Abstract
Recently, traces of double-positive FoxP3+RORγt+ T cells were identified and viewed as dual programming differentiation intermediates geared towards development into T regulatory (Tregs) or Th17 cells. Herein, we report that FoxP3+RORγt+ intermediates arise in the NOD mouse T cell repertoire prior to inflammation and can be expanded with tolerogen without further differentiation. Furthermore, FoxP3+RORγt+ cells express both CD62L and membrane-bound TGFβ (mTGFβ) and utilize the former to traffic to the pancreas and the latter to suppress effector T cells both in vitro and in vivo. The cells perform these functions as FoxP3+RORγt+ intermediates, despite being able to terminally differentiate into either FoxP3+RORγt− Tregs or FoxP3−RORγt+ Th17 cells upon polarization. These previously unrecognized observations extend plasticity to both differentiation and function and indicate that the intermediates are poised to traffic to sites of inflammation and target diverse pathogenic T cells, likely without prior conditioning by effector T cells, thus broadening efficacy against autoimmunity.
Introduction
Naïve CD4+ helper T (Th) cells differentiate into one of several CD4+ T cell lineages including Th1, Th2, Th17 and T regulatory (Treg)5 cells depending on environmental stimuli. This process offers flexibility that the type of antigen and the cytokine milieu exploit to coordinate measured immune responses (1). Lately, it has become clear that a delicate cellular and programming interplay between Tregs and Th17 cells dictates the balance between health and autoimmunity (2).
Naïve Th cells stimulated with antigen in the presence of TGFβ up-regulate expression of the transcription factor FoxP3 (3) and the phenotypic cell surface marker CD25 and develop into Tregs (4, 5). FoxP3 up-regulation diverts the cell from activated to regulatory status by disrupting normal NFAT:AP-1 binding and instead forming an NFAT:Foxp3 complex (6). These cells display effective suppressive function against pathogenic self-reactive T cells (7–9). In contrast, when naïve T cells are stimulated with antigen in the presence of both TGFβ and IL-6, they up-regulate the transcription factor RORγt (10) and develop into Th17 cells (11, 12), a distinct lineage from Th1 and Th2 cells (13, 14) that produces the cytokine IL-17, hence the designation Th17 (15). Th17 cells proved pathogenic in several autoimmune disease models (16, 17), including type 1 (TID) or autoimmune diabetes (18).
Given that IL-6 diverts differentiation from Treg to Th17 to give rise to cells with opposite function, emphasis was put on the plasticity of Th programming (19–22). Recently, it was reported that the differentiation of naïve Th cells transits through a FoxP3/RORγt intermediate stage to provide further flexibility for the development of measured responses (2, 19). There is even evidence that fully differentiated Tregs can be reprogrammed to a Th17 phenotype (19), while fully differentiated Th17 cells can convert to Th1 lymphocytes (20–22). Because intermediates represent a transitional state, only traces of cells are available at any given time and isolating a sufficient number of cells to investigate molecular programs or in vivo function has not been feasible.
Herein, we were able to expand FoxP3+RORγt+ cells in vivo and demonstrate that these cells are fully functional. Indeed, Ig-GAD1, an immunoglobulin molecule genetically engineered to incorporate the glutamic acid decarboxylase (GAD) 524–543 diabetogenic amino acid sequence (designated GAD1) expands Tregs that protect against type I or autoimmune diabetes (23). Examination of these cells showed an increased number of FoxP3+RORγt+ cells. Interestingly, these intermediates are present within the natural repertoire and express CD62L, which is required for trafficking as well as active membrane-bound TGFβ (mTGFβ), through which they suppress pathogenic T cells. Importantly, the FoxP3+RORγt+ Tregs did not secrete IL-17, in agreement with previously published results (24–26). However, under Th17-polarizing conditions, they were able to fully differentiate into RORγt+ cells capable of producing IL-17 cytokine. CD62L and TGFβ were expressed on the FoxP3+RORγt+ Tregs in the natural repertoire prior to disease onset, which likely guides the Tregs to the site of inflammation to target diverse effector T cells. These previously unrecognized findings indicate that FoxP3+RORγt+ intermediates are fully functional, broadening Th plasticity to both programming and function. Moreover, as the cells express CD62L and mTGFβ prior to inflammation, they do not require conditioning by effector cells but are able to traffic to sites of inflammation and target diverse T cell specificities.
Materials and Methods
Mice
NOD (H-2g7), NOD.scid, NOD.BDC2.5 (27) and NOD.FoxP3:GFP mice were used according to the guidelines of the University of Missouri Columbia Animal Care and Use Committee. NOD.FoxP3:GFP reporter mice were generated by breeding C57BL/6.FoxP3:GFP knock-in animals (3) into NOD mice for 10 backcross generations.
Tolerogen
Ig-GAD1 (23) is an Ig chimeras carrying GAD1 peptide corresponding to aa residues 524–543 (SRLSKVAPVIKARMMEYGTT) of GAD65 (28). This was done by inserting GAD1 nucleotide sequence into the heavy chain veriable region of 91A3 IgG2b molecule and transfecting the resulting 91A3H-GAD1 chimeric gene along with the parental 91A3 κ chain gene into a non-Ig-secreting SP2/0 myleoma B cells (23). Transfectoma cells were then grown large-scale in DMEM media with 10% iron-enriched calf serum (HyClone) and Ig-GAD1 was purified using columns of CNBr-activated 4B sepharose (GE Healthcare) conjugated to rat-anti-mouse κ light chain mAb. Ig-GAD1 was aggregated by precipitation with 50% saturated (NH0)2SO4 as was previously described (29).
Expansion of FoxP3 expressingT cells by Ig-GAD1
NOD and NOD.FoxP3-GFP reporter mice are given i.p. 300 µg aggregated (agg) Ig-GAD1 in saline at wk 4, 5, and 6. The mice are sacrificed at the end of week 6 which is 5 days after the last injection. For analysis of FoxP3 T cells at wk 5 the mice receive two injections only, one at week 4 and one at week 5 and the animals are sacrificed 5 days later.
Assessment of diabetes
Assessment of blood glucose levels used Accu-Chek Advantage monitoring system. A mouse was considered diabetic when the blood glucose levels were above 300 mg/dL for 2 consecutive measures.
Purification of pancreatic cells
Islets and infiltrating cells were purified according to a standard procedure (30). Briefly, the pancreata were digested with collagenase type IV (Invitrogen, Carlsbad, CA) and islets and infiltrating cells were separated on a ficoll gradient (GE Healthcare, Waukesha, WI).
Neutralization of CD62L in vivo
Cells producing MEL-14 anti-CD62L antibody were grown large-scale in DMEM media containing 10% iron-enriched calf serum (HyClone, Thermo Fisher Scientific, Waltham, MA). Anti-CD62L antibody was then purified using columns containing CNBr-activated sepharose linked to mouse-anti-rat antibody. For neutralization of CD62L during expansion of FoxP3 expressing cells, 1 mg anti-CD62L antibody in PBS was administered i.p. along with each agg Ig-GAD1 injection. For neuralization of CD62L during transfer of FoxP3 expressing cells, 1 mg anti-CD62L antibody in PBS was administered i.p. along with the i.v. adoptive transfer of FoxP3int Tregs.
Flow cytometery analyses
Cell surface staining
Splenocytes, pancreatic cells, pancreatic lymph node cells or purified CD4+ T cells were incubated with the test antibodies for 30 min at 4°C, and then washed with staining buffer. The cells were then fixed with 2% formaldehyde and analyzed. For Biotin coupled antibodies the cells were stained with PE-conjugated streptavidin for 30 min at 4°C prior to fixation with formaldehyde. The antibodies were anti-CD4 antibody (clone RM4-5, ) Biotin-conjugated anti-TGF-β1 antibody (clone BAF240) and its isotype control chicken IgY (R&D Systems Minneapolis, MN); anti-CD25-APC (clone 2A3) and Rat IgG1 as isotype control (BD Pharmingen, San Jose, CA); anti-CD62L-FITC (clone MEL-14) and its isotype control rat IgG2a (BD Pharmingen, San Jose, CA); anti-ICOS-APC antibody (clone C398.4A) and its isotype control Armenian Hamster IgG1 (BD Pharmingen, San Jose, CA); anti-GITR-FITC antibody (clone TNFRSF18) and its Rat IgG2b isotype control (eBiosciences, San Diego, CA) .
