Abstract
OBJECTIVES
Our aims were to estimate the efficacy of hospital phototherapy for neonatal jaundice and the number needed to treat to prevent one infant from reaching the exchange transfusion level.
METHODS
From a cohort of 281 898 infants weighing ≥2000 g born at ≥35 weeks’ gestation at 12 Northern California Kaiser hospitals from 1995 to 2004, we identified 22 547 who had a “qualifying total serum bilirubin level” within 3 mg/dL of the American Academy of Pediatrics 2004 guideline phototherapy threshold. We used multiple logistic regression to estimate the efficacy of hospital phototherapy within 8 hours at preventing the bilirubin level from exceeding the 2004 guideline’s exchange transfusion threshold within 48 hours. We combined this efficacy estimate with other predictors of risk to estimate the numbers needed to treat at different values of covariates.
RESULTS
Of the 22 547 eligible newborns, 5251 (23%) received hospital phototherapy within 8 hours of their qualifying bilirubin level. Only 354 (1.6%) ever exceeded the guideline exchange transfusion threshold; 187 (0.8%) did so within 48 hours. Among infants who did not have a positive direct antiglobulin test, hospital phototherapy within 8 hours was highly effective (adjusted odds ratio, 0.16; 95% confidence interval, 0.07–0.34). For infants with bilirubin levels 0–0.9 mg/dL above the phototherapy threshold, the estimated number needed to treat at mean values of covariates was 222 (95% CI: 107–502) for boys and 339 (95% CI: 154–729) for girls, ranging from 10 (95% CI: 6–19) for <24-hour-old, 36-week gestation boys to 3,041 (95% CI: 888–11 096) for ≥3-day-old 41-week girls. Hospital phototherapy was less effective for infants direct antiglobulin test-positive infants (adjusted odds ratio 0.55; 95% CI: 0.21–1.45; P = 0.01 for the direct antiglobulin test × phototherapy interaction).
CONCLUSIONS
While hospital phototherapy is effective, the number needed to treat according to current guidelines varies considerably across different infant subgroups.
Keywords: hyperbilirubinemia, jaundice, phototherapy, exchange transfusion, clinical guidelines, cohort studies, kernicterus, risk assessment
The authors of the 2004 American Academy of Pediatrics (AAP) clinical practice guideline for the management of hyperbilirubinemia in newborns conceded that there was little evidence on which to base their recommendations and that they relied on “uncertain estimates and extrapolations.”1 One gap in evidence is the number needed to treat (NNT) with phototherapy when it is used as currently recommended. The few previous randomized trials of phototherapy2–4 found that, among infants without hemolysis, 6 to 10 infants needed to be treated with phototherapy to prevent 1 from developing a total serum bilirubin (TSB) level ≥20 mg/dL.5 However, these trials included newborns with lower TSB levels than those at which the AAP recommends phototherapy and had as their end points lower TSB levels6 than those at which the AAP recommends exchange transfusion.
It is unknown how many infants need to be treated at bilirubin levels at which the AAP guideline recommends phototherapy to prevent 1 from reaching a level at which the AAP recommends exchange transfusion. A randomized trial to address this question is not feasible, because the outcome is rare, and there are ethical obstacles to randomly assigning newborns not to receive treatment for bilirubin levels at which phototherapy is believed to be needed. The objectives of this study were to estimate the efficacy of phototherapy in a modern setting, to determine the NNT to prevent 1 infant from reaching a TSB level at which the AAP recommends exchange transfusion, and to identify predictors of that NNT.
METHODS
Subjects, Setting, and Institutional Review Board Approval
The Northern California Kaiser Permanente Medical Care Program (NC-KPMCP) is a group-model managed care organization that covers ~3.3 million members, ~30% of the insured population in northern California. The cohort from which the subjects were identified included all infants born alive in 12 NC-KPMCP hospitals from January 1, 1995, to December 31, 2004, whose birth weight was ≥2000 g and whose gestational age was ≥35 weeks (N = 281 898). We obtained laboratory tests, diagnoses, procedures, and basic demographic data on the entire cohort from NC-KPMCP electronic databases, as described previously.7,8 This project was approved by the NC-KPMCP Institutional Review Board for the Protection of Human Subjects and by the University of California San Francisco Committee on Human Research.
