Abstract
In these studies the immunotoxicity of arsenic trioxide (ATO, As2O3) was evaluated in mice following 14 days of inhalation exposures (nose only, 3 hrs per day) at concentrations of 50 μg/m3 and 1 mg/m3. A biodistribution analysis performed immediately after inhalation exposures revealed highest levels of arsenic in the kidneys, bladder, liver, and lung. Spleen cell levels were comparable to those found in the blood, with the highest concentration of arsenic detected in the spleen being 150 μg/mg tissue following the 1 mg/m3 exposures. No spleen cell cytotoxicity was observed at either of the two exposure levels. There were no changes in spleen cell surface marker expression for B cells, T cells, macrophages, and natural killer (NK) cells. There were also no changes detected in the B cell (LPS-stimulated) and T cell (Con A-stimulated) proliferative responses of spleen cells, and no changes were found in the NK-mediated lysis of Yac-1 target cells. The primary T-dependent antibody response was, however, found to be highly susceptible to ATO suppression. Both the 50 μg/m3 and 1 mg/m3 exposures produced greater than 70% suppression of the humoral immune response to sheep red blood cells. Thus, the primary finding of this study is that the T-dependent humoral immune response is extremely sensitive to suppression by ATO and assessment of humoral immune responses should be considered in evaluating the health effects of arsenic containing agents.
INTRODUCTION
Arsenic is a naturally occurring element that exists in numerous chemical forms in three stable oxidation states as AsIII (arsenite), AsV (arsenate), and AsIII- (arsenide). The health effects of arsenic have been studied for numerous years and have recently been reviewed (Carter et al., 2003; NRC, 1999). In general, arsenite (AsIII) in drinking water is known to be carcinogenic in humans and exerts numerous noncarcinogenic actions affecting kidneys, skin, liver, lungs, and other organs in animals and humans (ATSDR, 2007). Both methylated and nonmethylated forms of arsenic have been associated with cell toxicity (Styblo et al., 2000; Thomas et al., 2001). Several forms of arsenic are known to be carcinogenic in humans with recent attention being focused on the methylated forms (Cohen et al., 2006). Arsenate is generally less well-absorbed into cells than arsenite, although the cellular uptake of arsenate is dependent on the extracellular concentrations of phosphate (Carter et al., 2003). Once arsenate is absorbed into cells it can be converted to arsenite by arsenate reductases in animals (Radabaugh et al, 200, 2002; Waalkes and Liu, 2005). Arsenic has been hypothesized to exert its toxicologic effects in cells and tissue via oxidative stress mechanisms (Pi et al., 2002; Shi et al., 2004), although the role of ROS in arsenic-induced apoptosis has also been questioned (Morales et al., 2009).
Arsenic trioxide (ATO, As2O3) is an important environmental dust that is a common contaminant around copper smelters (Milham and Strong, 1974, Hysong et al., 2003). Children living near copper smelters in Arizona were found to have elevated concentrations of arsenic in their urine, with an analysis of house dust containing ATO measured in excess of 300 μg/g (Morse, et al., 1979). Glass workers have been reported to be exposed to up 5 ug/m3 of ATO in their workplace (Apostoli et al., 1999). Airborne arsenic levels around some smelters have been estimated to be in excess of 50 μg/m3 (Smith et al., 1974). Environmental ATO particles are well absorbed following inhalation, although there are insoluble components that have long residence times in the lung (ATSDR, 2007). Particle size, pH, and temperature determine the amount of absorption following inhalation exposures. Sodium arsenite is believed to be the active form of arsenic liberated from ATO following inhalation or other exposures exposures (Carter et al., 2003). Sodium arsenite present in drinking water has been correlated with a suppression of human peripheral blood lymphocyte activation and responses (Soto-Peña et al., 2006). ATO suppresses the activity of human myeloid and mononuclear cells (Lemarie et al.. 2006 and is being developed as a treatment for myelodysplastic syndromes and certain leukemias (Kantarjian et al. 2008). ATO is considered to be a promising new therapy in the treatment of autoimmune diseases (Bobe et al., 2006).
There have been few studies on the immunotoxicity of ATO following inhalation exposures. In one study, short term inhalation exposures to ATO in mice were found to suppress the host response to bacteria (Aranyi et al., 1985). Intratracheal exposure of mice to ATO has been found to cause fibrosis, a response also seen to a lesser degree with the semiconductor material gallium arsenide (Webb et al., 1986). Gallium arsenide has been evaluated for immunotoxicity in mice, where it was found to alter macrophage and lymphocyte activities (Sikorski et al., 1989, 1991a,b; Burns et al., 1991, 1993).
The purpose of the present studies was to determine the immunotoxicity of ATO following two week inhalation exposure in mice. Our hypothesis was that ATO results in systemic immunotoxicity following inhalation exposures in mice, as evidenced by suppression of spleen cell immune function assays. The biodistribution of arsenic in murine tissues was analyzed to determine the concentration of arsenic in various tissues, especially in the spleen. Results show that ATO at daily inhalation exposures of 50–1000 ug/m3 over 14 days is immunosuppressive to spleen cells of mice.