FoxP3 and RORγt intracellular staining
The test cells were stained for surface molecules as indicated above and then blocked with 1µg/million cells mouse IgG and subsequently permeabilized with Fix/Perm buffer (eBioscience, San Diego, CA) and stained with anti-FoxP3-FITC antibody (clone FJK-16s) or isotype control Rat IgG2a-FITC and/or anti-RORγt antibody (clone AFKJS-9) or isotype control Rat IgG2a-PE antibody for 30 min at 4°C. The cells were then washed and analyzed. Both antibodies were purchased from eBiosciences (San Diego, CA).
Cell analyses used a FACScan (Becton Dickson, San Jose, CA) or Cyan ADP (Dako, Denmark) flow cytometers and the FlowJo software (Tree Star, Inc., Ashland, OR). All staining data is representative of at least three replicates.
Suppression of passive diabetes by Tregs
10 million splenocytes from diabetic NOD mice were injected i.v. into NOD.scid recipient mice along with 5×105 of suppressor cells. FoxP3int, FoxP3hi and FoxP3− T cells were sorted to 95% purity based on GFP expression from NOD FoxP3:GFP reporter mice on a MoFlo cell sorter (Dako, Denmark).
Proliferation Assays
This was done as previously described (23). Briefly, allogenic effector NOD T cells were incubated in a 96-well plate (200,000/well) with CD11c+ C57BL/6 APC (100,000/well) that were purified from bulk splenocytes on anti-CD11c-coupled Miltenyi microbeads. Sorted (95% purity) FoxP3int or FoxP3hi Tregs were added at the indicated ratios and the . The culture was incubeted for 3 days with addition of 1 µCi of [3H]thymidine during the last 8 hours of culture. The cells were then harvested on a Trilux 1450 Microbeta Wallac Harvester, and incorporated [3H]thymidine was counted using the Microbeta 270.004 software (Wallac, Walltham, MA).
T cell activation and polarization
Purified T cells were incubated in a 96-well round-bottom plate for 72 hours along with plate-bound anti-CD3 (2C11) (10µg/mL), soluble anti-CD28 (PV1) (1µg/mL) and 100U rIL-2 (212-12; Peprotech Inc, Rocky Hills, NJ).
Th1 polarization
Purified T cells were incubated under activating conditions and polarized with: 10ng/mL rIL-12 (210-12; Peprotech Inc., Rocky Hills, NJ) and 10µg/mL anti-IL-4 (11B11).
Th17 polarization
Purified T cells were incubated under activating conditions and polarized with: 3 ng/mL rhTGFβ (100-21C; Peprotech Inc., Rocky Hills, NJ), 10ng/mL rIL-6 (216-16; Peprotech Inc., Rocky Hills, NJ), 10 µg/mL anti-IFNγ (R4-6A4) and 10µg/mL anti-IL-4 (11B11).
Treg polarization
Purified T cells were incubated under activating conditions and polarized with: 3 ng/mL rhTGFβ (100-21C; Peprotech Inc., Rocky Hills, NJ), 10 µg/mL anti-IFNγ (R4-6A4) and 10µg/mL anti-IL-4 (11B11).
Cytokine detection
Intracellular cytokine
After 5 days polarization the T cells were stimulated for 6 hours with 50 ng/mL PMA and 500 ng/mL ionomycin in the presence of Brefeldin A. The cells were then permeabilized with 0.2% saponin and intracellular cytokines were detected by staining with anti-cytokine antibodies .
Secreted cytokines
Detection of cytokine in culture supernatant used ELISA as described (18, 23, 29). Also, cytokine secretion were detected on the cell surface to identify IL-10 producing cells using Miltenyi bioconjugate mouse IL-10 secretion assay according to the manufacture’s instruction (Miltenyi biotec, Bergisch Gladbach, Germany).
RT-PCR analysis
FoxP3hi or FoxP3int cells were sorted to at least 95% purity using a MoFlow cell sorter (Dako, Denmark). RNA was isolated using Trizol extraction and ethanol precipitation. RT-PCR for RORγt and FoxP3 was performed using the QuantiTect Reverse Transcription kit from Qiagen and the StepOne Instrument cycler (Applied Biosystems). The forward primers were 5’TACACCCAGGAAAGACAGCAACCT3’for FoxP3 and 5’ACAGCCACTGCATTCCCAGTTT3’ for RORγt. The reverse primers were 5’TCTGCTTGGCAGTGCTTGAGAA3’ for FoxP3 and 5’TCTCGGAAGGACTTGCAGACAT3’ for RORγt .
Statistical analysis
P values were calculated using the two-tailed Student’s t test.
RESULTS
Protective antigen therapy against TID expands discrete FoxP3 Tregs
We have previously described a protective treatment regimen with Ig-GAD1, which expands splenic Tregs when administered into non-obese diabetic (NOD) mice at week (wk) 4 to 6 of age (23). The same regimen, however, was unable to protect against TID when administered at wk 8 of age, despite effective expansion of splenic Tregs (23) (Supplementary Fig. 1A). The 8-wk-old splenic Tregs, though, had diminished levels of mTGFβ (Supplementary Fig. 1B), which is indispensable for suppression of the disease (23).
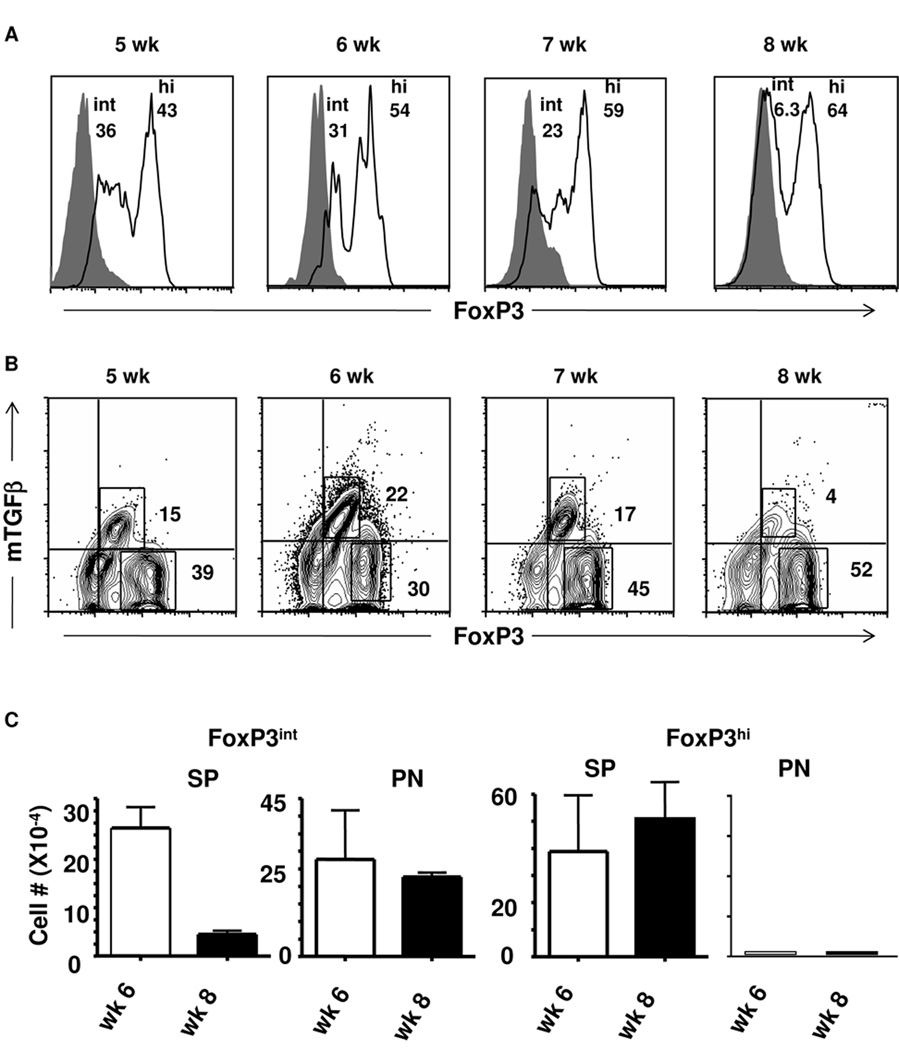
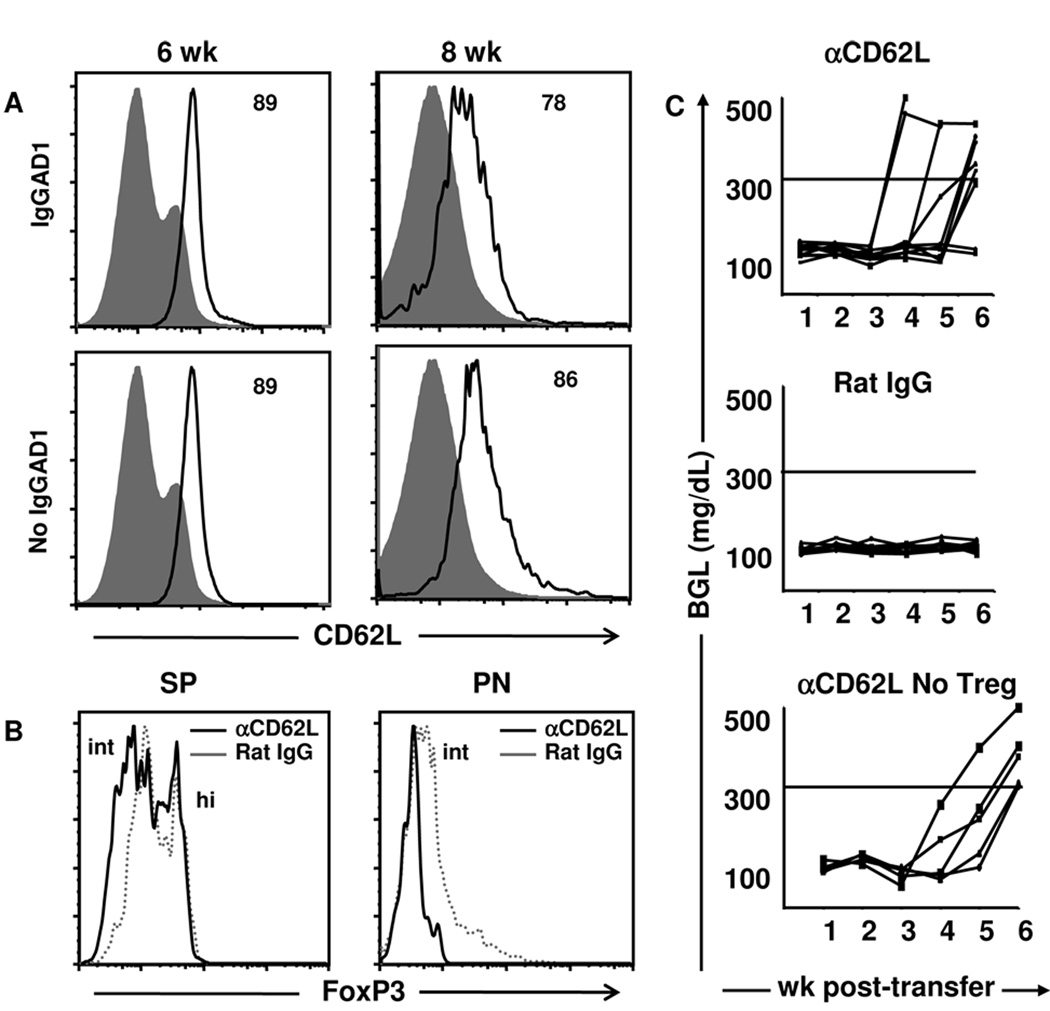
Figure 1. Intermediate but not high level FoxP3 correlates with expression of mTGFβ.
