Abstract
Most healthy individuals display a subtle spatial attentional bias, exhibiting relative inattention for stimuli on one side of the visual field, a phenomenon known as pseudoneglect. Prior work in animals and patients has implicated dopamine in spatial attention asymmetries. The current study therefore examined - in healthy individuals - the relationship between the attentional bias and spontaneous eye-blink rate (EBR), a putative measure of central dopaminergic function. We found that those individuals, who blinked more often under resting conditions, displayed greater preference for the right side of the visual display in a subsequent attention task. This finding may support the idea that the observed attentional bias in healthy individuals reflects asymmetries in dopaminergic circuits, and corroborates previous findings implicating dopamine in spatial attention.
Introduction
Following unilateral brain damage, patients often display a spatial attentional bias towards the side of the lesion, and reduced awareness of stimuli on the other side of space (Heilman, Watson, & Valenstein, 1985). Interestingly, healthy individuals display similar, although generally more subtle attentional biases, reflecting the phenomenon of pseudoneglect (Nicholls, Bradshaw, & Mattingley, 1999). For example, the greyscales (GS) task, which has been shown to be sensitive to pathological attentional biases in patients, also revealed biases in spatial attention in healthy individuals (Mattingley, Berberovic, Corben, Slavin, Nicholls & Bradshaw, 2004). In this task, individuals are required to indicate which of two, mirror reversed, incrementally shaded rectangles, from dark on one side to light on the other, appears overall darker. Although the two rectangles are equal in luminance, healthy individuals designate the rectangle that is darker on the left-hand side as darker overall on about two thirds of trials (Nicholls et al., 1999). This consistent leftward bias is thought to reflect the preferential activation of the right hemisphere, due to the spatial component of the brightness comparison, which induces a contralateral bias of attention (Kinsbourne, 1993). Notably, although overall, healthy individuals show a leftward bias, there is large individual variability in the magnitude and direction of the attentional bias, with a small percentage of individuals (about 15%) showing a bias towards the right side of space (Nicholls et al., 1999; Tomer, 2008). Using the GS task, Tomer (2008) recently reported a consistent magnitude and direction of attentional bias across two separate testing sessions in healthy individuals, suggesting that this bias may reflect an individual trait.
Based on findings in patient populations and animal studies, it has been suggested that the observed attentional bias in healthy individuals may reflect asymmetries in dopaminergic circuits (Tomer, 2008). First, improvement of neglect behavior has been reported in patients treated with a dopamine (DA) agonist (Fleet, Valenstein, Watson & Heilman, 1987). Second, it has been shown that intact rats consistently orient contralaterally to the striatum with higher DA activity and that, following unilateral DA depletion induced by 6-OHDA, both rats (Glick & Shapiro, 1985) and marmoset monkeys (Milton et al., 2004) exhibit an acute unilateral syndrome, similar to contralesional spatial neglect resulting from an experimentally induced middle cerebral artery stroke. Third, studies in both healthy children and children with attention deficit hyperactivity disorder (ADHD) have reported associations with variants of the dopamine transporter gene (DAT1) and the control of spatial attention across the hemifields (Bellgrove et al., 2007; Bellgrove, Hawi, Kirly, Gill, & Robertson, 2005). Lastly, in ADHD, left-sided inattention is improved by treatment with methylphenidate, which inhibits the DAT1 (Sheppard, Bradshaw, Mattingley, & Lee, 1999). These demonstrations that modulations of, and disturbances in striatal DA function influence the attentional bias provide support for the suggestion that the observed attentional bias in healthy individuals reflects asymmetries in dopaminergic circuits (Tomer, 2008).