METHODS
Animals
Male C57Bl/6N mice were ordered from Charles River Laboratories at 8 weeks of age and were quarantined for two weeks upon arrival. All animal procedures were approved by Lovelace Respiratory Research Institute (LRRI) and University of New Mexico (UNM) Institutional Animal Care and Use Committees. All animal housing and husbandry at LRRI and UNM was performed in accordance with The Guide for the Care and Use of Laboratory Animals (National Academy of Sciences 1996). After the 2 week quarantine period, all animals were conditioned to nose-only exposure tubes on at least 3 occasions. Exposure groups consisted of 12 animals each and were exposed to control air, or concentrations of 50 μg/m3 or 1 mg/m3 ATO. Mice were exposed 3 hrs/day for 14 consecutive days. Immediately after exposure completion (day 14), 5 animals per group were sacrificed for biodistribution and histopathology endpoints. The morning after (day 15), approximately 18 hrs after exposure completion, the remaining 7 animals per group were sacrificed to assess Arsenic Trioxide effects on various organ systems. Animals were euthanized by CO2 narcosis and spleens were collected using aseptic technique.
Chemicals and Reagents
Arsenic Trioxide (As2O3) was purchased from Sigma Aldrich (Cat # 202673, CAS# 1327-53-3). This material was aerosolized without the use of a vehicle.
Exposure System
Inhalation exposures were conducted at Lovelace Respiratory Research Institute, the inhalation exposure system consists of an aerosol generator and an exposure plenum (chamber) fitted with filter sampler and cascade impactor sample ports. Test article aerosols were generated with a Wright Dust Feeder. Arsenic trioxide (ATO) was loaded into a cylinder and packed at 3,000 psi for 2 hrs. The cylinder rotates at a constant speed around a stationary scraper blade. The rotation speed was controlled to adjust the aerosol concentration. Filtered, compressed air passed the blade and picked up the dust that had been scraped from the packed cylinder. The dust then passed through a 3 μM cutoff cyclone to reduce the number of large particles. The resultant aerosol entered the nose-only 36 port exposure chamber plenum (In-Tox Products, Moriarity, NM) and was therefore present in the breathing zone of animals in nose-only restraint tubes connected to the exposure chamber. Chamber pressure was maintained slightly negative relative to the exposure room and oxygen (%) was monitored throughout the exposure to ensure that it did not drop below 19%.
Aerosol Concentration Monitoring
Particle size was measured using a 7 stage Mercer impactor (In-Tox Products, Moriarty, NM). The aerosol concentration was determined by gravimetric analysis of filter samples collected during the exposure. Filter samples were collected from the exposure plenum. Aerosols were collected with 47-mm Palflex filters (Pall Life Sciences, Ann Arbor, MI) at a flow rate of 0.5 l/min. After collection, the filters were removed from the filter holders, placed in Petri dishes, and weighed on a microbalance (Mettler MX-5, Columbus, OH).
Estimation of Pulmonary Dose
Respiratory minute volume (RMV; liters per min) is calculated using the following allometric equation: RMV = 0.499BW0.809, where BW is average exposure day body weight in kilograms (Bide et al., 2000). The estimated dose is calculated using the following formula: Dose = (C × RMV × T × DF)/BW, where C is the average concentration of the test article in the exposure atmosphere, T is exposure time, and the deposition fraction (DF) is assumed to be 10% (Guyton et al., 1974).
Collection of tissue for biodistribution and immune function assays
After euthanasia, blood was collected into heparinized syringes. Plasma was then separated by centrifugation and transferred into an appropriately labeled sample vial and stored frozen (approximately −80 °C) for biodistribution analysis.
Tissues
Tissues collected for Biodistribution
Tissues acquired for biodistribution analysis, skin, brain, kidney, liver, lungs, spleen, and bladder were collected into labeled cryovials and snap frozen in liquid nitrogen.
Analysis of Arsenic Tissue Levels
Three mg of tissue and 200 μl blood samples were taken from the arsenic exposed mice. Samples were digested, using 2 ml nitric acid, at 90°C. After digestion was completed, digests were brought up to 10 ml final volume and transferred into ICP plastic tubes. Inductively Coupled Plasma-Mass Spectrometer (ICP/MS) was used to analyze the samples following US EPA method 200.8. Results were then calculated using the starting weight of tissue (g) or volume (blood) and the final volume after digestion. Results were expressed as μg/mg for tissue and μg/ml for blood.
Spleen Harvest and Cell Isolation
Spleens were harvested into sterile Hank's Balanced Salt Solution (HBSS) (Cambrex, Walkersville, MD) in a sterile 15 ml centrifuge tube on ice. Using sterile forceps and gauze; spleens were blotted to remove excess liquid and weighed. Once weights were recorded, spleens were bathed in approximately 5ml of supplemented RPMI media in a sterile culture dish. Supplemented RPMI media contained 10% fetal bovine serum (Hyclone, Logan, UT), 1% penicillin/Streptomycin (Cambrex, Walkersville, MD), and 1% L-glutamine (Cambrex, Walkersville, MD). Microscope slides were flame sterilized and frosted ends were used to homogenize the spleen, complete cell isolation was determined by a white spleen capsule. Isolated splenocytes remained on ice until all samples had been isolated, then centrifuged at 280 × g for 10 minutes. Splenocyte pellets were resuspended in 2 ml of supplemented RPMI media. Lymphocytes were counted and viability determined for each sample by Trypan Blue exclusion on a hemacytometer. Counts and viabilities were recorded and used for normalizing lymphocyte number during cell plating for immune function assays.