AAP Guidelines, Risk Groups, and Qualifying TSB Levels
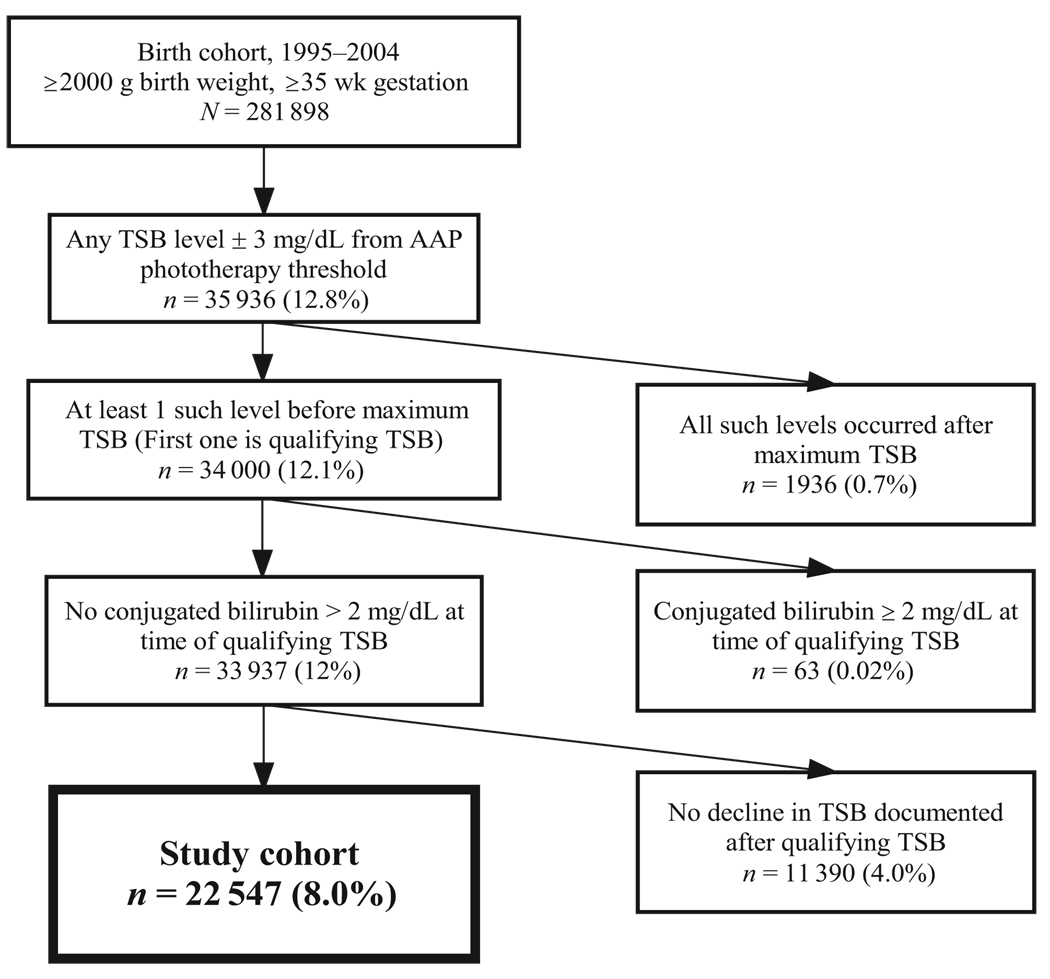
The AAP treatment guidelines are summarized in 2 figures, 1 for phototherapy and 1 for exchange transfusion. Each figure has TSB treatment threshold lines for infants in 3 different risk groups, defined by gestational age (<38 weeks or ≥ 38 weeks) and the presence of hemo-lysis or other signs of significant illness.1 By determining a newborn’s risk group and plotting the TSB level and age on the graph, clinicians can determine whether the AAP recommends phototherapy or consideration of exchange transfusion. We assigned infants to risk groups as follows: (1) low-risk: gestational age ≥38 weeks and no positive direct antiglobulin test (DAT); (2) medium-risk: either gestational age <38 weeks or a positive DAT result; and (3) high-risk: gestational age <38 weeks and a positive DAT. For each infant we then compared all of the TSB levels to the treatment guidelines. To obtain a large sample size of infants in whom we expected to find variability in phototherapy use, we decided a priori to include infants with a TSB level within 3 mg/dL of the AAP phototherapy threshold for their age and risk group. For each newborn, the first TSB level that met these criteria was considered the qualifying TSB. We excluded infants if their TSB was already declining, if a conjugated or direct bilirubin level at the time of the qualifying TSB was ≥2.0 mg/dL, or if they did not have a subsequent documented decline in their TSB.
FIGURE 1.
Creation of the study cohort.
Predictor Variables
We derived all of the predictor variables from electronic sources. These included the difference between the newborn’s qualifying TSB and the TSB level at which the AAP recommends phototherapy for infants of that age and risk group and the rate of rise of the TSB between the qualifying TSB level and the most recent previous TSB level ≥2 hours earlier (assumed to be 1.5 mg/dL at birth9 if there was no previous TSB). We considered home phototherapy to have been given within 1 day of the qualifying TSB level if a home phototherapy unit was delivered on the same day or the day after the qualifying TSB level.
Because timing of hospital phototherapy was not available electronically, we assumed that it began 1 hour after admission for readmissions with a procedure code for phototherapy. We assumed that all phototherapy during the birth hospitalization began within 8 hours of the qualifying TSB level, our a priori point for dichotomizing timely phototherapy. (We chose 8 hours as a reasonable limit for the time between obtaining an out-patient TSB level and getting an infant readmitted to the hospital and started on phototherapy.) We compared procedure codes for phototherapy with chart review in 307 subjects from a companion study.10 To capture readmissions for phototherapy that might be missing phototherapy codes, we created an alternative definition for hospital phototherapy that included all of the infants readmitted within 8 hours of the qualifying TSB level for any reason, whether or not that admission included a procedure code for phototherapy.
Outcome Variable
Our outcome variable was a TSB level that reached the AAP exchange transfusion threshold within 48 hours of the qualifying TSB. (We chose 48 hours, reasoning that if the TSB crossed the exchange line later, there would still have been time to start phototherapy the day after the qualifying TSB level.) For TSB levels that exceeded the exchange threshold >48 hours after the qualifying TSB level, we estimated that the time the threshold was crossed by assuming a linear increase in TSB levels between the last TSB level below the exchange threshold and the first TSB level above the threshold. We ascertained exchange transfusions using procedure code 99.01, verified by chart review.
