Abstract
The physics of mass transport within body compartments and across biological barriers differentiates cancers from healthy tissues. Variants of nanoparticles can be manufactured in combinatorially large sets, varying only one transport-affecting design parameter at a time. Nanoparticles can also be used as building blocks for systems that perform sequences of coordinated actions, in accordance to a prescribed logic. These are referred to as Logic-Embedded Vectors “(LEV)” in the following. Nanoparticles and LEVs are ideal probes for the determination of mass transport laws in tumors, acting as imaging contrast enhancers, and can be employed for the lesion-selective delivery of therapy. Their size, shape, density and surface chemistry dominate convective transport in the blood stream, margination, cell adhesion, selective cellular uptake, as well as sub-cellular trafficking and localization. As argued here, the understanding of transport differentials in cancer, termed ‘transport oncophysics’ unveils a new promising frontier in oncology: the development of lesion-specific delivery particulates that exploit mass transport differentials to deploy treatment of greater efficacy and reduced side effects.
Introduction
In a seminal paper, Hanahan and Weinberg [1] identified six fundamental acquired capabilities as the shared-trait identifiers of the very diverse family of heterogeneous diseases we collectively term “cancer”. These are: tissue invasion and metastasis; sustained angiogenesis; self-sufficiency in growth signals; limitless replicative potential; evasion of apoptosis; and insensitivity to anti-growth signals. Tissue invasion and metastasis are exquisitely cancer-defining transport phenomena at the cellular level (Box 1). All cancer hallmark mechanisms are based on a complex set of defects in the regulatory circuitry that governs normal cell homeostasis and proliferation. Regulatory circuitries at the cell level, in turn, are based on information flow pathways that involve mass transport at the molecular level, since their effectors are cascades of interaction among molecules that are endowed with a coordinated set of mutual recognition specificities. For instance, extra-cellular transport properties crucially impact cell proliferation through signals received by the cell via transmembrane receptors that bind diffusible growth factor, cell adhesion molecules, and extra-cellular matrix components. The hallmark of acquired self-sufficiency in growth signals is known to be related to over-expression of growth factor receptors (e.g. EGF, HER2/neu) or receptor type switching (integrins), and might be impacted by disruptions in the signal transport chain, such as by independent or enhanced signaling resulting from receptor over-expression, or other causes. Dys-regulation of the downstream receiving and processing of signals emitted by ligand-activated growth factor receptors and integrins [1] is a transport-based type of mechanism for the induction of self-sufficiency in growth signals (e.g. SOS-Ras-Raf-MAPK pathway). The acquisition of insensitivity to antigrowth signals, which characterizes a large majority of cancers, comprises molecular processing components, which are interrelated with transport properties and the emergence of dys-regulated transport barriers to signaling molecules. Apoptosis controls are largely embedded in cell-to-cell contact signals that mandate the preservation of ‘health’ architectural configurations, and are therefore impacted by transport differentials in cells and molecules.
BOX 1.
Differentials in mass and information transport are defining characteristics of cancer
Tissue invasion and metastasis are the most significant transitions in the evolution of cancer, and the threshold events that govern its outcome and clinical intervention. By definition, these events are based on mass transport at the cellular level, and in particular the acquired ability of cancer cells to penetrate across biological barriers that are normally impassable. Tissue invasion entails violation of the encapsulating boundaries between proliferating cancer cells and their host stromal environment. Metastasis comprises a yet more complex set of steps that include acquired gains in cellular motility, as well as penetration of vascular endothelia at proximal sites (intravasation) and at the distant lesion-forming sites (extravasation). Differentials in transport properties that underlie this hallmark property of invasion and metastasis include changes in motility, mechanical properties at the cell level, stromal transport, and permeation across the vascular endothelium through a set of coordinated mechanisms that in turn involve ‘permeabilizing’ modifications of the endothelial barrier as well [54-59]. Angiogenesis also profoundly impacts transport on multiple levels. Neo-vascular networks have enhanced permeability owing to the substantial presence of fenestrations, or endothelial gaps with sizes ranging from tens of microns to hundreds of nanometers [21, 23].
Further differential transport properties are acquired by a growing cancer lesion through angiogenesis owing to the architectural features, such as anastomoses and atypical vessel morphologies that profoundly affect the dynamics of blood flow, the transport from the vascular compartment, and extravasation into the surrounding tissue [21]. The mechanics of molecular fractionation along radial directions, of cell margination, and endothelial adhesion in conditions of slow flow through lacunae-like angiogenic vessels differ greatly from transport dynamics of ‘normal’ vasculature. Competitive advantages for preferential transport in tumor-associated vasculature might be attained by modifications in cell size, shape, and mechanical properties [60, 61]. Additionally, the endothelial surfaces of tumor-associated angiogenic vasculature present with bimolecular markers [62] that mediate targeted adhesion and localization by cells with conjugate surface recognition moieties [63]. Angiogenic processes engender increased mass transport in cancer lesion. Together with unregulated proliferation growth, the insufficient co-development of lymphatic drainage and constitutive differentials in biomolecular permeabilities of neovascular endothelia, they generate imbalances in hydrostatic osmotic and interstitial fluid pressures during cancer growth [3]. These form a biophysical barrier to mass transport from the vascular into the tissue compartment as the resulting pressure typically directs convection into the opposing direction, and therefore tends to isolate the lesion from blood-borne large molecules and cellular infiltration.
Collectively, these observations suggest a novel framework for the understanding of cancer, that is based on aberrations of mass transport at all levels, from molecules to the full organism. This paper is dedicated to identifying fundamental elements of this framework, and proposing novel, transport oncophysics-based diagnostic and therapeutic strategies. Nanotechnologies naturally emerge as the fundamental enabling platforms for this approach – i.e., the novel frontier of cancer nanomedicine.
