Abstract
Animals develop robust learning and long lasting taste aversion memory once they experience a new taste that is followed by visceral discomfort. A large body of literature has supported the hypothesis that basolateral amygdala (BLA) plays a critical role in the acquisition and extinction of such conditioned taste aversions (CTA). Despite the evidence that BLA is crucially engaged during CTA training, it is unclear how BLA neural activity represents the conditioned tastes. Here, we incorporated a modified behavioral paradigm suitable for single unit study, one which utilizes a sequence of pulsed saccharin and water infusion via intraoral cannulae. After conditioning, we investigated BLA unit activity while animals experience the conditioned taste (saccharin). Behavioral tests of taste reactivity confirmed that the utilized training procedure produced reliable acquisition and expression of the aversion throughout test sessions. When neural activity was compared between saccharin and water trials, half of the recorded BLA units (77/149) showed differential activity according to the types of solution. 76% of those cells (29/38) in the conditioned group showed suppressed activity, while only 44% of taste reactive cells (17/39) in controls showed suppressed activity during saccharin trials (relative to water trials). In addition, the overall excitability of BLA units was increased as shown by altered characteristics of burst activity after conditioning. The changes in BLA activity as a consequence of CTA were maintained throughout test sessions, consistent with the behavioral study. The current study suggests that the neuronal activity evoked by a sweet taste is altered as a consequence of CTA learning, and that the overall change might be related to the learning induced negative affect.
Keywords: Taste aversion, Reactivity, Single unit, Suppression, Burst
Introduction
Taste aversion learning is a form of classical conditioning in which an animal associates a taste with a treatment that produces nausea or illness. As a consequence of conditioning, a preferred taste becomes disliked and avoided. The learning is potent and adaptive, as it protects animals from ingestion of lethal toxins (Rozin & Kalat, 1971; Garcia et al., 1974). The robustness of conditioned taste aversions (CTAs) and their rapid acquisition makes this paradigm an ideal model for studying the neural basis of learning (Bermúdez-Rattoni, 2004). In such single trial learning, discrete, all-or-none changes must occur which provides an opportunity to identify key neural alterations during learning.
Fos-like immunoreactivity (FLI) has been used for identifying patterns of neuronal activation after animals are exposed to particular experiences. Lateralized pathways of the amygdala and insular cortex have been identified as neural substrates of CTA learning using expression of the immediate early gene, c-Fos (Koh & Bernstein, 2005). This technique, however, relies on strong and sustained activation of neurons (Dragunow & Faull, 1989) and provides no temporal information. In contrast, single cell recordings in awake and behaving animals can address neural responsiveness over time, and provide new insight into the nature of the neural code underlying learning.
Work aimed at characterizing how individual neurons in the amygdala change their responsiveness to a taste after aversion conditioning (Burešová et al., 1979; Yashosima et al., 1995; Grossman et al., 2008) has not yielded consistent patterns. This may be due, in part, to technical difficulties and limitations of the CTA paradigms, which has not been designed for characterizing taste-responsive unit activity. CTAs are acquired in a single trial, which does not provide much time for unit sampling and characterization. Several neurobehavioral studies have implemented brief infusions of solution via an intraoral (I/O) cannula to examine the neural activity corresponding to behavioral reactivity (Roitman et al., 2005; Grossman et al., 2008; Wheeler et al., 2008). We adapted this methodology to the CTA learning paradigm to make it suitable for unit recording. This protocol incorporated pulsed fluids (saccharin solution as the conditioned stimulus (CS) or water) delivered through two chronically implanted I/O cannulae that enforced stimulus sampling, and allowed for the assessment of time-locked unit activity over multiple stimulus presentations. This protocol successfully resulted in taste aversions following a single conditioning session. Next, this paradigm was utilized during recording sessions to compare basolateral amygdala (BLA) unit responses to a CS taste in conditioned and control animals. It is well accepted that BLA is important for responding to the emotional salience of sensory stimuli (Aggleton & Mishkin, 1986). Previous work with FLI expression has revealed that BLA is differentially activated after CS-US (unconditioned stimulus) pairing during taste aversion training (Koh & Bernstein, 2005). In addition, delivery of mild electrical stimulation to the amygdala when CTA trained rats lick a CS disrupts the discrimination of a learned taste from water (Brožek et al., 1979). Therefore, it was hypothesized that BLA neurons would show differential responsiveness to a CS taste as a function of whether an aversion had been conditioned to it.