The present study aimed at gaining a better understanding of the neuropharmacology underlying the attentional bias in healthy individuals. Specifically, it examined the relationship between the direction and degree of attentional bias, as measured using the GS task, and spontaneous eye-blink rate (EBR), a putative measure of tonic DA level (Karson, 1983). Convergent evidence shows that EBR, or the frequency of blinks per minute under resting conditions, is regulated in part by DA. Most importantly, EBR is elevated by DA agonists and reduced by DA antagonists (Kleven & Koek, 1996; Lawrence & Redmond, 1990). Furthermore, EBR is reduced in Parkinson’s disease (PD) and this reduction is correlated with disease severity and occurs even when the signs and symptoms of the illness are mild (Karson, Burns, LeWitt, Foster, & Newman, 1984). Since severity of motor signs in PD is correlated with the degree of loss of DA activity in the striatum (Tatsch et al., 1997), the significant relationship between EBR and motor signs in PD supports the notion that EBR may serve as an index of dopaminergic activity in the striatum. This notion is further supported by experimental manipulation of the dopaminergic system in monkeys; Taylor et al. (1999) reported that in MPTP-treated monkeys, severity of Parkinsonism was inversely correlated with EBR, and EBR significantly correlated with concentration of DA in the caudate nucleus. Several other lines of research provide additional support for a link between EBR and striatal dopaminergic activity. First, recreational cocaine users, who display reduced D2 receptor activity, show reduced EBRs (Colzato, van den Wildenberg, & Hommel, 2008b). Second, individuals with repetitive behaviour disorders, which are associated with lower levels of plasma concentrations of the dopamine metabolite homovanillic acid (HVA) (Lewis et al. 1996), generally show lower EBR (Bodfish, Powell, Golden, & Lewis, 1995; MacLean et al., 1985). Third and lastly, a genetic study in humans demonstrated a strong association between EBR and the DRD4/7 genotype, which is related to the control of striatal DA release (Dreisbach et al., 2005). Thus, convergent evidence from different lines of research indicates that striatal DA activity regulates EBR.
Since spontaneous EBR reflects striatal DA activity, and given that the attentional bias has been linked to asymmetries in the nigrostriatal DA circuits, the current study examined the relationship between EBR and the direction and degree of attentional bias in healthy individuals. As mentioned earlier, Bellgrove and colleagues (2005; 2007) reported that children who were homozygous for the 10-repeat allele of the 3′-UTR VNTR of the DAT gene, had an attenuated leftward attentional bias as compared to children who were heterozygotes for this allele. Based on in vitro studies showing association between the 10-repeat DAT1 allele and increased expression level of the transporter (Fuke et al., 2001; Mill et al., 2002), the authors interpreted their finding as suggesting that the 10-repeat allele is associated with decreased dopamine signaling at the level of the right striatum (Bellgrove et al., 2005), or the right parietal lobe (Bellgrove et al., 2007). If attention is biased ipsilaterally to the hemisphere with lower dopamine signaling, this decreased dopaminergic signaling in the right hemisphere (associated with the homozygous 10-repeat allele) should be associated with rightward attentional bias. Taken together with evidence (described earlier) suggesting that EBR may be used as an index of tonic DA activity, we predicted a correlation between EBR and attentional bias, with higher blink rate associated with greater bias toward the right hemispace.
2. Methods
2. 1. Participants
23 right-handed participants (12 women, 18 to 29 years old, median age 20.0 years) were paid $10 per hour for their participation. They had no clinically significant medical disease, no history of mental or neurological illness, and normal or corrected-to-normal vision. The study was approved by the university’s ethics committee.
2.2. Eyeblink rate recordings
Eye blinks were recorded with two vertical Ag–AgCl electrodes above and below the left eye, for 6-min eyes-open segments under resting conditions (cf. Colzato, van Wouwe,, & Hommel, 2007; Colzato, Slagter, Spape, & Hommel, 2008; Colzato, Slagter, van den Wildenberg, & Hommel, 2009). A ground electrode was placed on the forehead. Impedances were kept below 20 kΩ. Given that spontaneous EBR is stable during daytime, but increases in the evening (Barbato et al., 2000), data were never collected after 5 p.m. In addition, we asked participants to avoid alcohol and nicotine consumption and to sleep sufficiently the day before the recording. During recordings, participants did not wear contact lenses, were alone in the room, and sat upright and silent. They were asked to look straight ahead, to not visually fixate on a particular object, and were not instructed in any manner about blinking.