Cancer as a multi-scale mass- transport pathology
Cancer is s a disease of the cell, which is inextricably linked to its surrounding biological milieu through a complexity of interactions with its microenvironment and distant sites within the host organism [1, 2]. Both, the proximal contact and the remote interactions, centrally involve mass transport-based information transfer, in the form of molecular signals, the directed movement of cells, and the dynamic elaboration of tissue. The higher-scale portrait of cancer that emerges is that of a complex adaptive system that competitively exploits phenotype and genotype differentials to gain growth advantage, employing mass transport as a fundamental mechanism of coordination and communication. The multi-scale understanding of mass transport dys-regulation including the molecular, cellular, micro-environmental, and systemic levels, would provide the elements of a unifying framework for the diverse set of hallmarks, or common traits of cancer.
Biological barriers dominate transport differentials in cancer
A coarse classification of mechanisms of mass transport includes transport within defined compartments (e.g. vascular, cytoplasm, stromal, etc.) and between different compartments, in processes governed by elements of separation collectively termed “biological barriers” or “biobarriers”. These comprise defined biological surfaces (epithelia, endothelia, cell, nuclear, endosomal membranes etc), which are actually themselves multi-cellular and/or multi-compartmental, respectively, at a more granular scale of investigation. Biobarrier determinants of trans-compartment transport also include the activated monocytes and macrophages of the reticulo-endothelial system (RES), which provide for the circulatory clearance of aged red blood cells and blood-borne particulates into RES organs. From the perspective of the systemic administration of drugs and contrast agents, the biobarriers [3-6] as determinants of local concentration comprise, sequentially, from the point of intravascular injection: enzymatic degradation; sequestration by the phagocytes of the RES [7, 8]; the vascular endothelium [9]; adverse oncotic and interstitial pressures in the tumor [10, 11]; the tumor interstitium [12-18]; the membranes the cell themselves or subcellular structures such as the nucleus consist of, the endosomes and finally, ionic, or molecular efflux pumps for the elimination of toxins such as active therapeutic agents [19, 20]. The delivery of injected cancer-selective therapeutic agents requires successful negotiation of these barriers in order to attain a sufficient therapeutic index. For oral delivery into the systemic circulation through the gastro-intestinal tract, further barriers that need to be negotiated are pH gradients in the gastro-intestinal tract, enzymatic and chemical degradation, mucosal and endothelial (tight junctions) barriers. A prominent example of dys-regulation of transport across biological barriers in cancer is the hyper-permeability of tumor-associated neo-vascular endothelia, which manifests itself through the presence of fenestrations in the range of 100 nm to 10 microns [21-23]. Remarkably, the tumor associated vascular fenestrations themselves vary from patient to patient, from lesion to lesion, as well as over time in the course of therapy [24, 25]. While this variability poses a problem for therapeutic delivery, at the same time it presents an opportunity for lesion-specific targeting, and it suggests that this targeting strategy could be extended to broader combinations of biological barriers in cancer.
Nanotechnology tools are necessary for an investigation of mass transport differentials – and are the ideal tool for exploiting these differentials for lesion-specific therapeutics
The enhanced fenestrations in tumor-associated neovascular endothelia, and the resulting hyper-permeability of cancer-feeding angiogenic vessels gives rise to the so-called enhanced permeation and retention ‘EPR’ effect (see Box 2) of systemically-injected nanoparticles, which in turn leads to their preferential concentration at cancer sites. The EPR mechanism is considered the main reason underlying the therapeutic index advantages that have warranted the FDA approval of the first “nanomedicines”, i.e. liposomes for the delivery of doxorubicin and antifungal agents [26, 27], in the mid Nineties.
BOX 2.
Transport oncophysics and biomolecular targeting
A great conundrum in cancer therapeutics is that transport across biological barriers is typically more difficult for biologically-targeted molecules. This is the case for therapeutic molecules as well as for nanoparticles. For instance, in therapies involving molecular targeting, typically only one injected molecule in 1,000-100,000 reaches its intended destination [64, 65], despite the higher concentration that might be expected when considering the dissociation constant of complex formation alone. As a consequence of this, undesired collateral effects are often large and become unacceptable as is the case for example for trastuzumab, which was shown to have cardiac toxicity [66]. The targeting of nanoparticles via the surface conjugation of high-affinity ligands, antibodies, aptamers, peptides and other biological recognition moieties and the subsequent drug release at the target site has been widely investigated as a possible strategy to selectively deliver therapeutics to cancer lesions. This quest has however been complicated by the fact that nanoparticles with surface targeting moieties typically experience increased difficulty in mass transport across biobarriers. This is the main reason why all nanotherapeutics in clinical use to date are passively targeted, that is, they localize in tumor by means of the EPR effect rather than via biomolecular recognition. Specifically, the addition of biological targeting moieties to the surface renders the equivalent cross section of nanoparticles considerably larger and they are therefore much less likely to be able to exploit the beneficial EPR effect. EPR-based targeting is really a form of exploitation of the cancer differentials in biobarriers for localized delivery – the main concept in this article. Another biobarrier mitigation strategy that was first pioneered in liposomes, is the use of surface PEGylation, which results in a delay of nanoparticle uptake by the filtering organs of the RES. Adding further complexity, despite modest successes PEGylation and biological targeting have proven to be mutually exclusive for nanoparticles, forcing a decision between longer circulation life and biological recognition. Both the discussion of EPR targeting and the avoidance of RES uptake by PEGylation point to the fact that that the addition of biomolecular surface targeting moieties actually renders the problem of transport across biobarriers substantially more complex. The combination of novel transport-by-design vectors and biomolecular targeting thus emerges as a necessary, and potentially advantageous, strategy for new generations of therapeutic delivery carriers.
With the further FDA approval of albumin nanoparticulates for the delivery of Cremaphor®-free taxanes [28, 29] nanotherapeutics have become substantial players in oncology for well over a decade (see Box 3). Many novel classes of ‘second generation’ nanoparticles are currently under development for potential use in cancer therapeutics (see Box 3 and references therein). At the same time, nanoparticles, such as liposomes and quantum dots, have proven to be the tool of choice investigating the hyperpermeable vascular endothelia in cancer-associated angiogenesis [23-25, 30]. This illustrates the ‘dual use’ of nanoparticulates as instruments of investigation of the biological barriers that define cancer oncophysics, and their natural transition to use as vectors of selective therapeutic strategies that take advantage of transport differentials.
