fig. 5O,P and data not shown). Depletion of epithelial β-catenin resulted in loss of Shh and Lef1 expression in dental epithelial cells (Fig. 5I-N). Interestingly, expression of Lef1 was also downregulated in dental mesenchymal cells of the mutant at E13.5 (Fig. 5M,N), and mesenchymal Bmp4 was reduced, although not entirely absent (Fig. 5A-D). Expression of Msx1 and Msx2 was reduced in mesenchymal cells immediately adjacent to dental epithelium of the β-catenin depleted mutant (Fig. 5E-H). As β-catenin is a cell autonomous molecule, these data suggest that epithelial β-catenin regulates mesenchymal expression of Lef1, Bmp4, Msx1 and Msx2 indirectly. However, these results do not rule out the possibility that β-catenin signaling is additionally required within the mesenchyme for expression of these genes and tooth development.
Fig. 5. Epithelial deletion of β-catenin affects expression of regulatory genes in dental epithelium and mesenchyme.
(A-B) Mandibles dissected from littermate control (A) and K14-Cre; Ctnnb1fl/fl (β-catenin LOF) (B) E13.5 embryos and subjected to in situ hybridization with digoxygenin-labeled probe for Bmp4. (C-H) Transverse sections of oral cavities from littermate control (C,E,G) and K14-Cre Ctnnb1fl/fl (β-catenin LOF) (D,F,H) E13.5 embryos subjected to in situ hybridization with the probes indicated. (I, J). Mandibles dissected from littermate control (I) and K14-Cre Ctnnb1fl/fl (β-catenin LOF) (J) E13.5 embryos and subjected to in situ hybridization with digoxygenin-labeled probe for Shh. (K-P) Transverse sections of oral cavities from littermate control (K,M,O) and K14-Cre Ctnnb1fl/fl (β-catenin LOF) (L,N,P) E13.5 embryos subjected to in situ hybridization with the probes indicated. Arrows indicate positive signals (brown or purple). A dashed line indicates the epithelial-mesenchymal boundary in (M,N). Sections are counterstained with methyl green. Size bar in (H) applies to (C-H); size bar in (L) applies to (K,L); size bar in (P) applies to (M-P).
Wnt/β-catenin signaling is not required for induction of Msx1 and Msx2 by exogenous BMP4
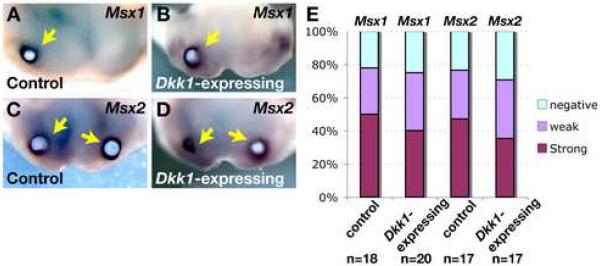
Bmp4 is known to regulate Msx gene expression in the developing tooth, and can also induce expression of Lef1 (Kratochwil et al., 1996). Thus Bmp4 could mediate the effects of Wnt signaling on Lef1 and Msx gene expression. Alternatively, Wnt and Bmp4 could coordinately regulate expression of Lef1 and/or Msx genes. In line with the latter suggestion, LEF/TCF as well as SMAD binding sites are present in Msx gene promoter regions, and BMP and WNT factors synergistically activate Msx gene expression in human embryonic carcinoma cells and murine embryonic stem cells (Hussein et al., 2003; Willert et al., 2002). To distinguish among these scenarios in dental development, we asked whether Dkk1 could block induction of Msx expression by exogenous BMP4. BMP4 protein coated beads were implanted into control and Dkk1-expressing mandibles. The tissue explants were cultured for 24 hours in medium containing doxycycline to maintain Dkk1 induction, and then subjected to in situ hybridization with Msx1 and Msx2 probes. BMP4- but not control BSA-coated beads caused robust Msx gene expression in immediately adjacent tissue in both control and Dkk1-expressing mandibles (Fig. 6A-E and data not shown). In additional experiments, beads for implantation were coated with BMP4 and BSA, or with BMP4 and recombinant DKK1. Recombinant DKK1 had no effect on the ability of BMP4 to induce Msx gene expression in control or Dkk1- expressing mandibles (data not shown). These observations indicate that Dkk1 is not able to block the effects of exogenous BMP4, consistent with a model in which Bmp4 functions downstream of Wnt activation to promote Msx gene expression, rather than acting synergistically with Wnt.