2.3. The greyscales task
After the EBR recordings, participants performed the computerized version of the greyscales task (Nicholls et al., 1999). This task requires participants to judge which of two brightness gradients (greyscales) appears darker overall. Each stimulus pair includes one greyscale shaded (in 80 increments) from black on the left to white on the right and one greyscale shaded in the reverse direction (Figure 1). The horizontal midlines of the stimuli are aligned with the center of the display screen, and the stimuli are aligned vertically (one above the other), such that choices (top vs. bottom) are orthogonal to the direction of the gradients, reducing the potential influence of response biases. They are 79 pixels high. Their lengths vary between 320, 400, 480, 560, 640, and 720 pixels (the same for both stimuli in a pair). Each pair of stimuli is presented on the screen until a response is made and maximally for 5000 ms. Following a response, the screen is cleared and a central fixation cross is shown on which participants have to fixate their gaze for 1000 ms before the next pair of greyscales is presented. Stimuli were shown using Eprime 2.0.
Figure 1.
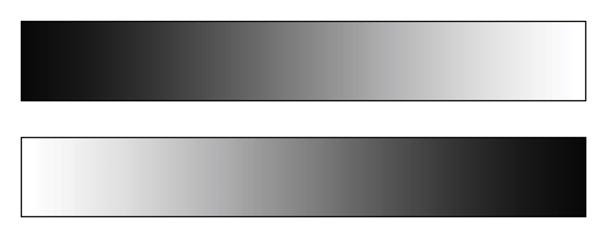
Example of one of the greyscale stimuli.
During an initial practice block, participants familiarized themselves with the task by performing 12 trials in which the relative luminance of the two stimuli was adjusted so that one stimulus was clearly darker than the other. In a subsequent “test” block, they performed 96 trials in which the relative luminance of two stimuli was adjusted so that one stimulus was only slightly darker than the other. Without any notice or break, they then continued to do another 72 trials in which greyscales within a pair were identical in overall luminance, but left–right mirror-reversed. Thus, in this “bias” block, the two stimuli were physically identical, but one greyscale was darker at the right end and the other was darker at the left end. This permitted us to determine each individual’s attentional bias. Stimuli of each length were presented 12 times in pseudorandom order, with the positions of the rectangles (top/bottom) counterbalanced.
Participants were asked to align their midlines with the center of the screen, and the viewing distance was 65 cm. They were asked to press the up or down arrow key on the computer keyboard to indicate the top or bottom rectangle, respectively. Accuracy of response was stressed as important rather than speed, but participants were told to respond while the stimuli are present on the screen. Responses were categorized as either left or right according to whether participants selected the rectangle that was dark on its ‘left’ or ‘right’ side, respectively.
3. Analysis
Based on the behavioral data from the “bias” block, an asymmetry index (AI) was calculated for each subject, according to the following formula: AI = [number of right responses−number of left responses]/total. The values of this index can vary between −1.0 and +1.0, with negative scores indicating a leftward bias and positive scores indicating a rightward bias. We also calculated and compared the percentage of left vs. right errors in the test block to determine the presence of a left attentional bias in this block. Each individual’s EBR was computed according to automatic and manual procedures using Matlab. First, a voltage threshold was determined that appeared to capture most blinks, and little artifacts (e.g., muscle-related artifacts) in the data. Then, 20-sec epochs were visually inspected for detection accuracy, i.e., the presence/absence of blinks. To test our main hypothesis, that a higher blink rate is associated with a greater bias toward the right hemispace, a Pearson correlation test was run which examined the relationship between EBR and the AI.