Box 3.
Nanotechnologies and cancer therapeutics
The administration of nanoparticles, which allow therapeutic agents to preferentially locate at desired lesion sites, has emerged as an attractive treatment modality with greater therapeutic index than the conventional formulations of the same agents [67-71]. The first nanodrugs were liposomal encapsulations of antifungals [72] and doxorubicin [73, 74], which were approved in the mid-Nineties. Another nanotherapeutic agent that has secured FDA approval for breast cancer in 2005 employs albumin nanoparticles that have been conjugated with Cremaphor®-free taxanes [28, 29]. In this formulation, the mass transport advantage that is credited with improved therapeutic index with respect to taxane is the chaperoning effect of the albumin, which enhances extravasation into the target tumor. This form of nanotherapy has experienced very rapid post-approval growth in clinical use, largely based on the fact that it does not require pretreatment with steroidal anti-inflammatory drugs. Nanoparticles currently command a market in excess of $ 5.4 Billion per year [77], making them a substantial reality in medicine by any metric. Hundreds of clinical trials are currently underway for the use of these nanoformulations, in combination with established individual drugs (see: http://www.cancer.org/ClinicalTrials.gov) in novel applications beyond the current approved indications of breast, ovarian, head and neck cancer, and Kaposi's sarcoma.
The clinically-approved liposomes and the albumin nanoparticles have no biomolecular target recognition specificity and are classified as ‘first generation’ nanotherapeutics. Second-generation nanoparticles, by definition, should perform other functions in addition to passive localization by EPR and drug release. A highly desired ‘second-generation’ function is ‘active targeting’, i.e., the biomolecular targeting to cancer cell surface epitopes by means of nanoparticle surface-appended recognition moieties, such as antibodies, peptides, or aptamers. Many years of research have been dedicated to developing actively-targeting nanoparticles, through the attachment of biorecognition moieties to their surfaces. Before the days of nanoparticles, this strategy has been pursued for the receptor-specific delivery of immunotherapy [78], radiopharmaceuticals [79] antisense oligonucleotides [80], liposomal drug carriers [81], imaging contrast agents [82], and finally has been the object of research for the intended targeting of novel generation nanovectored therapeutic agents [82-95] to cancer cells.
Nevertheless, the approach of decorating the surface of nanoparticles with targeting moieties has been met with very limited success as any injected nanoparticles must be PEGylated to warrant sufficient circulation half-lifes to be able to localize in the tumor [96].
PEG, however, tends to shield not only nanoparticles from the RES, but also their targeting agent from its target, resulting in a dramatic reduction of the probability of biorecognition [97]. Thus, to date, there are no nanovectors with biomolecular targeting strategy that have received regulatory approval [98]. A promising alternative strategy for selective localization employs nanoparticles as ‘antennae’ of externally supplied energy. Upon receiving an external signal, nanoparticles are activated to release heat, which results in thermal ablation of the surrounding tissue. Localization can be achieved by combination of EPR and the directed nature of the exogenous energy beam.
Important current applications of this strategy include the remote activation of gold nanoshells by near infrared radiation [36, 99], which is now in phase 1 clinical trials for head-and-neck cancer. Other approaches to induce localized nanoparticles activation involve the use of a rapidly switching magnetic field [100, 101] (in clinical trials for glioblastoma multiforme), or radiofrequencies [102, 103]. Remotely-triggered thermal ablation obviously bypasses the problem of the ‘emergence’ of resistant clones, which is a major concern of cancer therapeutics, since no clonal variants can develop that are resistance to heat.
Individualizing treatment by the selection of logic-embedded vectors with lesion-specific transport oncophysical properties that optimize therapeutic index
Without an effective strategy to negotiate the sequence of barriers, any current or novel therapeutic agents based on enhanced biomolecular selectivity might yield sub-optimal utility, simply because only very small fractions of the agent typically might reach their intended targets, with the vast majority being captured or eliminated in biobarriers. This problem is further compounded by the fact that the barriers themselves vary from patient to patient, from lesion to lesion, and over time in the course of therapy, as eloquently shown for instance in the case of tumor-associated vascular fenestrations [24, 25]. However, the differential expression of biological barriers offers an opportunity to ‘personalize’ treatment based on the observable characteristics of the biological barriers themselves. To illustrate: liposomes of suitable size concentrate preferentially in cancer lesions only by means of EPR, thus exploiting the hyper-permeability of tumor-associated vasculature in the angiogenic phase of cancer growth [26, 27]. The vascular endothelial anomalies (fenestrations) that cause this increase in permeability change with patient, cancer type, in the course of the disease, and in response to therapy. This offers the conceptual opportunity to tailor nanoparticle size to the specific characteristic of the fenestrations, thus ‘personalizing’ treatment based on the pathological disruptions of a biobarrier: the vascular endothelium.
Thus, in summary, the overall challenge is to optimize therapeutic indices for multi-agent, targeted treatments. This requires the attainment of biological specificity of treatment, while simultaneously addressing the conundrum of the sequence of biological barriers – and this is not only the case for a single biomolecular drug, but also true for the broad spectrum of agents that can address the multitude of different cancer presentations.
Molecular oncology is advancing toward a more complete understanding of the biological pathways and molecular expressions that identify cancers. New therapies will doubtlessly emerge from these efforts. Our philosophy is that these must be aided by concurrent, synergistic programs on the complementary problem of the avoidance of biological barriers, and the development of delivery strategies that will permit the co-localization of multiple, cooperative agents at the same target location. This is where nanotechnology can provide a crucial benefit. The search for molecules that can provide the triad of multiplexed functions: biorecognition, cytotoxicity or specific pathway modulation, and biobarrier avoidance for several synergistic agents might be too much to ask of drugs, present and future alike, that may be employed to address the full, time-dynamic diversity of cancer presentations.