Spleen Cell Surface Marker Analysis
Subsets of murine spleen cells were determined as previously described (Gao et al, 2008; Mitchell et al., 2009). Briefly, after spleen cells were harvested, 3 ×106 cells were aliquoted into each of three 12 × 75 mm tubes, and their surface marker expression was analyzed using a FACS Calibur Flow Cytometry system (Becton Dickinson Immunocytometry Systems, San Jose, CA). Three combinations of custom rat anti-mouse monoclonal antibody cocktails were custom ordered from BD Biosciences (BD Pharmingen, San Diego, CA). Splenic cells were first incubated with purified rat anti-mouse CD16/CD32 monoclonal antibody (Fc block antibody) (BD Pharmingen, San Diego, CA) for 10 min at room temperature in the dark. To detect the spleen cell subpopulations, 20 μl of antibody cocktail containing IgG1+ IgG2a-FITC/IgM-PE/CD45-PerCP/IgG2a-APC, or CD3- FITC/CD8a-PE/CD45 -PerCP/CD4-APC or CD3+CD19-FITC/Pan NK-PE/ CD45-PerCP/CD11b- APC was added to the appropriate sample tubes.. After 30 min incubation in the dark, 2 ml of 1× fresh ammonium chloride was added to each sample to lyse red blood cells, samples were then incubated at room temperature for 10 min in the dark. Samples were centrifuged at 275 × g for 10 min, supernatants were aspirated and the pellets were washed with 2 ml of the DPBS wash buffer (Sigma Chemical Co, St. Louis, MO) [contains sodium azide and fetal bovine serum] and then centrifuged as above. Cells were then resuspended in 400 μl of PBS wash buffer, tubes were capped and covered by aluminum foil for transport to the Flow Cytometry Facility for analysis. Data were acquired by gating on CD45 positive cells and acquiring 10,000 gated events. CellQuest software was used to analyze the data.
Natural Killer Cell Assay
Yac-1 (ATCC, Manassas, VA), a mouse lymphoma cell line, was used as a target cell in this assay because they are sensitive to the cytotoxicity of NK cells. Yac-1 cells were incubated with radio labeled Chromium-51 (Perkin Elmer, Boston ma) (50 μCi/1×105 Yac-1 cells) for 1 hr prior to plating. Labeled Yac-1 cells were then plated at a concentration of 5×103 cells per well in round bottom 96 well plates. Splenocytes were plated at 1×106 cells/well and serially diluted across the plate for effector to target ratios (E:T) of 200:1, 100:1, 50:1, and 25:1. In order to measure maximum Chromium release, Triton X-100 was added at a concentration of 5% to control wells and spontaneous chromium release was measured by analyzing wells that only contained target and no effector cells. Splenocytes and Yac-1 cells were co-incubated for 4 hours at which point plates were centrifuged at 200 × g for 10 minutes. In order to measure chromium release as a result of Yac-1 lysis, supernatants were collected into tubes (Perkin Elmer, Boston, MA) and analyzed on a gamma counter.
Mitogenesis Assay
For each spleen sample cells were suspended in 5mL of supplemented RPMI media at a concentration of 1×106 cells/ml. Each spleen sample was tested in replicates of 6 for 3 mitogens for a total of 18 wells per sample. 200 μl (2×105 cells) were placed in corresponding wells of sterile flat bottom 96 well plates. Concanavalin A (Con A) (Sigma) was added to the appropriate wells at a final concentration of 1 μg/ml. Lippopolysaccharide (LPS) (Alexis, San Diego, CA) was added to appropriate wells at a final concentration of 10 μg/ml. Supplemented RPMI media was added to control wells. Plates were briefly mixed on a plate shaker and then placed in a 5% CO2, 37°C incubator for 48 hours. Following the 48 hour incubation, cells were pulsed with 1 μCi/well of 3H-thymidine (MP Biomedicals, Irvine, CA) and incubated for an additional 18 hours. Cells were harvested using a Brandel Model 24V cell harvester onto filter paper (Whatman, Maidstone, England) and lysed with a 0.05% Tween 20 solution (Fisher). Once filter paper samples are completely dry, samples were placed in liquid scintillation vials containing 3mL ScintiVerse Scintillation Cocktail (Fisher), and counted on a liquid scintillation beta counter. Results are expressed as percent control based on counts per minute (CPM).
Jerne-Nordin Plaque Assay
Spleen cells were suspended in 3 ml of supplemented RPMI media at a concentration of 4×106 cells/ml. For the Jerne-Nordin Plaque assay supplemented media contained heat inactivated fetal bovine serum (Hyclone, Logan, UT), 1% penicillin/streptomycin (Cambrex, Walkersville, MD), 1% L-glutamine (Cambrex, Walkersville, MD), 0.09% 55mM 2-Mercaptoethanol (Gibco, Grand Island, NY), 1% 100mM Sodium Pyruvate (Cambrex, Walkersville, MD), 0.5% gentamycin (Gibco, Grand Island, NY). 1% sheep red blood cells (SRBC) (Colorado Serum, Denver, Colorado) were washed and suspended in supplemented RPMI media and added to the appropriate wells of a 48 well plate. Each sample was immunized with SRBC in duplicate. Media was used for non-immunized control wells for each sample. Cells were incubated for 4 days in a 5% CO2, 37°C incubator. Following spleen cell immunization, cells were washed and resuspended in supplemented media. A 0.8% solution of SeaPlaque agarose (Cambrex, Rockland, ME) in 2× RPMI medium (Gibco, Grand Island, NY) was warmed to 43°C in glass tubes. SRBC and spleen cells were added to the tubes and then spread on agarose coated microscope slides and incubated face down on custom slide trays in a humidified plastic box at 37°C for 1 hour. Guinea pig complement (Colorado Serum, Denver, Colorado) was diluted 1:20 in Dulbecco's Phosphate Buffered Saline containing Ca2+ and Mg2+ (Sigma) and warmed to 37°C in a water bath. Slides were flooded with diluted complement following the 1 hr incubation and then incubated an additional 2 hours. Slides were removed from the incubator and stored in a cold 0.85% sodium chloride solution. SRBC lysis was quantified by counting plaques in the SRBC/agar lawn using a dissecting microscope. Results are expressed as percent control.