4. Results
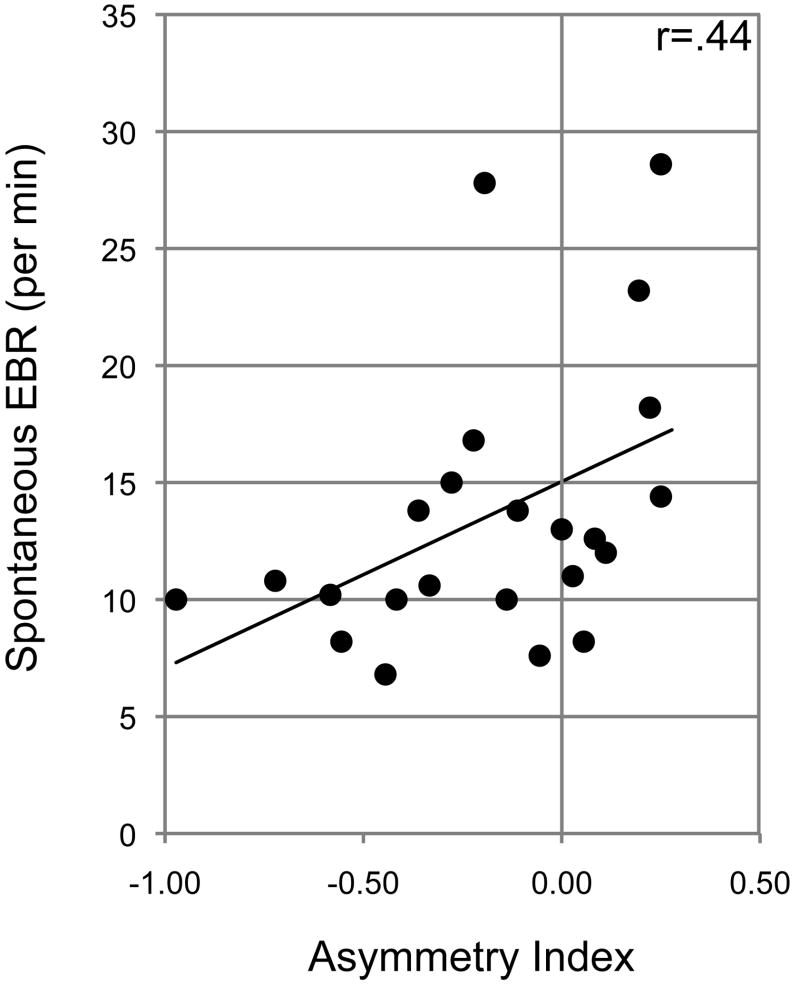
Replicating previous findings, on average, participants displayed a slight bias towards the left hemispace (mean AI = −0.18; standard deviation (SD) = 0.33; Nicolls et al., 1999; Tomer, 2008) and blinked 13.6 times per minute (SD = 5.9; Colzato et al., 2008, 2009; Depue et al., 1994). A one sample t-test, comparing the mean AI to zero, confirmed the presence of a left attentional bias at the group level (p=.011). Furthermore, as in previous studies, large variability was observed across individuals both in the magnitude and direction of the attentional bias and in EBR. Importantly, as predicted, those individuals who blinked relatively often, generally showed a stronger bias towards the right hemispace, as reflected by a significant correlation between EBR and AI (r(n=23) = 0.44; p=.036) (Figure 2).
Figure 2.
Relationship between the magnitude and degree of attentional bias and central dopaminergic activity, as indexed by EBR.
The mean correct responses during the test block was 71.7% (±12.2). As would be expected, participants as a group made slightly more errors choosing left when right was the correct response (left errors; 58.2% ± 21.96) than when choosing right when left would have been correct (right errors; 41.8% ± 21.96) (p=.087). This error bias was also reflected in a modest left AI for the errors (i.e., (right errors − left errors)/total number of errors) in the test block: −0.16 (differed at trend level from zero; p=.08). Thus, the test block also revealed a left attentional bias, although this bias was somewhat weaker than the bias that was observed in the bias block. The correlation between the Error AI and the Bias AI was highly significant (r(23)=.73; p<.0001), suggesting that the attentional bias is reflected in both measures.
