Abstract
Background
For more than 100 years, group A Streptococcus has been identified as a cause of severe and, in many cases, fatal infections of the female urogenital tract. Due to advances in hospital hygiene and the advent of antibiotics, this type of infection has been virtually eradicated. However, within the last three decades there has been an increase in severe intra- and post-partum infections attributed to GAS.
Methodology
We hypothesized that GAS alters its transcriptome to survive in human amniotic fluid (AF) and cause disease. To identify genes that were up or down regulated in response to growth in AF, GAS was grown in human AF or standard laboratory media (THY) and samples for expression microarray analysis were collected during mid-logarithmic, late-logarithmic, and stationary growth phases. Microarray analysis was performed using a custom Affymetrix chip and normalized hybridization values derived from three biological replicates were collected at each growth point. Ratios of AF/THY above a 2-fold change and P-value <0.05 were considered significant.
Principal Findings
The majority of changes in the GAS transcriptome involved down regulation of multiple adhesins and virulence factors and activation of the stress response. We observed significant changes in genes involved in the arginine deiminase pathway and in the nucleotide de novo synthesis pathway.
Conclusions/Significance
Our work provides new insight into how pathogenic bacteria respond to their environment to establish infection and cause disease.
Introduction
Group A Streptococcus (Streptococcus pyogenes, GAS) is an exclusively human, Gram-positive pathogen that causes a broad variety of diseases from mild pharyngitis and skin infections to necrotizing fasciitis, streptococcal toxic shock syndrome, and non-suppurative sequelae such as acute rheumatic fever or glumerulonephritis (for a review see [1]). Since the 1980's, GAS has re-emerged as an important cause of severe invasive infections and is estimated to cause approximately 500,000 deaths each year globally despite available antibiotic treatment [2]. In the late 1800's, GAS was identified as a causative factor of puerperal sepsis – a severe invasive infection in post partum women [3]. Due to advances in hospital hygiene, namely physicians washing their hands between deliveries, these types of GAS infections became less frequent. However, in the last three decades there has been a resurgence in GAS infections of the female urogenital tract and vulvovaginatis in pre-pubescent females, and invasive postpartum disease now accounts for approximately 2.2% of invasive GAS diseases [4]. Women with pre-existing throat infections or disrupted mucosal and skin barriers during pregnancy and delivery are particularly susceptible [4].
GAS strains are classified based on the aminoterminal sequence of the M protein, a polymorphic cell surface adhesin and anti-phagocytic factor [1]. Among the various GAS serotypes, serotype M28 strains are a common cause of invasive disease and have an unusual propensity to cause vaginitis and postpartum infections [4]–[6]. Full-genome sequence analysis of an M28 strain provided the first insight into the underlying molecular mechanism for the ability of these strains to cause a disproportionate number of postpartum infections [5]. Green et al. identified a mobile genetic element, named Region of Difference (RD) 2, that was present in all serotype M28 strains analyzed and a subset of other M serotype strains that have been linked epidemiologically to maternal-neonatal infections [5]. Interestingly, RD2 shares extensive similarity with regions in group B Streptococcus (Streptococcus agalactiae, GBS) [5], [7], which is a common cause of female urogenital tract infections, and genes in groups C and G Streptococcus (Sitkiewicz I., Green NM., and Musser JM, unpublished) that can also cause severe invasive infections [8], [9].
As mentioned previously, GAS is capable of causing a wide range of diseases by successfully colonizing a variety of anatomical sites. Transcriptome analyses of GAS grown in blood, saliva, epithelial cells, and polymorphonuclear leukocytes (PMNs) have revealed that GAS is extremely adaptable and modifies its transcriptome based on environmental signals [10]–[13]. To establish infection in the female urogenital tract, GAS must survive and replicate in a bacteriostatic host environment. The ability of GBS to persist and replicate in human amniotic fluid (AF) [14] is an important contributing factor to its ability to cause severe pre- and postpartum infections, despite AF having antibiotic properties towards other bacterial species [15]. Because M28 GAS strains contain the RD2 element and cause a disproportionate number of postpartum infections, we hypothesized that serotype M28 GAS would be able to survive and persist in human AF, similarly to GBS. To initially characterize the interaction of pathogenic GAS within this specific host niche, we used an ex vivo strategy to analyze the global transcriptional response of GAS grown in human AF compared to GAS grown under standard laboratory conditions.
Materials and Methods
Bacterial strains and routine growth
GAS strains MGAS6180 (serotype M28, RD2+) [5], and MGAS 5005 (serotype M1, RD2-) [16] were grown in Todd Hewitt medium with 0.5% yeast extract or on TSA II plates supplemented with 5% sheep blood (BD Diagnostics) at 37°C in 5% CO2.
Growth of bacteria in AF
Human (AF) was collected from pregnant women seen at The Methodist Hospital, Houston, Texas, or Weill Medical College of Cornell University in New York City. Samples were collected in accordance with an exempt human subjects protocol approved by the Institution Review Board of each institution. The study involved collection of existing diagnostic specimens routinely collected during clinical procedures as amniocenteses and would have been otherwise discarded. Specimens were stripped of all identifiers and processed in a manner that subjects cannot be directly or indirectly identified.
AF samples were tested to determine if they supported bacterial growth. GAS cells were grown overnight in THY, washed twice in sterile PBS, and re-suspended in PBS to 100×. Ten µl of the 100× bacteria suspension were diluted further in PBS and were used to inoculate each 250 µl sample of heat inactivated (95°C, 5 min) AF, resulting in a final average inoculum of ∼104 CFU/ml. Samples were incubated at 37°C with 5% CO2 for 24 h. To avoid THY carryover that might bias the growth results, bacteria were diluted 1∶50 or 1∶25 into a fresh aliquot of AF after the first 24 h. Aliquots were removed, serially diluted, and plated on TSA II plates (BD Diagnostics) every hour for the first 12 h and every 12 h thereafter for CFU enumeration. AF samples that supported growth of GAS were pooled and used for the microarray analysis. Samples were collected from three pooled AF cultures (biological replicates) after 3.5, 5, and 9 hours of growth in AF, which corresponds to bacteria in mid log (ML) growth phase, late log (LL, time point corresponding with the transition from log to stationary phase) and stationary (S) phase, respectively. Bacteria grown in THY laboratory medium were collected in ML, LL and S phase from three independent cultures (biological replicates).
RNA isolation
The bacteria used for RNA isolation were mixed with 2 volumes of RNA Protect reagent (Qiagen) and cells were collected by centrifugation and stored at −80°C until processing. RNA from GAS was isolated as described previously [17]. All samples were processed at the same time to minimize experimental error. Reverse transcription, cDNA fragmentation, and labeling for all samples were performed as described previously [17].
Microarray analysis
Microarray analysis was performed using a custom-made Affymetrix chip [18] that contained 1929 redundant probes representing the core GAS chromosome and 289 redundant probes unique for MGAS6180. A total of 1765 MGAS6180 genes were represented on the array. Chip hybridization and data acquisition and processing were performed as described [19]. Samples used for microarray analysis were collected from cultures in THY and AF at mid logarithmic (ML), late logarithmic (LL) and stationary (S) growth phases. The average expression values for each transcript in AF in each growth phase was divided by the average expression in THY to generate AF/THY ratios and degree of changes. Genes that had a two-fold change of expression or greater in AF and were statistically significant (P<0.05) were included in the analysis.
The data is deposited in MIAME-compliant GEO database under accession number GSE19985.
Results and Discussion
Characterization of GAS growth in AF
Human AF is a nutritionally poor environment. AF is composed primarily of water and low amounts of sugars and proteins, levels of which decrease as the pregnancy nears term [20]. Recent detailed compositional analysis of AF revealed the presence of multiple proteins (serum albumin, transferrin, α-I-Antitripsin, α-fetoprotein, calpain 6, pinin, type XIII collagen, immunoglobulins) [21], glucose, fructose, lipids, hormones (estrogen and progesterone), and epithelial cells. Sources of AF are predominantly fetal urine (∼900 ml per day influx at term), tracheal fluid, fetal lung fluid, and water and solvents transferred between AF and fetal blood in placenta [22].
