Abstract
Spinal cord injury (SCI) results in immediate disruption of neuronal membranes followed by extensive secondary neurodegenerative processes. A key approach for repair of SCI is sealing the damaged membranes early. Here we show that axonal membranes injured by compression can be effectively repaired by using self-assembled monomethoxy poly(ethylene glycol)-poly(D,L-lactic acid) di-block copolymer micelles (60 nm diameter). Injured spinal tissue incubated with micelles showed rapid restoration of compound action potential and reduced calcium influx into axons. Much lower micelle concentration is required for treatment than the positive control, polyethylene glycol. Intravenously injected micelles effectively recovered the locomotor function and reduced the volume and inflammatory response of the lesion in SCI rats. The micelles showed no adverse effects after systemic administration to live rats. Our results suggest that copolymer micelles can interrupt the spread of primary SCI damage with minimal toxicity.
Most SCI cases involve a primary injury and subsequent secondary damage 1, 2. During the primary injury, the acute mechanical stress to the spinal cord breaks neuronal membranes and causes Ca2+ influx into cells. The latter triggers a series of secondary biological events including inflammation, free radical release, and apoptosis 3, 4, which further exacerbate the damage. Among various SCI treatments 5, a key approach is to seal the damaged membrane at the early stage of SCI. Poly(ethylene glycol) (PEG) and Poloxamer 188 (P188), known as membrane sealing agents 6, 7, have been used to restore compound action potential (CAP) in ex vivo tissues 8 and recover behavioral functions in vivo 9–13. Topical delivery of PEG (≥ 30% w/w in water) to the site of injury was shown to be effective in alleviating the secondary injuries including oxidative stress and free radical production 14, 15. Systemic delivery methods including intravenous injection and subcutaneous injection were also utilized for the ease of clinical use. However, intravenous injection of 30% PEG only increased the locomotor rating score by 0.7 out of a 21-point-scale compared to the control group receiving isovolumetric dose of saline solution 16, whereas subcutaneously injected P188 has shown to be less effective than PEG in improvement of cutaneous trunci muscle reflection 12. The limited behavioral recovery is partly due to the difficulty in delivering sufficient amount of agents to the injured site via systemic circulation. For polymers, it was shown that unimers had a circulation half-life of less than 10 min 17.
In this paper we present a new approach that allows effective membrane repair and functional recovery in SCI animals. Instead of using individual polymers, we choose PEG-polyester micelles which are spherical assemblies of di-block copolymers, containing a hydrophilic PEG shell and a hydrophobic inner core 18. These polymeric micelles, sizing from 10 nm to 100 nm, possess unique properties such as biocompatibility and long blood residence time 19, and have been widely investigated as nanocarriers of water insoluble drugs 20. By recognizing the high-density coronal PEG and amphiphilic structure in a polymeric micelle, we demonstrate a new application of copolymer micelles as a membrane repair agent to treat SCI.
Restoration of CAP in ex vivo spinal tissue
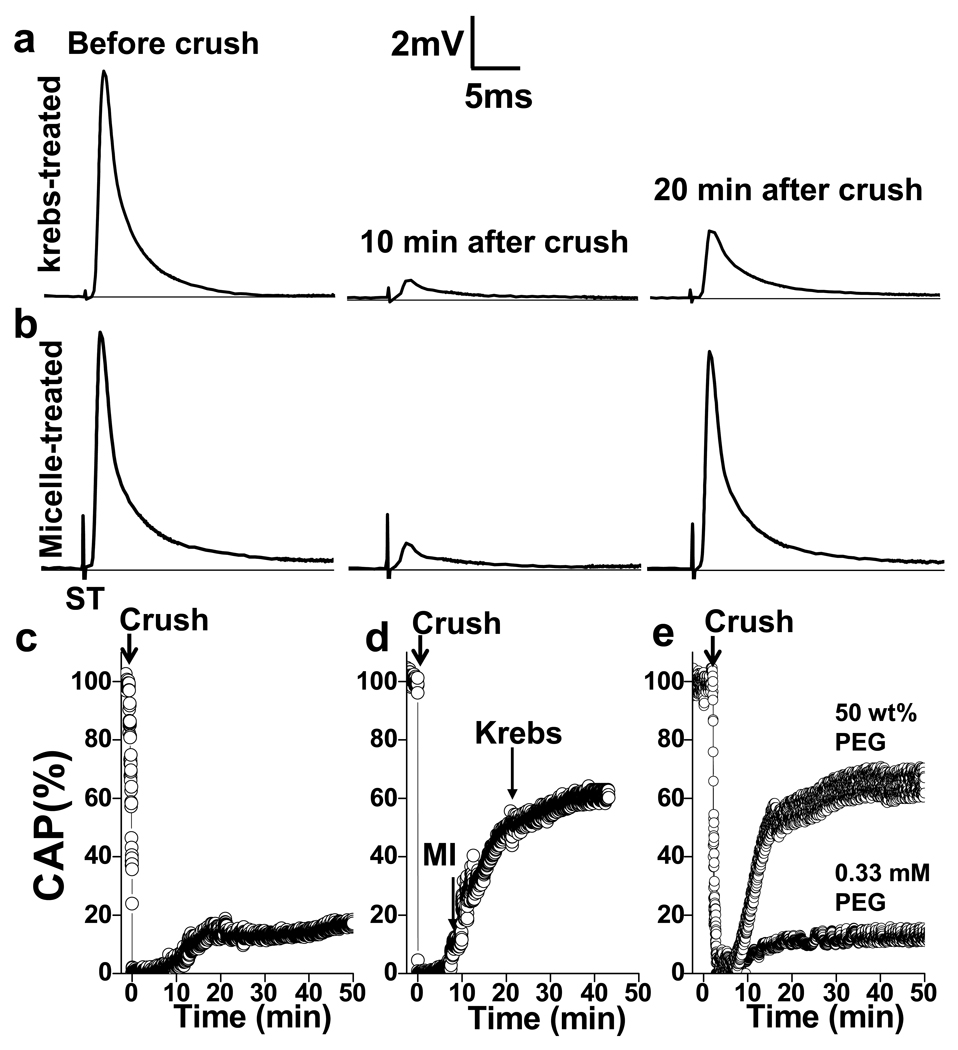