(A, B) Splenic cells from Ig-GAD1-treated mice were harvested at wk 5, 6, 7, and 8 of age and stained for surface CD4, CD25 and mTGFβ and intracellular FoxP3. CD4+CD25+ T cells were analyzed for (A) FoxP3 and (B) TGFβ expression by flow cytometry. Isotype control is represented by filled histograms and quadrants, respectively. In (A) the numbers indicate the percent of cells expressing intermediate (int) and high (hi) level FoxP3. In (B) the numbers represent the percent of cells expressing only FoxP3 or both FoxP3 and TGFβ. (C) FoxP3int and FoxP3hi Treg cell numbers were quantified in both the spleen (SP) and pancreas (PN) at wk 6 and 8 of age. The bars indicate the number of FoxP3 cells per million of CD4+CD25+ cells. Data are presented as mean of three replicates ± SD. The experiments illustrated in this figure were repeated 3 times.
To gain further insight on the specific loss of mTGFβ+ Tregs during the transition from 6 to 8 wks, which coincides with ongoing progressive insulitis in NOD mice, we opted to compare the phenotype of the two populations. The initial experiments were focused on FoxP3 protein expression in 5, 6, 7, and 8-wk-old splenic Tregs from Ig-GAD1-treated mice. Much to our surprise, these splenic Tregs comprised two distinct populations with one displaying intermediate FoxP3 (FoxP3int) expression and the second with high FoxP3 (FoxP3hi) expression (Fig. 1A). Intriguingly, there was a gradual decrease of the FoxP3int population and, by wk 8, most of the Tregs had a FoxP3hi phenotype (Fig. 1A). Indeed, 5-wk-old Tregs comprised 36% FoxP3int and 43% FoxP3hi cells. However, by wk 8 of age, 64% of Tregs were FoxP3hi and the FoxP3int population was significantly reduced to 6.3%. Interestingly, the decrease in FoxP3int Tregs coincides with the loss of mTGFβ in Ig-GAD1-expanded 8-wk-old splenic Tregs (23), which prompted us to determine the dynamics of mTGFβ expression by the two populations. The results illustrated in Figure 1B clearly show that mTGFβ was predominantly expressed on the FoxP3int population but was significantly reduced when the FoxP3int cells were no longer detectable by wk 8. Indeed, the FoxP3hi population, which represented 39% and 52% of total Tregs on wk 5 and 8, respectively had no significant mTGFβ expression at any time point. However, the FoxP3intmTGFβ+ population went down from 15% of total Tregs at wk 5 to 4% by wk 8 (Fig. 1B). Similar patterns of FoxP3 and TGFβ expression was observed in untreated mice (Supplementary Fig. 2). Additionally, a control Ig-HEL molecule, incorporating the I-Ag7-restricted non-diabetogenic hen egg lysozyme (HEL) peptide instead of GAD1, was unable to expand any T regulatory cells in this model (23).
To determine which of the two populations (FoxP3int and FoxP3hi) might be involved in protection against diabetes during treatment with Ig-GAD1, we searched for Tregs in both the spleen (SP) and pancreas (PN) at wk 6 and 8, time points where insulitis is progressive. As illustrated in Figure 1C, FoxP3int Tregs dropped from 22 ×104 (per 106 counted CD25+ cells) at wk 6 to 4 ×104 at wk 8 in the SP but were maintained at high numbers in the PN (27 and 22×104 per 106 counted CD25+ cells at wk 6 and 8, respectively). Interestingly, FoxP3hi Tregs were present at high numbers in the SP at both wk 6 and 8 (39 and 50×104 per 106 CD25+ counted cells, respectively) but were minimal in the PN at both time points (Fig. 1C). These results indicate that Ig-GAD1 expands phenotypically distinct Tregs that reside in different organs during the onset of disease. In untreated mice FoxP3int and FoxP3hi T cells were present, though at smaller numbers, than Ig-GAD1-treated mice (supplemental Fig 2B and C). Furthermore, their residential patterns at week 6 and 8 of age were similar to Ig-GAD1-expaneded T cells, possibly indicating that both FoxP3int and FoxP3hi T cells arise in the normal repertoire and can be expanded by Ig-GAD1.
FoxP3int T cells express the Th17 signature RORγt transcription factor
Given their differential expression of mTGFβ and location during insulitis, we sought to further characterize the two populations of Tregs for expression of trafficking molecules, transcription factors, and effector cytokines. We performed these analyses at 6-wks of age in the SP, before the cells begin trafficking and become subject to local environmental changes. Figure 2 shows that 73.2% of CD4+CD25+ FoxP3int cells express mTGFβ while only 12.4% of the CD4+CD25+FoxP3hi Tregs had mTGFβ (Fig. 2A). Given that the 2 populations had different localization patterns during progressive insulitis and that CD62L is known to regulate Treg trafficking and function in NOD mice (31–33), the cells were tested for CD62L expression. Not surprisingly, 87.3% of splenic FoxP3int Tregs expressed CD62L while only 3.2% of FoxP3hi Tregs had CD62L. These findings may explain the presence of FoxP3int but not FoxP3hi Tregs in the PN during insulitis (32). GITR protein was, however, expressed to a similar extent on both FoxP3int and FoxP3hi Tregs (92.5 and 93.8%, respectively) (Fig. 2A).