GAS rarely causes septic abortions [4]; however, after disruption of membranes, release of AF can change the environment of the female genital tract by increasing pH from acidic to neutral/slightly alkaline and affect balance of natural bacterial vaginal flora. GAS grows better in neutral than acidic pH [23], therefore pH change can promote growth of colonizing pathogens and postpartum infection. Because GAS is able to cause postpartum infections suggests that it is able to survive in an environment containing AF.
Individual specimens of AF collected from women at various stages of pregnancy can vary in their bacteriostatic properties, primarily due to the activity of lysozyme, immunoglobins, and β−lysin [24]–[26]. In general, samples from early stage pregnancies are less bacteriostatic than samples from late stage pregnancies [27]. In addition, bacteriostatic properties of AF also depend on the presence of meconium and iron availability [28]. Often the presence of meconium in AF is used to predict the likelihood of the mother developing a postpartum or intrapartum bacterial infection [29].
Therefore we wanted to determine if GAS can survive in bacteriostatic AF and if so, what transcriptional changes it undergoes to achieve this.
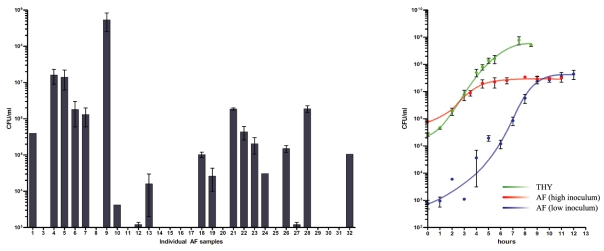
To determine if AF had antimicrobial properties towards GAS, we tested samples collected from separate individuals at different stages of pregnancy. About half of the tested specimens did not support the growth of GAS (Fig 1A). Observed inhibition of GAS growth did not correlate with the gestation period (data not shown), and was instead patient specific and influenced by unknown factors. Visual inspection of AF specimens did not allow determination of the presence of meconium. Growth of GAS in AF was not restricted to the serotype M28 strain, as serotype M1 strain MGAS5005 grew at a comparable density to the M28 strain in one of the individual growth-positive specimens (data not shown). In conclusion, unlike many other bacteria, GAS can survive and grow in some AF specimens, which could have important implications for its ability to cause postpartum infections.
Figure 1. Characterization of growth of GAS in AF.
A. Individual specimens of AF support growth of GAS at various levels after 24 h of incubation; initial inoculum ∼104 CFU/ml. B. Growth densities of GAS (CFU/ml) in THY and AF.
To further characterize the growth of GAS in AF, we pooled all AF specimens that supported growth of GAS and performed growth curves with two different starting inocula and compared it to GAS grown in laboratory medium (THY) (Fig. 1B). Interestingly, GAS grown in AF reached similar density (around 2×107 CFU/ml) in stationary phase, despite a three-fold difference in starting inocula. Similar cell density was observed after 48h of incubation in AF. This suggests that the limited nutrient availability of AF could not sustain higher density growth.
Microarray analysis: general quantitative trends in response to AF
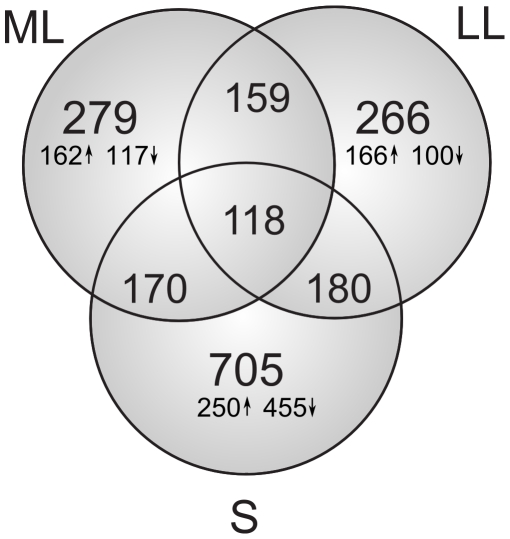
To characterize the transcriptional response of GAS grown in AF we utilized an ex vivo microarray approach that has been used previously to characterize the interactions of GAS with various environments such as blood and saliva [10], [11]. We detected 859 differentially expressed genes fulfilling the criteria of differentially expressed gene (∼49% of genes present on the array). Over 250 genes were differentially expressed in ML and LL phases and the number of differentially expressed genes increased and reached maximum in S phase (Figures 2 and 3). The majority of these genes in ML and LL phases were up regulated rather than down regulated. This differs from the response of GBS grown in AF [19], in which the majority of genes are down regulated when compared to laboratory conditions and the bulk of changes are observed during the transition from logarithmic to stationary phase [19]. The complete list of transcriptional changes in GAS is shown in Table S1 and comparison of gene expression between GAS and GBS in Table S2.
Figure 2. Number of genes differentially expressed in response to AF in ML, LL and S phases.
Upward arrows represent the number of transcripts with increased expression in AF in ML, LL and S phases; the downward arrows represent the number of transcripts with increased expression in THY (down regulated in AF) in ML, LL and S phases. Intersections of the Venn diagram indicate number of differentially expressed genes during more than one growth phase.
Figure 3. Dynamics of gene expression of GAS genes in ML, LL, and S phase in response to AF.
Each dot represents a single transcript and its coordinates are the average level of expression in THY (x-axis) and AF (y-axis). The dotted lines denote 2-fold change in transcription. Transcripts below the lower dotted line are more highly expressed in THY, transcripts above the upper dotted line are more highly expressed in AF. Thick black lines denote 10-fold differences in transcript level between the studied conditions.
Expression of virulence factors
Because GAS causes severe invasive postpartum diseases, we were interested in determining which virulence factors exhibited a change in expression in response to AF. Surprisingly, with the exception of CAMP factor, streptodornase, and one of the enterotoxins (Table 1), we did not detect up regulation of virulence factor expression. However, we did detect massive down regulation of genes encoding proteins involved in adhesion, such as multiple fibronectin, collagen and laminin binding proteins, M protein, the gene encoding R28 protein (M28_Spy1336), and streptolysin S. Down regulation of multiple adhesins and cell surface proteins could be a mechanism to evade the host immune response. The genes encoding two peptidases that interact with the host immune response, C5A peptidase and SpyCEP, were expressed at much lower levels in AF than in THY (Table 1). C5A peptidase has been also shown to facilitate fibronectin-independent invasion of epithelial cells [30]. Similar down regulation of adhesins and capsule was observed in GBS when grown in AF [19].
Table 1. Differential expression of genes encoding known GAS virulence factors upon contact with AF.
| 6180 | SF370 | Locus | ML | LL | S | Descriptions |
| M28_Spy0105 | −4.20 | −12.76 | −28.84 | Fibronectin-binding protein | ||
| M28_Spy0107 | - | −2.49 | −9.14 | −7.83 | Fibronectin-binding protein | |
| M28_Spy0109 | SPy0128 | - | −2.33 | −5.79 | −5.36 | Fibronectin-binding protein |
| M28_Spy0113 | −2.49 | −2.64 | −16.21 | Collagen adhesion protein | ||
| M28_Spy0754 | SPy1054 | - | −2.41 | −1.82 | Collagen-like surface protein | |
| M28_Spy1675 | sclA | −167.84 | −13.82 | −2.83 | Collagen-like surface protein | |
| M28_Spy1696 | SPy2007 | lmb | −2.90 | −5.03 | Laminin-binding surface protein | |
| M28_Spy1702 | SPy2018 | emm | −21.63 | −3.84 | M protein | |
| M28_Spy1715 | −53.54 | −8.97 | −3.47 | Fibronectin-binding protein | ||
| M28_Spy1716 | −75.85 | −15.05 | −5.01 | Fibronectin-binding protein | ||
| M28_Spy1336 | −53.13 | −90.92 | −133.85 | R28 protein | ||
| M28_Spy0139 | SPy0167 | slo | −45.03 | 2.68 | Streptolysin O | |
| M28_Spy0540 | SPy0738 | sagA | −2.06 | Streptolysin S precursor | ||
| M28_Spy0541 | SPy0739 | sagB | −12.20 | Streptolysin S biosynthesis protein | ||
| M28_Spy0542 | SPy0740 | sagC | −18.65 | Streptolysin S biosynthesis protein | ||
| M28_Spy0543 | SPy0741 | sagD | −15.04 | Streptolysin S biosynthesis protein | ||
| M28_Spy0544 | SPy0742 | sagE | −15.65 | Streptolysin S biosynthesis protein | ||
| M28_Spy0545 | SPy0743 | sagF | −15.06 | Streptolysin S biosynthesis protein | ||
| M28_Spy0546 | SPy0744 | sagG | −18.88 | Streptolysin S export ATP-binding protein | ||
| M28_Spy0547 | SPy0745 | sagH | −16.89 | Streptolysin S export protein | ||
| M28_Spy0548 | SPy0746 | sagI | −14.21 | Streptolysin S export protein | ||
| M28_Spy0180 | SPy0212 | speG | −2.59 | Enterotoxin | ||
| M28_Spy0969 | 2.53 | Enterotoxin | ||||
| M28_Spy0953 | SPy1273 | cfa | 15.18 | 2.52 | cAMP factor | |
| M28_Spy0968 | SPy1436 | spd3 | 2.26 | Streptodornase (EC 3.1.21.1) | ||
| M28_Spy0329 | SPy0416 | Spy CEP | −16.21 | −2.50 | Endopeptidase lactocepin | |
| M28_Spy1700 | SPy2010 | scpA | −47.34 | −2.64 | C5A peptidase precursor (EC 3.4.21.-) |
Values represent fold change in expression in AF at ML, LL, and S growth phases compared to expression in THY medium.