We prepared monomethoxy poly(ethylene glycol)-poly(D,L-lactic acid) (mPEG-PDLLA) micelles (Fig. S1) using dialysis, and applied them to compression-injured ventral white matter strips isolated from adult guinea pigs, a tissue model widely used in neuronal membrane repair studies 8, 14, 15. The effect of mPEG-PDLLA micelles on compression injured white matter was examined by the CAP amplitude, an indicator of the proportion of the axons conducting action potential. The viability of the white matter was affirmed by initial CAP measurements using a double sucrose gap recording chamber (Fig. S2). A constant displacement of white matter strips by 5 to 30 sec compression with modified forceps possessing a 0.8 mm spacer was implemented to reduce the CAP amplitude to 0 mV 14. Without micelle treatment, the CAP was self-recovered to 18.5 ± 5.1% (n=5) of the pre-compression amplitude during the 10 to 20 min post-compression period (Fig. 1a). With a Krebs’ solution containing 0.67 mg/mL micelles, the white matter strips (n=8) recovered to 66.5 ± 14.8% of the original CAP within 20 min after incubation with micelles (Fig. 1b). The time-dependent increase of CAP is shown in Fig. 1c–e. Without treatment, the CAP remained stable after self-recovery (Fig. 1c). For samples treated with micelles from 10 to 24 min after the compression injury, the CAP exhibited a continuous increase during the 40 min post-injury period (Fig. 1d). The difference of CAP recovery between the control and micelle-treated white matter was significant (p<0.001, Student’s t-test).
Figure 1.
Immediate CAP restoration in compression injured spinal cords after treatment with mPEG-PDLLA diblock copolymer micelles. Electrophysiological records show typical responses to acute injury without (a) and with (b) micelle treatment. CAPs before compression injury (left), acute reduction of CAP by compression (middle) and self-recovery after injury (right) are shown. ST: stimulus. c–e, Three representative experiments demonstrate CAP restoration in control (c), micelle-treated (d, 0.67 mg/mL), and PEG-treated (e, 250 mM and 0.33 mM) spinal cords after compression injury. Arrows indicate the time of crushing, addition of micelles (MI), Krebs’ washing. c, Without micelles, CAP self-recovered to 14.4% at 20 min post-compression and remained at the same level in the next 30 min. d, Adding 500 µL of 2.0 mg/mL micelles to the recording chamber increased CAP to 62.3% of the pre-compression amplitude within 30 min. e, Addition of 0.33 mM PEG at 10 min post-compression increased (up to 11.8%) CAP to the level of self-recovery. A 2 min administration of 50 wt% (250 mM) PEG(2000) significantly restored CAP.
In the above experiment, the weight concentration of the mPEG-PDLLA block copolymer was 2.0 mg/mL, corresponding to 0.33 mM copolymer based on the average molecular weight of 6,101 g/mol (2,000 from PEG and 4,101 from PDLLA). In comparison, the 50 wt% PEG (2,000 molecular weight) used in previous studies has a molar concentration of 250 mM 8, 10. To evaluate the effectiveness of PEG in membrane repair, we performed the same CAP restoration experiment using a solution of PEG (2000) at 250 mM and 0.33 mM. The 0.33 mM PEG solution did not result in additional increase of CAP (Fig. 1e) in comparison with the control group (Fig. 1c). With 0.33 mM PEG, the average recovery (n=4) was 18.2 ± 7.0%, which was at the same level of self-recovery (p>0.05, Student’s t-test). Meanwhile, the 50% PEG produced the same CAP restoration as the micelle did (Fig. 1e). These results suggest that the self-assembled mPEG-PDLLA micelle could restore CAP in spinal cord tissues more efficiently and at a concentration much lower than free PEG.