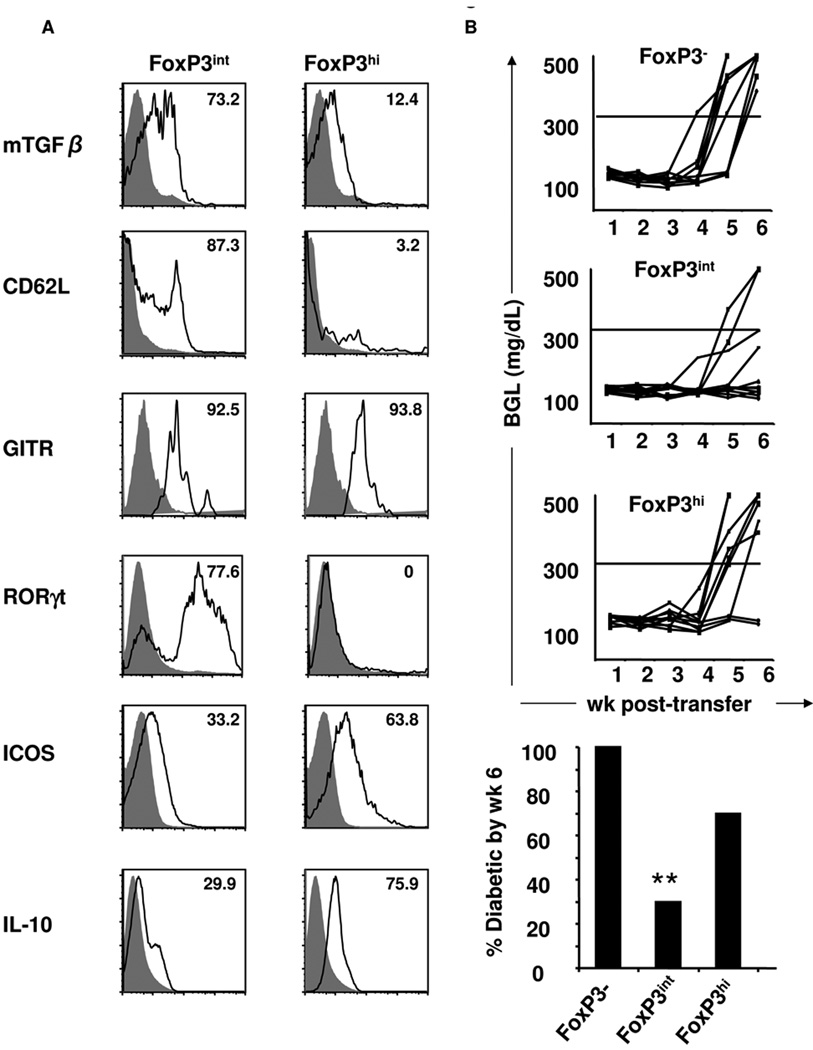
Figure 2. FoxP3int cells co-express RORγt and protect against diabetes.
(A) Splenic cells from Ig-GAD1-treated mice were harvested at wk 6 of age and stained for CD4, CD25 and FoxP3 along with the indiacted proteins. The numbers represent percent of FoxP3int and FoxP3hi cells expressing the marker of interest relative to isotype control (filled histograms). Stainings are representative of at least three experiments. (B) Splenic FoxP3-, FoxP3int or FoxP3hi CD4 T cells from Ig-GAD1-treated FoxP3:GFP reporter mice were co-transferred with diabetogenic splenocytes into NOD.scid recipients and the hosts were monitored for blood glucose levels. Lines represent individual mice (N= 10/group). The bars represent the percent of diabetic mice at wk six post-cell transfer. ** p = 0.0016 when compared to mice recipient of FoxP3− cells.
The most surprising finding in these analyses is that FoxP3int mTGFβ+CD62L+ but not FoxP3himTGFβ−CD62L− cells had an impressive level of the Th17 signature transcription factor RORγt (Fig. 2A). Indeed, 77.6% of FoxP3int cells expressed RORγt protein while FoxP3hi cells had no detectable RORγt. FoxP3+RORγt+ double-positive T cells have been described recently and were thought to represent transitional differentiation intermediates geared toward single-positive RORγt+ Th17 or FoxP3+ Tregs (2, 19). These intermediates did not produce IL-17 under non-polarizing conditions in vitro (Supplementary Fig. 3), which is in good agreement with reports showing that FoxP3+RORγt+ intermediates are unable to secrete IL-17 due to inhibition by FoxP3 (24, 25).
The two other molecules that were analyzed were ICOS and IL-10. Similar to what was observed in humans (34), FoxP3intmTGFβ+ Tregs express lower levels of ICOS (33.2%) than FoxP3hiTGFβ− cells (63.8%) (Fig. 2A). Also, in agreement with what has been demonstrated in human Tregs (34), FoxP3hi cells, which are highly ICOS+ (Fig. 2A), secrete greater amounts of IL-10 (75.9%) than their FoxP3int counterparts (29.9%) (Fig. 2A). Overall, the FoxP3intRORγt+ intermediates express CD62L and mTGFβ while the FoxP3hi Tregs express ICOS and produce IL-10 and both populations have GITR (Fig. 2A).
Given that both the FoxP3intRORγt+ intermediates and FoxP3hi Tregs express suppressive molecules, it is possible that both could display suppressive function in vitro. This premise was tested in an allogenic system where effector NOD cells were incubated with C57BL/6 dendritic cells in the presence or absence of highly purified FoxP3intRORγt+ intermediates or FoxP3hi Tregs and proliferation of effector T cells was measured. The results indicated that both populations displayed significant suppression of NOD effector T cell responses in vitro (Supplementary Fig. 4).
Splenic FoxP3intRORγt+ T cells protect against passive TID
mTGFβ serves as a suppressive molecule on Tregs (23, 35–37). In fact, disease suppression by Ig-GAD1-expanded Tregs was dependent on mTGFβ as a blocking anti-TGFβ antibody abrogated protection (23). Before testing the in vivo function of highly purified FoxP3int cells, we used the NOD.FoxP3-GFP reporter mouse and ensured that Ig-GAD1 treatment expands these intermediates and yields sufficient cells for testing against diabetes. Subsequently, the mice were treated with Ig-GAD1 and FoxP3−, FoxP3int (which highly co-express RORγt), or FoxP3hi (RORγt−) T cells were sorted on the basis of FoxP3 expression. To test for protection against diabetes, the sorted cells were adoptively transferred into NOD.scid recipient mice along with splenocytes from recently diabetic mice. Blood glucose levels were then monitored for 6 wks post-transfer. The results indicate that by wk 6 after transfer, 100% of the mice that received the control FoxP3− T cells were diabetic (Fig. 2B). Mice recipient of both FoxP3int and FoxP3hi Tregs showed protection from diabetes as 70% and 30% were protected from disease by wk 6, respectively (Fig. 2B). Statistical analyses indicated that FoxP3int display significant protection relative to FoxP3− T cells (p = 0.0016) while FoxPhi were less protective at this time point after transfer (p = 0.0778). Overall, this data indicates that FoxP3intRORγt+ intermediates, which are found in the PN during the onset of insultis, exercise suppressive function in vivo, to a better extent than FoxP3hi counterparts.