Statistical Analysis
We used SAS (SAS Institute, Inc, Cary, NC) to create data sets from NC-KPMCP databases and Stata 9.2 for all of the analyses (Stata Corp, College Station, TX). We obtained bivariate and multivariate odds ratios (ORs) using logistic regression, with SEs adjusted for clustering by hospital.11 We used indicator variables for the age at qualifying TSB level (in 24-hour categories), gestational age (in weeks), and difference between the qualifying TSB level and the AAP phototherapy threshold (in 1-mg/dL categories). Because previous studies had suggested reduced efficacy of phototherapy in infants with a positive DAT,5 we included a term for interaction between the first DAT result (positive versus either negative or missing) and phototherapy in the model. Discrimination was assessed using the area under the model receiver operating characteristic curve, and goodness of fit was assessed according to Hosmer and Lemeshow.12
To estimate the NNT, we used the logistic model to estimate the probability of the outcome for infants who were and were not treated with hospital phototherapy within 8 hours of their qualifying TSB level. The estimated NNT is the reciprocal of the difference between these 2 probabilities. These calculations were done stipulating the following: (1) birth weight of 3.3 kg (the mean); (2) the DAT was not positive; and (3) the TSB level was 0.0 to 0.9 mg/dL above the phototherapy threshold. We estimated confidence intervals (CIs) for the NNT using a cluster-resampled (by facility), bias-corrected, and accelerated bootstrap in Stata.13
RESULTS
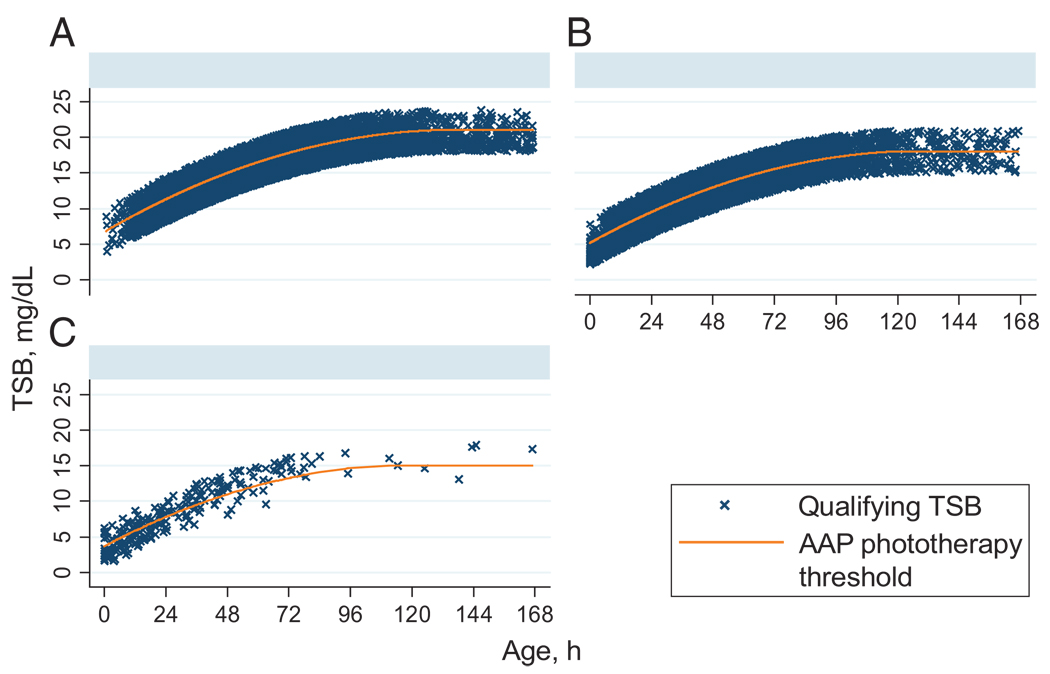
Figure 1 and Figure 2 show the selection of the study cohort and their qualifying TSB levels in relation to the AAP phototherapy threshold. The 22 547 included infants are compared with those of the birth cohort in Table 1. The mean birth weight and gestational age of the study infants were lower, whereas the maternal age, birth hospitalization length of stay, and proportion of boys were higher than in the other newborns. Asian infants were overrepresented and black infants underrepresented, and the distribution of AAP risk groups was shifted toward higher risk.
FIGURE 2.
Qualifying TSB levels of the study subjects compared with the AAP phototherapy threshold for the 3 risk groups of the AAP. A, low risk (N = 14591); B, medium risk (N = 7716); C, high risk (N = 240). Graphs by AAP Bilirubin Risk Group.
TABLE 1.
Characteristics of Infants in the Study Cohort Compared With All Other Infants
| Characteristics | Study Cohort (n = 22 547) |
All Others (n = 259 351) |
P |
|---|---|---|---|
| Birth weight, mean (SD), g | 3332.0 (557.0) | 3473.0 (499.0) | <.001a |
| Gestational age, mean (SD), wk | 38.4 (1.7) | 39.0 (1.3) | <.001a |
| Maternal age, mean (SD), y | 29.4 (6.0) | 28.8 (6.0) | <.001a |
| Length of stay, birth hospitalization, mean (SD), h | 70.7 (99.8) | 45.5 (59.8) | <.001b |
| Length of stay, birth hospitalization if no phototherapy, mean (SD), h |
55.3 (73.1) | 44.9 (58.8) | <.001b |
| Male, n (%) | 12 875 (57) | 131 123 (51) | <.001c |
| Gestational age, n (%), wk | <.001b | ||
| 35 | 1522 (7) | 2735 (1) | |
| 36 | 2158 (10) | 6462 (2) | |
| 37 | 2700 (12) | 14 743 (6) | |
| 38 | 3918 (17) | 36 788 (14) | |
| 39 | 5302 (24) | 71 113 (27) | |
| 40 | 4954 (22) | 84 882 (33) | |
| ≥41 | 1993 (9) | 42 628 (16) | |
| Race, n (%) | <.001c | ||
| White | 9145 (41) | 118 750 (46) | |
| Asian | 6008 (27) | 58 854 (23) | |
| Hispanic | 4972 (22) | 43 889 (17) | |
| Black | 1039 (5) | 22 473 (9) | |
| Other | 1002 (4) | 11 882 (5) | |
| Unknown | 381 (2) | 5697 (2) | |
| AAP risk group, n (%) | <.001c | ||
| Low risk:≤38 wk and not DAT positive | 14 591 (65) | 232 677 (90) | |
| Medium risk:<38 wk and not DAT positive | 6140 (27) | 23 609 (9) | |
| Medium risk:≥38 wk and DAT positive | 1576 (7) | 2734 (1) | |
| High risk:<38 wk and DAT positive | 240 (1) | 331 (0) | |
| DAT, n (%) | <.001c | ||
| Negative | 12 553 (56) | 50 801 (20) | |
| Positive | 1816 (8) | 3065 (1) | |
| Not done | 8178 (36) | 205 485 (79) |
P-value calculated by t test.