Materials and Methods
Subjects
Long-Evans male rats (N = 19; Charles River, Raleigh, NC) were the subjects of this study. Twelve rats were used for behavioral testing and seven for unit recording. They were housed individually in Plexiglas cages and handled daily upon arrival. Due to the prolonged period of unit recording experimentation, it was desirable to mildly food restrict all subjects to test rats of comparable body weight, such that weights were maintained at ~390g throughout the entire experiment. Water was available ad libitum. All experiments were conducted during the light phase of a 12 hr light/dark cycle. Animals were treated according to the University of Washington Institutional Animal Care and Use Committee and National Institutes of Health guidelines for the care and use of animals in research.
Behavioral assessment of CTA
Surgery
Immediately prior to surgery, Ketofen (analgesic 5 mg/kg) and Baytril (antibiotic 5 mg/kg) were administered subcutaneously. Under isoflurane anesthesia (Summit Anesthesia Solutions, Bend, OR), each rat was implanted with an intraoral (I/O) cannula constructed with PE-100 tubing. The cannula was inserted through the right cheek, caudal to the eye, and exited at the scapular area behind the head. One week was given for recovery from I/O surgery. The I/O cannula was rinsed daily beginning three days after surgery.
CTA training and testing
The training protocol consisted of daily sessions of water habituation, followed by conditioning and subsequently testing. Behavioral testing sessions were carried out in a transparent, cylindrical Plexiglas chamber (12” high). During the five days of water habituation, all rats received 30 trials of water infusion daily for half an hour. Each trial consisted of 10 sec water infusion at a rate of 0.5 ml/min with an intertrial interval of 40~60 sec (average 50 sec). On the sixth day, 0.2% saccharin solution (CS) was introduced for 60 trials utilizing the same infusion schedule as described for the water habituation phase. Six rats were randomly assigned to a conditioned group and six rats to an unpaired (control) group. For the conditioned group, 0.15 M LiCl (1% body weight; an agent to induce nausea as the US) was injected intraperitoneally immediately after the final saccharin infusion; for controls, LiCl was given the following day. Five daily sessions of testing (extinction) started the day after LiCl injection in controls and on the second day after conditioning in the paired group. During test sessions, saccharin solution was delivered continuously for ten min at a rate of 0.5 ml/min. CTA acquisition was assessed by viewing and scoring behavioral taste reactivity to saccharin infusion for both groups. A mirror-image of the animals’ responses was videotaped during continuous infusions for the testing phase. When orofacial expressions were closely examined within the 10 min of saccharin infusion, animals exhibited various types of negative responses, such as chin rubbing, head shaking, and forelimb flailing. Due to the prevalence of flails and the reliability of behavioral coding among scorers, the number of episodes of flailing forelimbs was quantified to measure negative taste reactivity. The taste reactivity was quantified by counting the number of brief chains of flailing forelimbs. The behavior was counted as a valid flail when both forelimbs were wide open with fast flails. Experimenters were blind to the group membership of the animal being scored.
Unit Recording
Surgical preparation and procedures
Seven male Long-Evans rats were used for unit recordings. Rats were implanted with double I/O cannulae, which allowed for alternating water and tastant delivery. This was modified from the single I/O cannula described in the behavioral assessment section. Each cannula was inserted with a sharpened stainless steel probe anterolateral to the first maxillary molar. The probe passed through the right cheek, caudal to the eye and exited at the scapular region behind the head. Insertions were made one after the other and the two tubes were positioned close in distance on the same side of the cheek. Daily rinsing of I/O cannulae began a day after surgery.