Ig-GAD1 treatment sustains migration of FoxP3intRORγt+ T cells to the pancreas during insulitis
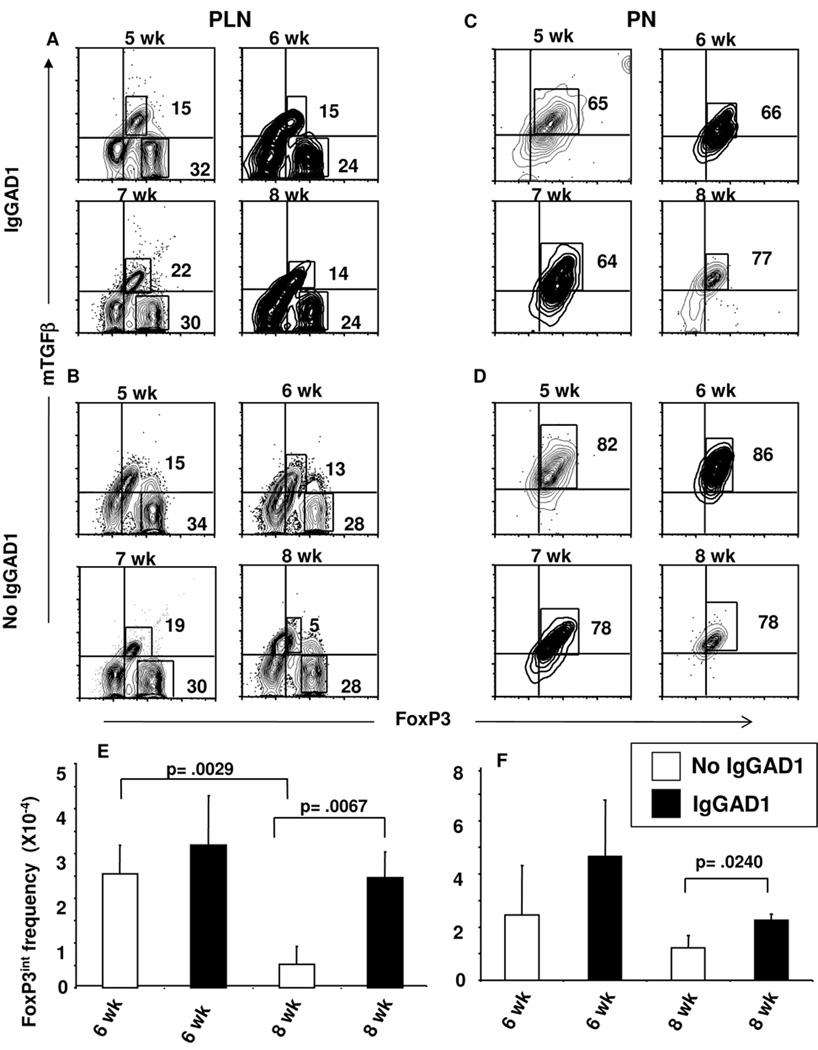
To protect against passive or spontaneous diabetes, splenic FoxP3int Tregs likely migrate to the pancreatic lymph node (PLN) and the PN, where they can suppress effector cells (Fig. 1C). Since these cells express RORγt (Fig. 2A) there is potential to exercise their function as FoxP3intRORγt+ intermediate cells. Alternatively, the cells could complete their differentiation on the way to the inflammatory site and perform suppression as fully differentiated FoxP3intRORγt− Tregs. To test this premise, we began by analyzing the dynamics of the FoxP3 cells during the onset of insulitis. The results indicate that in the Ig-GAD1-treated mice the FoxP3int cells are located both in the PLN (Fig. 3A) and PN (Fig. 3C) at wk 6 as well as wk 8 of age. However, in untreated mice, the cells are found in the PLN and PN at wk 6 but by wk 8 only a very small number reside in the PLN and the majority of the cells are located in the PN (Fig. 3B, D). Quantification of FoxP3+mTGFβ+ T cell numbers indicated that Ig-GAD1 therapy increases the frequency of these cells both in the PLN and PN during progressive insulitis relative to untreated mice (Figure 3E, F). Indeed, the frequency of FoxP3+mTGFβ+ Tregs at wk 8 of age increased form 0.5×104 per 106 CD4+ T cells in the untreated mice to 2.4×104 per 106 CD4+ T cells in Ig-GAD1 treated animals. The accumulation of these suppressive cells at the site of inflammation likely suggests a protective role against diabetes and offers a mechanism by which Ig-GAD1 therapy delays the disease (23).
Figure 3. FoxP3int Tregs expressing mTGFβ traffic and accumulate in the sites of inflammation upon treatment with tolerogen.
Cells were harvested from (A, B) pancreatic lymph nodes (PLN) or (C, D) PN of (B, D) untreated or (A, C) agg Ig-GAD1-treated NOD mice at wk 5, 6, 7, and 8 of age. The cells were stained for CD4, CD25, FoxP3 and TGFβ and analyzed by flow cytometry. The contour plots show mTGFβ and FoxP3 expression on CD4+CD25+ cells. Quadrant lines represent isotype staining levels. (E, F) illustrate the number of CD25+FoxP3int Tregs at wk 6 and 8 with in the (E) PLN and (F) PN of (open bars) untreated and (filled bars) Ig-GAD1-treated mice described in (A–D). Bars represent the mean of three experiments ± SD. P values were generated using the student’s t test.
Pancreatic FoxP3intRORγt+ T cells protect against disease without further differentiation
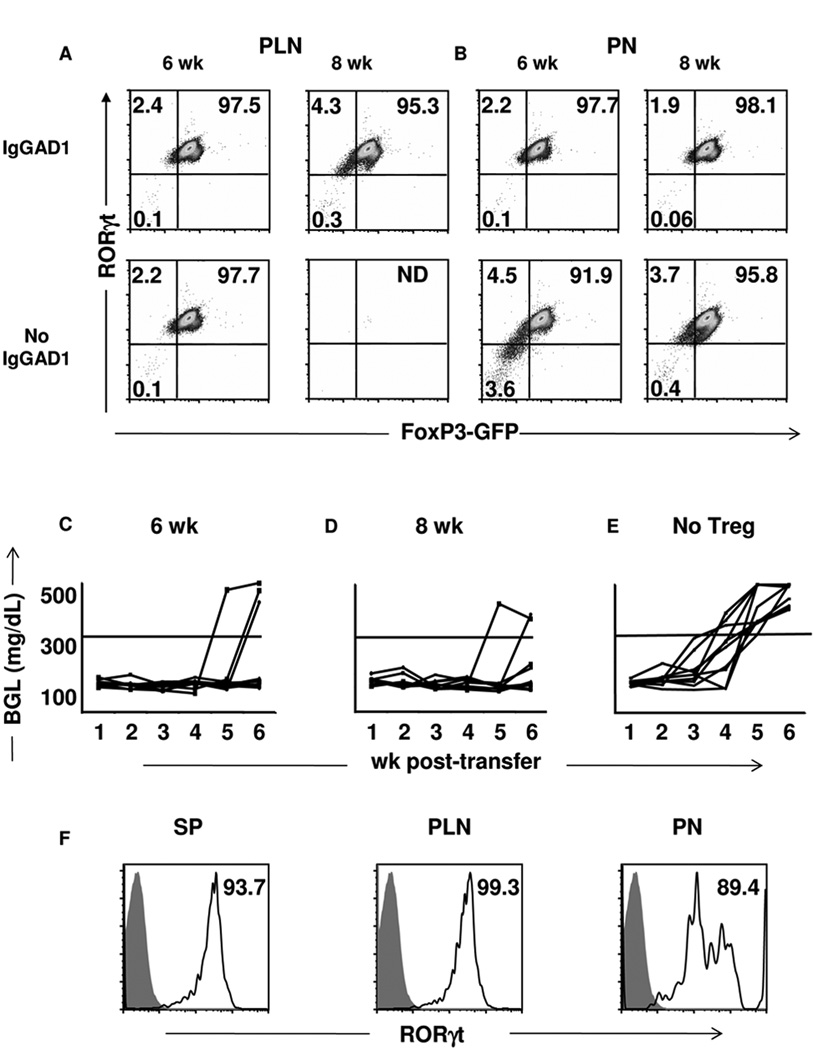
To determine whether these regulatory cells locate to the site of inflammation and perform suppressive function as FoxP3int intermediates bearing RORγt or rather as fully differentiated FoxP3intRORγt− Tregs, we tested the PLN and PN resident FoxP3intTGFβ+ cells for expression of RORγt at wk 6 and 8 of age in both untreated as well as Ig-GAD1 treated NOD mice. The findings indicate that, in the Ig-GAD1 treated mice, the majority of FoxP3intmTGFβ+ Tregs strongly maintain their RORγt+ phenotype both in the PLN (97.5% and 95.3% at wk 6 and 8, respectively) (Fig. 4A) and the PN (97.7% and 98.1% at wk 6 and 8, respectively) (Fig. 4B). No detectable single-positive FoxP3intRORγt− cells were found in either organ, indicating that conversion among the TGFβ+ Treg population was minimal. Similarly, in the untreated mice, 97.7% of the PLN (Fig. 4A) and 91.9% of the PN (Fig. 4B) FoxP3intmTGFβ+ Tregs express RORγt at wk 6 with no detectable single-positive FoxP3intRORγt− cells. Also, at wk 8 when FoxP3intmTGFβ+ Tregs were minimally present in the PLN (Fig. 3B) the majority (95.9%) of the cells available in the PN express RORγt, indicating that conversion was not operative. These results indicate that FoxP3intTGFβ+ T cells express RORγt and maintain their intermediate (FoxP3intTGFβ+ RORγt+) phenotype upon migration to the inflammatory sites.
Figure 4. Pancreatic FoxP3int residents maintain RORγt expression.
(A) PLN and (B) PN cells were harvested from untreated and Ig-GAD1-treated NOD.FoxP3:GFP reporter mice at wk 6 and 8 and CD4+CD25+mTGFβ+ cells were analyzed for RORγt and FoxP3 expression. Quadrants represent isotype staining levels. Stainings are representative of three experiments. (C, D) Pancretic FoxP3int Tregs from Ig-GAD1-treated (C) 6- or (D) 8–wk-old FoxP3:GFP reporter mice were co-transferred with diabetogenic splenocytes into NOD.scid recipients and the hosts were monitored for blood glucose levels. Lines represent individual mice (N= 10/group). (E) control NOD.scid mice recipient of diabetogenic splenocytes but not Tregs (N= 10/group). (F) FoxP3int cells were recovered from host NOD.scid SP, PLN and PN 72 hours after transfer and were analyzed for RORγt expression. Numbers represent percent of cells expressing RORγt compared to isotype (filled histograms). ND= Not Detected.