Fig.6. Ectopic Dkk1 does not block the ability of exogenous BMP4 to induce expression of Msx1 and Msx2.
(A-D) Representative samples of mandibles dissected from littermate control (A,C) and K5-rtTA tetO-Dkk1 (Dkk1-expressing) (B,D) embryos doxycycline treated from E0.5, sacrificed at E11.5, implanted with BMP4-coated beads, cultured in the presence of doxycycline for 24 hours, and subjected to in situ hybridization with digoxygenin-labeled probes for Msx1 (A,B) and Msx2 (C,D). Arrows indicate positive signals (purple). (E) Summary of Msx1 and Msx2 expression data from multiple experiments.
Disruption of Wnt/β-catenin signaling from the early bell stage inhibits the formation of molar cusps
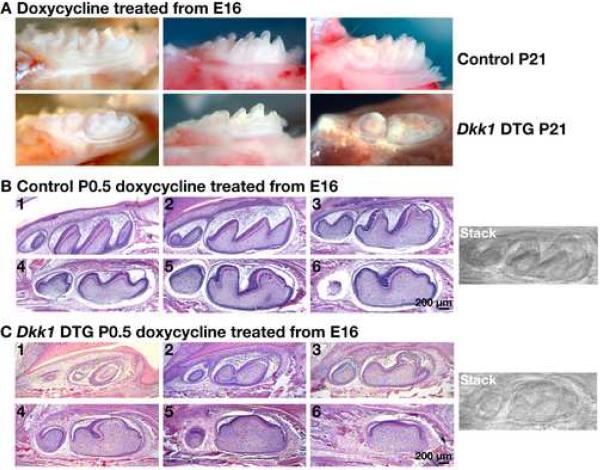
The TOPGAL and BAT-gal Wnt reporter transgenes are activated specifically in the secondary enamel knots (Fig. 1), and beads coated with the Mfrzb1 Wnt inhibitor decrease molar tooth size in ex vivo experiments (Sarkar and Sharpe, 2000), suggesting involvement of Wnt/β-catenin signaling in molar cusp development. To determine the requirements for Wnt/β-catenin signaling in cusp development in vivo, K5-rtTA tetO-Dkk1 embryos and control littermates were exposed to doxycycline via treatment of the pregnant mothers starting at E16, a time point at which the molar teeth are at the late cap - early bell stages and secondary enamel knots are just beginning to form in the more advanced (first and second) molars. At these stages the K5 promoter is active in inner and outer enamel epithelial cells and developing ameloblasts (Cascallana et al., 2005). Doxycycline treatment was continued for the remainder of pregnancy and pups were maintained on doxycycline after birth via their mothers' milk. Light microscopic analysis of mandibular molar teeth at P21 revealed that Dkk1 induction from E16 caused development of cusps that were dramatically flattened, smaller and irregular compared with those of control littermate molars (Fig. 7A). The second molars were more severely affected than the first molars, consistent with the later timing of second compared with first molar development.
Fig. 7. Wnt/β-catenin signaling is required for normal development of molar cusps.