The required individualization might well prove to be at the single lesion or cell clone, even beyond ‘personalization’ at the level of a single patient. The emerging frontier is thus the development of therapeutic multi-component constructs, in which the functions of biorecognition, cytotoxicity and biobarrier avoidance are decoupled, yet act in efficacious operational harmony. In view of the sequential nature of biobarriers, it is further necessary to develop therapeutic agents that have the ability to perform time-sequential function, thus confronting biobarriers in their accurate time-sequential order. Therefore, the need emerges for a ‘logic’ of actions in negotiating the barriers, which, ultimately, would result in therapeutic drug release only at the desired lesion site. Obviously, such logic cannot be coded in the sense of electronic programs. Instead, it must be embedded into the material properties of the delivery vectors and their payloads. We have recently introduced a first example of ‘logic-embedded vectors’ (LEVs). These are multi-stage carriers (Figure 1), whereby each stage performs part of the journey from the site of administration toward the target lesion, negotiating one or more biological barriers, and adding a degree of targeting selectivity in the process. As biological selectivities, we used here cell target recognition by different classes of surface molecules, but as discussed further below, we employed mathematics-based rational design of the size, shape, and physical properties of the vector particles to multiply manifold the probability of recognition of the target antigen – a synergy between molecular biology, physics, engineering and mathematics.
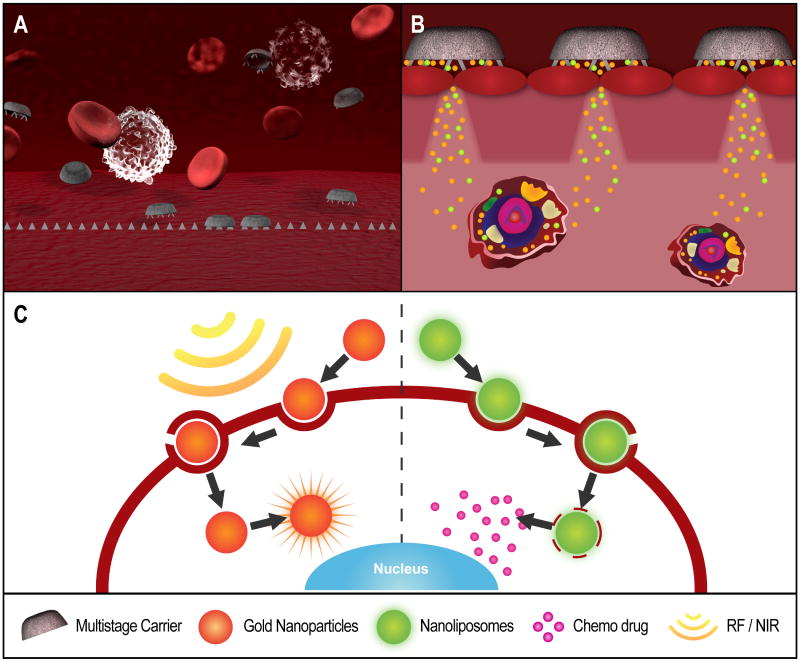
Figure 1. Schematic illustration of the action of a multi-stage vector.
A) A non-toxic, biodegradable first-stage carrier is optimally designed to evade RES and have margination, adhesion, internalization properties that allow it to attain preferential concentration on the target tumor vascular endothelium. (B) The first-stage particle co-releases second-stage carrier nanoparticles with agents that facilitate their permeation through the vascular endothelium into the tumor tissue. (C) The second-stage nanoparticles penetrate the cellular membrane and deploy different, synergistic therapeutic agents into the cytoplasm, the nucleus, or other subcellular targets. The particles themselves can serve as a means for physical therapy, e.g. by converting external radiation (light, radiofrequency, ultrasounds) into heat, for a localized form of thermal ablation.
Biological action at the target cells is then carried out by the vector-embedded molecule. By way of example, a system may be envisioned that comprises a first stage vector that localizes preferentially to tumor-associated vasculature, and there directionally releases penetration enhancer agents together with one or more species of second stage particles that permeate into the tumor and reach the target cells with biological specificity, where they cross the cell and nuclear membranes, and deliver a single or multiple payload at different subcellular locations, with potentially different time release profiles (Figure 1). The vectors can be optimally designed and synthesized following direct observation of the individual characteristics of the target lesions, and the barriers to reaching it: For instance, imaging techniques can be used to determine vascular permeability [23, 30], intratumor flow characteristics [31, 32], and target antigen densities on the tumor-associated vascular endothelium and the cancer cells [33-36]. The information can be entered into suitable mathematical design codes, which then yield the optimal, ‘individualized’ vector characteristics. Synthesis of the multi-stage vector follows. A suitable combination of multiple agents of therapy is loaded into the vector, which is further decorated with one or more targeting moieties. The individualization of therapy is thus built in the carrier vector, following direct imaging observation of the lesion, and is present regardless of the drug delivered, though obviously optimized when molecularly targeted drugs are delivered. The time dynamics of the evolution of the lesion do not necessarily require a change in cytotoxic or otherwise bio-modulating payload – the response to the evolution of the lesion and its microenvironment might be built in the individualization of carrier, simply by designing the vectors to permeate preferentially across the biological barriers of the target lesion at the specific time point, in which therapy is administered.
We recently established such a multi-stage technology platform, and have essentially demonstrated the fundamental components of the above-cited vision. The optimal mathematical design of first-stage vector particles with respect to margination [37-39], firm cellular adhesion [39, 40], internalization [41, 42] was demonstrated. We also developed comprehensive carrier “design maps” [43], providing a direction for the solution to the “targeting versus. RES-uptake conundrum” by way of rational design of the carrier geometry [44, 45]. Furthermore, a framework for the translation of these results to full drug biodistribution models was also presented [46, 47].