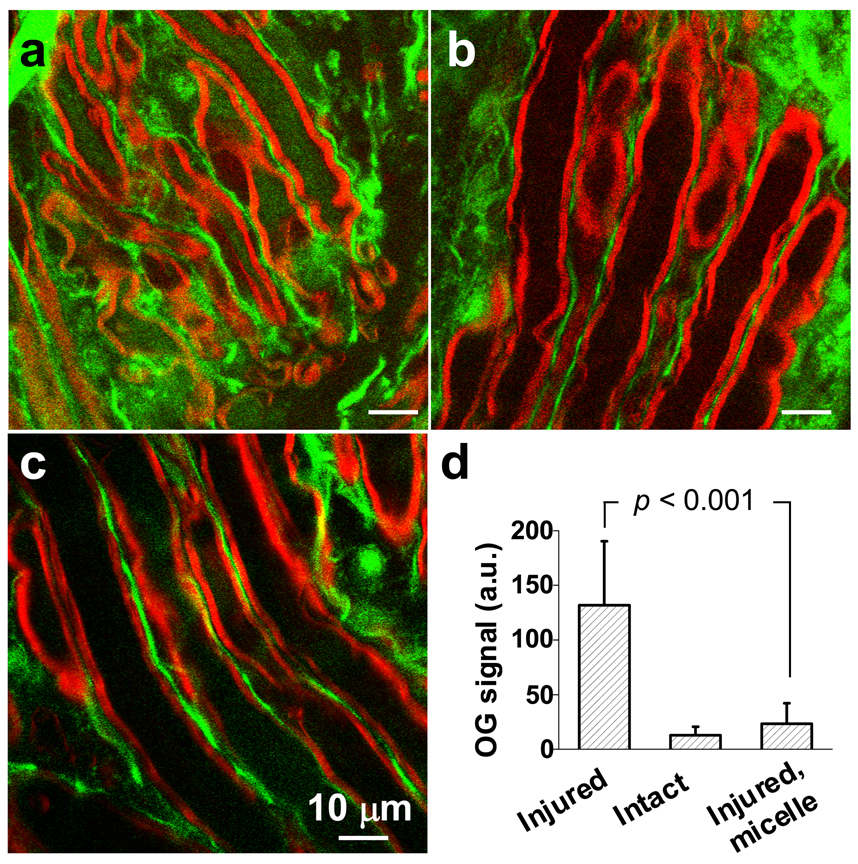
Because high-concentration PEG is known to protect the intracellular environment by sealing cell membrane breaches 21, we tested whether the mPEG-PDLLA micelles could reduce Ca2+ influx into axons in injured white matter. The Ca2+ concentration was evaluated by two photon excited fluorescence (TPEF) imaging of a calcium indicator Oregon Green 488 (OG). A coherent anti-Stokes Raman scattering (CARS) microscope (Fig. S3) that permits label-free vibrational imaging of intact myelin sheath 22 was used to define the intra- and extra-axonal space in healthy, compression-injured, and injured/micelle-treated white matter. Strong TPEF signals from OG were observed in extra-axonal space in all cases, but the intra-axonal OG intensities were different, as shown in Fig. 2. Without any treatment, the OG intensity inside injured axons (shown in green) at 1 h after compression injury was 10 times higher (131.89 ± 58.47 a.u., measured from 57 axons) than that in healthy axons (13.02 ± 7.55 a.u., measured from 64 axons). In contrast, by incubating the white matter with 0.67 mg/mL micelles immediately after compression injury, the intra-axonal OG intensity (23.42 ± 18.78 a.u., measured from 55 axons) was only 2 times higher than that in healthy axons (p<0.001, Student’s t-test). These results demonstrate that calcium influx could be effectively diminished by micelle-mediated repair of axonal membrane.
Figure 2.
Calcium influx into axons. TPEF images of Oregon Green 488 (green) and CARS images of myelin (red) show intra-axonal free Ca2+ level in (a) compression injured, (b) healthy, and (c) compression-injured and micelle-treated spinal cords. Images were acquired at 1 h after the compression injury. Statistical analysis is shown in (d). Without micelle treatment, the TPEF intensity from Oregon Green 488 inside the injured axons was 10 times greater than healthy axons. The intensity was only twice that of healthy axons when 0.67 mg/mL micelles were added immediately after compression injury.
To elucidate the distinctive roles of the PEG shell and the hydrophobic core in micelle-mediated membrane repair, we prepared micelles with different polyester cores and varied PEG chain lengths. Administration of mPEG-poly(ε-caprolactone) (PCL) micelles (20.9 ± 11.7%, n=3) or mPEG-poly(L-lactic acid) (PLLA) micelles (28.8 ± 3.8%, n=4) did not significantly increase the CAP compared with the control of Krebs’ solution only (18.5 ± 5.1%, n=5) (Fig. 3a). On the other hand, administration of mPEG-PDLLA micelles with two different PEG molecular weights, 2,000 and 5,000, produced the same level of efficacy, 66.5 ± 14.8% (n=5) and 66.2 ± 21.2% (n=3), respectively. These results indicate a critical role of the hydrophobic chains in membrane repair. The mPEG2,000-PDLLA4,000 was selected for the rest of our study.
Figure 3.
Efficiency of CAP restoration depends on micelle structure and concentration. a, Application of 0.67 mg/mL mPEG5,000-PCL2,000 did not result in significant increase in CAP amplitude compared to the Krebs control (p>0.05, Student’s t-test). Application of mPEG2,000-PLLA4,000 micelles only slightly restored the CAP compared to the control. Application of mPEG2,000-PDLLA4,000 and mPEG5,000-PDLLA5,000 resulted in the same level of CAP restoration (p>0.05, Student’s t-test). b, Application of 0.0067, 0.067, and 0.67 mg/mL mPEG-PDLLA micelles resulted in significant CAP restoration compared with the control group with no micelle supplement. *, p<0.001, ANOVA test.
Considering the challenge that membrane repair agents are significantly diluted during in vivo administration, we examined the CAP restoration efficiency at lower micelle concentrations. Krebs’ solutions containing 0, 0.0067, 0.067, and 0.67 mg/mL micelles were applied to separate white matter strips which were compressed using the same procedure. As shown in Fig. 3b, 0.67 mg/mL micelle resulted in a CAP increase of 66.5 ± 14.8%. This value was more than three times of the CAP increase (18.5 ± 5.1%) in the control group (no micelles). Micelles at 0.067 mg/mL and 0.0067 mg/mL caused a CAP increase of 55.1 ± 8.2% (n=5) and 44.8 ± 7.1% (n=5), respectively. These results indicate that higher concentration of micelles resulted in a larger CAP increase, while the restoration remained efficient when the concentration was lowered by 100 times from 0.67 to 0.0067 mg/mL. It is worth noting that 0.0067 mg/mL corresponds to 1.1 µM PEG in the solution, which is about 105 times dilution of the PEG concentration (250 mM or 50 wt%) previously used for treatment of injured white matter 8. The effective restoration of CAP by diluted micelles shows the great potential of micelles for in vivo repair of SCI.