P-value calculated by rank-sum test.
P-value calculated by χ2 test.
Of the study infants, 6960 (30.9%) had any hospital admissions with procedure codes for phototherapy. Compared with chart review, the electronic data were 90.5% sensitive (200 of 221) and 100% (86 of 86) specific for hospital phototherapy. Broadening the definition of hospital phototherapy to include all of the readmissions within 7 days of the qualifying TSB level, whether or not there was a procedure code for phototherapy, did not affect efficacy estimates, so only those based on phototherapy codes are shown. We estimated that the phototherapy began within 8 hours of the qualifying TSB level in 5251 (75.4%) of the infants who ever received phototherapy. Approximately 4% of the cohort received home phototherapy.
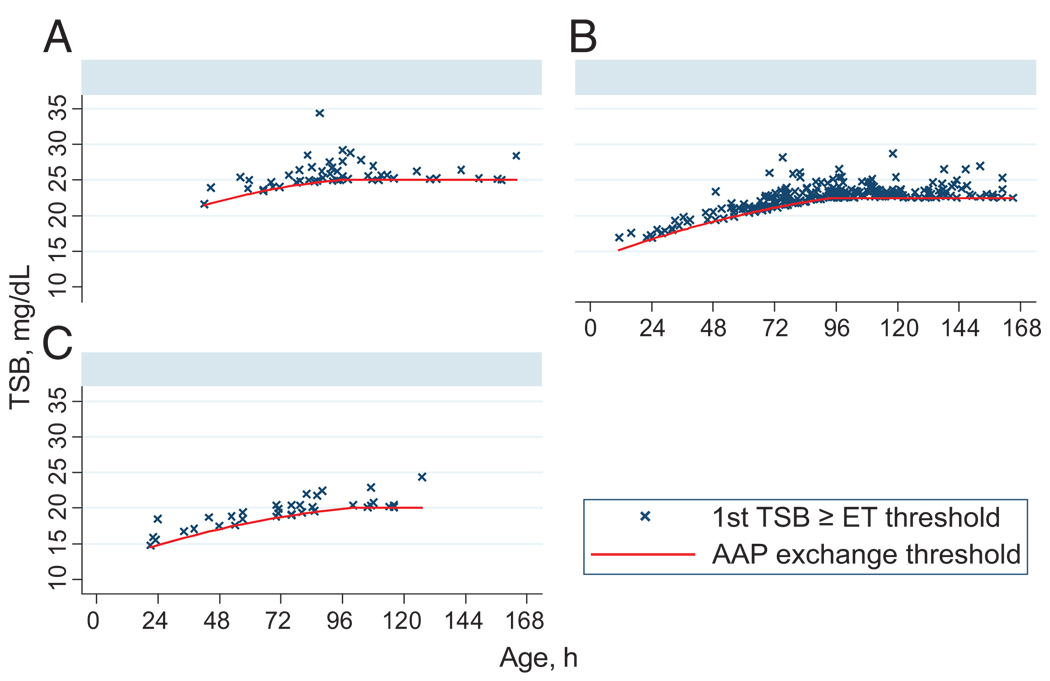
Only 354 infants (1.6%) ever exceeded the AAP exchange threshold; (Fig. 3) 187 (53%) did so within 48 hours of the qualifying TSB. Of these 354, 278 (79%) had maximum TSB levels <25 mg/dL, and 350 (99%) had maximum TSB levels <30 mg/dL. Just 3 infants received exchange transfusions. In bivariate analyses, the risk of exceeding the AAP exchange threshold within 48 hours varied markedly by gestational age, AAP risk group, age, and the difference between the qualifying TSB level and the AAP phototherapy threshold (Table 2).
FIGURE 3.
TSB levels (ever) reaching or exceeding the AAP exchange transfusion levels by risk group. ET, exchange transfusion. A, low risk (N = 54); B, medium risk (N = 247); C, high risk (N = 53). Graphs by AAP Bilirubin Risk Group.
TABLE 2.