The design maps provide a graphic summary of the probabilities that certain transport functions associated with the delivery of therapeutic payload will be realized, as function of the design parameters of the carriers. Examples of such transport functions are margination in tumor-associated capillaries, firm adhesion onto vascular endothelial cells, and uptake by phagocytes. Design parameters for the vectors include size, shape, surface charge, affinity for endothelially expressed cancer antigens, and deformability. The design maps can act as selection criteria for of the particular nanotherapeutics to be chosen for clinical translation [47] in analogy to the use of in silico criteria in conventional drug development, and of course with the same requirement of experimental validation in relevant biological tests. Once the optimal design properties of a candidate are determined from mathematical analysis, it is necessary to develop the manufacturing of particles that can attain them. We have elected to use photolithographic synthesis protocols, which can yield first-stage particles of essentially any size and shape, so that the entire space within the design maps can be realized [48]. We have applied these methods to nanoporous silicon, which is fully biodegradable with degradation rates that can be tuned from hours to years and which has no residual toxicity as the degradation product is orthosilicic acid in concentrations that are far below the daily dietary intake. Finally, we were also able to demonstrate loading and release of multiple nanoparticles types (e.g. quantum dots, single-walled nanotubes) from micron-sized nanoporous silicon carriers [48].
Drug and nanoparticle release from these multi-stage particles can be tuned to take place at different times, and through different pathways. Particles can also be designed to be intracellularly internalized [49, 50] in order to deliver their payloads to different subcellular components. We were able to show that optimized carrier sizes and shapes enhanced the probability of complex formation between cell surface moieties and the conjugate targeting molecules [40, 51]. Furthermore, it was shown in animal cancer models that the biodistribution of the carriers was a function of their geometry and materials [52]. Consequently, by tailoring the particle size and shape, an injected dose of carriers could be concentrated in the animal tumors, without the need to add any biological targeting moieties to these particles [52]. Far from being just passive carriers, nanoparticles thus directly impact on cancer cell proliferation and apoptosis proliferation through the Akt and MAPK pathways, and they are governed only by their size and shape [46], thus suggesting the potential of ‘physics-based’ cancer therapy.
Conclusions
Mass transport differentials are defining characteristics of cancer. Biological barriers are determinants of such relevance in mass transport differentials that a new operational (“oncophysics”) classification of individual lesions might be envisioned based on the characteristics of transport through biobarriers. Nano- and micro-particulates are ideal probes for the study of mass transport differentials in cancer, and, as an immediate corollary, are perfectly suited for the preferential transport of therapeutic agents to cancer sites, and the realization of treatments of enhanced therapeutic index. The unfortunate reality is that the addition of biomolecular recognition moieties on the surface of nanoparticles renders their penetration across biological barriers more difficult to attain. In view of the sequential nature of biobarriers, the development of Logic-Embedded Vectors (LEV), i.e. particulates that can act in a time-sequential fashion to exploit biobarrier vulnerabilities is a necessary new frontier on cancer therapeutics. ‘Multi-stage vectors are a first class of such LEVs and comprise a system of nested nanoparticles, each of which is intended to carry out part of the travel from site of intravascular injection to subcellular localization in target cells, each adding a degree of targeting specificity. Through the development of a set of mathematical tools, which can aid in the rational design of nanoparticulates and LEV, their properties such as margination and firm adhesion to target vascular endothelia can be optimized and their preferential cellular uptake might thus be achievable.
BOX 4.
Future Questions
The biological barriers to mass transport are many, and evolve dynamically. Novel methods for overcoming their diverse and sequential presentation are necessary. Nanotechnology provides an ideal set of tools to meet this challenge, and in particular to develop approaches to the central problem of interstitial transport, against an adverse hydrostatic pressure field. Given the substantial reliance of rational design codes for nanoparticles and LEVs, it will be necessary to expand the available mathematical codes to incorporate effects beyond vascular transport and endothelial adhesion. Most pressing needs are the incorporation of models of transport across the interstitium, and the identification of the transport differentials in healthy and neoplastic interstitial, at different stages of the evolution of the pathology. The physics of transport within the cytoplasm, such as trafficking to different subcellular locations, also requires much deeper understanding, from both the viewpoint of mathematical codes, and the development of nanoparticulate probes and transport vehicles. Great improvements on the experimental methods for the validation of all components of the rational design codes for mass transport are required.