Behavioral recovery and tissue repair in SCI rats
To determine whether micelles could improve functional outcomes in live animals, we performed a behavioral study using Long-Evans rats with compression-injured spinal cords and evaluated the hindlimb functional recovery using the Basso Beattie Bresnahan (BBB) locomotor rating scale. Within 10 min post injury, we injected through tail vein either 1.0 mL saline containing 1.8 mg/mL micelles, 1.0 mL saline containing 30 wt% PEG or 1.0 mL pure saline as control. The presence of the micelles at the injury site was demonstrated by confocal fluorescence signals of fluorescein isothiocyanate (FITC)-conjugated mPEG-PDLLA micelles (Fig. S4). The first BBB test was carried out at 6 h post injury when all the rats were able to ambulate with forelimbs. The BBB scores are shown in Fig. 4. At 6 h (day 0), day 1 and day 7 after treatment, we did not observe a significant difference in BBB scores between the micelle group and the PEG group. However, subsequent improvement in the locomotor function in the micelle treated group was evident by a more rapid increase of BBB scores in the first 14 days and continuation of improvement over the following two weeks. Specifically, at 28 days post-injury, the BBB scores were 12.5 ± 3.1, 7.0 ± 4.3 and 7.1 ± 2.8 for the micelle-treated, PEG-treated, and saline-treated control animals, respectively, with the micelle-treated group having a significantly higher score than the other two groups (p<0.01) and no difference between the PEG-treated and the control group (p>0.05). Our result is consistent with an earlier comparison between 30% PEG and saline control 16, neither of which showed axonal transduction through the lesion site. From a clinical perspective, an animal with a BBB score equal to or less than 11 lacks hindlimb and forelimb coordination, whereas a score of 12 to 13 corresponds to occasional to frequent forelimb and hindlimb coordination. Reaching a BBB score of 12 is significant in that it is a sign of axonal transduction through the lesion site 23. In summary, the hindlimb function measurements demonstrate that mPEG-PDLLA micelles are significantly more effective than high-concentration PEG in functional recovery of SCI.
Figure 4.
Recovery of locomotor function in rats after compression injury. After spinal cord injury, Long-Evans rats received saline, 1.0 mL of mPEG-PDLLA micelles (1.8 mg/mL) or 30% PEG by tail vein injection. Intravenous administration of mPEG-PDLLA micelles resulted in an average BBB score of 12.5 ± 3.1 (n=12) at day 28, which was significantly higher than 7.1 ± 2.8 by saline control (n=10) and 7.0 ± 4.3 by 30% PEG (n=9) (ANOVA test, * p<0.05, ** p<0.01, *** p<0.001).
The effective recovery of locomotor functions by the copolymer micelles is partly owing to the efficient systemic delivery of PEGylated nanostructures. It was shown that the PEG chains on the nanoparticle surface create a highly water bound barrier which blocks the adhesion of opsonins24. Moreover, the nanoscale particle size was known to prevent the glomerular filtration which further extends the retention of the micelles in blood 24. These two factors allowed prolonged blood residence of mPEG-PDLLA micelles (t1/2 = 2.66 h 18). In contrast, it has been shown that unimers only possess blood half time around 10 min 17. We note that the micelles can be gradually destabilized by the lipophilic agents in vivo as shown recently25, 26. However, it is possible that a fraction of polymer micelles remain intact and circulate for hours, resulting in an effective delivery of the agents to the injured site.
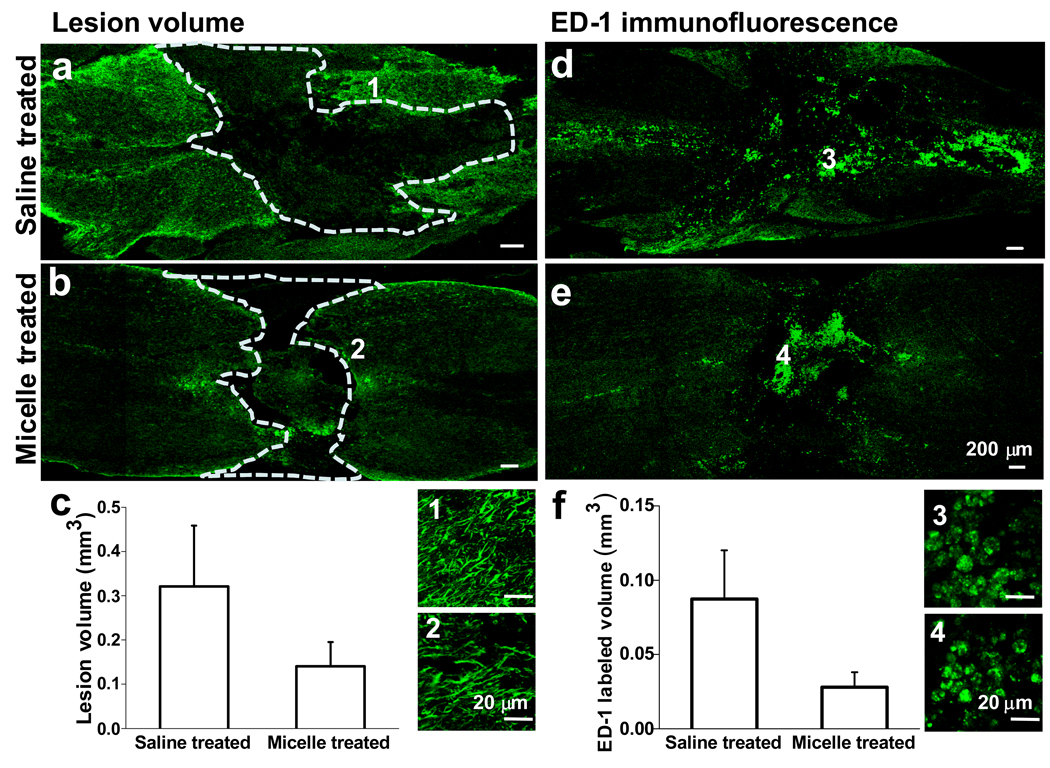