(A) Right mandibles dissected at P21 from control littermate and K5-rtTA tetO-Dkk1 (Dkk1 DTG) mice doxycycline treated from E16. Note blunted cusp formation in Dkk1 DTG molar teeth compared with controls. (B,C) Right mandibles from P0.5 control littermate (B) and K5-rtTA tetO-Dkk1 (Dkk1 DTG) (C) mice doxycycline treated from E16.5, serially sectioned at 5μ-m in a sagittal plane from lingual to buccal side. Sections were stained with hematoxylin and eosin. The photographs shown were taken every 8 sections; each photo in each series is of a section 40μ-m from the previous photo. The images on the right represent stacked images of the serial sections, compiled using the Stack function of ImageJ software.
Histological analysis of serial sections showed that this phenotype was apparent in un-erupted Dkk1-expressing molars at birth (Fig. 7B,C). Ameloblast differentiation and enamel deposition were detected in Dkk1-expressing molars, but developing cusps were blunted and lacked the anterior-posterior asymmetry that was apparent in controls. These results indicate that Wnt inhibition from E16 represses development of molar cusps.
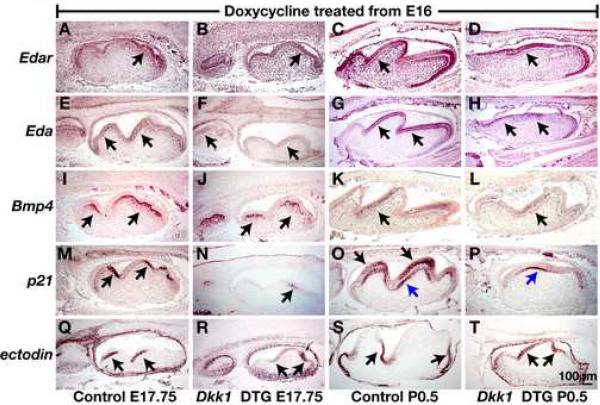
To determine the molecular basis for the effects of Wnt inhibition on molar cusp development, we examined the expression of signaling factors known to be important for cusp patterning. EDA/EDAR signaling has been shown to play a key role in cusp formation, and mice deficient in this signaling pathway display small enamel knots and decreased cusp sizes (Courtney et al., 2005; Laurikkala et al., 2001; Pispa et al., 1999). Edar mRNA is detected in inner enamel epithelium, stratum intermedia, and stellate reticulum at the bell stage (Tucker et al., 2000). We did not detect significant differences in the level or pattern of Edar expression in molars induced to express Dkk1 from E16, compared with control littermate molars at E17.75 or P0.5 (Fig. 8A-D). Using a probe recognizing all Eda-A1 mRNA isoforms (Schmidt-Ullrich et al., 2006), we detected Eda expression in preameloblasts and outer enamel epithelium in control E17.5 and newborn mice (Fig. 8E,F). Consistent with previous reports that Eda expression is Wnt-regulated (Laurikkala et al., 2001), we found that Eda expression was downregulated, although not absent, in molars induced to express Dkk1 from E16 compared with control molars (Fig. 8E-H). Thus downregulation of EDA/EDAR signaling in Dkk1-expressing molars may contribute to the abnormal cusp phenotype.
Fig. 8. Wnt/β-catenin signaling is required for the maintenance of secondary enamel knots.
Sagittal sections of oral cavities from control littermate (A,C,E,G,I,K,M,O,Q,S) and K5-rtTA tetO-Dkk1 (Dkk1 DTG) (B,D,F,H,J,L,N,P,R,T) mice doxycycline treated from E16 and sacrificed at E17.75 (A,B,E,F,I,J,M,N,Q,R) or P0.5 (C,D,G,H,K,L,O,P,S,T) hybridized with digoxygenin-labeled probes for Edar (A-D), Eda (E-H), Bmp4 (I-L), p21 (M-P) and ectodin (Q-T). Positive signals (purple-brown) are indicated by arrows. In (O,P) black arrows indicate epithelial cells; blue arrows indicate developing odontoblasts. Size bar in (T) applies to (A-T).