R28 is an adhesin encoded by the RD2 element present in MGAS6180 and several other GAS strains [5]. It is a known virulence factor that increases attachment of GAS to cervical cells [31]. Its down regulation might be connected to down regulation of M28_Spy1337, which encodes a putative regulator; however, this has yet to be tested experimentally. In addition to R28, we observed an increase in transcription levels for multiple genes encoded by RD2 (Table 2). The RD2 element has been suggested to be involved in pathogenesis and adaptation to specific environments [5]. Interestingly, a large number of RD2 genes differed in their expression among biological replicates; therefore the calculated P value in many cases was greater than 0.05.
Table 2. Differential expression of RD2 genes in response to AF.
| Gene | ML | P | LL | S | Putative function | note | ||
| M28_Spy1304 | 10.19 | 6.76 | 16.10 | + | Hypothetical Protein | |||
| M28_Spy1305 | 2.39 | + | 2.43 | + | 1.75 | + | Hypothetical membrane protein | |
| M28_Spy1306 | 10.06 | + | 7.60 | + | 2.47 | Cell surface protein | Virulence | |
| M28_Spy1307 | 5.13 | 3.98 | + | 1.08 | Hypothetical exported protein | Virulence | ||
| M28_Spy1308 | 5.52 | 2.65 | 3.81 | + | Hypothetical exported protein | Virulence | ||
| M28_Spy1309 | 1.54 | + | 1.71 | + | 1.70 | + | Hypothetical protein | |
| M28_Spy1310 | 3.41 | 3.13 | + | 1.78 | Membrane protein | |||
| M28_Spy1311 | Not detected | |||||||
| M28_Spy1312 | Not detected | |||||||
| M28_Spy1313 | 8.70 | 6.23 | 2.28 | Hypothetical membrane protein | ||||
| M28_Spy1314 | 5.17 | 4.37 | + | 3.00 | hypothetical protein | |||
| M28_Spy1315 | 5.69 | 7.31 | + | 3.42 | Hypothetical protein | |||
| M28_Spy1316 | 9.67 | 8.45 | 6.03 | Hypothetical protein | ||||
| M28_Spy1317 | 35.90 | 12.95 | 3.67 | Hypothetical protein | ||||
| M28_Spy1318 | 3.81 | 2.69 | 6.31 | + | Hypothetical protein | |||
| M28_Spy1319 | Not detected | |||||||
| M28_Spy1320 | 11.20 | 36.55 | + | 10.89 | + | Hypothetical cytosolic protein | ||
| M28_Spy1321 | Not detected | |||||||
| M28_Spy1322 | 6.26 | 4.41 | 2.37 | FtsK SpoIIIE family | ||||
| M28_Spy1323 | 9.05 | + | 6.88 | + | 1.61 | Hypothetical cytosolic protein | ||
| M28_Spy1324 | 5.80 | 7.41 | + | 2.68 | Hypothetical cytosolic protein | |||
| M28_Spy1325 | 3.99 | + | 2.28 | 1.72 | Cell surface protein | Virulence | ||
| M28_Spy1326 | 2.18 | + | 1.08 | −1.29 | + | M like protein | Virulence | |
| M28_Spy1327 | 18.65 | 6.43 | 4.84 | Hypothetical cytosolic protein | ||||
| M28_Spy1328 | Not detected | |||||||
| M28_Spy1329 | 12.91 | 5.16 | 2.98 | Transcriptional Cro CI regulator | ||||
| M28_Spy1330 | 1.80 | + | 1.75 | + | 2.23 | + | Transcriptional Cro CI regulator | |
| M28_Spy1331 | 2.22 | + | 2.51 | + | 4.28 | + | Hypothetical cytosolic protein | |
| M28_Spy1332 | 2.42 | + | 2.50 | + | 8.66 | + | Hypothetical exported protein | Virulence |
| M28_Spy1333 | 1.89 | + | 1.69 | + | 5.46 | + | Hypothetical cytosolic protein | |
| M28_Spy1334 | 1.74 | + | 2.35 | + | 6.34 | + | DNA-damage-inducible protein J | |
| M28_Spy1335 | 1.20 | 1.36 | 3.20 | Transposase | ||||
| M28_Spy1336 | −53.13 | + | −90.92 | + | −133.85 | + | R28 Cell surface protein | Virulence |
| M28_Spy1337 | −5.18 | + | −8.50 | + | −7.93 | + | Transcriptional regulator | |
Values represent fold change in expression in AF at ML, LL, and S growth phases compared to expression in THY medium. +: P<0.05.
Stress response of GAS and GBS to AF
In contrast to GBS, which does not increase transcription of genes encoding proteins involved in the stress response [19], GAS activates multiple genes involved in this process. We observed increased transcription of the genes encoding proteolytic complex composed of ATPase and catalytic subunits ( clpP, clpE, clpX, and clpL) known to be stress effectors in streptococci [32], [33], the GroEL and GroES chaperonins, members of the Gls24 family of stress proteins (stress and starvation inducible genes in Enterococcus faecalis, [34]), and a putative GTP pyrophosphokinase (Table 3). Expression of the gene M28_Spy1562 that encodes a putative stress protein, was dramatically decreased in AF; however, its function in GAS is unknown. We also observed lowered expression of the relA gene which is major regulator of stringent control in GAS [35].
Table 3. Differential expression of stress response genes in response to AF.
| 6180 | SF370 | Locus | ML | LL | S | Descriptions |
| M28_Spy0317 | SPy0395 | clpP | 3.01 | proteolytic subunit clpP | ||
| M28_Spy1179 | SPy1509 | clpE | 2.01 | 2.64 | 2.04 | ATP-binding subunit clpE |
| M28_Spy0659 | SPy0873 | - | 2.05 | GTP pyrophosphokinase | ||
| M28_Spy0671 | clpX | −6.23 | ATP-binding subunit clpX | |||
| M28_Spy0674 | SPy0888 | clpL | 3.29 | 6.32 | ATP-binding subunit clpL | |
| M28_Spy0943 | SPy1260 | - | 2.66 | General stress protein, Gls24 family | ||
| M28_Spy0945 | SPy1262 | - | 2.59 | General stress protein, Gls24 family | ||
| M28_Spy1286 | SPy1557 | msrA | 3.73 | 2.41 | Peptide methionine sulfoxide reductase msrA | |
| M28_Spy0755 | SPy1055 | msrB | 5.36 | 4.95 | 8.23 | Peptide methionine sulfoxide reductase msrB |
| M28_Spy1503 | SPy1780 | - | 2.71 | Universal stress protein family | ||
| M28_Spy1562 | - | −102.42 | −17.90 | Universal stress protein family | ||
| M28_Spy0659 | SPy0873 | - | 2.05 | GTP pyrophosphokinase | ||
| M28_Spy1674 | SPy1981 | relA | −2.11 | −2.15 | GTP pyrophosphokinase | |
| M28_Spy1747 | SPy2070 | groEL | 2.34 | 60 kDa chaperonin GROEL | ||
| M28_Spy1748 | SPy2072 | groES | 2.36 | 10 kDa chaperonin GROES | ||
| M28_Spy1751 | SPy2077 | csp | 2.24 | 8.50 | Cold shock protein |
Values represent fold change in expression in AF at ML, LL, and S growth phases compared to expression in THY medium.