We further examined whether intravital micelle treatment would result in tissue protection. By immunofluorescence imaging of sliced spinal tissues, we measured the total lesion volume and immunoreactivity in micelle- and saline-treated rats sacrificed at week 4 after compression injury. As shown in Fig. 5a and b, the compression injury created a cavity (outlined by dashed lines), an area depleted of both neurons and astrocytes, at the spinal cord tissue. The lesion volume was calculated as the volume of the cavity. It was found that micelle treatment resulted in a significantly decreased lesion volume, 0.14 ± 0.03 mm3, as compared to that by saline treatment, 0.32 ± 0.08 mm3, at 4 weeks post injury (Fig. 5c). Using tissues from the same animals, we also mapped macrophages and/or activated microglia by ED-1 immunofluorescence. Representative distributions of ED-1+ cells in the injured spinal cord following treatment with saline and micelles are shown in Fig. 5d and e, respectively. The ED-1+ cells in the micelle-treated group were more confined than those in the control group. The volume that ED-1+ cells spread was found to be significantly reduced in the micelle treated group (0.03 ± 0.01 mm3) compared with the control group (0.09 ± 0.02 mm3) (Fig. 5f). The significant reduction in both lesion volume and inflammatory response at week 4 after injury could be considered as a consequence of membrane repair and inhibition of Ca2+ influx by the micelles administered in the acute phase. To determine whether micelles could repair the cell membrane in vivo, we injected a cell-impermeable dye into the cerebral spinal fluid prior to SCI, and quantified the number of cell bodies taking up the dye using confocal fluorescence microscopy. We found that micelle treatment significantly reduced the number of damaged cells in both grey matter and white matter (Fig. S5).
Figure 5.
Lesion volume and immunoreactivity analysis of rat tissue. a–b, Immunostaining for GFAP highlights the lesion area 28 days post compression injury in a saline treated (a) and micelle treated (b) spinal cord, respectively. The images represent longitudinal sections at 350 µm from the dorsal surface. Images of high magnification highlight the branched astrocytes at the border of the scar. c, Quantitative comparison of the lesion volume between the micelle-treated and the saline-treated group shows a smaller lesion volume in the micelle-treated spinal tissue. d–e, ED-1 immunofluorescence shows the distribution of macrophages/reactive microglia in the saline- (d) and micelle-treated (e) spinal cords. The representative images of the longitudinal sections at 500 µm from the dorsal surface show a more confined area of immunoreactivity in the micelle-treated spinal tissue. Images of high magnification highlight individual ED-1+ cells inside the lesion area. f, Quantitative comparison of the ED-1+ volume between the micelle-treated and the saline-treated group.
Toxicity analysis of micelles in rats
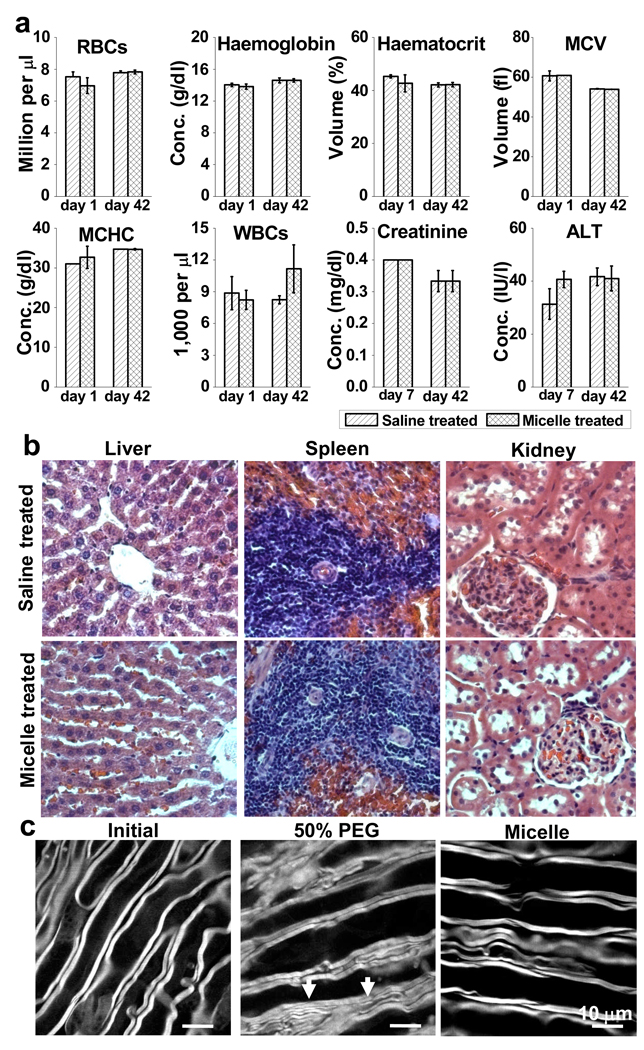
We examined both acute and chronic toxicity of the micelles administered to Long-Evans male rats. The animals were randomized into a micelle-treated group (n=3) or a saline-treated group (n=3). Each animal received 1 mL saline containing either 1.8 mg/mL mPEG-PDLLA micelles or 1 mL saline through tail vein injection. After the treatment, blood samples were drawn through the jugular vein at day 1 and day 7 for acute toxicity analysis, and at day 42 for chronic toxicity analysis. The results are shown in Fig. 6a. There was no significant difference between the micelle group and the saline group in terms of red blood cell (RBC) counts, haemoglobin, haematocrit, mean corpuscular volume (MCV), or mean corpuscular haemoglobin concentration (MCHC). The total number of white blood cells (WBCs) in the micelle group at day 42 was within the normal range, as well as the saline group. In particular, the levels of creatinine and alanine transaminase (ALT) in serum were not increased, indicating no damage to either the kidneys or the livers. Morphologically, linear hepatic cords were observed in both the micelle and saline groups (Fig. 6b). No sign of splenic changes such as hematopoietic cell proliferation or hemosiderin deposition occurred in either group. Moreover, the kidney tubules were regularly oriented in good shape and glomerulus was not attached to the surroundings in both micelle and saline treated animals. To summarize, the above study observed no adverse effect to healthy animals after systemic administration of mPEG-PDLLA micelles.