Recording tetrodes were constructed from 25 µm Teflon-coated platinum wires and were mounted on a microdrive that was prepared as described in Gill and Mizumori (2006). After tetrode tips were gold plated to an impedence of 0.2 – 0.4 MΩ, measured at 1 kHz, recording tetrodes were surgically implanted into the brain. Rats were fixed onto a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) under isoflurane anesthesia. Isoflurane anesthetic was provided through a nozzle over the nose to provide a constant level of anesthesia. Twelve holes were drilled for ten skull anchor screws to secure the microdrive, a ground screw, and placement of a reference electrode in a quiet brain area. Then, two holes were drilled so that two tetrodes (per hemisphere) could be placed bilaterally above basolateral amygdala (AP: −2.9 mm from bregma, ML: ±4.9 mm from the midsagittal suture, DV: −7.0 mm from dura). Dental cement was applied to mount the drive assembly to the skull. Ketofen and Baytril were administrated perioperatively as described for the intraoral surgery. Rats were not disturbed for a week, except to rinse the double cannulae.
CTA training and testing
Daily sessions were performed in a dimly lit recording room, with a low level of white noise. Recordings and screening of units were performed while rats were placed on a Plexiglas platform (9” × 9” × 2”) that was situated 30” above a table top. The experimental procedure was similar to that used during behavioral testing, with daily sessions of water habituation, followed by conditioning, and then testing. All daily sessions consisted of 60 trials of 10 sec infusions followed by an average 50 sec intertrial interval over the course of an hour. During sessions of water habituation, all rats received 60 daily infusions of water delivered to alternating cannulae, which were individually connected to separate infusion pumps. On the conditioning day, 0.2% saccharin was introduced for all animals. A LiCl injection was administered immediately after the 60th trial of saccharin infusion for the conditioned group (n = 4), whereas the injection was given a day later for the unpaired (control) group (n = 3). Rats remained on the platform for 20 min following the LiCl injection. Daily test sessions began the next day following LiCl injections for controls and on the second day after conditioning in the conditioned group. For each test session, trials of water and saccharin presentation were randomly alternated. Behaviors exhibited by the rats were monitored outside the recording room via a video camera.
Data acquisition
The chronically mounted drive assembly was connected to a preamplifier. Output signals from the preamplifier were amplified 6,000 – 8,000 times, filtered between 0.6 and 6.0 kHz, digitized at 32 kHz, and stored on a PC (Neuralynx, Bozeman, MT). The location of electrode tips was initially estimated based on the traveling distance of the electrode and basic firing properties of the recorded cells. When one of the four tetrodes obtained at least one reliable and well isolated unit that remained stable for over 10 min, the daily recording session started. Acceptable signals were at least twice the amplitude as the background activity. At the end of each daily session, the electrodes were lowered at least 50 µm to target new units for the following day. Experimental sessions continued until the tips of the electrodes passed through the BLA region. Onset and termination infusion times were flagged as an event timestamp during the acquisition of neural data. Unit recordings were obtained for all experimental sessions. Although unit data were obtained throughout all the phases of the experiment, we report here only data obtained during test sessions. Units recorded during habituation and conditioning consisted of too few units for analysis. Spike sorting was performed with Offline Sorter (Plexon Inc., Dallas, TX), which is based on a variety of waveform features. Custom analysis of sorted units was executed with MATLAB (The MathWorks, Inc., Natick, MA). All statistical analyses were performed with SPSS.
Construction of spike arrays
Once spikes were sorted, units emitting more than one spike per trial for at least 45 of the 60 trials were included for analysis. Timestamps of spikes for each unit were extracted beginning with the first trial and ending with the last trial of each recording session. Since the sequence of trials was intermixed randomly with water and saccharin infusions, spike timestamps were extracted according to the trial type. A spike count array (SCA) was constructed for 10 sec beginning at each infusion onset with the order of trials maintained. Subsequent to the construction of the first SCA, the 10 sec period slid 5 sec to construct the next SCA. These steps were repeated to obtain ten SCAs in total. By applying this method to the type of trials separately, two sets of ten SCAs were obtained per cell (SCA #1 to #10 for saccharin and water trials, respectively).