Regulatory events during growth in AF
We observed a large number of significant changes in transcription of regulatory genes (Table S1). Because the exact functions of many of these are unknown, it is impossible to predict the significance of these changes. However, we did detect changes in expression of known GAS regulators (Table 4) such as RofA/RALP that are involved in the regulation of virulence factor expression, namely adhesins [36]. Down regulation of rofA in response to AF might be partially responsible for the observed decreases in the transcription of the genes encoding fibronectin binding proteins. The decreased gene expression of the regulator ahrC.2 may be responsible for the observed changes in expression of genes involved in arginine metabolism (see below). Two other differentially expressed regulators were recently shown to be involved in GAS pathogenesis. The first, MtsR, is a regulator of ion transport and is involved in the development of necrotizing fasciitis [37], [38] The second, CcpA, links GAS virulence and carbohydrate metabolism [18]. In addition to individual regulators, we detected differential expression of seven (of 13) two component systems (TCSs) encoded by GAS. In the absence of regulation by alternative sigma factors in GAS, it is believed that concerted activity of regulons and TCSs is responsible for reaction of GAS to the environment. FasBCA and ihk/irr were shown to be involved in regulation of GAS pathogenesis. FasBCA controls expression of streptokinase, streptolysin S, and fibronectin binding proteins [39], [40]. The Ihk/Irr system is involved in GAS survival upon contact with human PMNs [12]. Circuits regulated by two other two component systems, M28_Spy0761/2 (SPy1061/2 in SF370 strain) and M28_Spy0807/8 (SPy1106/7 in SF370 strain), were studied by microarray analysis [17] and were shown to be involved in regulation of genes involved in carbohydrate and malate utilization, respectively. M28_Spy0919/20, M28_Spy1346/7 and M28_Spy1373/4 (SPy1236/7, SPy1587/8, SPy1621/2 in SF370 strain, respectively) have not been characterized thus far.
Table 4. Differential expression of selected regulatory systems in response to AF.
| 6180 | SF370 | Locus | ML | LL | S | Descriptions |
| M28_Spy0104 | SPy0124 | rofA | −2.09 | −3.56 | −2.69 | Transcriptional regulator RofA |
| M28_Spy0122 | SPy0146 | sloR | −11.53 | −13.76 | Transcriptional regulator pfoR | |
| M28_Spy0198 | SPy0242 | fasB | −3.31 | Sensory transduction protein kinase FasB | ||
| M28_Spy0199 | SPy0244 | fasC | −2.08 | −2.09 | −4.77 | Sensory transduction protein kinase FasC |
| M28_Spy0200 | SPy0245 | fasA | −3.61 | Response regulator FasA | ||
| M28_Spy0356 | SPy0450 | mtsR | 3.96 | Iron-dependent repressor | ||
| M28_Spy0412 | SPy0514 | ccpA | −3.65 | Catabolite control protein A | ||
| M28_Spy0465 | SPy0584 | ptsK | 2.66 | HPR(SER) kinase | ||
| M28_Spy0761 | SPy1061 | - | −4.83 | Two-component sensor kinase yesM | ||
| M28_Spy0762 | SPy1062 | - | −3.19 | Two-component response regulator yesN | ||
| M28_Spy0807 | SPy1106 | dpiA | 2.09 | Transcriptional regulatory protein dpiA | ||
| M28_Spy0808 | SPy1107 | dpiB | 2.16 | Sensor kinase dpiB | ||
| M28_Spy0919 | SPy1236 | ciaH | −3.35 | Sensor protein ciaH | ||
| M28_Spy0920 | SPy1237 | ciaR | −2.58 | Transcriptional regulatory protein ciaR | ||
| M28_Spy1215 | SPy1549 | ahrC.2 | −16.22 | Arginine repressor, argR | ||
| M28_Spy1346 | SPy1587 | - | −4.45 | Two-component response regulator yesN | ||
| M28_Spy1347 | SPy1588 | - | −3.03 | Two-component sensor kinase yesM | ||
| M28_Spy1373 | SPy1621 | yvqC | 2.05 | Two-component response regulator yvqC | ||
| M28_Spy1374 | SPy1622 | yvqE | 2.28 | 3.93 | Two-component sensor protein yvqE | |
| M28_Spy1708 | SPy2026 | ihk | −1.96 | Two component system histidine kinase | ||
| M28_Spy1709 | SPy2027 | irr | −1.58 | Two-component response regulator |
Values represent fold change in expression in AF at ML, LL, and S growth phases compared to expression in THY medium.
Metabolic adaptation to AF environment
A recently performed transcriptional analysis of GBS grown in AF revealed dramatic changes in expression of genes that control metabolism of carbohydrates, amino acids, and nucleotides [19], which presumably reflects an adaptive response to limited nutrient availability. We observed similar trends in the metabolic response of GAS to AF, with the majority of differences occurring in expression of genes involved in carbohydrate utilization (Table S2). In contrast to GBS that up regulates genes involved in carbohydrate utilization, GAS down regulates genes belonging to this category. Clear examples are the M28_Spy1036-1048 (mal genes), M28_Spy1349-1354, M28_Spy1438-1445 (lac.1 genes), and M28_Spy1622-1629 (lac.2 genes) loci (Table 5). The Mal locus has been shown to be involved in GAS persistence in the oropharynx [41], and lac loci are not only involved in fermentation and energy production via the tagatose pathway, but also link metabolism and the regulation of virulence [42].
Table 5. Differential expression of selected genes responsible for transport and metabolism of carbohydrates.
| 6180 | SF370 | Locus | ML | LL | S | Descriptions |
| M28_Spy0757 | SPy1057 | - | −3.86 | −11.47 | −18.53 | PTS system, mannose fructose family |
| M28_Spy0759 | SPy1059 | ptsC | −4.04 | −7.65 | −13.42 | PTS system, mannose fructose family |
| M28_Spy0958 | SPy1280 | glmS | −2.80 | −2.01 | Glucosamine–fructose-6-phosphate aminotransferase | |
| M28_Spy1036 | SPy1291 | glgP | −2.16 | Maltodextrin phosphorylase | ||
| M28_Spy1037 | SPy1292 | malM | −14.96 | 4-alpha-glucanotransferase | ||
| M28_Spy1039 | SPy1294 | malE | −4.94 | Maltose maltodextrin-binding protein | ||
| M28_Spy1041 | SPy1296 | malG | 2.19 | Maltose transport system permease protein | ||
| M28_Spy1044 | SPy1299 | malD | −34.73 | Maltodextrin transport system permease protein | ||
| M28_Spy1045 | SPy1301 | malC | −38.81 | Maltodextrin transport system permease protein | ||
| M28_Spy1046 | SPy1302 | amyA | −82.63 | Cyclodextrin glucanotransferase | ||
| M28_Spy1047 | SPy1304 | amyB | 2.06 | −24.86 | Neopullulanase | |
| M28_Spy1048 | SPy1306 | malX | −37.14 | Maltose maltodextrin-binding protein | ||
| M28_Spy1349 | SPy1592 | - | −42.74 | Sugar-binding protein | ||
| M28_Spy1350 | SPy1593 | - | −13.77 | Sugar transport system permease protein | ||
| M28_Spy1351 | SPy1595 | - | −7.07 | Sugar transport system permease protein | ||
| M28_Spy1354 | SPy1599 | - | −2.17 | Beta-glucosidase | ||
| M28_Spy1438 | SPy1704 | lacD.1 | −49.45 | Tagatose-bisphosphate aldolase | ||
| M28_Spy1440 | SPy1707 | lacB.1 | −170.54 | Galactose-6-phosphate isomerase lacB subunit | ||
| M28_Spy1441 | SPy1708 | lacA.1 | −100.49 | Galactose-6-phosphate isomerase lacA subunit | ||
| M28_Spy1442 | SPy1709 | - | 2.40 | PTS system, galactose-specific IIC component | ||
| M28_Spy1443 | SPy1710 | - | −26.62 | PTS system, galactose-specific IIB component | ||
| M28_Spy1444 | SPy1711 | - | −57.34 | PTS system, galactose-specific IIA component | ||
| M28_Spy1445 | SPy1712 | lacR.1 | −2.47 | Lactose phosphotransferase system repressor | ||
| M28_Spy1622 | SPy1916 | lacG | −67.36 | 6-phospho-beta-galactosidase | ||
| M28_Spy1624 | SPy1918 | lacF | −8.85 | PTS system, lactose-specific IIA component | ||
| M28_Spy1625 | SPy1919 | lacD.2 | −122.01 | Tagatose-bisphosphate aldolase | ||
| M28_Spy1626 | SPy1921 | lacC.2 | −55.33 | Tagatose-6-phosphate kinase | ||
| M28_Spy1627 | SPy1922 | lacB.2 | −21.89 | Galactose-6-phosphate isomerase lacB subunit | ||
| M28_Spy1628 | SPy1923 | lacA.2 | −69.34 | Galactose-6-phosphate isomerase lacA subunit | ||
| M28_Spy1629 | SPy1924 | lacR.2 | −3.72 | Lactose phosphotransferase system repressor | ||
| M28_Spy1668 | SPy1972 | pulA | −5.30 | Pullulanase | ||
| M28_Spy1767 | SPy2096 | dexS | −6.18 | −39.01 | Trehalose-6-phosphate hydrolase | |
| M28_Spy1768 | SPy2097 | - | −8.81 | −43.06 | PTS system, trehalose-specific IIBC component |
Values represent fold change in expression in AF at ML, LL, and S growth phases compared to expression in THY medium.