Bivariate Predictors of TSB Reaching the Exchange Transfusion Level Within 48 Hours
| Characteristics | TSB Level Reached in <48 h at Which Exchange Transfusion Recommended |
|||
|---|---|---|---|---|
| No. (%) of Cohort | No. (%) With Outcome | OR | Pa | |
| Gender | ||||
| Female | 9672 (43) | 68 (0.70) | Ref | |
| Male | 12 875 (57) | 119 (0.92) | 1.3 | .06 |
| Gestational age, wk | ||||
| 35 | 1522 (7) | 16 (1.10) | 3.09 | .008 |
| 36 | 2158 (10) | 50 (2.20) | 6.88 | <.001 |
| 37 | 2700 (12) | 54 (2.00) | 5.92 | <.001 |
| 38 | 3918 (17) | 23 (0.59) | 1.71 | .15 |
| 39 | 5302 (24) | 21 (0.40) | 1.15 | .64 |
| 40 | 4954 (22) | 17 (0.34) | Ref | |
| ≥41 | 1993 (9) | 6 (0.30) | 0.88 | .76 |
| Race | ||||
| White | 9145 (41) | 72 (0.79) | Ref | |
| Asian | 6008 (27) | 47 (0.78) | 0.99 | .99 |
| Hispanic | 4972 (22) | 43 (0.88) | 1.13 | .53 |
| Black | 1039 (5) | 16 (1.54) | 1.97 | .008 |
| Other | 1002 (4) | 4 (0.40) | 0.51 | .16 |
| Unknown | 381 (2) | 4 (1.05) | 1.34 | .29 |
| AAP risk group | ||||
| Low | 14 591 (65) | 32 (0.22) | Ref | |
| Medium | 7716 (34) | 131 (1.70) | 7.86 | <.001 |
| High | 240 (1) | 24 (10.0) | 50.6 | <.001 |
| Age at qualifying TSB level, h | ||||
| <24 | 2196 (10) | 44 (2.00) | 1.63 | .10 |
| 24 to <48 | 6801 (30) | 84 (1.24) | Ref | |
| 48 to <72 | 6563 (29) | 36 (0.55) | 0.44 | .001 |
| 72 to <96 | 4665 (21) | 15 (0.32) | 0.26 | <.001 |
| ≥96 | 2322 (10) | 8 (0.34) | 0.28 | <.001 |
| TSB minus AAP phototherapy threshold, mg/dL | ||||
| −3 to less than − 2 | 5750 (26) | 3 (0.05) | 0.06 | <.001 |
| −2 to less than − 1 | 5343 (24) | 12 (0.22) | 0.27 | <.004 |
| −1 to <0 | 4347 (19) | 21 (0.48) | 0.59 | .05 |
| 0 to <1 | 3186 (14) | 26 (0.82) | Ref | |
| 1 to <2 | 2347 (10) | 55 (2.34) | 2.92 | <.001 |
| 2 to <3 | 1574 (7) | 70 (4.45) | 5.66 | <.001 |
| Hospital phototherapy | ||||
| Not within 8 h | 17 296 (77) | 146 (0.84) | Ref | |
| Within 8 h | 5251 (23) | 41 (0.78) | 0.80 | .66 |
| Home phototherapy | ||||
| Not within 1 d | 22 167 (98) | 186 (0.84) | Ref | |
| Within 1 d | 380 (2) | 1 (0.26) | 0.29 | .25 |
Ref indicates reference group for OR; TSB indicates total serum bilirubin adjusted for clustering by hospital.
All of the P values were calculated from χ2 test.
There was a significant interaction between hospital phototherapy and the DAT result, consistent with phototherapy being less effective in newborns with a positive DAT (P = .01). Controlling for confounders, the adjusted OR for hospital phototherapy among infants who were not DAT positive was 0.16, indicating about 84% efficacy (Table 3). The ORs for phototherapy and the phototherapy × DAT interaction were unchanged when the 8178 infants whose DAT was missing were excluded from the model. The discrimination and fit of the logistic model were excellent (area under the receiver operating characteristic curve: 0.907; Hosmer-Lemeshow χ2 [8 degrees of freedom]: 6.3; P = .6). The small number of infants receiving home phototherapy led to a wider CI around the estimate of efficacy (adjusted OR: 0.29 [95% CI: 0.03–3.15]).
TABLE 3.
Multivariate Predictors of TSB Level Reaching Exchange Transfusion Level Within 48 Hours
| Variable | OR (95% CI) | P |
|---|---|---|
| Male gender | 1.53 (1.12–2.11) | .008 |
| Gestational age, wk | ||
| 35 | 8.37 (3.38–20.69) | <.001 |
| 36 | 11.77 (6.95–19.94) | <.001 |
| 37 | 7.29 (4.57–11.62) | <.001 |
| 38 | 3.09 (1.64–5.83) | .001 |
| 39 | 1.44 (0.78–2.63) | .24 |
| 40 | Ref | |
| ≥41 | 0.72 (0.35–1.47) | .36 |
| Birth weight, per kg | 1.34 (0.97–1.83) | .07 |
| Age at qualifying TSB level, h | ||
| <24 | 1.93 (1.32–2.82) | <.001 |
| 24 to <48 | Ref | |
| 48 to <72 | 0.30 (0.17–0.51) | <.001 |
| 72 to <96 | 0.14 (0.08–0.25) | <.001 |
| ≥96 | 0.14 (0.07–0.28) | <.001 |
| TSB minus AAP phototherapy threshold, mg/dL |
||
| −3 to less than − 2 | 0.06 (0.02–0.14) | <.001 |
| −2 to less than − 1 | 0.25 (0.09–0.64) | .004 |
| −1 to <0 | 0.55 (0.30–1.00) | .05 |
| 0 to <1 | Ref | |
| 1 to <2 | 3.17 (1.97–5.09) | <.001 |
| 2 to <3 | 7.35 (5.08–10.63) | <.001 |
| DAT positive (if no phototherapy) | 2.16 (1.18–3.94) | .01 |
| Home phototherapy within 1 d | 0.29 (0.03–3.15) | .31 |
| Hospital phototherapy within 8 h If DAT negative or missing | 0.16 (0.07–0.34) | <.001 |
| Interaction DAT positive and phototherapy | 3.46 (1.38–8.69)a | .01 |
Ref indicates reference group for OR; TSB indicates total serum bilirubin.
The OR of 3.46 for the interaction term means the estimated OR for phototherapy when the DAT is positive is 3.46 times higher than when the DAT is negative, that is, 0.158 × 3.46 = 0.547 (95% CI: 0.21–1.45).