The accumulation of FoxP3intTGFβ+RORγt+ intermediates in the PN of mice treated with Ig-GAD1, which are protected from diabetes, suggest that these cells are potentially functional and contribute to resistance against the disease. To test this premise, FoxP3int Tregs were purified from the PN of 6- and 8-wk-old mice on the basis of GFP expression and transferred to NOD.scid recipients along with diabetogenic splenocytes and the recipients were monitored for blood glucose levels. The results indicated that 6- as well as 8-wk-old pancreatic FoxP3intTGFβ+RORγt+ Tregs were strongly protective as 70% (Fig. 4C) and 80% (Fig. 4D) of the mice were protected from diabetes, respectively, while all (100%) of the control mice without Treg transfer developed diabetes (Fig. 4E). To determine whether the cells were further differentiated in the recipient mice, we harvested the SP, PLN and PN from the hosts and analyzed the cells for FoxP3 and RORγt expression. As indicated in Figure 4E, 93.7% of FoxP3int cells in the SP express RORγt. Interestingly, the 99.3% of the FoxP3int cells that migrated to the PLN also maintain expression of RORγt (Fig. 4E, median panel). Equally important, 89.4% of the FoxP3int cells that migrated to the PN still co-express RORγt (Fig. 4E, right panel). Overall, these findings indicate that FoxP3intTGFβ+RORγt+ cells migrate to the site of inflammation and protect against diabetes without loss of RORγt or full differentiation to FoxP3+RORγt− Tregs.
CD62L is required by FoxP3intRORγt+ Treg for migration to the pancreas and protection against diabetes
Both FoxP3hi and FoxP3int Tregs are present in the SP but only FoxP3int express CD62L (Fig. 2A) and migrate to the PN (Fig. 1C). Given that CD62L was previously shown to facilitate trafficking of Tregs to the PLN (32), it is possible that FoxP3int cells preserve CD62L expression to migrate to the site of inflammation and confer resistance against diabetes. To address this point, we began by determining whether Ig-GAD1-expanded FoxP3intRORγt+ cells that migrate to the PN maintain CD62L expression. The results presented in Fig. 5a indicate that pancreatic FoxP3intRORγt+ Tregs maintain high levels of CD62L expression at both wk 6 and wk 8 of age in untreated as well as Ig-GAD1-treated mice. Indeed, at wk 6 of age, 89% of FoxP3int Tregs from both Ig-GAD1-treated and untreated mice express CD62L (Fig. 5A). Similarly, at wk 8, most (86%) of the cells preserved CD62L expression in Ig-GAD1-treated mice while 78% had CD62L in the untreated mice (Fig. 5A). This data suggests that FoxP3intTGFβ+RORγt+ Tregs might rely on CD62L to traffic to the PN, the site of inflammation in the NOD mouse model. To determine whether CD62L is required for migration of FoxP3intTGFβ+RORγt+ T cell to the PN, mice were given Ig-GAD1 therapy (to expand Tregs) accompanied with a neutralizing anti-CD62L antibody and migration of the cells to the PN was evaluated. The results in Figure 5B indicate that in vivo neutralization of CD62L retains FoxP3int Tregs in the SP that can no longer migrate to the PN (Fig. 5B). This indicates that CD62L is required for trafficking of FoxP3intRORγt+ Tregs to the site of inflammation.
Figure 5. Protection against diabetes by FoxP3int RORγt intermediate cells requires CD62L.
(A) Pancreatic cells were harvested at wk 6 and 8 from 8 to 12 untreated or agg Ig-GAD1-treated NOD mice and stained for CD4, CD25, FoxP3 and CD62L and analyzed by flow cytometry. Cells were gated on CD4+CD25+FoxP3int and analyzed for CD62L expression. The numbers represent the percent of cells expressing CD62L compared to isotype levels (filled histograms). (B) 1mg anti-CD62L (MEL-14) (black lines) or isotype-matched Rat IgG antibody (dashed lines) was administered along with Ig-GAD1 at wk 4, 5, and 6. Mice were sacrificed at wk 6 and intracellular FoxP3 levels were measured on splenic (left panel) or pancreatic (right panel) CD4+CD25+ cells. Stainings are representative of at least three experiments. (C) Pancretic FoxP3int Tregs from 6-wk-old Ig-GAD1-treated FoxP3:GFP mice were co-transferred with diabetogenic splenocytes into NOD.scid recipients along with a neutralizing antibody to CD62L or an isotype control and the hosts were monitored for blood glucose levels. A control group that received only the diabetogenic splenocytes along with neutralizing CD62L antibody was also included Lines represent individual mice (N= 10/group).
To determine whether CD62L-mediated trafficking of FoxP3intRORγt+ Tregs to the PN is required for protection against diabetes, NOD.scid mice were adoptively transferred with pancreatic FoxP3intRORγt+ Tregs along with diabetogenic splenocytes and the hosts were given anti-CD62L antibody or an isotype control. The mice were then monitored for blood glucose levels. The results show that neutralization of CD62L nullifies the suppressive function of FoxP3intRORγt+ Tregs as the mice were no longer protected against diabetes (Fig. 5C). Indeed, 80% of the mice given anti-CD62L were diabetic by wk 6 post cell transfer (Fig. 5C top panel). However, all mice (100%) recipient of Rat IgG instead of anti-CD62L remained free from diabetes (Fig. 5C median panel). Neutralization of CD62L protein had no effect on the ability of effector diabetogenic splenocytes to induce TID in this adoptive transfer model. This conclusion is drawn from the observation that transfer of diabetic splenocytes without FoxP3intRORγt+ Tregs, leads to diabetes when the mice are given anti-CD62L antibody. In fact, 100% of the mice in this control group became diabetic by wk 6 post-cell transfer (Fig. 5C, bottom panel), which parallels observations reporting that diabetogenic T cells enter the pancreas through an L-selectin independent pathway (38). Though, other studies have shown that anti-CD62L antibody can interfere with migration of diabeteogenic T cells and protect against diabetes (39). This however, required frequent injection of a higher dose of the antibody and used wild type NOD rather than NOD.scid mice. Overall, the results demonstrate that FoxP3intRORγt+ Tregs require CD62L to traffic to the PN and perform suppressive function, which bodes well with reports indicating that NOD.CD62L−/ − animals develop robust TID (40).
FoxP3intRORγt+ T cells retain potential for differentiation into either Th17 or FoxP3hi Tregs
FoxP3int T cells, despite their high co-expression of RORγt, function as Tregs since they suppress effector T cells in vitro, migrate to the PN in vivo and confer resistance against passive diabetes. The question then is whether these cells represent a new subset of Tregs with a fixed phenotype or retain RORγt to preserve program plasticity with potential for differentiation into Th17 cells as was previously suggested (19). To test this premise, we sought to determine whether FoxP3int Tregs could be polarized towards a Th1, Th17, or FoxP3hi Treg phenotype. Accordingly, FoxP3int and FoxP3hi Tregs were sorted from NOD.FoxP3-GFP reporter mice and expression of FoxP3 was analyzed prior to investigation of polarization. Figure 6A shows that the two populations had the expected FoxP3 level and were highly pure. Subsequently, the cells were polarized under Th1 (Fig. 6B), Th17 (Fig. 6C), and Treg (Fig. 6D) conditions and analysed for IL-17 and IFNγ cytokine production. The results show that the FoxP3int population, which strongly expresses the transcription factor RORγt, could easily be polarized towards a Th17 phenotype as 37% of the cells produced high levels of IL-17 cytokine (Fig. 6C). Interestingly, this population was unable to differentiate towards a Th1 phenotype as measured by IFNγ production and no detectable cells were positive for this cytokine (Fig. 6B). Under Treg polarizing conditions, FoxP3int Tregs did not secrete significant levels of IL-17 or IFNγ (0.91%) (Fig. 6D). FoxP3hi Tregs appear to be terminally differentiated and were unable to produce IL-17 or IFNγ under Th1, Th17 or Treg – polarizing conditions (Fig. 6B–D). It seems, then, that Tregs that highly express FoxP3 are unable to polarize towards Th17 and the earlier reports of Treg to Th17 polarization may have included cells expressing low level of FoxP3 Tregs than can convert to T17 cells (19).