Statistics
Data were analyzed by SigmaStat software (Systat Software, Inc., San Jose, CA). The statistical differences among groups were determined by one-way analysis of variance and/or Student's t test. The values were expressed as mean ± S.E.M. A p value <.05 indicated a statistically significant difference.
RESULTS
Exposure Atmospheres
The target concentrations for exposure in these studies were 50 μg/m3 and 1 mg/m3. The measured final inhalation exposure concentrations fell into the target range at 64 ± 28 μg/m3 and 1.0 ± 0.15 mg/m3, as measured from chamber monitor filters collected daily (range 35–123 ug/m3 for low dose and 927–1159 ug/m3 for high dose)The mass median diameter particle size at these two levels was 2.5μ and 2.3μ respectively, and the geometric standard deviation was 1.7μ for the 50 μg/m3 and 3.5μ for the 1 mg/m3 exposure levels.
14-Day inhalation exposures to ATO does not affect murine spleen cell recoveries
Two sets of male C57Bl/6 mice 12 weeks of age (7 mice per group for functional assays and 5 mice per group for biodistribution studies) were exposed to ATO at concentrations of 50 μg/m3 or 1 mg/m3 3 hrs per day in nose only exposure tubes for 14 days. There was no treatment-related mortality or morbidity associated with these exposures. Table 1 demonstrates that there were no treatment-related changes in the number of viable white blood cells recovered from the spleens for either the 50 μg/m3 or the 1 mg/m3 groups as compared to control mice who received clean air exposures.
TABLE 1.
Spleen Cell Recoveries
| Treatment | Cell recovery per mg spleen ± SEM* |
|---|---|
| Control | 1,280,000 ± 73,485 |
| 50 μg/m3 ATO | 1,937,500 ± 347,407 |
| 1 mg/m3 ATO | 1,628,571 ± 161,414 |
There were no statistical differences between groups; p < .05
Tissue levels of ATO after 14 days inhalation exposure
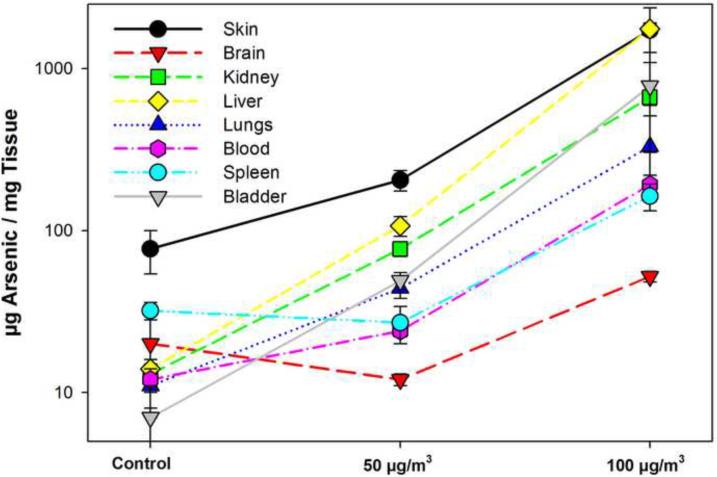
Mice exposed to ATO at two concentrations and control mice exposed to fresh air were used to measure the tissue and organ levels of ATO immediately after the last exposure on Day 14. The results showed that the highest levels of arsenic were present in the liver and the skin. The high skin levels were likely due to surface contamination that occurred during exposures due to occasional loss of the seal between the nose and the exposure tubes. The organs with the next highest concentrations of arsenic on a g/mg basis were bladder and kidney, followed by lung, blood (μg/ml), and spleen (Figure 1). Minimal levels of arsenic were detected in the brain. The results suggest that following inhalation exposure, ATO is rapidly excreted via the kidney into the bladder, which is consistent with previous reports (Aranyi et al., 1985; Webb et al., 1986; Gentry et al., 2004).
Figure 1.
Tissue analysis of total arsenic in tissues by ICP/MS immediately following the last nose-only inhalation exposure. There were 5 mice per group that received air only (CA), low ATO (LA, 50 μg/m3), and high ATO (HA, 1 mg/m3) 14 days (3 hrs per day). Results are expressed as the average level of arsenic (μg/mg tissue) ± standard deviation.