For infants who were not DAT positive, with qualifying TSB levels 0.0 to 0.9 mg/dL above the AAP phototherapy threshold, the mean gestational age was 384/7 weeks, the mean birth weight was 3.3 kg, and the mean age at the time of the qualifying TSB level was 63 hours. Using these values, the estimated NNT from the multivariate model was 222 for boys and 339 for girls.
The results from the multivariate model agree with those obtained by simple stratification, although the sample size becomes small when stratifying by several variables. For example, of the 3186 infants with qualifying TSB levels 0.0 to 0.9 mg/dL above the AAP guideline, 1374 were in the AAP low-risk group and ≥48 hours old at the time of their qualifying TSB levels. Only 3 crossed the exchange line within 48 hours, and all 3 were among the 1132 who had not received hospital phototherapy within 8 hours. Thus, in this group, phototherapy seemed 100% effective, but the NNT was still 377 (1132/3).
The estimated NNT varied widely with gestational age, gender, and age at the time of the qualifying TSB level, ranging from 10 (95% CI: 6–19) for a 36-week gestational age boy <24 hours old to 3041 (95% CI: 888–11,096) for a 41-week gestational age girl >72 hours old (Table 4). For infants with TSB levels 0.1 to 3.0 mg/dL below or ≥1 mg/dL above the phototherapy threshold, NNT can be calculated by dividing numbers in Table 4 by the corresponding ORs in Table 3. Inclusion of the 11 390 infants without a documented decline in TSB had little effect on adjusted ORs but led to lower absolute risks and higher NNT.
TABLE 4.
Estimated NNT With Inpatient Phototherapy (95% CI) for 3.3-kg Infants Who Were Not DAT Positive, by Gender, Gestational Age, and Age at Qualifying TSB
| Gestational Age, wk |
NNTs (95% CI) | |||
|---|---|---|---|---|
| Age at Qualifying TSB: <24 h |
Age at Qualifying TSB: 24 to <48 h |
Age at Qualifying TSB: 48 to <72 h |
Age at Qualifying TSB:≥72 h |
|
| Boys | ||||
| 35 | 14 (7–40) | 26 (14–57) | 83 (36–190) | 171 (70–426) |
| 36 | 10 (6–19) | 19 (12–39) | 59 (31–101) | 122 (68–236) |
| 37 | 16 (10–28) | 29 (20–58) | 95 (52–168) | 196 (100–407) |
| 38 | 35 (14–100) | 67 (31–215) | 222 (107–502) | 460 (196–1352) |
| 39 | 74 (31–244) | 142 (62–554) | 476 (197–1385) | 989 (373–3607) |
| 40 | 106 (44–256) | 204 (98–487) | 682 (367–1294) | 1419 (634–3755) |
| ≥41 | 148 (54–428) | 284 (127–780) | 953 (366–3017) | 1983 (676–8408) |
| Girls | ||||
| 35 | 21 (12–49) | 40 (21–86) | 126 (50–267) | 261 (105–585) |
| 36 | 15 (11–26) | 28 (20–51) | 90 (43–146) | 186 (102–347) |
| 37 | 23 (16–39) | 44 (31–75) | 145 (73–243) | 300 (146–671) |
| 38 | 53 (23–134) | 102 (43–236) | 339 (154–730) | 705 (314–2016) |
| 39 | 113 (58–342) | 217 (103–713) | 729 (272–1730) | 1516 (614–4520) |
| 40 | 162 (75–400) | 312 (164–704) | 1046 (491–2136) | 2176 (922–6107) |
| ≥41 | 226 (92–702) | 435 (183–1140) | 1461 (510–4842) | 3041 (888–11096) |
The NNT is estimated as the reciprocal of the difference between predicted probabilities of the outcome in those who did and did not receive hospital phototherapy within 8 hours of their qualifying TSB level.
DISCUSSION
The existence of large electronic databases and considerable practice variation14 in this managed care organization allowed us to study the efficacy of phototherapy in 22 547 newborns. We found that hospital phototherapy was effective at preventing TSB levels from rising to levels at which the AAP recommends exchange transfusion, but the NNT is high and may vary as much as 300-fold depending on infant age, gender, and gestational age. This suggests that the guidelines could be made more internally consistent with additional refinement.
Although this study had a different design and outcome measure from the companion case-control study of TSB levels ≥25 mg/dL in the same population,10 our finding of 84% efficacy of phototherapy for those without a positive DAT was similar to the overall 85% efficacy found in that study. A difference is that, in the current study, we found an interaction with the DAT result, with lower efficacy in those who were DAT positive. The difference may be related to greater power in the current study (187 vs 62 outcomes) or the fact that, in this study, the outcome variable was crossing the AAP exchange transfusion line rather than developing a TSB level ≥25 mg/dL. Because the exchange line is <25 mg/dL in DAT-positive infants, phototherapy may not have had enough time to prevent that outcome. Finally, although the lower efficacy of phototherapy in DAT-positive infants is consistent with results from the National Institute of Child Health and Human Development Phototherapy Trial,2,3 potential biases toward no effect (discussed below) suggest that the true efficacy of phototherapy in DAT-positive infants is probably better than the estimate from this study.
There are several limitations of this study. First, we relied on procedure codes for hospital phototherapy to determine who had received it and admission times to determine its timing. This may have led to misclassification of phototherapy exposure. The effect of such misclassification would be to diminish the apparent efficacy of hospital phototherapy. Our 84% efficacy estimate for infants who were not DAT positive suggests that there could not have been much misclassification, consistent with what we found with chart review. The misclassification of phototherapy may have been greater in DAT-positive infants in whom the efficacy estimate was lower.