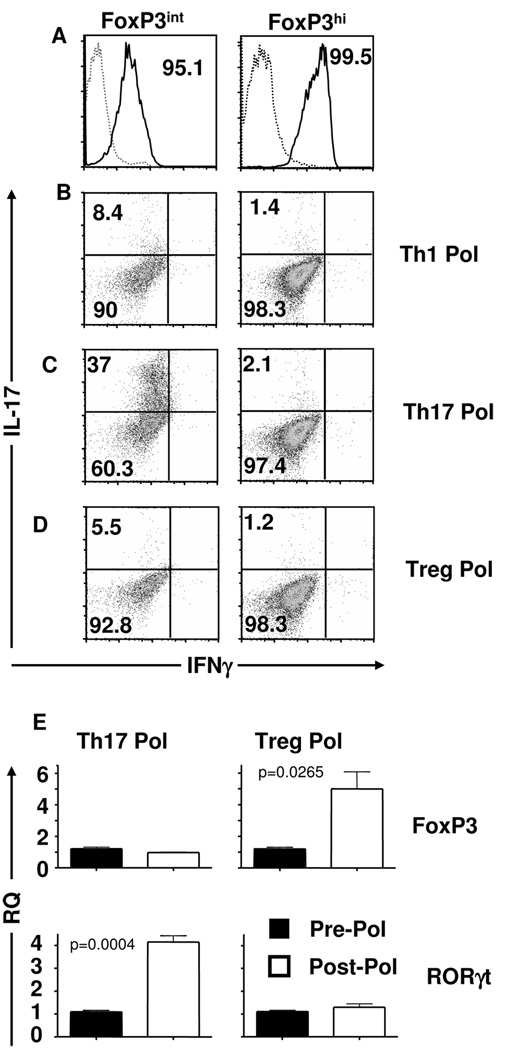
Figure 6. FoxP3int cells can differentiate into FoxP3hi and IL-17 producing Th17 cells upon polarization.
(A) FoxP3int (left panel) and FoxP3hi (right panel) T cells were sorted from 8 to 12 mice and polarized for 72 hours under (B) Th1 (Th1 pol), (C) Th17 (Th17 pol), or (D) Treg (Treg pol) polarizing conditions. Following polarization, IFNγ and IL-17 protein expression were measured by intracellular staining. In (B–D) black quadrant lines represent isotype controls. Staining is representative of at least three experimental replicates. (E) FoxP3 and RORγt transcript levels were measured in FoxP3int cells pre- (pre-pol) and post- (post-pol) Treg or Th17 polarization. Bars represent the mean of three replicates ± SD.
The FoxP3int cells were further analysed for FoxP3 and RORγt mRNA upon polarization to Tregs and Th17 cells. Upon Treg polarization, FoxP3 mRNA levels rose from 1 relative quantity (RQ) pre-polarization to 5 RQ after polarization while RORγt remained the same (Fig. 6E). However, under Th17 polarizing conditions while FoxP3 was similar pre- and post-polarization, RORγt rose from 1 to 4.1 RQ pre- to post-polarization (Fig. 6E). Similar results were obtained at the protein level (not shown). Overall, these findings indicate that the FoxP3intRORγt+ population represents a differentiation intermediate stage that can further develop into either FoxP3hi Tregs or Th17 cells.
FoxP3intRORγt intermediate Tregs arise in the periphery
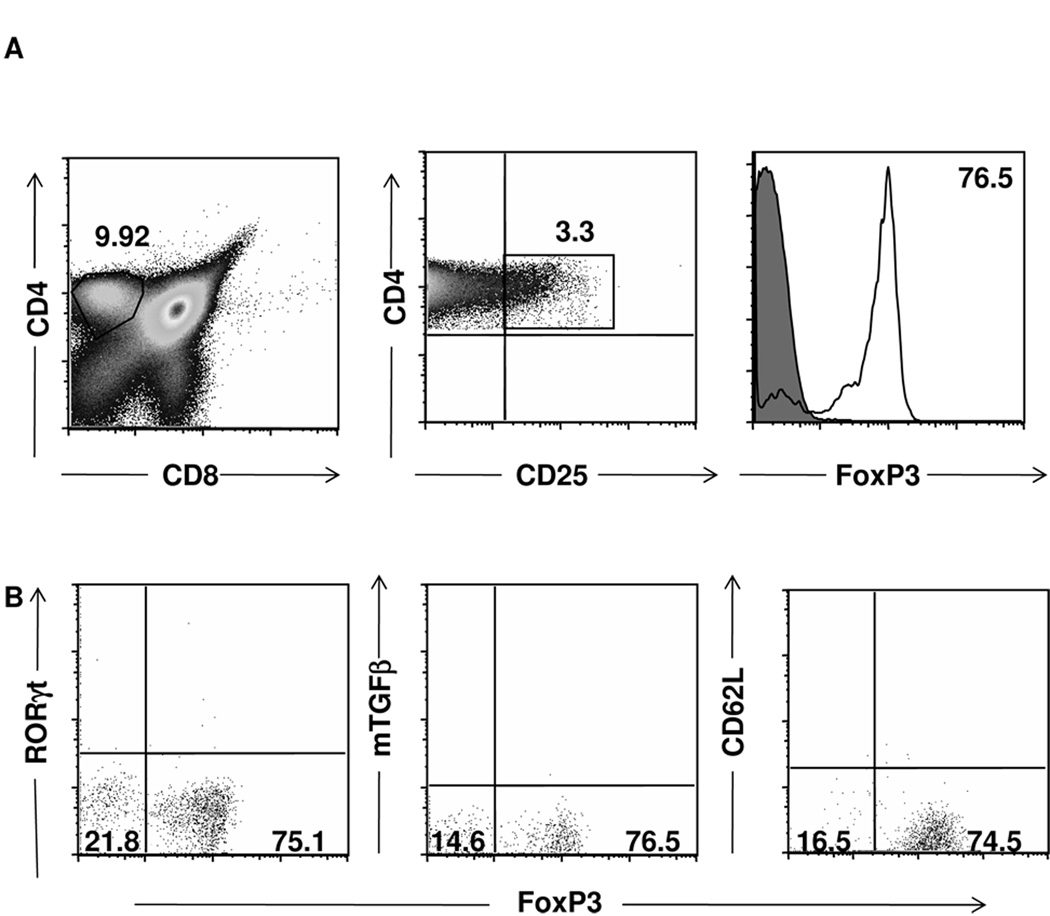
To better understand the origin of these suppressive FoxP3intRORγt+ Tregs, we sought to determine whether or not this population originates from the thymus in its FoxP3int form or arises in the periphery. To address this question, thymi from 6-wk-old NOD mice were harvested and FoxP3 protein level was measured on the CD4+CD25+ population, which represents 3.3% of all CD4+ single-positive T cells (Fig. 7A). The majority (76.5%) of these cells were FoxP3hi cells with the rest not expressing FoxP3 and FoxP3int could be observed. Further phenotypic analyses indicated that these thymic FoxP3 cells did not express RORγt, mTGFβ, or CD62L (Fig. 7B). These results indicate that the FoxP3intRORγt+ cells, which are observed in the SP, PLN and PN do not develop their FoxP3int RORγt+ phenotype while in the thymus but rather in the periphery, which is in agreement with the idea that this population is a differentiation intermediate of peripheral CD4+ T cells (2, 19).
Figure 7. FoxP3+ but not FoxP3+RORγt+ cells are detectable in the thymus.
(A, B) Thymi were harvested from 6wk-old NOD mice and were stained for CD4, CD8, CD25, FoxP3 and either RORγt, mTGFβ or CD62L. (A) Gated CD4 single-positive cells were analyzed for CD25 and FoxP3 expression. (B) gated CD4+CD25+ thymocytes were analyzed for FoxP3 and RORγt, mTGFβ or CD62L expression. Isotype staining levels are represented by quadrant bars in dot plots and by filled gray lines in histograms. Stainings are representative of three experiments.