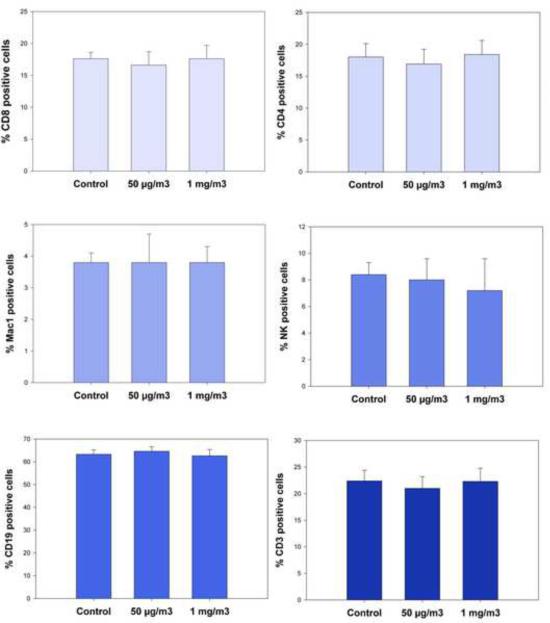
ATO inhalation did not alter spleen cell subsets
Spleen cells obtained immediately after the last inhalation exposure to ATO were analyzed for changes in cell subsets for B (CD19), T (CD3), T helper cells (CD4), cytotoxic T cells (CD8), natural killer cells (CD16), and macrophages (Mac-1). Results demonstrated that there were no changes in any cell subsets (Figure 2) at either the 50 ug/m3 or 1 mg/m3 ATO exposure levels as compared to controls.
Figure 2.
Cell surface phenotype analysis following 14 day ATO inhalation exposures (3 hrs per day) at 50 μg/m3 and 1 mg/m3 compared to air in male C57BL/6 mice. CD45+ spleen cells were examined for surface markers for cytotoxic T cells (CD8+), helper T cells (CD4+), macrophages (Mac-1), NK cells (CD16+), total B cells (CD19+), and total T cells (CD3+). Results shown are the averages for individual determinations from 7 mice ± SEM.
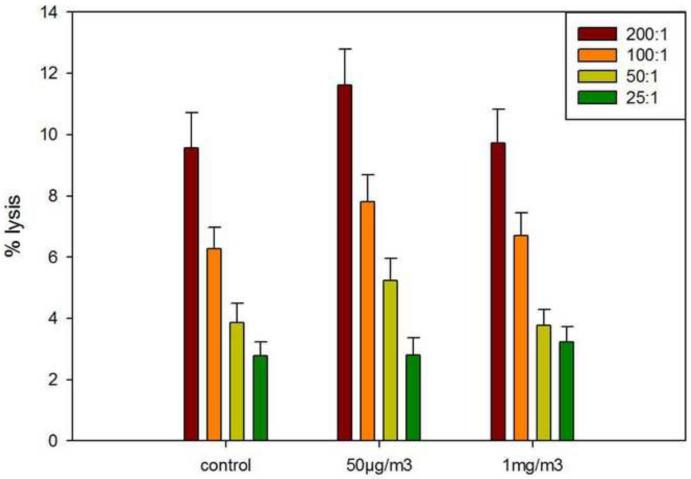
ATO inhalation exposures did not alter splenic natural killer cell activity
Mice exposed to ATO by inhalation were assessed for their innate immune responses by measuring natural killer (NK) cell activities. The NK cell activity assay, especially when combined with cell surface markers has previously been found to detect a large percentage of immunosuppressive xenobiotics (Luster et al, 1992). Neither the 50 ug/m3 nor the 1 mg/m3 exposure levels exerted any effect on the response of mice to Yac-1 target cells (Figure 3). These results suggest the ATO is not a nonspecific immunotoxic agent.
Figure 3.
Natural Killer (NK) cell activity analysis following 14 day ATO inhalation exposures ( 3 hrs per day) of 50 μg/m3 and 1 mg/m3 compared to air. Results are shown for different effector cell (NK) to target cell (Yac-1) ratios ranging from 200:1 to 25:1, each obtained from individual mice (n=7) compared to control (air only exposure) expressed as the average percent specific lysis + SEM. No differences between treatment and control groups were detected.
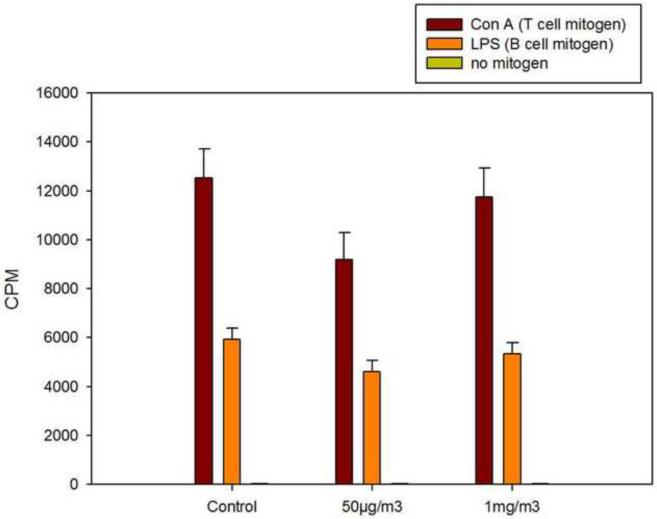
ATO inhalation did not alter splenic B and T cell proliferation induced by mitogens
Another routine assay that is commonly used for an assessment of immunotoxicity in murine spleen cells is the proliferative response of B and T cells to mitogens. ATO was not found to suppress either the B cell proliferative response induced by LPS or the T cell proliferative response to Con A (Figure 4). Again, these results suggest that ATO does not nonspecifically alter B and T cell activity.
Figure 4.
Assessment of cell B cell proliferation induced by LPS and T cell proliferation induced by Con A in murine spleen cells following 14 day inhalation exposures (3 hrs per day) to ATO at 50 μg/m3 and 1 mg/m3 compared to air (control). Results are shown as the amount of 3H-thymidine incorporated into DNA after a 72 hr period for 6 replicate cultures from each of 7 mice per group. Values are the average CPM ± SEM.