Figure 6.
Toxicity analysis. a, Following injection of 1 mL mPEG-PDLLA micelle or saline solution, neither complete blood count of Long-Evans rats at day 1 and day 42 or serum analysis at day 7 and day 42 shows adverse effects between the two groups. b, Histological analysis of explanted liver, spleen and kidney with haematoxylin and eosin staining between the control group and the micelle treated group indicates no signs of cellular or tissue damage. Magnification: 400×. c, Morphological analysis. After incubating a healthy spinal cord strip (left) in 50% PEG in Krebs’ solution for 17 min, the axons became attached to each other (middle). The white arrows indicate possible fusion of adjacent axonal myelin. In contrast, after incubation with 0.67 mg/mL mPEG-PDLLA micelles for 180 min (right), a spinal cord strip displays no obvious morphological changes of myelin (right).
Using CARS microscopy, we further examined the morphological changes of myelinated axons invoked by PEG and micelle treatments (Fig. 6c). After incubating a non-compressed white matter strip with 50% PEG for 17 min, the adjacent parallel axons appeared to become attached to each other, implying the occurrence of axonal fusion. In contrast, the axons maintained normal morphology after incubation with 0.67 mg/mL micelles for 3 h. We have also evaluated functional toxicity (Fig. S6) by circulating Krebs’ solution containing 0.67 mg/mL micelles through the white matter strip for 45 min, and found the CAP remained at the same level. In contrast, by incubating the white matter with 50% PEG, the CAP exhibited a decrease to 40% of the initial value in 45 min. These observations indicate that micelles can effectively restore the CAP in an injured white matter with minimal toxicity.
Conclusions
The current study demonstrated that polymeric micelles could effectively repair a traumatically injured spinal cord. Administration of mPEG-PDLLA micelles immediately after traumatic injury leads to significant electrophysiological recovery in spinal tissue and functional recovery in live animals with unnoticeable toxicity. Because of the small number of animals used and the fixed dosage of mPEG-PDLLA micelles, our work should be considered a pilot study. Further studies using a larger pool of animals are to be carried out to determine how dosage, administration frequency, and timing of micelle treatment affect the clinical outcome. Moreover, follow-up studies will aim to elucidate how the micelles interact with a damaged membrane. In summary, our work provides an exciting example of how unique properties of nanomaterials can be utilized for treatments of traumatic injuries.
Methods
Synthesis and characterization of mPEG-PDLLA micelles
The mPEG(2000)-PDLLA block copolymer (Fig. S1) was synthesized by ring opening polymerization of D,L-lactide 34. The mPEG-PDLLA micelles with a weight concentration of 2.0 mg/mL were prepared by dialysis. Details can be found in Supplemental Information.
Spinal sample preparation and CAP recording
The procedure for isolation of the spinal cord white matter was described previously 8 and can be found in Supplementary Information. CAPs were recorded using a double sucrose gap recording chamber shown in Fig. S2.
Compression injury and local micelle treatment
The compression injury was inflicted by a constant displacement of 5–30 sec compression of the spinal cord using modified forceps possessing a spacer until the CAP dropped to 0 mV 14. For local application of micelles, immediately after injury, the spinal cord white matter strips were kept in perfusing Krebs’ solution at the speed of 2.0 mL/min controlled by a peristaltic pump for 10 min. Then the perfusion was stopped and 500 µL of 2.0 mg/mL micelle solution was added gently to the 1.0 mL Krebs’ solution in the central compartment, leading to a final concentration of 0.67 mg/mL. Following the micelle treatment, the spinal cord strips were thoroughly rinsed with Krebs’ solution. All the solutions were bubbled with 95% O2/5% CO2 throughout the experiment.
CARS and TPEF imaging of spinal tissues
CARS and TPEF imaging was carried out on a multimodal nonlinear optical microscope shown in Fig. S3. The CARS signal was generated by two synchronized 2.5-ps lasers. The TPEF signal was generated by a mode-locked 200-fs Ti:Sapphire oscillator (Mira 900, Coherent Inc., Santa Clara, CA, USA). Details can be found in Supplementary Information.
In vivo spinal cord injury and micelle administration
All protocols for this experiment were approved by the Purdue Animal Care and Use Committee. Adult male Long-Evans rats (Hilltop Lab Animals, Inc., Scottdale, Pennsylvania, USA) weighing 300–350 g were used. The tenth thoracic (T10) spine was removed by laminectomy and the T10 spinal cord segment was constantly compressed for 15 sec by the modified forceps under aseptic condition as described previously 9. Prior to the compression injury, all rats were anesthetized deeply with the mixture of 90 mg/kg ketamine and 5 mg/kg xylazine. After the compression injury, the laminectomy was closed by suturing the muscle with 3-0 prolene followed by the use of 7.5 mm Michel wound clips (Fine Science Tools, Foster City, CA, USA) to close the skin incision.