Another limitation is confounding: the possibility that clinicians used variables not included in our model to make phototherapy decisions. For example, we did not have data on breastfeeding, which, in our case-control study, was associated with twice the risk of developing a TSB level ≥25 mg/dL.10 However, unless these unknown confounders were associated with more phototherapy but lower risk of the outcome (which would not make sense clinically), the effect of this unmeasured confounding would be to reduce the apparent efficacy of phototherapy. Because our efficacy estimate in infants who were not DAT positive was high, this probably did not occur to a great extent in that group.
This study reveals some high NNTs with phototherapy to prevent 1 infant from crossing the exchange threshold. The technical report that accompanied the AAP guideline5 reported an NNT of 6 to 10 infants to prevent 1 infant from developing a TSB level ≥20 mg/dL. For infants 0.0 to 0.9 mg/dL above the AAP phototherapy threshold, we found an NNT that low only for 36-weekgestation boys <24 hours old; typical NNT were in the hundreds and reached the thousands in later-gestation newborns ≥3 days old. However, the NNT drops significantly as the qualifying TSB level rises above the phototherapy threshold.
Our finding of high NNTs for older and more mature infants does not imply that such infants do not need to be followed or treated. Their low risk may be partly because they received other interventions, such as lactation support or addition of formula. The results do suggest that those with TSB levels below the phototherapy threshold do not need phototherapy. For infants ≥48 to 72 hours old with TSB levels ≤2 mg/dL above the threshold, perhaps alternatives to immediate rehospitalization for phototherapy should be considered, such as lactation support, formula substitution, or supplementation4; home phototherapy; or repeating the TSB after several hours to assess its trajectory. In considering these options, it is important to remember that, whereas exchange transfusions are risky, a TSB level exceeding the AAP exchange level is a surrogate outcome that is generally benign15,16 and that phototherapy is associated with costs and the possibility of as-yet-unconfirmed potential harms.17–21
The marked variation in the estimated NNT is particularly noteworthy. Presumably, the thresholds chosen for exchange transfusion were those at which the risks of hyperbilirubinemia first exceed the risks of exchange transfusion. This suggests that the number of infants worth treating with phototherapy to prevent 1 from having a TSB level exceed the exchange threshold should be uniform. The fact that the NNT to prevent this outcome varies by a factor of >300 suggests that the guidelines are not internally consistent. For example, if it is worth treating 100 infants with phototherapy to prevent 1 from exceeding the exchange level, then phototherapy thresholds should be lower in younger, less mature infants (so we treat more of them) and/or higher in others (so we treat fewer). Similarly, current guidelines for treating boys and girls are the same, although boys are consistently overrepresented among cases of extreme hyperbilirubinemia2,22–25 and bilirubin encephalopathy. 23,25,26 Our findings suggest that phototherapy thresholds should be lowered in boys or raised in girls.
Although the current AAP guideline provides age-specific bilirubin treatment thresholds for only 3 risk groups, it acknowledges this oversimplification by suggesting that practitioners may individualize treatment decisions in infants of 35 to 376/7 weeks’ gestational age according to the actual gestational age. Our results suggest that this sort of individualization would also be desirable by gender and for gestational ages of ≥38 weeks. An electronic version of the guideline that accepts user input, either Web based or for a handheld device, such as is available at www.bilitool.org, would make this more practical for clinicians.
Finally, our results highlight important areas for future investigation. Decisions about phototherapy necessarily involve a tradeoff between avoiding dangerous bilirubin levels and avoiding unnecessary treatment. Clearly, the TSB level at which phototherapy should be done depends on the NNT one is willing to accept. Additional research on the costs and possible adverse effects of phototherapy is needed. However, an even more important knowledge gap is defining a “dangerous bilirubin level.” We know that the risk of kernicterus at TSB levels above the AAP exchange transfusion thresholds is low,15 but we do not know how low. Unless we can quantify how bad an outcome is, it is hard to know how many people it is worth treating to prevent it.
What’s Known on This Subject
American Academy of Pediatrics’ guidelines suggest bilirubin levels at which to initiate phototherapy and exchange transfusion. However, the guidelines are based on “uncertain estimates and extrapolations” from previous studies and do not provide estimates of numbers needed to treat.
What This Study Adds
We estimate the efficacy of phototherapy and the numbers needed to treat to prevent one infant from developing a bilirubin level at which the AAP recommends exchange transfusion. This information could increase the internal consistency of the guidelines.
ACKNOWLEDGMENTS
This study was supported by grant RO1 HD047557 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr Kuzniewicz was support by a grant from the Glaser Pediatric Research Network.