Another category of metabolic genes affected by growth in AF were genes involved in protein and amino acid utilization. We observed differential expression of multiple proteases, and di- and oligopeptide transport systems (Table 6). Similarly to GBS, we observed up regulation of genes involved in uptake and utilization of branched chain amino acids (BCAA, valine, leucine, and isoleucine). GBS is auxotrophic in respect to BCAA and mobilizes all transport systems to maximize utilization from AF [19]; GAS could possibly employ the same mechanism to maximize nutrient utilization.
Table 6. Differential expression of genes involved in the transport and metabolism of amino acids, peptides, and amines.
| 6180 | SF370 | Locus | ML | LL | S | Descriptions |
| Proteases | ||||||
| M28_Spy0463 | SPy0581 | - | −2.95 | Metallopeptidase, SprT family | ||
| M28_Spy0470 | SPy0590 | - | −2.23 | −2.82 | Peptidase family U32 | |
| M28_Spy0471 | SPy0591 | - | −2.10 | Peptidase family U32 | ||
| M28_Spy0572 | SPy0775 | - | 2.31 | Neutral zinc metallopeptidase family | ||
| M28_Spy1136 | SPy1402 | - | 34.77 | Peptidase family S11 | ||
| M28_Spy1397 | SPy1651 | pepC | −1.98 | Aminopeptidase C | ||
| M28_Spy1565 | SPy1858 | pepXP | 2.31 | 4.45 | Xaa-Pro dipeptidyl-peptidase | |
| M28_Spy1744 | SPy2066 | - | −4.28 | −22.44 | Dipeptidase A (EC 3.4.13.-) | |
| M28_Spy1766 | SPy2095 | pepO | −2.78 | Oligoendopeptidase O (EC 3.4.24.-) | ||
| Arginine metabolism | ||||||
| M28_Spy0575 | SPy0778 | - | −2.35 | Arginine-binding protein | ||
| M28_Spy1176 | SPy1506 | artP | 2.95 | 2.12 | 2.07 | Arginine transport ATP-binding protein |
| M28_Spy1177 | SPy1507 | artQ | 2.50 | 2.08 | Arginine transport system permease protein | |
| M28_Spy1208 | SPy1541 | arcC | −8.01 | −877.27 | Carbamate kinase | |
| M28_Spy1209 | SPy1542 | - | −199.49 | Xaa-His dipeptidase | ||
| M28_Spy1210 | SPy1543 | - | −2.55 | −191.92 | Arginine ornithine antiporter | |
| M28_Spy1211 | SPy1544 | arcB | −4.96 | −212.09 | Ornithine carbamoyltransferase | |
| M28_Spy1213 | SPy1547 | arcA | −8.87 | −712.24 | Arginine deiminase | |
| Branched chain amino acids | ||||||
| M28_Spy0266 | SPy0323 | braB | 5.13 | 5.26 | 2.21 | Branched-chain amino acid carrier protein |
| M28_Spy0693 | SPy0911 | bcaT | 2.13 | 3.48 | Branched-chain amino acid aminotransferase | |
| Di- and oligopeptide transport | ||||||
| M28_Spy1689 | SPy2000 | dppA | −4.23 | −2.68 | Dipeptide-binding protein | |
| M28_Spy1690 | SPy2001 | dppB | −3.39 | −2.70 | Dipeptide transport system permease protein | |
| M28_Spy1691 | SPy2002 | dppC | −4.57 | −2.82 | −2.61 | Dipeptide transport system permease |
| M28_Spy1692 | SPy2003 | dppD | −4.19 | −2.58 | −2.46 | Dipeptide transport ATP-binding protein |
| M28_Spy1693 | SPy2004 | dppE | −4.21 | −2.76 | Dipeptide transport ATP-binding protein | |
| M28_Spy0245 | SPy0294 | oppB | 2.55 | Oligopeptide transport system permease | ||
| M28_Spy0246 | SPy0295 | oppC | 2.01 | Oligopeptide transport system permease | ||
| M28_Spy0247 | SPy0296 | oppD | 1.75 | Oligopeptide transport ATP-binding | ||
| M28_Spy0248 | SPy0297 | oppF | 1.89 | Oligopeptide transport ATP-binding | ||
Values represent fold change in expression in AF at ML, LL, and S growth phases compared to expression in THY medium.
The most dramatic changes in gene expression were observed in genes encoding enzymes of the arginine deiminase pathway, which were down regulated almost 900-fold upon contact with AF (Table 6). The arginine deiminase pathway allows utilization of arginine as a carbon and energy source [43] and plays a role in the modification of the environmental acidity [44], [45]. Arginine deiminase from the GAS Manfredo strain is also a potent inhibitor of the proliferation of peripheral blood mononuclear cells [46]. However, arginine deiminase is not only an intracellular enzyme involved in the aforementioned processes. It can be also found on the GAS cell surface [47] and antibodies against this protein have been recently reported to protect mice against GAS infection (Walker MJ, Henningham A, Cork A, Cole JN, Ramachandran V et al. (2008) Group A Anchorless Surface proteins. XVII Lancefield International Symposium on Streptococci and Streptococcal Diseases O9.2; Henningham A, Batzloff M, Cole JN, Gillen C, Hartas J et al. (2008) Protection Against lethal Streptococcus pyogenes challenge following vaccination with anchorless cell wall-associated proteins. XVII Lancefield International Symposium on Streptococci and Streptococcal Diseases P54). It is possible that the lack of arginine deiminase on the surface is linked to decreased recognition by the host immune response, which would be consistent with the observed down regulation of adhesins and other major surface proteins. Interestingly, fetal urine (which is a major component of AF) flow and composition is modulated by arginine levels [22], therefore bacterial arginine metabolism could be linked to AF dynamics.
Arginine metabolism is linked via carbamate kinase to another pathway – nucleotide synthesis (Figure 4). We observed dramatic up regulation of genes involved in purine and pyrimidine nucleotide biosynthesis pathways and down regulation of genes encoding enzymes involved in salvage pathways (Table 7). The extent of changes in GAS correlates well with changes detected in GBS [19], and almost all of the enzymes in the nucleotide metabolic pathways undergo the same directional changes (Figure 4), what suggests direct influence of the AF environment.