Acknowledgments
Biana Godin, Paolo Decuzzi, Rita Serda and Tong Sun are gratefully acknowledged for their insightful discussion of the manuscript, and assistance in its preparation. Matt Landry is gratefully recognized for his artistry in the preparation of Figure 1. The author acknowledges a financial support from the following sources: DODW81XWH-09-1-0212, DODW81XWH-07-2-0101; NASA NNJ06HE06A; NIH RO1CA128797, NIH – R33 CA122864, NIH U54CA143837 and State of Texas, Emerging Technology Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–63. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 4.Jain RK. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst. 1989;81:570–6. doi: 10.1093/jnci/81.8.570. [DOI] [PubMed] [Google Scholar]
- 5.Sakamoto J, Annapragada A, Decuzzi P, Ferrari M. Antibiological barrier nanovector technology for cancer applications. Expert Opin Drug Deliv. 2007;4:359–69. doi: 10.1517/17425247.4.4.359. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari M. Cancer Nanotechnology. In: Bast R, Frei E, Holland JF, editors. Cancer Medicine e.8. BC Decker Inc; 2009. In press. [Google Scholar]
- 7.Moghimi SM, Davis SS. Innovations in avoiding particle clearance from blood by Kupffer cells: cause for reflection. Crit Rev Ther Drug Carrier Syst. 1994;11:31–59. [PubMed] [Google Scholar]
- 8.Caliceti P, Veronese FM. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev. 2003;55:1261–77. doi: 10.1016/s0169-409x(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 9.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 10.Less JR, Posner MC, Boucher Y, Borochovitz D, Wolmark N, Jain RK. Interstitial hypertension in human breast and colorectal tumors. Cancer Res. 1992;52:6371–4. [PubMed] [Google Scholar]
- 11.Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60:4251–5. [PubMed] [Google Scholar]
- 12.Pluen A, Boucher Y, Ramanujan S, McKee TD, Gohongi T, di Tomaso E, Brown EB, Izumi Y, Campbell RB, Berk DA, Jain RK. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc Natl Acad Sci U S A. 2001;98:4628–33. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–6. [PubMed] [Google Scholar]
- 14.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–503. [PubMed] [Google Scholar]
- 15.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 16.McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–13. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 17.Mok W, Boucher Y, Jain RK. Matrix metalloproteinases-1 and -8 Improve the distribution and efficacy of an oncolytic virus. Cancer Res. 2007;67:10664–10668. doi: 10.1158/0008-5472.CAN-07-3107. [DOI] [PubMed] [Google Scholar]
- 18.Mok W, Stylianopoulos T, Boucher Y, Jain RK. Mathematical modeling of herpes simplex virus distribution in solid tumors: implications for cancer gene therapy. Clin Cancer Res. 2009;15:2352–60. doi: 10.1158/1078-0432.CCR-08-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng. 2006;8:343–75. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- 20.Majumdar S, Mitra AK. Chemical modification and formulation approaches to elevated drug transport across cell membranes. Expert Opin Drug Deliv. 2006;3:511–27. doi: 10.1517/17425247.3.4.511. [DOI] [PubMed] [Google Scholar]
- 21.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–51. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 22.Undevia SD, Gomez-Abuin G, Ratain MJ. Pharmacokinetic variability of anticancer agents. Nat Rev Cancer. 2005;5:447–58. doi: 10.1038/nrc1629. [DOI] [PubMed] [Google Scholar]
- 23.Hobbs SK, Monsky WL, Yuan F, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–12. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellian M, Yuan F, Trubetskoy VS, Torchilin VP, Jain RK. Vascular permeability in a human tumour xenograft: molecular charge dependence. Br J Cancer. 2000;82:1513–8. doi: 10.1054/bjoc.1999.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun C, Jain RK, Munn LL. Non-uniform plasma leakage affects local hematocrit and blood flow: implications for inflammation and tumor perfusion. Ann Biomed Eng. 2007;35:2121–9. doi: 10.1007/s10439-007-9377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 27.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–8. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, Beals B, Figg WD, Hawkins M, Desai N. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol) Clin Cancer Res. 2005;11:4136–43. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 29.Gradishar WJ. Albumin-bound nanoparticle paclitaxel. Clin Adv Hematol Oncol. 2005;3:348–9. [PubMed] [Google Scholar]
- 30.Stroh M, Zimmer JP, Duda DG, Levchenko TS, Cohen KS, Brown EB, Scadden DT, Torchilin VP, Bawendi MG, Fukumura D, Jain RK. Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nat Med. 2005;11:678–82. doi: 10.1038/nm1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosen MA, Schnall MD. Dynamic contrast-enhanced magnetic resonance imaging for assessing tumor vascularity and vascular effects of targeted therapies in renal cell carcinoma. Clin Cancer Res. 2007;13:770s–776s. doi: 10.1158/1078-0432.CCR-06-1921. [DOI] [PubMed] [Google Scholar]
- 32.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 33.Hoshii T, Nishiyama T, Toyabe S, Akazawa K, Komatsu S, Kaneko M, Hara N, Takahashi K. Evaluation of magnetic resonance imaging-based prostate-specific antigen density of the prostate in the diagnosis of prostate cancer. Int J Urol. 2007;14:305–10. doi: 10.1111/j.1442-2042.2007.01686.x. [DOI] [PubMed] [Google Scholar]
- 34.Koyama Y, Barrett T, Hama Y, Ravizzini G, Choyke PL, Kobayashi H. In vivo molecular imaging to diagnose and subtype tumors through receptor-targeted optically labeled monoclonal antibodies. Neoplasia. 2007;9:1021–9. doi: 10.1593/neo.07787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett T, Koyama Y, Hama Y, Ravizzini G, Shin IS, Jang BS, Paik CH, Urano Y, Choyke PL, Kobayashi H. In vivo diagnosis of epidermal growth factor receptor expression using molecular imaging with a cocktail of optically labeled monoclonal antibodies. Clin Cancer Res. 2007;13:6639–48. doi: 10.1158/1078-0432.CCR-07-1119. [DOI] [PubMed] [Google Scholar]
- 36.Riehemann K, Schneider SW, Luger T, Godin B, Ferrari M, Fuchs H. Nanotechnology - challenge and opportunity in clinical applications. Angew Chem Int Ed. 2009;48:872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decuzzi P, Lee S, Bhushan B, Ferrari M. A theoretical model for the margination of particles within blood vessels. Ann Biomed Eng. 2005;33:179–90. doi: 10.1007/s10439-005-8976-5. [DOI] [PubMed] [Google Scholar]