Rats were randomized into three groups: 1 mL micelle (1.8 mg/mL in saline; n=14), 1 mL PEG (30% in saline n=12), and isovolumetric doses of saline (n=12). Treatments were given within 10 min post injury by intravenous tail vein injection. Animals were caged individually 1 week before the surgery and then 4 weeks post surgery. For post-operation pain management, the analgesics buprenorphine (0.05–0.10 mg/kg) were given every 12 h through subcutaneous injection during anesthesia recovery and for the first 3 days post surgery. One rat in the micelle treated group and one rat in the PEG group were excluded from the study due to the abnormally high BBB score on day 0. Two rats in the PEG group, one rat in the micelle group and two rats in the control group suffered from kidney fail during the first week post surgery thus were sacrificed.
Behavioral testing
The locomotor recovery of the animals was determined by using the 21-point BBB open-field locomotor scale 23. The test was conducted by two observers, with one observer being blind to the treatments. Two observers scored independently and made an agreement on the score before the scores were finalized. Locomotor scoring was conducted on day 0 at 6 h post-injury, day 1 and subsequently once per week for 4 weeks.
Histological assessment
Three micelle-treated and three saline-treated rats were used for histology. Samples were immuno-processed with glial fibrillary acidic protein (GFAP) to detect reactive astrocytes and ED-1 to detect activated microglial cells and macrophages using standard procedures 37, 38. Details can be found in Supplementary Information.
Toxicity assessment
Long-Evans adult male rats were randomized into micelle treated group (n=3) or saline treated group (n=3). Each animal received 1 mL micelle (1.8 mg/mL suspended in saline) or saline solution through tail vein injection. Blood samples (1 mL) were drawn through the jugular vein at day 1, day 7 and day 42 post treatment. Hematology study and serum analysis were performed by the Purdue University Veterinary Clinical Pathology Lab in a blind manner. The rats were then sacrificed and tissues including liver, kidney, and spleen were fixed in 10% neutral buffered formalin for at least 48 h, embedded into paraffin. Sections of 5 µm thickness were stained with haematoxylin and eosin in Purdue University Histopathology Lab. The slides were then examined with a Nikon microscope equipped with a CCD camera.
Supplementary Material
Acknowledgments
The authors cordially acknowledge Woo Sun Shim for preparation of mPEG-PDLLA di-block copolymers, Gary Leung for isolation of spinal cord white matter strips from guinea pigs, Jianming Li for fabrication of the CAP recoding chamber, Kevin Cheng and Hao Lou for help in immunostaining and image analysis, Yanshu Zhang for histological examination and intracerebroventricular injection of dextran-FITC, Xiao-Ming Xu and Xiaofei Wang for instructions of immunostaining, Debbie Bohnert for training of survival surgery, Carol Dowell, Melissa Bible, Gena Brock and Amy Peterson for help in blood draw. The work was supported by a Showalter Trust grant from Purdue University and an Indiana Spinal Cord and Brain Injury Research Fund from State of Indiana.
Footnotes
Author contributions
J.X.C. conceived and designed the experiments. Y.S., S.K. and T.B.H. performed the experiments. Y.S. analyzed the data. K.P. contributed copolymer micelles. R.S. contributed spinal cord white matter tissues and the CAP recording chamber. R.B.B. contributed training of survival surgery. Y.S. and J.X.C. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Additional Information
Supplementary information accompanies this paper at www.nature.com/naturenanotechnology. Reprints and permission information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury EJ, McMahon SB. Opinion - Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- 3.Young W. Role of calcium in central-nervous-system injuries. J Neurotrauma. 1992;9:S9–S25. [PubMed] [Google Scholar]
- 4.Klussmann S, Martin-Villalba A. Molecular targets in spinal cord injury. J Mol Med. 2005;83:657–671. doi: 10.1007/s00109-005-0663-3. [DOI] [PubMed] [Google Scholar]
- 5.Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006;23:318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- 6.Krause TL, Bittner GD. Rapid morphological fusion of severed myelinated axons by polyethylene-glycol. Proc Natl Acad Sci U S A. 1990;87:1471–1475. doi: 10.1073/pnas.87.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RC, River LP, Pan FS, Ji L, Wollmann RL. Surfactant-induced sealing of electropermeabilized skeletal-muscle membranes in vivo. Proc Natl Acad Sci U S A. 1992;89:4524–4528. doi: 10.1073/pnas.89.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi R, Borgens RB, Blight AR. Functional reconnection of severed mammalian spinal cord axons with polyethylene glycol. J Neurotrauma. 1999;16:727–738. doi: 10.1089/neu.1999.16.727. [DOI] [PubMed] [Google Scholar]
- 9.Borgens RB, Shi R. Immediate recovery from spinal cord injury through molecular repair of nerve membranes with polyethylene glycol. FASEB J. 2000;14:27–35. doi: 10.1096/fasebj.14.1.27. [DOI] [PubMed] [Google Scholar]
- 10.Borgens RB, Bohnert D. Rapid recovery from spinal cord injury after subcutaneously administered polyethylene glycol. J Neurosci Res. 2001;66:1179–1186. doi: 10.1002/jnr.1254. [DOI] [PubMed] [Google Scholar]
- 11.Laverty PH, et al. A preliminary study of intravenous surfactants in paraplegic dogs: Polymer therapy in canine clinical SCI. J Neurotrauma. 2004;21:1767–1777. doi: 10.1089/neu.2004.21.1767. [DOI] [PubMed] [Google Scholar]