Expression in developing molars of the TGFβ inhibitor follistatin, a key regulator of cusp development (Wang et al., 2004), was unaffected by induced Dkk1 (data not shown). Bmp4 induces expression of the enamel knot marker p21, a cyclin-dependent kinase inhibitor (Jernvall et al., 1998), and is also implicated in ameloblast and odontoblast differentiation (Bloch-Zupan et al., 1998). Bmp4 mRNA was detected in the dental papilla and preodontoblasts of control molars at E17.75, and in ameloblasts and odontoblasts at P0.5 (Aberg et al., 1997) (Fig. 8I,J). Expression of Bmp4 at either stage was unaffected by Dkk1 induction from E16 (Fig. 8K,L). By contrast, in controls the enamel knot marker p21 was expressed in inner enamel epithelium on the anterior side of each cusp at E17.5 and in terminally differentiating ameloblasts and odontoblasts at P0.5 (Bloch-Zupan et al., 1998) (Fig. 8M,N). In Dkk1-expressing teeth induced from E16, p21 expression was downregulated in inner enamel epithelium at E17.5 and was absent from ameloblasts at P0.5. Interestingly, Dkk1 did not affect expression of p21 in odontoblasts, indicating that epithelial but not mesenchymal expression of p21 is Wnt-dependent (Fig. 7O,P). Thus, decreased expression of p21 in Dkk1-induced molars could contribute to failure of enamel knot and cusp development.
The BMP antagonist ectodin is expressed as a “negative image” of enamel knots and plays a key role in regulating cusp patterning (Kassai et al., 2005; Laurikkala et al., 2003). In ectodin-deficient mice, p21 expression domains are expanded, molar tooth crowns are broader than normal and buccal cusps are fused (Kassai et al., 2005). We found that in control molars the expression patterns of p21 and ectodin were complementary in molar epithelial cells (Fig. 8M,O,Q,S), in agreement with previous data. In molars induced to express Dkk1 from E16, domains expressing ectodin were expanded to include both sides of the developing molar cusps at E17.75, and the tips of the blunted cusps at P0.5 (Fig. 8R,T).
Discussion
Wnt/β-catenin signaling plays key roles in the development of multiple ectodermal appendages including teeth (Mikkola and Millar, 2006). However, precisely how this pathway intersects with the complex interplay of signaling factors regulating tooth development has been unclear. Here, using immunofluorescence detection of nuclear β-catenin, and three independent Wnt/β-catenin reporter transgenic lines, we demonstrate that Wnt/β-catenin signaling is active throughout tooth development. A gain of function mutation in epithelial β-catenin results in expanded expression of several key regulatory genes. Conversely, expression of these key dental regulators is disrupted when epithelial and mesenchymal Wnt/β-catenin signaling is inhibited soon after tooth initiation in Dkk1-expressing embryos, resulting in arrested development at the early bud stage. Depletion of epithelial β-catenin produces a similar, albeit less severe, phenotype, demonstrating a requirement for epithelial Wnt/β-catenin signaling at early stages of tooth development. The stronger phenotypes resulting from ectopic Dkk1 expression compared with β-catenin depletion could be due to more efficient and earlier Wnt inhibition in Dkk1-expressing embryos, and/or to additional roles for β-catenin signaling within mesenchymal cells. We further demonstrate that inducible Wnt inhibition during molar cusp development results in defective cusp formation and loss of molar tooth polarity.
Arrested tooth development at the early bud stage in Wnt-inhibited mutants is unlikely to be due to the observed loss of Shh expression, as deletion of Shh at a similar stage permits much more extensive morphogenesis than that observed here (Dassule et al., 2000). Despite prior evidence suggesting Pitx2 and Eda as possible direct Wnt/β-catenin targets (Durmowicz et al., 2002; Kioussi et al., 2002; Laurikkala et al., 2001), in situ hybridization signals for these mRNAs are similar in control and Wnt-inhibited epithelium at E12.5-E13.5 and NF-κB signaling is unaffected by Wnt inhibition at this stage. Expression levels of Pax9 are also similar in control and Wnt-inhibited dental mesenchyme. Instead, the mechanism underlying arrested development in Dkk1-expressing embryos appears to involve loss of expression of Bmp4, Msx1 and Msx2. Consistent with this model, tooth development arrests at a similar stage in Msx1-/- Msx2-/- mice (Bei and Maas, 1998) and Wnt-inhibited mice.