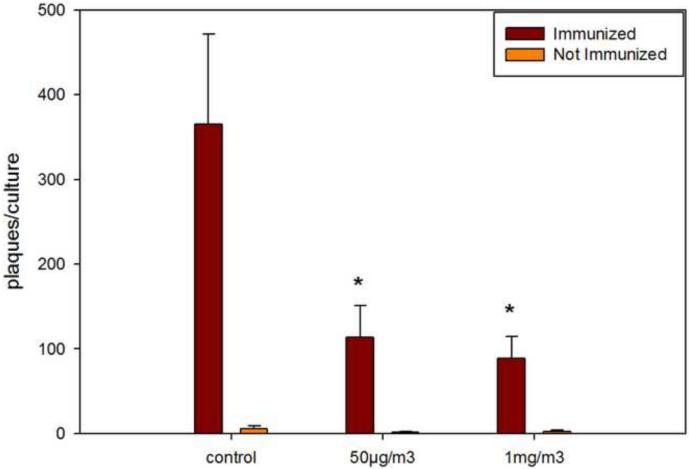
The T-dependent antibody response to SRBCs is suppressed by ATO inhalation
In contrast to results obtained in innate immunity (NK activity), cell surface marker analysis, and B/T cell mitogenesis assays, ATO was found to produce greater than 70% suppression of the T-dependent antibody response to sheep red blood cells when spleen cells were analyzed from mice receiving the 50 μg/m3 exposure for 14 days (Figure 5). The exposure to 1 mg/m3 ATO produced a further increase in suppression. We could not determine from these studies a no effect level for suppression of the T-dependent antibody response following 14 day inhalation exposures to ATO.
Figure 5.
Determination of the plaque forming cell (PFC) response to sheep red blood cells (SRBC) following 4 days in culture of spleens cells obtained from mice receiving 14 day inhalation exposure (3 hrs per day) to air (control) , 50 μg/m3 ATO, or 1 mg/m3 ATO. Results shown are the average number of PFC + SEM obtained for triplicate cultures of 7 individual mice per group. Significant suppression (p < .05) of the PFC response was seen at both the 50 μg/m3 and 1 mg/m3 levels of exposure.
DISCUSSION
During recent years there has been increasing interest in inorganic arsenic and various arsenicals. ATO has proved to have clinical applications in treatment of myelodysplastic syndromes and leukemia (Bobe et al., 2006; Kantarjian et al. 2008). Several traditional herbal medicines that have been utilized for the treatment of cancers have been found to contain arsenic (Wang et al., 2008). The main environmental particulate form of arsenic is arsenic trioxide (ATO), which following inhalation and absorption in the lungs containing an aqueous environment forms arsenious acid (As(OH)3) or the anion of its salt arsenite (AsO2-) (Carter et al., 2003). Many of the biologic effects of ATO have been attributed to AsIII, such as thiol binding or oxidative stress, although methylated forms of arsenic are receiving increasing Toxicologic attention. In vivo, the most sensitive target organ of ATO in mammals appears to be the kidney, although many other organs and tissues are affected by exposure (ATSDR, 2007). In the kidney, pyruvate dehyrdogenase (PDH) appears to have the lowest Ki for inhibition (ATSDR, 2007). More recently it was found that the monomethylarsonate (MMAIII), which is formed in many mammalian cells, had an even lower Ki for inhibition of PDH (Petrick et al., 2000). Thus, both inorganic and organic forms of arsenic are important in its toxicity.
The present studies were aimed at evaluating the two week inhalation immunotoxicity of ATO. ATO is known to form sodium arsenite (or arsenious acid) in aqueous solutions (Carter et al, 2003). Previous studies have shown that AsIII in drinking water is associated with immunosuppression in humans (Soto-Peña et al. 2006). Lemarie et al (2006) have shown that human macrophages are sensitive to inhibition of antigen presentation following exposure to sodium arsenite. In addition, there is a significant immunotoxicity literature on the effects of gallium arsenide (GaAs, see below), a AsIII- form that is converted to AsIII in aqueous systems (Carter et al., 2003). Sikorski et al (1989, 1991 a,b) have shown that GaAs produces a dose-dependent suppression of humoral and cell mediated immunity in mice following a single intratracheal (IT) exposure to concentration of 2.5–200 mg/kg. There was significant cytotoxicity at exposure levels greater than 100 mg/kg. GaAs was also found to suppress humoral immunity in vitro, producing a 97% decrease in the SRBC response at exposure concentrations of 25 μM (Sikorski et al., 1991a). Burns et al (1991) demonstrated that in vitro exposure to GaAs at concentrations of 25 μM were immunosuppressive and were similar to exposures to NaAsO2. Burns and Munson (1993) demonstrated that in vitro GaAs exposure alters T cell activation and suppresses T cell proliferation.
Our current studies show that the most sensitive immune assay for assessment of ATO immunotoxicity in mice is the T-dependent antibody response to SRBCs. We found that a 14 day exposure (3 hrs per day) to 50 μg/m3 nose-only exposure produced >70% suppression of this response. This is interesting because we found a negligible increase in the measured levels of total arsenic in the spleen compared to control mice. Increasing the exposure level to 1 mg/m3 produced only a small increase in immunotoxicity. There were no other observed immune effects following the 1 mg/m3 exposure ATO, including assessment of spleen cell recovery, cell subset recovery, NK cell activity, and B/T cell proliferation. The peak level of ATO exposure seen in the spleen was approximately 150 μg/mg of tissue for the1 μg/m3 exposure group. This is a significantly lower concentration than required to suppress humoral immunity in mice following a single intratracheal administration of GaAs (Sikorski et al, 1991a,b), although it is difficult to compare the total AsIII exposures that would be achieved due to the complexity of arsenic metabolism in vivo.