5. Discussion
The current study examined the relationship between spontaneous EBR, a functional marker of central DA function, and the attentional bias in healthy individuals. Our main finding was that spontaneous EBR predicted the magnitude and direction of the attentional bias: Those individuals who blinked relatively often, showed a stronger attentional bias towards the right hemispace. Although the correlative nature of our finding does not directly speak to the underlying causal relations, together with prior work in animals and patients (discussed below), it supports the idea that asymmetries in striatal DA activity may influence the tendency of a healthy individual to attend to one versus the other side of space (Tomer, 2008).
As mentioned in the introduction, spontaneous EBR provides a well-established, albeit indirect, measure of striatal DA function. Yet, at present, it is still unclear to what extent this measure reflects left versus right striatal DA function. There is indirect evidence from two lines of research suggesting that higher DA activity in the left striatum may drive both higher EBR and a rightward attentional bias. First, in schizophrenic patients, both increased EBR (Karson, 1990; Mackert, Flectner, Woyth & Frick, 1991) and greater left than right dopaminergic activity in the striatum have been reported (Farde et al.,1990; Nozaki et al., 2009). Second, children with left, but not right, focal epileptic activity displayed significantly lower EBR than those with a right focus (Caplan et al., 1998), and epilectic patients show lower DA D2 receptor binding in the left basal ganglia (Ring et al., 1992). These findings provide indirect support for the idea that greater activity in left striatal structures may drive both higher EBR and a rightward attentional bias (Tomer, 2008). Neuroimaging studies using positron emission tomography (PET) are necessary to further establish the link between asymmetries in striatal DA activity, EBR, and the tendency of a healthy individual to attend to one versus the other side of space.
Other neurotransmitters likely also play a role in spatial attention. Indeed, contemporary models of attention emphasize the importance of cholinergic and noradrenergic mechanisms in modulating spatial attention (e.g., Coull, 1998; Posner & Peterson, 1990). Yet, as argued above, and as others have previously argued (Bellgrove et al., 2007), there are good reasons to expect a role for dopamine in directed attention, in particular across hemifields. First, clinical neglect in humans improves by treatment with dopamine agonists (Fleet et al., 1987). Second, following unilateral DA depletion induced by 6-OHDA, rats (Glick & Shapiro, 1985) and monkeys (Milton et al., 2004) display neglect of the hemispace contralateral to the lesion. Third, studies in both healthy children and children with attention deficit hyperactivity disorder (ADHD) have reported associations with variants of the dopamine transporter gene (DAT1) and the control of spatial attention across the hemifields (Bellgrove et al., 2007; Bellgrove, Hawi, Kirly, Gill, & Robertson, 2005). Also, in ADHD, left-sided inattention is improved by treatment with methylphenidate, which inhibits the DAT1 (Sheppard, Bradshaw, Mattingley, & Lee, 1999). The current observation that spontaneous eye blink rate, a functional marker of central DA activity, predicts spatial attention asymmetries in healthy adults adds to this body of work supporting a role for dopamine in directing spatial attention. Clearly, future studies using dopamine neuromodulators or PET are needed to determine more precisely the role of dopamine in spatial attention asymmetries and spatial attention in general.