Although we observe nuclear β-catenin in dental mesenchymal cells immediately underlying the epithelium at E12.5-E14, this region only partially overlaps with the more extensive domains of Bmp4, Msx1 and Msx2 in dental mesenchyme, suggesting that the requirement for Wnt signaling in regulation of these genes may be at least in part indirect. In support of this model, depletion of epithelial β-catenin results in decreased expression of mesenchymal Lef1 and Bmp4, and reduced expression of Msx genes in mesenchymal cells immediately adjacent to the epithelium. Furthermore, ectopic Dkk1 fails to block the ability of exogenous BMP4 to induce Msx gene expression, consistent with a mechanism in which Bmp4 mediates Msx expression downstream of Wnt, rather than acting synergistically with Wnt.
Wnt/β-catenin pathway activation in the secondary enamel knots of developing molar teeth suggested that in addition to its roles at the lamina-early bud stage, Wnt signaling is also important for the development of molar cusps. Consistent with this hypothesis, induction of Dkk1 expression in epithelial cells at the early bell stage resulted in blunted cusp formation. Interestingly, in contrast to the effects of Wnt inhibition at the early bud stage, Dkk1 expression in these later stage teeth did not significantly affect expression of Bmp4. Expression of Eda, a known regulator of cusp development, was reduced, although not eliminated, by ectopic Dkk1, suggesting that reduced Eda may contribute to the cusp phenotype. In addition, we observed pronounced effects of Wnt inhibition on expression of the enamel knot marker p21 and the Bmp and Wnt inhibitor ectodin. p21 expression was markedly downregulated in the dental epithelium, although expression was observed in odontoblasts in newborn mice. Conversely, expression of ectodin, which is excluded from enamel knots, was expanded to include both sides and the tips of the blunted molar cusps. These results indicate that Wnt/β-catenin signaling is necessary for maintenance of the enamel knots and plays a major role in determining tooth shape. These data further suggest that ectodin expression is normally suppressed in cells where the Wnt/β-catenin signaling pathway is activated. Consistent with this, despite expanded ectodin expression in the β-catenin gain of function mutant, ectodin mRNA appears to be excluded from regions of the abnormal and ectopic teeth that exhibit expression of TOPGAL and Wnt10b (Fig. 2). As ectodin is known to inhibit Bmp4-mediated p21 expression, our data suggest a model in which Wnt/β-catenin signaling in the enamel knot normally suppresses ectodin expression in the knot, allowing Bmp4 to upregulate p21. In addition to its function as a BMP inhibitor, ectodin is also reported to block Wnt signaling in certain biological contexts (Itasaki et al., 2003). Thus ectodin and Wnt may compete to establish enamel knot and non-knot domains in the developing molar.
In summary, our data indicate that Wnt/β-catenin signaling operates at multiple stages of tooth morphogenesis. While Wnt reporter transgene expression data and gain of function phenotypes suggest that Wnt/β-catenin signaling can initiate the formation of dental placodes and pattern tooth development in the dental lamina, further experiments using earlier-acting promoters to drive expression of Dkk1 or delete β-catenin in dental lamina cells will be necessary to determine whether or not Wnt/β-catenin signaling is required for tooth initiation in vivo.