- 38.Gentile F, Chiappini C, Fine D, Bhavane RC, Peluccio MS, Cheng MM, Liu X, Ferrari M, Decuzzi P. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech. 2008;41:2312–8. doi: 10.1016/j.jbiomech.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Lee SY, Ferrari M, Decuzzi P. Design of bio-mimetic particles with enhanced vascular interaction. J Biomech. 2009;42:1885–90. doi: 10.1016/j.jbiomech.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Decuzzi P, Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27:5307–14. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Decuzzi P, Ferrari M. The role of specific and non-specific interactions in receptor-mediated endocytosis of nanoparticles. Biomaterials. 2007;28:2915–22. doi: 10.1016/j.biomaterials.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Decuzzi P, Ferrari M. The receptor-mediated endocytosis of nonspherical particles. Biophys J. 2008;94:3790–7. doi: 10.1529/biophysj.107.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Decuzzi P, Ferrari M. Design maps for nanoparticles targeting the diseased microvasculature. Biomaterials. 2008;29:377–84. doi: 10.1016/j.biomaterials.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 44.Godin B, Driessen WH, Proneth B, Lee SY, Srinivasan S, Rumbaut R, Arap W, Pasqualini R, Ferrari M, Decuzzi P. An integrated approach for the rational design of nanovectors for biomedical imaging and therapy. In: Pasqulini R, Arap W, editors. Advances in genetics. Elsevier; 2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: Does geometry really matter? Pharm Res. 2009;26:235–43. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 46.Sanga S, Sinek JP, Frieboes HB, Ferrari M, Fruehauf JP, Cristini V. Mathematical modeling of cancer progression and response to chemotherapy. Expert Rev Anticancer Ther. 2006;6:1361–76. doi: 10.1586/14737140.6.10.1361. [DOI] [PubMed] [Google Scholar]
- 47.Ferrari M. The mathematical engines of nanomedicine. Small. 2008;4:20–5. doi: 10.1002/smll.200701144. [DOI] [PubMed] [Google Scholar]
- 48.Tasciotti E, Liu XW, Bhavane R, Plant K, Leonard AD, Price BK, Cheng MMC, Decuzzi P, Tour JM, Robertson F, Ferrari M. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nature Nanotechnology. 2008;3:151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 49.Serda RE, Gu J, Bhavane RC, Liu X, Chiappini C, Decuzzi P, Ferrari M. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials. 2009;30:2440–8. doi: 10.1016/j.biomaterials.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 50.Serda RE, Ferrati S, Godin B, Tasciotti E, Liu X, Ferrari M. Mitotic trafficking of silicon microparticles. Nanoscale. 2009 doi: 10.1039/B9NR00138G. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Decuzzi P, Causa F, Ferrari M, Netti PA. The effective dispersion of nanovectors within the tumor microvasculature. Ann Biomed Eng. 2006;34:633–41. doi: 10.1007/s10439-005-9072-6. [DOI] [PubMed] [Google Scholar]
- 52.Decuzzi P, Godin B, Tanaka T, Lee SY, Liu X, Chiappini C, Ferrari M. Size and Shape Effects in the Biodistribution of nano-Particle Systems. J Control Rel. 2009 doi: 10.1016/j.jconrel.2009.10.014. 2009, in press. [DOI] [PubMed] [Google Scholar]
- 53.Ferrari M. Nanogeometry: Beyond drug delivery. Nature Nanotechnology. 2008;3:131–132. doi: 10.1038/nnano.2008.46. [DOI] [PubMed] [Google Scholar]
- 54.Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863–71. [PubMed] [Google Scholar]
- 55.Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12:89–96. doi: 10.1006/scbi.2001.0416. [DOI] [PubMed] [Google Scholar]
- 56.Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–31. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 57.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: The force journey of a tumor cell. Cancer Metastasis Rev. 2009 Jan 21; doi: 10.1007/s10555-008-9173-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnsen M, Lund LR, Rømer J, Almholt K, Danø K. Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr Opin Cell Biol. 1998;10:667–71. doi: 10.1016/s0955-0674(98)80044-6. [DOI] [PubMed] [Google Scholar]
- 59.Karadag A, Fedarko NS, Fisher LW. Dentin matrix protein 1 enhances invasion potential of colon cancer cells by bridging matrix metalloproteinase-9 to integrins and CD44. Cancer Res. 2005;65:11545–52. doi: 10.1158/0008-5472.CAN-05-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: The force journey of a tumor cell. Cancer Metastasis Rev. 2009 doi: 10.1007/s10555-008-9173-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato M, Arap W, Pasqualini R. Molecular targets on blood vessels for cancer therapies in clinical trials. Oncology. 2007;21:1346–52. [PubMed] [Google Scholar]
- 63.Tandle A, Hanna E, Lorang D, Hajitou A, Moya CA, Pasqualini R, Arap W, Adem A, Starker E, Hewitt S, Libutti SK. Tumor vasculature-targeted delivery of tumor necrosis factor-alpha. Cancer. 2009;115:128–39. doi: 10.1002/cncr.24001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Epenetos AA, Snook D, Durbin H, Johnson PM, Taylor-Papadimitriou J. Limitations of radiolabeled monoclonal antibodies for localization of human neoplasms. Cancer Res. 1986;46:3183–91. [PubMed] [Google Scholar]
- 65.Khawli LA, Miller GK, Epstein AL. Effect of seven new vasoactive immunoconjugates on the enhancement of monoclonal antibody uptake in tumors. Cancer. 1994;73:824–31. doi: 10.1002/1097-0142(19940201)73:3+<824::aid-cncr2820731312>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 66.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 67.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 68.Langer R. Drug delivery. Drugs on target. Science. 2001;293:58–9. doi: 10.1126/science.1063273. [DOI] [PubMed] [Google Scholar]
- 69.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 70.Peer D, Karp J, Hong S, Farokhzad O, Margalit R, Langer R. Nanocarriers: Emerging platforms for cancer therapy. Nature Nanotechnology. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka T, Decuzzi P, Cristofanilli M, Sakamoto JH, Tasciotti E, Robertson FM, Ferrari M. nanotechnology for breast cancer therapy. Biomedical Microdevices. 2009;11:49–63. doi: 10.1007/s10544-008-9209-0. [DOI] [PubMed] [Google Scholar]
- 72.Moen MD, Lyseng-Williamson KA, Scott LJ. Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs. 2009;69:361–92. doi: 10.2165/00003495-200969030-00010. [DOI] [PubMed] [Google Scholar]