Abbreviations
- AAP
American Academy of Pediatrics
- NNT
number needed to treat
- TSB
total serum bilirubin
- NC-KPMCP
Northern California Kaiser Permanente Medical Care Program
- DAT
direct antiglobulin test
- OR
odds ratio
- CI
confidence interval
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 2.Brown A, Kim M, Wu P, Bryla D. Efficacy of phototherapy in prevention and management of neonatal hyperbilirubinemia. Pediatrics. 1985;75(2 pt 2):393–400. [PubMed] [Google Scholar]
- 3.Maurer HM, Kirkpatrick BV, McWilliams NB, Draper DA, Bryla DA. Phototherapy for hyperbilirubinemia of hemolytic disease of the newborn. Pediatrics. 1985;75(2 pt 2):407–412. [PubMed] [Google Scholar]
- 4.Martinez JC, Maisels MJ, Otheguy L, et al. Hyperbilirubinemia in the breast-fed newborn: a controlled trial of four interventions. Pediatrics. 1993;91(2):470–473. [PubMed] [Google Scholar]
- 5.Ip S, Chung M, Kulig J, et al. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114(1) doi: 10.1542/peds.114.1.e130. Available at: www.pediatrics.org/cgi/content/full/114/1/e130. [DOI] [PubMed]
- 6.Watchko J, Oski F. Bilirubin 20 mg/dL = vigintiphobia. Pediatrics. 1983;71(4):660–663. [PubMed] [Google Scholar]
- 7.Selby JV. Linking automated databases for research in managed care settings. Ann Intern Med. 1997;127(8 pt 2):719–724. doi: 10.7326/0003-4819-127-8_part_2-199710151-00056. [DOI] [PubMed] [Google Scholar]
- 8.Newman TB, Escobar GJ, Gonzales V, Armstrong MA, Gardner M, Folck B. Frequency of neonatal bilirubin testing and hyperbilirubinemia in a large health maintenance organization. Pediatrics. 1999;104(5 pt 2):1198–1203. [PubMed] [Google Scholar]
- 9.Jacobson MP, Bernstein HH. Limited diagnostic value of routine cord blood bilirubin determinations. Clin Pediatr (Phila) 1982;21(10):610–612. doi: 10.1177/000992288202101009. [DOI] [PubMed] [Google Scholar]
- 10.Kuzniewicz M, Escobar G, Wi S, Liljestrand P, McCulloch C, Newman T. Risk factors for severe hyperbilirubinemia among infants with borderline bilirubin levels: a nested case-control study. J Pediatr. 2008;153(2):234–240. doi: 10.1016/j.jpeds.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stata Corp. Release 9. College Station, TX: Stata Corporation; 2005. Stata User’s Guide; p. 275. [Google Scholar]
- 12.Stata Corp. Stata, Release 9. Reference Manual. Volume 2 (K–Q) College Station, TX: Stata Corporation; 2005. p. 90. [Google Scholar]
- 13.Davison AC, Hinkley DV. Bootstrap Methods and Their Application. Cambridge, United Kingdom: Cambridge University Press; 1997. [Google Scholar]
- 14.Atkinson LR, Escobar GJ, Takayama JI, Newman TB. Phototherapy use in jaundiced newborns in a large managed care organization: do physicians adhere to the guideline? Pediatrics. 2003;111(5) doi: 10.1542/peds.111.5.e555. Available at: www.pediatrics.org/cgi/content/full/111/5/e555. [DOI] [PubMed]
- 15.Newman TB, Liljestrand P, Jeremy RJ. Outcomes among newborns with total serum bilirubin levels of 25 mg per deciliter or more. N Engl J Med. 2006;354(18):1889–1900. doi: 10.1056/NEJMoa054244. [DOI] [PubMed] [Google Scholar]
- 16.Newman TB, Maisels MJ. Does hyperbilirubinemia damage the brain of healthy full-term infants? Clin Perinatol. 1990;17(2):331–358. [PubMed] [Google Scholar]
- 17.Cnattingius S, Zack M, Ekbom A, Gunnarskog J, Linet M, Adami HO. Prenatal and neonatal risk factors for childhood myeloid leukemia. Cancer Epidemiol Biomarkers Prev. 1995;4(5):441–445. [PubMed] [Google Scholar]
- 18.Dahlquist G, Kallen B. Indications that phototherapy is a risk factor for insulin-dependent diabetes. Diabetes Care. 2003;26(1):247–248. doi: 10.2337/diacare.26.1.247-a. [DOI] [PubMed] [Google Scholar]
- 19.Matichard E, Le Henanff A, Sanders A, Leguyadec J, Crickx B, Descamps V. Effect of neonatal phototherapy on melanocytic nevus count in children. Arch Dermatol. 2006;142(12):1599–1604. doi: 10.1001/archderm.142.12.1599. [DOI] [PubMed] [Google Scholar]
- 20.Aycicek A, Erel O. Total oxidant/antioxidant status in jaundiced newborns before and after phototherapy [in Portuguese] J Pediatr (Rio J) 2007;83(4):319–322. doi: 10.2223/JPED.1645. [DOI] [PubMed] [Google Scholar]
- 21.Sola A. Turn off the lights and the oxygen, when not needed: phototherapy and oxidative stress in the neonate [in Portuguese] J Pediatr (Rio J) 2007;83(4):293–296. doi: 10.2223/JPED.1674. [DOI] [PubMed] [Google Scholar]
- 22.Newman TB, Xiong B, Gonzales VM, Escobar GJ. Prediction and prevention of extreme neonatal hyperbilirubinemia in a mature health maintenance organization. Arch Pediatr Adolesc Med. 2000;154(11):1140–1147. doi: 10.1001/archpedi.154.11.1140. [DOI] [PubMed] [Google Scholar]
- 23.Ebbesen F, Andersson C, Verder H, et al. Extreme hyperbilirubinaemia in term and near-term infants in Denmark. Acta Paediatr. 2005;94(1):59–64. doi: 10.1111/j.1651-2227.2005.tb01789.x. [DOI] [PubMed] [Google Scholar]
- 24.Sgro M, Campbell D, Shah V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. CMAJ. 2006;175(6):587–590. doi: 10.1503/cmaj.060328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manning D, Todd P, Maxwell M, Platt M. Prospective surveillance study of severe hyperbilirubinaemia in the newborn in the UK and Ireland. Arch Dis Child Fetal Neonatal Ed. 2007;92(5):342–346. doi: 10.1136/adc.2006.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhutani VK, Johnson L. Kernicterus in late preterm infants cared for as term healthy infants. Semin Perinatol. 2006;30(2):89–97. doi: 10.1053/j.semperi.2006.04.001. [DOI] [PubMed] [Google Scholar]