Figure 4. Changes in transcription of genes encoding predicted enzymes in the purine and pyrimidine biosynthetic pathway in GAS and GBS in response to AF.
Both GAS and GBS exhibit similar changes in the expression of genes encoding enzymes involved in nucleotide biosynthesis. Changes in transcription of GBS genes from [19], changes in transcription of GAS genes, this work. Light green: genes down regulated 2 to 5 fold upon contact with AF; dark green: above 5 fold. Light orange: genes up regulated 2 to 5 fold upon contact with AF; dark orange: above 5 fold. R5P, ribose 5-phosphate; PRPP, phosphoribosylpyrophosphate; PRA, 5-phospho-b-D-ribosylamine; GAR, 5′-phosphoribosylglycinamide; FGAR, 5′-phosphoribosyl-N-formylglycinamide; FGAM, 5′-phosphoribosyl-N-formylglycinamidine; AIR, 5′-phosphoribosyl-5-aminoimidazole; NCAIR, 5′-phosphoribosyl-5-carboxyaminoimidazole; CAIR, 5′-phosphoribosyl-5-aminoimidazole-4-carboxylate; SAICAR, 5′-phosphoribosyl-4-(N-succino-carboxamide)-5-aminoimidazole; AICAR, 5′-phosphoribosyl-4-carboximide-5-aminoimidazole ribonucleotide; FAICAR, 5′-phosphoribosyl-4-carboximide-5-formaminoimidazole; IMP, inosine 5′-monophosphate; XMP, xanthosine monophosphate; GMP, guanosine monophosphate; GTP, guanosine triphosphate; sAMP, adenylosuccinate; AMP, adenosine monophosphate; ATP, adenosine triphosphate.
Table 7. Differential expression of selected genes involved in nucleotide metabolism upon contact with AF.
| 6180 | SF370 | Locus | ML | LL | S | Descriptions |
| M28_Spy0022 | SPy0024 | - | 36.60 | 10.73 | 35.84 | Phosphoribosylaminoimidazole-succinocarboxamide synthase |
| M28_Spy0024 | SPy0026 | purF | 24.03 | 10.89 | 13.45 | Amidophosphoribosyltransferase |
| M28_Spy0026 | SPy0028 | purN | 37.86 | 18.63 | 17.69 | Phosphoribosylglycinamide formyltransferase |
| M28_Spy0027 | purH | 17.83 | 3.68 | 3.85 | Phosphoribosylaminoimidazolecarboxamide formyltransferase | |
| M28_Spy0029 | SPy0032 | purD | 15.56 | 9.89 | 11.31 | Phosphoribosylamine-glycine ligase |
| M28_Spy0030 | SPy0033 | purE | 16.52 | 7.50 | 11.42 | Phosphoribosylaminoimidazole carboxylase carboxyltransferase |
| M28_Spy0031 | SPy0034 | purK | 6.05 | 11.19 | Phosphoribosylaminoimidazole carboxylase NCAIR mutase | |
| M28_Spy0064 | SPy0074 | adk | −8.25 | Adenylate kinase | ||
| M28_Spy0134 | SPy0160 | purA | 3.17 | Adenylosuccinate synthetase | ||
| M28_Spy0193 | SPy0235 | - | −2.67 | Deoxyuridine 5 -triphosphate nucleotidohydrolase | ||
| M28_Spy0316 | SPy0392 | upp | −2.43 | Uracil phosphoribosyltransferase | ||
| M28_Spy0334 | SPy0425 | nrdF.1 | −5.27 | Ribonucleoside-diphosphate reductase beta chain | ||
| M28_Spy0335 | SPy0426 | nrdI | −4.58 | NrdI protein | ||
| M28_Spy0336 | SPy0427 | nrdE.1 | −2.37 | Ribonucleoside-diphosphate reductase alpha chain | ||
| M28_Spy0597 | SPy0803 | cmk | 2.59 | Cytidylate kinase | ||
| M28_Spy0620 | SPy0831 | pyrP | 6.66 | Uracil permease | ||
| M28_Spy0622 | SPy0833 | carA | 4.79 | −2.17 | Carbamoyl-phosphate synthase small chain | |
| M28_Spy0624 | SPy0835 | carB | 5.93 | −2.87 | Carbamoyl-phosphate synthase large chain | |
| M28_Spy0658 | SPy0872 | - | 68.19 | 15.18 | 11.50 | 5 -nucleotidase |
| M28_Spy0668 | SPy0882 | thyA | −3.66 | Thymidylate synthase | ||
| M28_Spy0679 | SPy0894 | deoD2 | −2.85 | Purine nucleoside phosphorylase | ||
| M28_Spy0683 | SPy0900 | pyrF | 3.45 | Orotidine 5 -phosphate decarboxylase | ||
| M28_Spy0684 | SPy0901 | pyrE | 4.03 | Orotate phosphoribosyltransferase | ||
| M28_Spy0689 | SPy0907 | pyrC | 2.82 | 2.39 | 2.51 | Dihydroorotase |
| M28_Spy0708 | SPy0927 | apt | −2.13 | Adenine phosphoribosyltransferase | ||
| M28_Spy0821 | SPy1123 | - | 2.04 | Ribose-phosphate pyrophosphokinase | ||
| M28_Spy0831 | SPy1135 | guaC | 68.20 | 23.60 | 95.96 | GMP reductase |
| M28_Spy0832 | SPy1136 | xpt | 40.85 | 90.15 | Xanthine phosphoribosyltransferase | |
| M28_Spy0833 | SPy1137 | - | 9.77 | 45.75 | 76.46 | Xanthine permease |
| M28_Spy0897 | SPy1211 | rnhB | 2.92 | 8.45 | Anaerobic ribonucleoside-triphosphate reductase | |
| M28_Spy0899 | SPy1213 | fhs.1 | 5.53 | 4.21 | Formate–tetrahydrofolate ligase | |
| M28_Spy0914 | SPy1228 | - | 2.11 | Nucleoside-binding protein | ||
| M28_Spy1109 | SPy1368 | udk | −2.04 | Uridine kinase | ||
| M28_Spy1118 | SPy1378 | nrdF.2 | 2.32 | Ribonucleoside-diphosphate reductase beta chain | ||
| M28_Spy1159 | SPy1432 | pyrD | 2.15 | Dihydroorotate dehydrogenase | ||
| M28_Spy1466 | SPy1736 | - | 5.01 | 3.02 | 5.14 | Guanine-hypoxanthine permease |
| M28_Spy1580 | SPy1869 | udp | −2.83 | Uridine phosphorylase | ||
| M28_Spy1599 | SPy1894 | pyrG | −2.64 | CTP synthase | ||
| M28_Spy1758 | SPy2085 | fhs.2 | 3.32 | 2.32 | Formate–tetrahydrofolate ligase | |
| M28_Spy1773 | SPy2105 | nrdG | −2.00 | −2.45 | Anaerobic ribonucleoside-triphosphate reductase activating protein | |
| M28_Spy1777 | SPy2110 | nrdD | −2.22 | −3.50 | Anaerobic ribonucleoside-triphosphate reductase | |
| M28_Spy1890 | SPy2206 | guaB | −2.28 | Inosine-5 -monophosphate dehydrogenase |
Values represent fold change in expression in AF at ML, LL, and S growth phases compared to the expression in THY medium.
Samant et al. [48] recently showed that multiple enzymes involved in nucleotide metabolism are essential for growth of Escherichia coli in human serum, and a bacterial growth defect can be rescued by the addition of nucleotides to the serum. To test if a lack of nucleotides or arginine/ornithine in the AF is a growth limiting factor, we tested growth of GAS in AF with the addition of selected amino acids and nucleotides (arginine, ornithine, adenine, xanthine, and uracyl at concentrations corresponding to minimal chemically defined media (CDM) concentrations for GAS [49]). The observed culture densities after overnight growth did not increase compared to the AF without any supplements (Figure 5). Therefore, the lack of arginine and nucleotides in AF is not a limiting factor for growth of GAS and up regulation of the nucleotide synthesis pathway has a distinct function from providing material for DNA synthesis. Multiple reports have suggested a connection between nucleotide metabolism and bacterial virulence [50]. Mereghetti et al. recently reported changes in nucleotide metabolism in GBS during growth in blood and as an effect of a temperature switch [51], [52].