- 12.Borgens RB, Bohnert D, Duerstock B, Spomar D, Lee RC. Subcutaneous tri-block copolymer produces recovery from spinal cord injury. J Neurosci Res. 2004;76:141–154. doi: 10.1002/jnr.20053. [DOI] [PubMed] [Google Scholar]
- 13.Cho Y, Shi R, Borgens R, Ivanisevic A. Repairing the damaged spinal cord and brain with nanomedicine. Small. 2008;4:1676–1681. doi: 10.1002/smll.200800838. [DOI] [PubMed] [Google Scholar]
- 14.Luo J, Borgens R, Shi R. Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. J Neurochem. 2002;83:471–480. doi: 10.1046/j.1471-4159.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- 15.Luo J, Borgens R, Shi R. Polyethylene glycol improves function and reduces oxidative stress in synaptosomal preparations following spinal cord injury. J Neurotrauma. 2004;21:994–1007. doi: 10.1089/0897715041651097. [DOI] [PubMed] [Google Scholar]
- 16.Ditor DS, et al. Effects of polyethylene glycol and magnesium sulfate administration on clinically relevant neurological outcomes after spinal cord injury in the rat. J Neurosci Res. 2007;85:1458–1467. doi: 10.1002/jnr.21283. [DOI] [PubMed] [Google Scholar]
- 17.Liu JB, Zeng FQ, Allen C. In vivo fate of unimers and micelles of a poly(ethylene glycol)-block-poly(caprolactone) copolymer in mice following intravenous administration. Eur J Pharm Biopharm. 2007;65:309–319. doi: 10.1016/j.ejpb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Burt HM, Zhang X, Toleikis P, Embree L, Hunter WL. Development of copolymers of poly(D,L-lactide) and methoxypolyethylene glycol as micellar carriers of paclitaxel. Colloids Surf B Biointerfaces. 1999;16:161–171. [Google Scholar]
- 19.Gref R, et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 20.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 21.Lentz BR. Polymer-induced membrane-fusion - Potential mechanism and relation to cell-fusion events. Chem Phys Lipids. 1994;73:91–106. doi: 10.1016/0009-3084(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang HF, Fu Y, Zickmund P, Shi R, Cheng JX. Coherent anti-stokes Raman scattering imaging of axonal myelin in live spinal tissues. Biophys J. 2005;89:581–591. doi: 10.1529/biophysj.105.061911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating-scale for open-field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 24.Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm. 2007;65:259–269. doi: 10.1016/j.ejpb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, et al. Fast release of lipophilic agents from circulating PEG-PDLLA micelles revealed by in vivo Forster resonance energy transfer imaging. Langmuir. 2008;24:5213–5217. doi: 10.1021/la703570m. [DOI] [PubMed] [Google Scholar]
- 26.Savic R, Azzam T, Eisenberg A, Maysinger D. Assessment of the integrity of poly(caprolactone)-b-poly(ethylene oxide) micelles under biological conditions: A fluorogenic-based approach. Langmuir. 2006;22:3570–3578. doi: 10.1021/la0531998. [DOI] [PubMed] [Google Scholar]
- 27.Cadichon SB, et al. Neuroprotective effect of the surfactant poloxamer 188 in a model of intracranial hemorrhage in rats. J Neurosurg. 2007;106:36–40. doi: 10.3171/ped.2007.106.1.36. [DOI] [PubMed] [Google Scholar]
- 28.Lee RC, Hannig J, Matthews KL. Pharmaceutical therapies for sealing of permeabilized cell membranes in electrical injuries. Ann N Y Acad Sci. 1999;888:266–273. doi: 10.1111/j.1749-6632.1999.tb07961.x. [DOI] [PubMed] [Google Scholar]
- 29.Lentz BR. PEG as a tool to gain insight into membrane fusion. Eur Biophys J. 2007;36:315–326. doi: 10.1007/s00249-006-0097-z. [DOI] [PubMed] [Google Scholar]
- 30.Chen HT, et al. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc Natl Acad Sci U S A. 2008;105:6596–6601. doi: 10.1073/pnas.0707046105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maskarinec SA, Hannig J, Lee RC, Lee KYC. Direct observation of poloxamer 188 insertion into lipid monolayers. Biophys J. 2002;82:1453–1459. doi: 10.1016/S0006-3495(02)75499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batrakova EV, et al. Mechanism of pluronic effect on P-Glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299:483–493. [PubMed] [Google Scholar]
- 33.Attwood D, Booth C, Yeates SG, Chaibundit C, Ricardo N. Block copolymers for drug solubilisation: Relative hydrophobicities of polyether and polyester micelle-core-forming blocks. Int J Pharm. 2007;345:35–41. doi: 10.1016/j.ijpharm.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 34.Liggins RT, Burt HM. Polyether-polyester diblock copolymers for the preparation of paclitaxel loaded polymeric micelle formulations. Adv Drug Deliv Rev. 2002;54:191–202. doi: 10.1016/s0169-409x(02)00016-9. [DOI] [PubMed] [Google Scholar]
- 35.Shim WS, et al. Novel pH sensitive block copolymer micelles for solvent free drug loading. Macromol Biosci. 2006;6:179–186. doi: 10.1002/mabi.200500182. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto Y, Yasugi K, Harada A, Nagasaki Y, Kataoka K. Temperature-related change in the properties relevant to drug delivery of poly(ethylene glycol)-poly(D,L-lactide) block copolymer micelles in aqueous milieu. J Control Release. 2002;82:359–371. doi: 10.1016/s0168-3659(02)00147-5. [DOI] [PubMed] [Google Scholar]
- 37.Hausmann ON, Fouad K, Wallimann T, Schwab ME. Protective effects of oral creatine supplementation on spinal cord injury in rats. Spinal Cord. 2002;40:449–456. doi: 10.1038/sj.sc.3101330. [DOI] [PubMed] [Google Scholar]
- 38.Chvatal SA, Kim YT, Bratt-Leal AM, Lee HJ, Bellamkonda RV. Spatial distribution and acute anti-inflammatory effects of Methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials. 2008;29:1967–1975. doi: 10.1016/j.biomaterials.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.