Interestingly, the effects of Wnt/β-catenin signaling at the early bud stage appear to be mediated primarily through Msx and Bmp4 gene expression, while suppression of molar cusp development does not involve Bmp4 downregulation, indicating that different mechanisms are involved. As Wnt signaling inhibition and gain of function both result in the development of aberrantly shaped teeth, our data further indicate that Wnt activity must be tightly regulated for normal dental morphogenesis. Strategies to utilize activation of this pathway for tooth regeneration must thus include techniques for temporally and spatially limiting pathway activity.
Supplementary Material
Acknowledgements
We thank Dr. Elaine Fuchs for TOPGAL mice; Dr. Daniel Dufort for TCF/Lef-LacZ mice; Dr. Adam Glick for K5-rtTA mice; and Drs. Gregory Shackleford, Brigid Hogan, Nobuyuki Itoh and Caroline Gibson for in situ hybridization probes. Work in S.E.M.'s laboratory is supported by R01-DE015342, RO1-AR47709, RO1-HD053829 and RO1-AR055241. E.Y.C. and N.M.G. were supported by T32-AR007465.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn. 1997;210:383–96. doi: 10.1002/(SICI)1097-0177(199712)210:4<383::AID-AJA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw EB, 3rd, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–68. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT Signals Are Required for the Initiation of Hair Follicle Development. Dev Cell. 2002;2:643–53. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development. 1998;125:4325–33. doi: 10.1242/dev.125.21.4325. [DOI] [PubMed] [Google Scholar]
- Bloch-Zupan A, Leveillard T, Gorry P, Fausser JL, Ruch JV. Expression of p21(WAF1/CIP1) during mouse odontogenesis. Eur J Oral Sci. 1998;106(Suppl 1):104–11. doi: 10.1111/j.1600-0722.1998.tb02160.x. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Byrne C, Tainsky M, Fuchs E. Programming gene expression in developing epidermis. Development. 1994;120:2369–83. doi: 10.1242/dev.120.9.2369. [DOI] [PubMed] [Google Scholar]
- Cascallana JL, Bravo A, Donet E, Leis H, Lara MF, Paramio JM, Jorcano JL, Perez P. Ectoderm-targeted overexpression of the glucocorticoid receptor induces hypohidrotic ectodermal dysplasia. Endocrinology. 2005;146:2629–38. doi: 10.1210/en.2004-1246. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–44. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131:4819–29. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- Courtney JM, Blackburn J, Sharpe PT. The Ectodysplasin and NFkappaB signalling pathways in odontogenesis. Arch Oral Biol. 2005;50:159–63. doi: 10.1016/j.archoralbio.2004.11.019. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–85. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Dassule HR, McMahon AP. Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev Biol. 1998;202:215–27. doi: 10.1006/dbio.1998.8992. [DOI] [PubMed] [Google Scholar]
- Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–94. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- Durmowicz MC, Cui CY, Schlessinger D. The EDA gene is a target of, but does not regulate Wnt signaling. Gene. 2002;285:203–11. doi: 10.1016/s0378-1119(02)00407-9. [DOI] [PubMed] [Google Scholar]
- Furth PA, St Onge L, Boger H, Gruss P, Gossen M, Kistner A, Bujard H, Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994;91:9302–6. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Bei M, Maas R, Zhang XM, Linde A, McMahon AP. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development. 2002;129:5323–37. doi: 10.1242/dev.00100. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle Z, Mo R, Hui CC, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998;125:2803–11. doi: 10.1242/dev.125.15.2803. [DOI] [PubMed] [Google Scholar]
- Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Morphogenesis. Cell. 1999;96:225–33. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Hussein SM, Duff EK, Sirard C. Smad4 and beta-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. J Biol Chem. 2003;278:48805–14. doi: 10.1074/jbc.M305472200. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 2003;130:4295–305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2006;103:18627–32. doi: 10.1073/pnas.0607289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J, Aberg T, Kettunen P, Keranen S, Thesleff I. The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development. 1998;125:161–9. doi: 10.1242/dev.125.2.161. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol. 1994;38:463–9. [PubMed] [Google Scholar]
- Jowett AK, Vainio S, Ferguson MW, Sharpe PT, Thesleff I. Epithelial-mesenchymal interactions are required for msx 1 and msx 2 gene expression in the developing murine molar tooth. Development. 1993;117:461–70. doi: 10.1242/dev.117.2.461. [DOI] [PubMed] [Google Scholar]
- Kassai Y, Munne P, Hotta Y, Penttila E, Kavanagh K, Ohbayashi N, Takada S, Thesleff I, Jernvall J, Itoh N. Regulation of mammalian tooth cusp patterning by ectodin. Science. 2005;309:2067–70. doi: 10.1126/science.1116848. [DOI] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–85. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Dull M, Farinas I, Galceran J, Grosschedl R. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–94. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(-/-) mice. Genes & Development. 2003;16:3173–85. doi: 10.1101/gad.1035602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurikkala J, Kassai Y, Pakkasjarvi L, Thesleff I, Itoh N. Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev Biol. 2003;264:91–105. doi: 10.1016/j.ydbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Laurikkala J, Mikkola M, Mustonen T, Aberg T, Koppinen P, Pispa J, Nieminen P, Galceran J, Grosschedl R, Thesleff I. TNF signaling via the ligand-receptor pair ectodysplasin and edar controls the function of epithelial signaling centers and is regulated by Wnt and activin during tooth organogenesis. Dev Biol. 2001;229:443–55. doi: 10.1006/dbio.2000.9955. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O'Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–82. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- Liu F, Thirumangalathu S, Gallant NM, Yang SH, Stoick-Cooper CL, Reddy ST, Andl T, Taketo MM, Dlugosz AA, Moon RT, Barlow LA, Millar SE. Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet. 2007;39:106–12. doi: 10.1038/ng1932. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lumsden AG. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development. 1988;103:155–69. doi: 10.1242/dev.103.Supplement.155. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–7. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola ML, Millar SE. The mammary bud as a skin appendage: unique and shared aspects of development. J Mammary Gland Biol Neoplasia. 2006;11:187–203. doi: 10.1007/s10911-006-9029-x. [DOI] [PubMed] [Google Scholar]
- Mina M, Kollar EJ. The induction of odontogenesis in non-dental mesenchyme combined with early murine mandibular arch epithelium. Arch Oral Biol. 1987;32:123–7. doi: 10.1016/0003-9969(87)90055-0. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A. 2005;102:8579–84. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucchielli ML, Mitsiadis TA, Raffo S, Brunet JF, Proust JP, Goridis C. Mouse Otlx2/RIEG expression in the odontogenic epithelium precedes tooth initiation and requires mesenchyme-derived signals for its maintenance. Dev Biol. 1997;189:275–84. doi: 10.1006/dbio.1997.8672. [DOI] [PubMed] [Google Scholar]
- Mustonen T, Ilmonen M, Pummila M, Kangas AT, Laurikkala J, Jaatinen R, Pispa J, Gaide O, Schneider P, Thesleff I, Mikkola ML. Ectodysplasin A1 promotes placodal cell fate during early morphogenesis of ectodermal appendages. Development. 2004;131:4907–19. doi: 10.1242/dev.01377. [DOI] [PubMed] [Google Scholar]