Acknowledgments
We would like to thank Lorenza Colzato for methodological advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbato G, Ficca G, Muscettola G, Fichele M, Beatrice M, Rinaldi F. Diurnal variation of spontaneous eye-blink rate. Psychiatry Research. 2000;93:145–151. doi: 10.1016/s0165-1781(00)00108-6. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Chambers CD, Johnson KA, Daibhis A, Daly M, Hawi Z, et al. Dopaminergic genotype biases spatial attention in healthy children. Molecular Psychiatry. 2007;12:786–792. doi: 10.1038/sj.mp.4002022. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Gill M, Hawi Z, Kirley A, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- BodWsh JW, Powell SB, Golden RN, Lewis MH. Blink rate as an index of dopamine function in adults with mental retardation and repetitive behavior disorders. Am J Ment Retard. 1995;99:335–344. [PubMed] [Google Scholar]
- Caplan R, Guthrie D, Komo S, Shields WD. Blink rate in pediatric complex partial seizure disorder. Journal of Child Psychology and Psychiatry. 1998;39:1145–1152. [PubMed] [Google Scholar]
- Colzato LS, Slagter HA, Spape MMA, Hommel B. Blinks of the eye predict blinks of the mind. Neuropsychologia. 2008;46:3179–3183. doi: 10.1016/j.neuropsychologia.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Slagter HA, van den Wildenberg WPM, Hommel B. Closing one’s eyes to reality: evidence for a dopaminergic basis of psychoticism from spontaneous eye blink rates. Personality and Individual Differences. 2009;46:377–380. [Google Scholar]
- Colzato LS, van den Wildenberg WPM, Hommel B. Reduced spontaneous eye blink rates in recreational cocaine users: evidence for dopaminergic hypoactivity. PLoS ONE. 2008b;2(11):e.1143. doi: 10.1371/journal.pone.0003461. http://dx.plos.org/10.1371/journal.pone.0003461. [DOI] [PMC free article] [PubMed]
- Colzato LS, van Wouwe NC, Hommel B. Spontaneous eye blink rate predicts the strenght of visuomotor binding. Neuropsychologia. 2007;45:2387–2392. doi: 10.1016/j.neuropsychologia.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Coull JT. Neural correlates of attention and arousal. Insights from electrophysiology, functional neuroimaging, and psychopharmacology. Progress in Neurobiology. 1998;55(4):343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Depue RA, Luciana M, Arbisi P, Collins P, Leon A. Dopamine and the structure of personality: Relation of agonist-induced dopamine activity on positive emotionality. Journal of Personality and Social Psychology. 1994;67(3):485–498. doi: 10.1037//0022-3514.67.3.485. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Müller J, Goschke T, Strobel A, Schulze K, Lesch K, Brocke B. Dopamine and cognitive control: the influence of spontaneous eyeblink rate and dopamine gene polymorphisms on perseveration and distractibility. Behav Neurosci. 2005;119:483–490. doi: 10.1037/0735-7044.119.2.483. [DOI] [PubMed] [Google Scholar]
- Farde L, Wiesel FA, Stone-Elander S, Halldin C, Nordström AL, Hall H, Sedvall G. D2 dopamine receptors in neuroleptic-naive schizophrenic patients. A positron emission tomography study with [11C] raclopride. Archives of General Psychiatry. 1990;47:213–219. doi: 10.1001/archpsyc.1990.01810150013003. [DOI] [PubMed] [Google Scholar]
- Fleet WS, Valenstein E, Watson RT, Heilman KM. Dopamine agonist therapy for neglect in humans. Neurology. 1987;37:1765–1770. doi: 10.1212/wnl.37.11.1765. [DOI] [PubMed] [Google Scholar]
- Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1:152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Glick SD, Shapiro RM. Functional and neurochemical mechanisms of cerebral lateralization in rats. In: Glick SD, editor. Cerebral lateralization in nonhuman species. New York: Academic Press; 1985. pp. 157–183. [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. New York: Oxford University Press; 1985. pp. 243–293. [Google Scholar]
- Karson CN. Spontaneous eye-blink rates and dopaminergic systems. Brain. 1983;106:643–653. doi: 10.1093/brain/106.3.643. [DOI] [PubMed] [Google Scholar]
- Karson CN. Blink rate in schizophrenia. Schizophrenia Bulletin. 1990;16:345–354. doi: 10.1093/schbul/16.2.345. [DOI] [PubMed] [Google Scholar]