Figure 5. Addition of arginine, ornithine, purines and pirymidines does not increase growth of GAS in AF.
Cell densities of GAS cultures grown in pooled AF for 24 hours with addition of amino acids (arginine, ornithine) and nucleotides (xanthine, uracil, and adenine) at the concentration that supports growth of GAS in minimal CDM [49].
Summary
Group A Streptococcus, a causative agent of postpartum invasive infections, is able to survive and multiply in AF. It is able to multiply and survive over 48h in the AF environment. The response of GAS is in many aspects similar to the response exhibited by GBS; however, on the contrary to GBS, GAS exhibits stronger stress response to the AF environment. GAS adapts to growth in AF by differential regulation of genes involved in arginine and nucleotide metabolism. In addition, GAS down regulates multiple cell surface proteins, presumably to escape host immune recognition.
Supporting Information
Differential expression of all GAS genes in response to AF. Changes in transcription of GAS genes upon contact with amniotic fluid. All changes detected in transcription of GAS in response to amniotic fluid. Values represent fold change in expression in amniotic fluid compared to expression in THY; ML, mid-logarithmic growth phase; LL, late-logarithmic growth phase; S, stationary growth phase; cut-off two fold change with P value less than 0.05. Positive values represent genes up-regulated in AF (orange), negative values represent down-regulated (green, better expressed in THY) genes. Rows marked grey denote transcripts not detected during the experiment.
(0.62 MB XLS)
Comparison of transcriptional changes between GAS and GBS grown in AF. Differential expression of GAS and GBS genes in response to AF - Changes in transcription of GAS and GBS genes upon contact with amniotic fluid. Values represent fold change in expression in amniotic fluid compared to expression in THY; ML, mid-logarithmic growth phase; LL, late-logarithmic growth phase; S, stationary growth phase; cut-off two fold change with P value less than 0.05. Positive values represent genes up-regulated in AF (orange), negative values represent down-regulated (green, better expressed in THY) genes. GBS data after reference [19].
(0.34 MB XLS)
Acknowledgments
The authors would like to thank Kathryn E. Stockbauer for critical reading of the manuscript and Pam McShane for the coordination of AF collection at TMHRI.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The project was internally funded by The Methodist Hospital Research Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 3.Pasteur L. Septicemie puerperale. Bulletin de l'Academie de Medecine. 1879;8:271–274. [Google Scholar]
- 4.Chuang I, Van BC, Beall B, Schuchat A. Population-based surveillance for postpartum invasive group A Streptococcus infections, 1995–2000. Clin Infect Dis. 2002;35:665–670. doi: 10.1086/342062. [DOI] [PubMed] [Google Scholar]
- 5.Green NM, Zhang S, Porcella SF, Nagiec MJ, Barbian KD, et al. Genome sequence of a serotype M28 strain of group A Streptococcus: potential new insights into puerperal sepsis and bacterial disease specificity. J Infect Dis. 2005;192:760–770. doi: 10.1086/430618. [DOI] [PubMed] [Google Scholar]
- 6.Green NM, Beres SB, Graviss EA, Allison JE, McGeer AJ, et al. Genetic diversity among type emm28 group A Streptococcus strains causing invasive infections and pharyngitis. J Clin Microbiol. 2005;43:4083–4091. doi: 10.1128/JCM.43.8.4083-4091.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome”. Proc Natl Acad Sci U S A. 2005;102:13950–13955. doi: 10.1073/pnas.0506758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mylvaganam H, Bruun T, Vindenes HA, Langeland N, Skrede S. Molecular epidemiological investigation of an outbreak of invasive beta-haemolytic streptococcal infection in western Norway. Clin Microbiol Infect. 2009;15:245–252. doi: 10.1111/j.1469-0691.2008.02664.x. [DOI] [PubMed] [Google Scholar]
- 9.Pinho MD, Melo-Cristino J, Ramirez M. Clonal relationships between invasive and noninvasive Lancefield group C and G streptococci and emm-specific differences in invasiveness. J Clin Microbiol. 2006;44:841–846. doi: 10.1128/JCM.44.3.841-846.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham MR, Virtaneva K, Porcella SF, Barry WT, Gowen BB, et al. Group A Streptococcus transcriptome dynamics during growth in human blood reveals bacterial adaptive and survival strategies. Am J Pathol. 2005;166:455–465. doi: 10.1016/S0002-9440(10)62268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shelburne SA, III, Sumby P, Sitkiewicz I, Granville C, DeLeo FR, et al. Central role of a bacterial two-component gene regulatory system of previously unknown function in pathogen persistence in human saliva. Proc Natl Acad Sci U S A. 2005;102:16037–16042. doi: 10.1073/pnas.0505839102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voyich JM, Sturdevant DE, Braughton KR, Kobayashi SD, Lei B, et al. Genome-wide protective response used by group A Streptococcus to evade destruction by human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 2003;100:1996–2001. doi: 10.1073/pnas.0337370100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldmann O, von Kockritz-Blickwede M, Holtje C, Chhatwal GS, Geffers R, et al. Transcriptome analysis of murine macrophages in response to infection with Streptococcus pyogenes reveals an unusual activation program. Infect Immun. 2007;75:4148–4157. doi: 10.1128/IAI.00181-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eidelman AI, Nevet A, Rudensky B, Rabinowitz R, Hammerman C, et al. The effect of meconium staining of amniotic fluid on the growth of Escherichia coli and group B Streptococcus. J Perinatol. 2002;22:467–471. doi: 10.1038/sj.jp.7210774. [DOI] [PubMed] [Google Scholar]
- 15.Pommerenke WT, Taylor PW. Antibacterial properties of vaginal and cervical secretions and amniotic fluid. Ann Ostet Ginecol. 1953;75:891–899. [PubMed] [Google Scholar]
- 16.Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J Infect Dis. 2005;192:771–782. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- 17.Sitkiewicz I, Musser JM. Expression microarray and mouse virulence analysis of four conserved two-component gene regulatory systems in group A Streptococcus. Infect Immun. 2006;74:1339–1351. doi: 10.1128/IAI.74.2.1339-1351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shelburne SA, III, Keith D, Horstmann N, Sumby P, Davenport MT, et al. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc Natl Acad Sci U S A. 2008;105:1698–1703. doi: 10.1073/pnas.0711767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sitkiewicz I, Green NM, Guo N, Bongiovanni AM, Witkin SS, et al. Transcriptome adaptation of group B Streptococcus to growth in human amniotic fluid. PLoS One. 2009;4:e6114. doi: 10.1371/journal.pone.0006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sozanskii A. The biochemical composition of amniotic fluid and of maternal and fetal blood at various periods of pregnancy. Biull Eksp Biol Med. 1961;51:323–326. doi: 10.1007/BF00783508. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson S, Ramstrom M, Palmblad M, Axelsson O, Bergquist J. Explorative study of the protein composition of amniotic fluid by liquid chromatography electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. J Proteome Res. 2004;3:884–889. doi: 10.1021/pr0499545. [DOI] [PubMed] [Google Scholar]
- 22.Modena AB, Fieni S. Amniotic fluid dynamics. Acta Biomed. 2004;75(Suppl 1):11–13. [PubMed] [Google Scholar]
- 23.Beck A, Bergner-Rabinowitz S, Ofek I. Effect of pH on in vitro phagocytosis of Streptococcus pyogenes. J Bacteriol. 1969;100:1204–1207. doi: 10.1128/jb.100.3.1204-1207.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galask RP, Snyder IS. Antimicrobial factors in amniotic fluid. Am J Obstet Gynecol. 1970;106:59–65. doi: 10.1016/0002-9378(70)90126-2. [DOI] [PubMed] [Google Scholar]
- 25.Larsen B, Snyder IS, Galask RP. Bacterial growth inhibition by amniotic fluid. I. In vitro evidence for bacterial growth-inhibiting activity. Am J Obstet Gynecol. 1974;119:492–496. doi: 10.1016/0002-9378(74)90207-5. [DOI] [PubMed] [Google Scholar]