DISCUSSION
The notion of Th cell plasticity (2, 19–22) suggests that the immune response is far more adaptable than was previously thought and is therefore able to respond more appropriately to environmental stimuli. Initially, the naïve Th cell was viewed as a pluripotent precursor that could engage in one of multiple pathways and differentiate into a terminal Th1, Th2, Th17 or Treg cell (1). More recently, however, observations were made indicating that fully differentiated Tregs can reverse into Th17 cells (19) and a Th17 cell can switch to a Th1 phenotype (20–22). Evidence is accumulating indicating that Th plasticity is even broader and Th precursors engage in more than one pathway during differentiation, generating intermediate phenotypes susceptible for further programming (19, 41, 42). However, since intermediate cells are thought of as having a transient phenotype available only at low frequency, analysis of the mechanism underlying the mixed phenotypes or their function was limited. Herein, we identified a FoxP3intRORγt+ intermediate population that arises in the natural T cell repertoire alongside FoxP3hi (RORγt−) Tregs that could be expanded without further differentiation. These cells do not represent artifacts of the NOD.FoxP3-GPF reporter mice because they are expandable with Ig-GAD1 in normal NOD mice and display similar residential pattern in both strains (Supplemental Fig. 5). The cells arise in vivo before and after expansion with Ig-GAD1 and evidence is provided indicating that they express mTGFβ that serves for suppression of effector T cells (Fig. 1B, Supplementary Fig. 2) and CD62L that facilitates trafficking to sites of inflammation (Fig. 5A–C). In the PN, which is the site of inflammation in NOD mice, the cells retained the FoxP3intRORγt+ intermediate phenotype (Fig. 4A, B) as well as CD62L (Fig. 5A) and TGFβ (Fig. 4A, B), likely suggesting that they contribute to protection against the disease as intermediate cells. In fact, when the expanded cells were isolated from both the SP and the PN and transferred to NOD.scid mice along with diabetogenic splenocytes, they were able to traffic again to the PLN and PN in the host animals (Fig. 4E) and confer resistance against diabetes (Fig. 4C, D). Surprisingly, upon migration to the PN the cells did not down-regulate RORγt and protected against disease as FoxP3intRORγt+ bearing both CD62L and TGFβ. However, if the cells are subject to polarization, they retain the ability to fully differentiate to Th17 cells and produce IL-17 cytokine (Fig. 6C), or Tregs with higher FoxP3 but significantly reduced RORγt (Fig. 6). It was previously shown that CD4+ T cells expressing CD62L protect against diabetes (43, 44). These suppressor cells may represent classical Tregs that utilize CD62L to traffic to the site of inflammation. However, it is not clear whether they display the differentiation intermediate phenotype.
Since CD62L and TGFβ were expressed on the intermediate Tregs in the natural repertoire prior to inflammation, it is unlikely that they were induced by factors related to effector T cells. Rather, it would be more logical to envision that they arise prior to the onset of disease to guide the Tregs to the site of inflammation and target the local effector T cells regardless of their phenotype. This indeed provides a functional plasticity that would add flexibility relative to effector dictated specific trafficking and suppression reported recently (41, 42).
Overall, the findings reported in this study indicated that Th intermediates display both differentiation (1, 19–22, 45) and functional plasticity that could sustain measured but broad effectiveness against autoimmunity.
Supplementary Material
Footnotes
This work was supported by RO1DK65748 and R21AI68746 (to H.Z.) from NIH. D.M.T. and J.A.C. were supported by Life Sciences Fellowships from the University of Missouri, Columbia. C. M. H. was supported by a training grant GM008396 from NIGMS.
Abbreviations: Abbreviations: Agg, aggregated; GAD, glutamic acid decarboxylase; mTGFβ, membrane-bound active TGFβ; PLN, pancreatic LN; PN, Pancreas; RQ, relative quantity; SP, spleen; TID, type I diabetes; Tregs, T regulatory cells
REFERENCES
- 1.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Ann. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 2.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberal G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ FoxP3+ RORγt+ T cells. J. Exp. Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor FoxP3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Jin W, Hardegen N, Lei K, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naïve T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor FoxP3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fantini MC, Becker C, Montelone G, Pallone F, Galle PR, Neurath MF. Cutting Edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through FoxP3 induction and down-regulation of smad7. J. Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Borde M, Heissmeyer V, Feurer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FoxP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 7.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor FoxP3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 8.Shevach ME. From vanilla to 28 flavors: Multiple varieties of T regulatory cells. Immunity. 2006;25:1996–2001. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Tang Q, Bluestone JA. The Foxp3+ regulatory T cells : a Jack of all trades, master of regulation. Nat. Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the Th17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 13.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphey TL, Murphey KM, T C. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 14.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang Y, Wang Y, Hood L, Zhu Z, Tian Q, Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: An effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–465. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J. Exp. Med. 2008:b: 811–b: 823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNγ induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J. Exp. Med. 2008;205:207–218. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng X-H, Jetten AM, Dong C. Molecular antagonism an plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lexberg MH, Taubner A, Forster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang H. Th memory for interleukin-17 expression is stable in vivo. Eur. J. Immunol. 2008;38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- 21.Bending D, La Pena HD, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J. Clin. Inves. 2009;119:565–573. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin-Orozco N, Chung Y, Chang SH, Wang Y, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion to Th1 cells. Eur. J. Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregg R, Jain R, Schoenleber SJ, Divekar R, Bell JJ, Lee HH, Yu P, Zaghouani H. A sudden decline in active membrane-bound TGF-β impairs both T regulatory cell function and protection against autoimmune diabetes. J.Immunol. 2004;173:7308–7316. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
- 24.Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. FoxP3 inhibitis RORγt-mediated IL-17A mRNA transcription through direct interaction with RORγt. J. Biol. Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Guangxun M, Strober W. Interactions among the transcription factors Runx1, RORγt and FoxP3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou L, Lopes JE, Chong MMW, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-β-induced FoxP3 inhibits Th17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 28.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in nonobese diabetic mice. Nature. 1993;366:72. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 29.Legge KL, Min B, Bell JJ, Caprio JC, Li L, Gregg RK, Zaghouani H. Coupling of peripheral tolerance to endogenous interleukin 10 promotes effective modulation of myelin-activate T cells and ameliorates experimental allergic encephalomyelitis. J. Exp. Med. 2000;191:2039–2048. doi: 10.1084/jem.191.12.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faveeuw C, Gagnerault MC, Lepault F. Isolation of leukocytes infiltrating the islets of langerhans of diabetes-prone mice flow cytometric analysis. J. Immunol. Methods. 1995;187:163–169. doi: 10.1016/0022-1759(95)00180-i. [DOI] [PubMed] [Google Scholar]
- 31.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFβ1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J. Exp. Med. 2005;201:1333–1346. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venturi GM, Conway RM, Steeber DA, Tedder TF. CD25+CD4+ Regulatory T cell migration requires L-selectin expression: L-selectin transcriptional regulation balances constitutive receptor turnover. J. Immnol. 2006;178:291–300. doi: 10.4049/jimmunol.178.1.291. [DOI] [PubMed] [Google Scholar]
- 33.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J. Exp. Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T, Hanabuchi S, Wang Y, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu Y. Two functional subsets of FoxP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-β1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J. Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 37.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsy H, O’Shea JJ, Shevach EM. CD4+FoxP3+ regulatory T cells confer infectious tolerance in a TGF-β-dependent manner. J. Exp. Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lepault F, Gagnerault MC, Faveeuw C, Bazin HH, Boitart C. Lack of L-selectin expression by cells transferring diabetes in NOD mice: insights into the mechanisms involved in diabetes prevention by Mel-14 antibody treatment. Eur. J. Immunol. 1995;25(6):1502–1527. doi: 10.1002/eji.1830250605. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Karin N, Tisch R, Steinman L, McDevitt H. Inhibition of insulitis and prevention of diabetes in nonobese diabetic mice by blocking L-selectin and very late antigen 4 adhesion receptors. Proc. Natl. Acad. Sci. 1993;90:10494–10498. doi: 10.1073/pnas.90.22.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mora C, Grewal IS, Wong FS, Flavell RA. Role of L-selectin in the development of autoimmune diabetes in non-obese diabetic mice. Int. Immunol. 2004;16:257–264. doi: 10.1093/intimm/dxh036. [DOI] [PubMed] [Google Scholar]
- 41.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JK, Chu T, Corcoran L, Treuting P, Klein U, Rudensky A. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control Th2 responses. Nature. 2009;29:351–357. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herbelin A, Gombert JM, Lepault F, Bach JF, Chatenoud L. Mature mainstream TCR alpha beta (+)CD4(+) thymocytes expressing L-selectin mediate 'active tolerance' in the nonobese diabetic mouse. J. Immunol. 1998;161(5):2620–2628. [PubMed] [Google Scholar]
- 44.Seddon B, Saoudi A, Nicholson M, Mason D. CD4+CD8− thymocytes that express L-selectin protect rats from diabetes upon adoptive transfer. Eur. J. Immunol. 1996;12:2702–2708. doi: 10.1002/eji.1830261123. [DOI] [PubMed] [Google Scholar]
- 45.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh T, Watford WT, Schones DT, Peng W, Sun H, Paul WE, O’Shea JJ, Zhao K. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in linieage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.