- Karson CN, Burns RS, LeWitt PA, Foster NL, Newman RP. Blink rates and disorders of movement. Neurology. 1984;34:677–678. doi: 10.1212/wnl.34.5.677. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Differential effects of direct and indirect dopamine agonists on eye blink rate in cynomolgus monkeys. The Journal of Pharmacology & Experimental Therapeutics. 1996;279:1211–1219. [PubMed] [Google Scholar]
- Lawrence MS, Redmond DE. MPTP lesions and dopaminergic drugs alter eye blink rate in African green monkeys. Pharmacology, Biochemistry and Behavior. 1990;38:869–874. doi: 10.1016/0091-3057(91)90255-z. [DOI] [PubMed] [Google Scholar]
- Lewis MH, BodWsh JW, Powell SB, Wiest K, Darling M, Golden RN. Plasma HVA in adults with mental retardation and stereo-typed behavior: biochemical evidence for a dopamine deWciency model. Am J Ment Retard. 1996;100:413–427. [PubMed] [Google Scholar]
- Mackert A, Flechtner KM, Woyth C, Frick K. Increased blink rates in schizophrenics. Influences of neuroleptics and psychopathology. Schizophrenia Research. 1991;4:41–47. doi: 10.1016/0920-9964(91)90008-f. [DOI] [PubMed] [Google Scholar]
- MacLean WE, Jr, Lewis MH, Bryson-Brockmann WA, Ellis DN, Arendt RE, Baumeister AA. Blink rate and stereotyped behavior: evidence for dopamine involvement? Biol Psychiatry. 1985;20:1321–1325. doi: 10.1016/0006-3223(85)90117-9. [DOI] [PubMed] [Google Scholar]
- Mattingley JB, Berberovic N, Corben L, Slavin MJ, Nicholls ME, Bradshaw DL. The greyscales task: A perceptual measure of attentional bias following unilateral hemispheric damage. Neuropsychologia. 2004;42(3):387–394. doi: 10.1016/j.neuropsychologia.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by The 3′ UTR VNTR: evidence from brain and lymphocytes using Quantitative RT-PCR. Am J Med Genet. 2002;114B:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Milton AL, Marshall JWB, Cummings RM, Baker HF, Ridley RM. Dissociation of hemi-spatial and hemi-motor impairments in a unilateral primate model of Parkinson’s disease. Behavioural Brain Research. 2004;150:55–63. doi: 10.1016/S0166-4328(03)00231-6. [DOI] [PubMed] [Google Scholar]
- Nicholls ME, Bradshaw DL, Mattingley JB. Free-viewing perceptual asymmetries for the judgement of brightness, numerosity and size. Neuropsychologia. 1999;37(3):307–314. doi: 10.1016/s0028-3932(98)00074-8. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Kato M, Takano H, Ito H, Takahashi H, Arakawa R, et al. Regional dopamine synthesis in patients with schizophrenia using L-[β-11C]DOPA PET. Schizophrenia Research. 2009;108:78–84. doi: 10.1016/j.schres.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Ring HA, Trimble MR, Costa DC, George MS, Verhoeff NP, Ell PJ. Effect of vigabatrin on striatal dopamine receptors: Evidence in humans for interactions of GABA and dopamine systems. Journal of Neurology, Neurosurgery and Psychiatry. 1992;55:758–761. doi: 10.1136/jnnp.55.9.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DM, Bradshaw JL, Mattingley JB, Lee P. Effects of stimulant medication on the lateralization of line bisection judgements of children with attention deficit hyperactivity disorder. Journal of Neurology, Neurosurgery, and Psychiatry. 1999;66:57–63. doi: 10.1136/jnnp.66.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsch K, Schwarz J, Mozley PD, et al. Relationship between clinical features of Parkinson’s disease and presynaptic dopamine transporter binding assessed with [123I]IPT and single-photon emission tomography. European Journal of Nuclear Medicine. 1997;24:415–21. doi: 10.1007/BF00881814. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Lawrence MS, Sladek JR, Roth RH, Redmond DE. Spontaneous blink rates correlate with dopamine levels in the caudate nucleus of MPTP-treated monkeys. Experimental Neurology. 1999;158:214–220. doi: 10.1006/exnr.1999.7093. [DOI] [PubMed] [Google Scholar]
- Tomer R. Attentional bias as trait: Correlations with novelty seeking. Neuropsychologia. 2008;46:2064–2070. doi: 10.1016/j.neuropsychologia.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]