The results of these studies clearly demonstrate that the alterations in the humoral immune response is a sensitive target for arsenic toxicity. It is unclear why the T-dependent antibody appears to be particularly sensitive to the effects of ATO. Previous studies in our lab have shown that agents that deplete glutathione, an observation made in many tissues following arsenic exposure (Carter et al., 2003), is associated with immunosuppression (Romero et al.,1997). Thus, oxidative stress may be an underlying for ATO-induced immunosuppression. Chronic inhalation exposures to arsenic dust in the form of ATO may be a significant source of environmental exposure, especially to people living in areas surrounding copper smelters. Human exposure levels in air samples near smelters have been reported in the 0.1 μg/m3 range (Binder et al., 1987) to 50 μg/m3 airborne levels (Smith et al., 1977). Our studies suggest that investigations of the environmental health effects of ATO should consider including immune function assays, and in particular tests that include an assessment of humoral immune responses.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Abduhl-Mehdi Ali in the UNM Dept of Earth and Planetary Sciences for his assistance in the analysis of arsenic by ICP/MS. These studies were supported by a supplement to NIEHS P30-012072 and by RO1 ES15826.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Apostoli P, Bartoli D, Alessio L, Buchet JP. Biological monitoring of occupational exposure to inorganic arsenic. Occup Environ Med. 1999;56:825–832. doi: 10.1136/oem.56.12.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR . Arsenic Toxicologic Profile. Aug, 2007. [Google Scholar]
- Aranyi C, Bradof JN, O'Shea WJ, Graham JA, Miller FJ. Effects of arsenic trioxide inhalation exposure on pulmonary antibacterial defenses in mice. J Toxicol Environ Health. 1985;15:163–172. doi: 10.1080/15287398509530643. [DOI] [PubMed] [Google Scholar]
- Bide RW, Armour SJ, Yee E. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J Applied Toxicol. 2000;V20:273–290. doi: 10.1002/1099-1263(200007/08)20:4<273::aid-jat657>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Binder S, Forney D, Kaye W, Paschal D. Arsenic exposure in children living near a former copper smelter. Bull Environ Contam Toxicol. 1987;39:114–121. doi: 10.1007/BF01691798. [DOI] [PubMed] [Google Scholar]
- Bobé P, Bonardelle D, Benihoud K, Opolon P, Chelbi-Alix MK. Arsenic trioxide: A promising novel therapeutic agent for lymphoproliferative and autoimmune syndromes in MRL/lpr mice. Blood. 2006;108:3967–3975. doi: 10.1182/blood-2006-04-020610. [DOI] [PubMed] [Google Scholar]
- Burns LA, Munson AE. Gallium arsenide selectively inhibits T cell proliferation and alters expression of CD25 (IL-2R/p55) J Pharmacol Exp Ther. 1993;265:178–186. [PubMed] [Google Scholar]
- Burns LA, Sikorski EE, Saady JJ, Munson AE. Evidence for arsenic as the immunosuppressive component of gallium arsenide. Toxicol Appl Pharmacol. 1991;110:157–169. doi: 10.1016/0041-008x(91)90298-s. [DOI] [PubMed] [Google Scholar]
- Carter DE, Aposhian HV, Gandolfi AJ. The metabolism of inorganic arsenic oxides, gallium arsenide, and arsine: a toxicochemical review. Toxicol Appl Pharmacol. 2003;193:309–334. doi: 10.1016/j.taap.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Arnold LL, Eldan M, Lewis AS, Beck BD. Methylated arsenicals: the implications of metabolism and carcinogenicity studies in rodents to human risk assessment. Crit Rev Toxicol. 2006;36:99–133. doi: 10.1080/10408440500534230. [DOI] [PubMed] [Google Scholar]
- Gao J, Mitchell LA, Lauer FT, Burchiel SW. p53 and ATM/ATR regulate 7,12-dimethylbenz[a]anthracene-induced immunosuppression. Mol Pharmacol. 2008;73:137–46. doi: 10.1124/mol.107.039230. [DOI] [PubMed] [Google Scholar]
- Gentry PR, Covington TR, Mann S, Shipp AM, Yager JW, Clewell HJ., 3rd Physiologically based pharmacokinetic modeling of arsenic in the mouse. J Toxicol Environ Health A. 2004;67:43–71. doi: 10.1080/15287390490253660. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Measurement of the Respiratory Volumes of Laboratory Animals. American Journal of Physiology. 1974;150:70–77. doi: 10.1152/ajplegacy.1947.150.1.70. [DOI] [PubMed] [Google Scholar]
- Hysong TA, Burgess JL, Cebrián Garcia ME, O'Rourke MK. House dust and inorganic urinary arsenic in two Arizona mining towns. J Expo Anal Environ Epidemiol. 2003;13:211–218. doi: 10.1038/sj.jea.7500272. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, O'Brien S, Cortes J, Wierda W, Faderl S, Garcia-Manero G, Issa JP, Estey E, Keating M, Freireich EJ. Therapeutic advances in leukemia and myelodysplastic syndrome over the past 40 years. Cancer. 2008;113:1933–1952. doi: 10.1002/cncr.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarie A, Morzadec C, Bourdonnay E, Fardel O, Vernhet L. Human macrophages constitute targets for immunotoxic inorganic arsenic. J Immunol. 2006;177:3019–3027. doi: 10.4049/jimmunol.177.5.3019. [DOI] [PubMed] [Google Scholar]