- Neubuser A, Peters H, Balling R, Martin GR. Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell. 1997;90:247–55. doi: 10.1016/s0092-8674(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Noramly S, Freeman A, Morgan BA. beta-catenin signaling can initiate feather bud development. Development. 1999;126:3509–21. doi: 10.1242/dev.126.16.3509. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Hu Y, Schmidt-Ullrich R, Cao Y, Scheidereit C, Karin M, Sharpe PT. A dual role for ikkalpha in tooth development. Dev Cell. 2004;6:219–27. doi: 10.1016/s1534-5807(04)00024-3. [DOI] [PubMed] [Google Scholar]
- Oosterwegel M, van de Wetering M, Timmerman J, Kruisbeek A, Destree O, Meijlink F, Clevers H. Differential expression of the HMG box factors TCF-1 and LEF-1 during murine embryogenesis. Development. 1993;118:439–48. doi: 10.1242/dev.118.2.439. [DOI] [PubMed] [Google Scholar]
- Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–47. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pispa J, Jung HS, Jernvall J, Kettunen P, Mustonen T, Tabata MJ, Kere J, Thesleff I. Cusp patterning defect in Tabby mouse teeth and its partial rescue by FGF. Dev Biol. 1999;216:521–34. doi: 10.1006/dbio.1999.9514. [DOI] [PubMed] [Google Scholar]
- Sarkar L, Sharpe PT. Expression of Wnt signalling pathway genes during tooth development. Mech Dev. 1999;85:197–200. doi: 10.1016/s0925-4773(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Sarkar L, Sharpe PT. Inhibition of Wnt signaling by exogenous Mfrzb1 protein affects molar tooth size. J Dent Res. 2000;79:920–5. doi: 10.1177/00220345000790040601. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Ito Y, Xu X, Han J, Bringas P, Jr., Maeda T, Slavkin HC, Grosschedl R, Chai Y. LEF1 is a critical epithelial survival factor during tooth morphogenesis. Dev Biol. 2005;278:130–43. doi: 10.1016/j.ydbio.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Memet S, Lilienbaum A, Feuillard J, Raphael M, Israel A. NF-kappaB activity in transgenic mice: developmental regulation and tissue specificity. Development. 1996;122:2117–28. doi: 10.1242/dev.122.7.2117. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Tobin DJ, Lenhard D, Schneider P, Paus R, Scheidereit C. NF-kappaB transmits Eda A1/EdaR signalling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down growth. Development. 2006;133:1045–57. doi: 10.1242/dev.02278. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- St Amand TR, Zhang Y, Semina EV, Zhao X, Hu Y, Nguyen L, Murray JC, Chen Y. Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev Biol. 2000;217:323–32. doi: 10.1006/dbio.1999.9547. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Hurmerinta K. Tissue interactions in tooth development. Differentiation. 1981;18:75–88. doi: 10.1111/j.1432-0436.1981.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Vaahtokari A, Partanen AM. Regulation of organogenesis. Common molecular mechanisms regulating the development of teeth and other organs. Int J Dev Biol. 1995;39:35–50. [PubMed] [Google Scholar]
- Tucker AS, Headon DJ, Schneider P, Ferguson BM, Overbeek P, Tschopp J, Sharpe PT. Edar/Eda interactions regulate enamel knot formation in tooth morphogenesis. Development. 2000;127:4691–700. doi: 10.1242/dev.127.21.4691. [DOI] [PubMed] [Google Scholar]
- Vaahtokari A, Aberg T, Jernvall J, Keranen S, Thesleff I. The enamel knot as a signaling center in the developing mouse tooth. Mech Dev. 1996;54:39–43. doi: 10.1016/0925-4773(95)00459-9. [DOI] [PubMed] [Google Scholar]
- Vadlamudi U, Espinoza HM, Ganga M, Martin DM, Liu X, Engelhardt JF, Amendt BA. PITX2, beta-catenin and LEF-1 interact to synergistically regulate the LEF-1 promoter. J Cell Sci. 2005;118:1129–37. doi: 10.1242/jcs.01706. [DOI] [PubMed] [Google Scholar]
- Vainio S, Karavanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Jorgez CJ, Matzuk MM, Werner S, Thesleff I. Follistatin regulates enamel patterning in mouse incisors by asymmetrically inhibiting BMP signaling and ameloblast differentiation. Dev Cell. 2004;7:719–30. doi: 10.1016/j.devcel.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM. Wnt signalling: Antagonistic Dickkopfs. Curr Biol. 2001;11:R592–5. doi: 10.1016/s0960-9822(01)00360-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.