Identification of responsive cells and calculation of r-D values (baseline comparison)
To determine whether neuronal activity changed after infusions, spike activity before infusions was compared to activity post-infusions. Additional SCAs (according to trial type) were constructed with the number of spikes emitted for 10 sec prior to each infusion onset as the baseline. The baseline SCA was compared with every SCA (SCA #1 to #10) generated by the sliding window method following infusions, corresponding to the trial type. Wilcoxon signed-rank test was applied to detect a statistical difference. If at least one of ten tests resulted in p value less than 0.05, the cell was considered a responsive cell. The detection of responsiveness was separately applied to the types of solution (water or saccharin) for a cell. For each responsive cell, a differential value (r-D value) was calculated to reflect the magnitude of the differential firing between the baseline and post infusion spike activity. The r-D value for responsive cells considered the total spike count within the set of arrays where the lowest p value was observed. The r-D value was calculated as follows: [(# of spikes for baseline − # of spikes for infusion)]/[(# of spikes for baseline + # of spikes for infusion)].
Identification of taste responsive cells and calculation of t-D values (taste comparison)
To determine whether a cell showed taste responsiveness post infusion, spike activity was compared between saccharin and water. SCAs generated for post infusions (SCA #1 to #10) were compared between water and saccharin trials to test if a cell showed taste responsiveness. Each SCA of water trials was compared with the timely corresponding SCA of saccharin trials. Wilcoxon signed-rank test was applied. If at least one of ten tests resulted in p value less than 0.05, the cell was considered a taste responsive cell. For each taste responsive cell, a differential value (t-D value) was calculated to reflect the magnitude of the differential firing between trial types. The t-D value for responsive cells considered the total spike count within the arrays where the lowest p value occurred. The t-D value was calculated as follows: [(# of spikes for saccharin − # of spikes for water)]/[(# of spikes for saccharin + # of spikes for water)].
Analysis of burst activity
Interspike interval (ISI) histograms for individual cells revealed that the majority of spike intervals were less than 50 msec. Therefore, a spike burst was defined as more than two of consecutive spikes whose ISIs were shorter than 50 msec. Once a burst was identified, then each ISI of the burst was indexed according to the position in the burst (i.e. first ISI, second ISI, and third ISI, etc.) for further analysis.
Histology
After the completion of all recording sessions, rats were deeply anesthetized with sodium pentobarbital and an electrolytic current (25 µA, 15 sec) was applied to each electrode to produce a marking lesion. The animals were subsequently perfused transcardially with saline followed by 10% formalin. Coronal sections (50 µm thick) were cut on a vibratome and stained with Cresyl Violet. Electrode tracks and lesion sites were identified utilizing a light microscope.
Results
Behavioral CTA Expression