- 26.Larsen B, Snyder IS, Galask RP. Bacterial growth inhibition by amniotic fluid. 2. Reversal of amniotic fluid bacterial growth inhibition by addition of a chemically defined medium. Am J Obstet Gynecol. 1974;119:497–501. doi: 10.1016/0002-9378(74)90208-7. [DOI] [PubMed] [Google Scholar]
- 27.Evans HE, Levy E, Glass L. Effect of amniotic fluid on bacterial growth. Obstet Gynecol. 1977;49:35–37. [PubMed] [Google Scholar]
- 28.Ahn YJ, Park SK, Oh JW, Sun HY, Shin SH. Bacterial growth in amniotic fluid is dependent on the iron-availability and the activity of bacterial iron-uptake system. J Korean Med Sci. 2004;19:333–340. doi: 10.3346/jkms.2004.19.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran SH, Caughey AB, Musci TJ. Meconium-stained amniotic fluid is associated with puerperal infections. Am J Obstet Gynecol. 2003;189:746–750. doi: 10.1067/s0002-9378(03)00767-1. [DOI] [PubMed] [Google Scholar]
- 30.Purushothaman SS, Park HS, Cleary PP. Promotion of fibronectin independent invasion by C5a peptidase into epithelial cells in group A Streptococcus. Indian J Med Res. 2004;119(Suppl):44–47. [PubMed] [Google Scholar]
- 31.Stalhammar-Carlemalm M, Areschoug T, Larsson C, Lindahl G. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol Microbiol. 1999;33:208–219. doi: 10.1046/j.1365-2958.1999.01470.x. [DOI] [PubMed] [Google Scholar]
- 32.Nair S, Poyart C, Beretti JL, Veiga-Fernandes H, Berche P, et al. Role of the Streptococcus agalactiae ClpP serine protease in heat-induced stress defence and growth arrest. Microbiology. 2003;149:407–417. doi: 10.1099/mic.0.25783-0. [DOI] [PubMed] [Google Scholar]
- 33.Robertson GT, Ng WL, Foley J, Gilmour R, Winkler ME. Global transcriptional analysis of clpP mutations of type 2 Streptococcus pneumoniae and their effects on physiology and virulence. J Bacteriol. 2002;184:3508–3520. doi: 10.1128/JB.184.13.3508-3520.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giard JC, Rince A, Capiaux H, Auffray Y, Hartke A. Inactivation of the stress- and starvation-inducible gls24 operon has a pleiotrophic effect on cell morphology, stress sensitivity, and gene expression in Enterococcus faecalis. J Bacteriol. 2000;182:4512–4520. doi: 10.1128/jb.182.16.4512-4520.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malke H, Steiner K, McShan WM, Ferretti JJ. Linking the nutritional status of Streptococcus pyogenes to alteration of transcriptional gene expression: the action of CodY and RelA. Int J Med Microbiol. 2006;296:259–275. doi: 10.1016/j.ijmm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Kreikemeyer B, Beckert S, Braun-Kiewnick A, Podbielski A. Group A streptococcal RofA-type global regulators exhibit a strain-specific genomic presence and regulation pattern. Microbiology. 2002;148:1501–1511. doi: 10.1099/00221287-148-5-1501. [DOI] [PubMed] [Google Scholar]
- 37.Beres SB, Richter EW, Nagiec MJ, Sumby P, Porcella SF, et al. Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group A Streptococcus. Proc Natl Acad Sci U S A. 2006;103:7059–7064. doi: 10.1073/pnas.0510279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen RJ, Sitkiewicz I, Ayeras AA, Gonulal VE, Cantu C, et al. Decreased necrotizing fasciitis capacity caused by a single nucleotide mutation that alters a multiple gene virulence axis. Proc Natl Acad Sci U S A. 2010;107:888–893. doi: 10.1073/pnas.0911811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreikemeyer B, Boyle MD, Buttaro BA, Heinemann M, Podbielski A. Group A streptococcal growth phase-associated virulence factor regulation by a novel operon (Fas) with homologies to two-component-type regulators requires a small RNA molecule. Mol Microbiol. 2001;39:392–406. doi: 10.1046/j.1365-2958.2001.02226.x. [DOI] [PubMed] [Google Scholar]
- 40.Steiner K, Malke H. Dual control of streptokinase and streptolysin S production by the covRS and fasCAX two-component regulators in Streptococcus dysgalactiae subsp. equisimilis. Infect Immun. 2002;70:3627–3636. doi: 10.1128/IAI.70.7.3627-3636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shelburne SA, III, Keith DB, Davenport MT, Horstmann N, Brennan RG, et al. Molecular characterization of group A Streptococcus maltodextrin catabolism and its role in pharyngitis. Mol Microbiol. 2008;69:436–452. doi: 10.1111/j.1365-2958.2008.06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loughman JA, Caparon MG. A novel adaptation of aldolase regulates virulence in Streptococcus pyogenes. EMBO J. 2006;25:5414–5422. doi: 10.1038/sj.emboj.7601393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierce WA, White AG. Arginine and glucose metabolism in a strain of Streptococcus pyogenes. J Bacteriol. 1955;69:230–231. doi: 10.1128/jb.69.2.230-231.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Degnan BA, Fontaine MC, Doebereiner AH, Lee JJ, Mastroeni P, et al. Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect Immun. 2000;68:2441–2448. doi: 10.1128/iai.68.5.2441-2448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quivey RG, Kuhnert WL, Hahn K. Genetics of acid adaptation in oral streptococci. Crit Rev Oral Biol Med. 2001;12:301–314. doi: 10.1177/10454411010120040201. [DOI] [PubMed] [Google Scholar]
- 46.Degnan BA, Palmer JM, Robson T, Jones CE, Fischer M, et al. Inhibition of human peripheral blood mononuclear cell proliferation by Streptococcus pyogenes cell extract is associated with arginine deiminase activity. Infect Immun. 1998;66:3050–3058. doi: 10.1128/iai.66.7.3050-3058.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lei B, Mackie S, Lukomski S, Musser JM. Identification and immunogenicity of group A Streptococcus culture supernatant proteins. Infect Immun. 2000;68:6807–6818. doi: 10.1128/iai.68.12.6807-6818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samant S, Lee H, Ghassemi M, Chen J, Cook JL, et al. Nucleotide biosynthesis is critical for growth of bacteria in human blood. PLoS Pathog. 2008;4:e37. doi: 10.1371/journal.ppat.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Rijn I, Kessler RE. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980;27:444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pettersson J, Schrumpf ME, Raffel SJ, Porcella SF, Guyard C, et al. Purine salvage pathways among Borrelia species. Infect Immun. 2007;75:3877–3884. doi: 10.1128/IAI.00199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mereghetti L, Sitkiewicz I, Green NM, Musser JM. Extensive adaptive changes occur in the transcriptome of Streptococcus agalactiae (group B Streptococcus) in response to incubation with human blood. PLoS One. 2008;3:e3143. doi: 10.1371/journal.pone.0003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mereghetti L, Sitkiewicz I, Green NM, Musser JM. Remodeling of the Streptococcus agalactiae transcriptome in response to growth temperature. PLoS One. 2008;3:e2785. doi: 10.1371/journal.pone.0002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differential expression of all GAS genes in response to AF. Changes in transcription of GAS genes upon contact with amniotic fluid. All changes detected in transcription of GAS in response to amniotic fluid. Values represent fold change in expression in amniotic fluid compared to expression in THY; ML, mid-logarithmic growth phase; LL, late-logarithmic growth phase; S, stationary growth phase; cut-off two fold change with P value less than 0.05. Positive values represent genes up-regulated in AF (orange), negative values represent down-regulated (green, better expressed in THY) genes. Rows marked grey denote transcripts not detected during the experiment.
(0.62 MB XLS)
Comparison of transcriptional changes between GAS and GBS grown in AF. Differential expression of GAS and GBS genes in response to AF - Changes in transcription of GAS and GBS genes upon contact with amniotic fluid. Values represent fold change in expression in amniotic fluid compared to expression in THY; ML, mid-logarithmic growth phase; LL, late-logarithmic growth phase; S, stationary growth phase; cut-off two fold change with P value less than 0.05. Positive values represent genes up-regulated in AF (orange), negative values represent down-regulated (green, better expressed in THY) genes. GBS data after reference [19].
(0.34 MB XLS)