- 73.Campos S. Liposomal anthracyclines: adjuvant and neoadjuvant therapy for breast cancer. Oncologist. 2003;8 2:10–6. doi: 10.1634/theoncologist.8-suppl_2-10. [DOI] [PubMed] [Google Scholar]
- 74.Lyass O, Uziely B, Ben-Yosef R, Tzemach D, Heshing NI, Lotem M, Brufman G, Gabizon A. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer. 2000;89:1037–47. doi: 10.1002/1097-0142(20000901)89:5<1037::aid-cncr13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 75.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–53. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 76.Blum JL, Savin MA, Edelman G, Pippen JE, Robert NJ, Geister BV, Kirby RL, Clawson A, O'Shaughnessy JA. Phase II study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer. 2007;7:850–6. doi: 10.3816/CBC.2007.n.049. [DOI] [PubMed] [Google Scholar]
- 77.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–9. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 78.Lu Y, Sega E, Low PS. Folate receptor-targeted immunotherapy: induction of humoral and cellular immunity against hapten-decorated cancer cells. Int J Cancer. 2005;116:710–9. doi: 10.1002/ijc.21126. [DOI] [PubMed] [Google Scholar]
- 79.Muller C, Hohn A, Schubiger PA, Schibli R. Preclinical evaluation of novel organometallic 99mTc-folate and 99mTc-pteroate radiotracers for folate receptor-positive tumour targeting. Eur J Nucl Med Mol Imaging. 2006;33:1007–16. doi: 10.1007/s00259-006-0111-9. [DOI] [PubMed] [Google Scholar]
- 80.Yang X, Gorenstein DG. Progress in thioaptamer development. Curr Drug Targets. 2004;5:705–15. doi: 10.2174/1389450043345074. [DOI] [PubMed] [Google Scholar]
- 81.Pan X, Lee RJ. Tumour-selective drug delivery via folate receptor-targeted liposomes. Expert Opin Drug Deliv. 2004;1:7–17. doi: 10.1517/17425247.1.1.7. [DOI] [PubMed] [Google Scholar]
- 82.Shi X, Wang S, Meshinchi S, Van Antwerp ME, Bi X, Lee I, Baker JR., Jr Dendrimer-entrapped gold nanoparticles as a platform for cancer-cell targeting and imaging. Small. 2007;3:1245–52. doi: 10.1002/smll.200700054. [DOI] [PubMed] [Google Scholar]
- 83.Rivera E. Liposomal anthracyclines in metastatic breast cancer: clinical update. Oncologist. 2003;8 2:3–9. doi: 10.1634/theoncologist.8-suppl_2-3. [DOI] [PubMed] [Google Scholar]
- 84.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–60. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 85.Green JJ, Chiu E, Leshchiner ES, Shi J, Langer R, Anderson DG. Electrostatic ligand coatings of nanoparticles enable ligand-specific gene delivery to human primary cells. Nano Lett. 2007;7:874–9. doi: 10.1021/nl062395b. [DOI] [PubMed] [Google Scholar]
- 86.Cloninger MJ. Biological applications of dendrimers. Curr Opin Chem Biol. 2002;6:742–8. doi: 10.1016/s1367-5931(02)00400-3. [DOI] [PubMed] [Google Scholar]
- 87.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A. 2003;100:13549–54. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5:709–11. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 89.Yong KT, Qian J, Roy I, Lee HH, Bergey EJ, Tramposch KM, He S, Swihart MT, Maitra A, Prasad PN. Quantum rod bioconjugates as targeted probes for confocal and two-photon fluorescence imaging of cancer cells. Nano Lett. 2007;7:761–5. doi: 10.1021/nl063031m. [DOI] [PubMed] [Google Scholar]
- 90.Kam NW, O'Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102:11600–5. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan F, Kopelman R. The embedding of meta-tetra(hydroxyphenyl)-chlorin into silica nanoparticle platforms for photodynamic therapy and their singlet oxygen production and pH-dependent optical properties. Photochem Photobiol. 2003;78:587–91. doi: 10.1562/0031-8655(2003)078<0587:teomis>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 92.Martin FJ, Melnik K, West T, Shapiro J, Cohen M, Boiarski AA, Ferrari M. Acute toxicity of intravenously administered microfabricated silicon dioxide drug delivery particles in mice: preliminary findings. Drugs R D. 2005;6:71–81. doi: 10.2165/00126839-200506020-00002. [DOI] [PubMed] [Google Scholar]
- 93.Peng J, He X, Wang K, Tan W, Li H, Xing X, Wang Y. An antisense oligonucleotide carrier based on amino silica nanoparticles for antisense inhibition of cancer cells. Nanomedicine. 2006;2:113–20. doi: 10.1016/j.nano.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 94.Oyewumi MO, Mumper RJ. Engineering tumor-targeted gadolinium hexanedione nanoparticles for potential application in neutron capture therapy. Bioconjug Chem. 2002;13:1328–35. doi: 10.1021/bc025560x. [DOI] [PubMed] [Google Scholar]
- 95.Yan F, Xu H, Anker J, Kopelman R, Ross B, Rehemtulla A, Reddy R. Synthesis and characterization of silica-embedded iron oxide nanoparticles for magnetic resonance imaging. J Nanosci Nanotechnol. 2004;4:72–6. doi: 10.1166/jnn.2004.074. [DOI] [PubMed] [Google Scholar]
- 96.Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs. 1997;54 4:15–21. doi: 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- 97.Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid-PEG conjugates. Adv Drug Deliv Rev. 2004;56:1177–92. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 98.Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nature Nanotechnology. 2008;3:242–244. doi: 10.1038/nnano.2008.114. [DOI] [PubMed] [Google Scholar]
- 99.Cherukuri P, Gannon CJ, Leeuw TK, Schmidt HK, Smalley RE, Curley SA, Weisman RB. Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence. Proc Natl Acad Sci U S A. 2006;103:18882–6. doi: 10.1073/pnas.0609265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Derfus A, Von Maltzahn G, Harris T, Duza T, Vecchio K, Ruoslahti E, Bhatia S. Remotely triggered release from magnetic nanoparticles. Advanced Materials. 2007;19:3932–36. [Google Scholar]
- 101.Maier-Hauff K, Rothe R, Scholz R, Gneveckow U, Wust P, Thiesen B, Feussner A, von Deimling A, Waldoefner N, Felix R, Jordan A. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol. 2007;81:53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 102.Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10:338–47. doi: 10.1245/aso.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 103.Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, Weisman RB, Pasquali M, Schmidt HK, Smalley RE, Curley SA. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110:2654–65. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]