- Luster MI, Portier C, Pait DG, White KL, Jr, Gennings C, Munson AE, Rosenthal GJ. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam Appl Toxicol. 1992;18:200–210. doi: 10.1016/0272-0590(92)90047-l. [DOI] [PubMed] [Google Scholar]
- Milham S, Strong T. Human arsenic exposure in relation to a copper smelter. Environ Res. 1974;7:176–182. [Google Scholar]
- Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms of Inhaled Multiwalled Carbon Nanotube-Induced Systemic Immune Suppression in Mice. Nature Nanotechnol. 2009;4:451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AA, Gutman D, Cejas PJ, Lee KP, Boise LH. Reactive oxygen species are not required for an arsenic trioxide-induced antioxidant response or apoptosis. J Biol Chem. 2009;284:12886–12895. doi: 10.1074/jbc.M806546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse DL, Harrington JM, Housworth J, Landrigan PJ, Kelter A. Arsenic exposure in multiple environmental media in children near a smelter. Clin Toxicol. 1979;14:389–399. doi: 10.3109/15563657909010601. [DOI] [PubMed] [Google Scholar]
- National Research Council Report (NRC) Arsenic in Drinking Water. National Academy Press; Washington, DC: 1999. [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol. 2000;163:203–297. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Pi J, Yamauchi H, Kumagai Y, Sun G, Yoshida T, Aikawa H, Hopenhayn-Rich C, Shimojo N. Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water. Environmental Health Perspectives. 2002;110:331–336. doi: 10.1289/ehp.02110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radabaugh TR, Aposhian HV. Enzymatic reduction of arsenic compounds in mammalian systems: reduction of arsenate to arsenite by human liver arsenate reductase. Chem Res Toxicol. 2000;13:26–30. doi: 10.1021/tx990115k. [DOI] [PubMed] [Google Scholar]
- Radabaugh TR, Sampayo-Reyes A, Zakharyan RA, Aposhian HV. Arsenate reductase II. Purine nucleoside phosphorylase in the presence of dihydrolipoic acid is a route for reduction of arsenate to arsenite in mammalian systems. Chem Res Toxicol. 2002;15:692–698. doi: 10.1021/tx0101853. [DOI] [PubMed] [Google Scholar]
- Romero DL, Mounho BJ, Lauer FT, Born JL, Burchiel SW. Depletion of glutathione by benzo(a)pyrene metabolites, ionomycin, thapsigargin, and phorbol myristate in human peripheral blood mononuclear cells. Toxicol Appl Pharmacol. 1997;144:62–69. doi: 10.1006/taap.1997.8113. [DOI] [PubMed] [Google Scholar]
- Shi H, Hudson LG, Liu KJ. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radic Biol Med. 2004;37:5582–5593. doi: 10.1016/j.freeradbiomed.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Sikorski EE, Burns LA, Stern ML, Luster MI, Munson AE. Splenic cell targets in gallium arsenide-induced suppression of the primary antibody response. Toxicol Appl Pharmacol. 1991a;110:129–142. doi: 10.1016/0041-008x(91)90296-q. [DOI] [PubMed] [Google Scholar]
- Sikorski EE, Burns LA, McCoy KL, Stern M, Munson AE. Suppression of splenic accessory cell function in mice exposed to gallium arsenide. Toxicol Appl Pharmacol. 1991b;110:143–156. doi: 10.1016/0041-008x(91)90297-r. [DOI] [PubMed] [Google Scholar]
- Sikorski EE, McCay JA, White KL, Jr, Bradley SG, Munson AE. Immunotoxicity of the semiconductor gallium arsenide in female B6C3F1 mice. Fundam Appl Toxicol. 1989;13:843–858. doi: 10.1016/0272-0590(89)90338-2. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Crecelius EA, Reading JC. Airborne arsenic exposure and excretion of methylated arsenic compounds. Environ Health Perspect. 1977;19:89–93. doi: 10.1289/ehp.771989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Peña GA, Luna AL, Acosta-Saavedra L, Conde P, López-Carrillo L, Cebrián ME, Bastida M, Calderón-Aranda ES, Vega L. Assessment of lymphocyte subpopulations and cytokine secretion in children exposed to arsenic. FASEB J. 2006;20:779–781. doi: 10.1096/fj.05-4860fje. [DOI] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol. Appl. Pharmacol. 2001;176:127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Environmental Monitoring Systems Laboratory. Office of Research and Development; Cincinnati, OH 45268: Method 200.8. [Google Scholar]
- Waalkes MP, Liu J. Purine nucleoside phosphorylase: a fortuitous cytosolic arsenate reductase? Toxicol Sci. 2002;70:1–3. doi: 10.1093/toxsci/70.1.1. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhou GB, Liu P, Song JH, Liang Y, Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, Chen SJ, Chen Z. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci U S A. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DR, Wilson SE, Carter DE. Comparative pulmonary toxicity of gallium arsenide, gallium(III) oxide, or arsenic(III) oxide intratracheally instilled into rats. Toxicol Appl Pharmacol. 1986;82:405–16. doi: 10.1016/0041-008x(86)90276-0. [DOI] [PubMed] [Google Scholar]