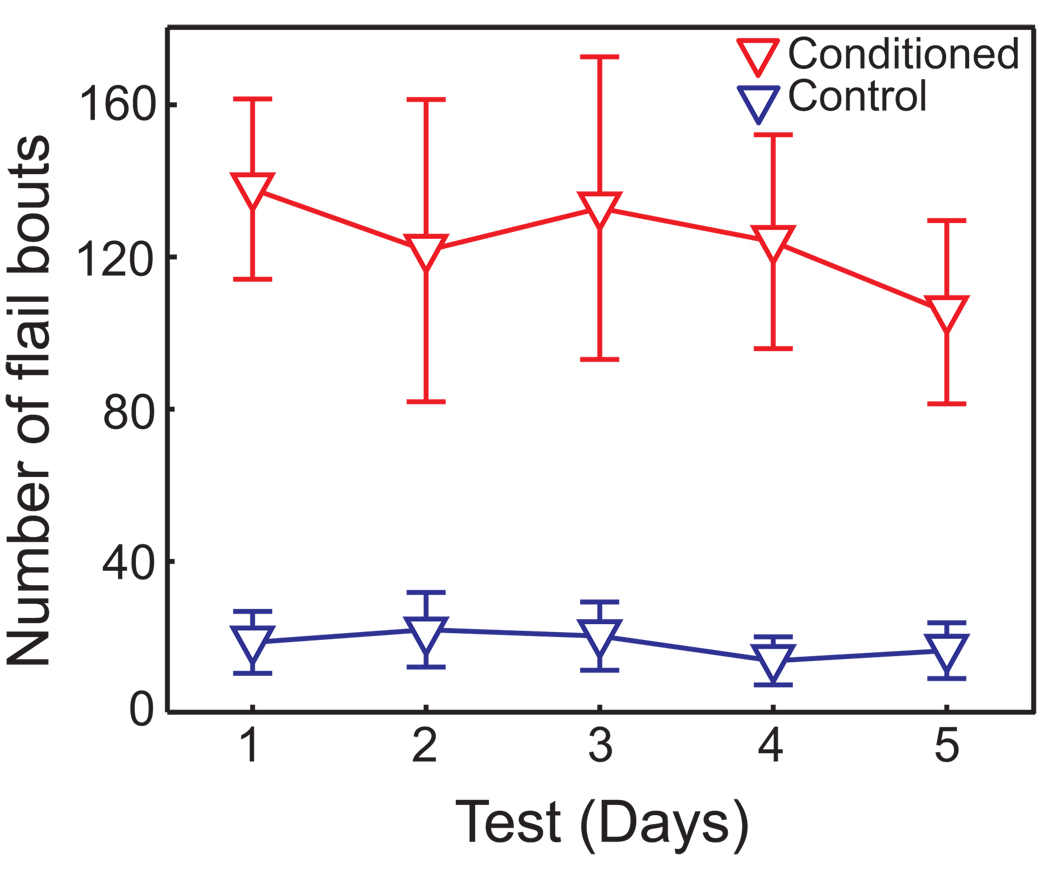
Behavioral assessments confirmed that reliable CTAs were induced using the modified behavioral protocol of 60 brief saccharin pulsed presentations (CS) followed by LiCl administration (US). Forelimb flails during test sessions were quantified during the 10 min of saccharin infusion as a measure of aversive taste reactivity. As shown in Figure 1, conditioned animals showed significantly more aversive taste reactivity than controls (ANOVA with repeated measures, F(1,5) = 10.34, p < 0.05) over the five days of testing. There was neither a main effect of days (F(4,20) = 0.91, p = 0.48) nor an interaction between groups and days (F(4,20) = 0.87, p = 0.50). This analysis suggests that significant aversion to saccharin developed and that little or no extinction occurred over the 5 test sessions.
Figure 1. Conditioning effect on taste reactivity.
0.15M LiCl (1% body weight) was injected immediately following 60 trials of 10 sec infusions of 0.2% saccharin in the conditioned group (n = 6), or 24 hrs later in the control group (n = 6). To determine the effectiveness of the modified behavioral paradigm, 5 ml of saccharin solution was continuously infused (I/O; 10 min per day) for five days. Aversive reactions were scored as the number of forelimb flail events during infusion. The conditioned group consistently displayed a high level of aversive reactivity to saccharin throughout test sessions, showing little to no sign of extinction.
Taste representation of the learned aversion
A total of 198 BLA cells were recorded from 7 rats during the test sessions. Of these 49 cells were excluded due to low levels of activity that precluded statistical analyses (See Material and Method). Of the remaining 149 cells, 81 were obtained from conditioned rats and 68 from controls. Table 1 summarizes the mean firing rate of BLA neurons from conditioned and control groups. The firing rate was calculated from the spikes obtained from the first trial to the last trial of each session and were computed separately for water and saccharin trials. No significant difference was found in the firing rate between groups, nor as a function of whether water or saccharin had been infused (ANOVA, all ps > 0.50).
Table 1.
Mean firing rate of BLA cells
| Mean firing rate (Hz) |
|
|---|---|
| Conditioned (n = 81) | 0.51 ± 0.66 |
| Water trials | 0.53 ± 0.68 |
| Taste trials | 0.49 ± 0.64 |
| Control (n = 68) | 0.53 ± 1.28 |
| Water trials | 0.53 ± 1.29 |
| Taste trials | 0.53 ± 1.27 |
The primary goal of this study was to characterize alterations in the representation of taste by BLA unit activity as a consequence of conditioning. Unlike primary sensory cortical neurons that display a reliable response to a given stimulus (Yamamoto et al., 1988), peristimulus time histograms (PSTH) of BLA unit activity in this study revealed very diverse response profiles after infusions. Therefore, to identify cell responsiveness, the differential activity between the baseline and post infusion, and between water and saccharin trials (for details, see Material and Method, and Figure 2) was computed. Subsequently, a group comparison was performed on the differential activity of cells to characterize the effect of conditioning.
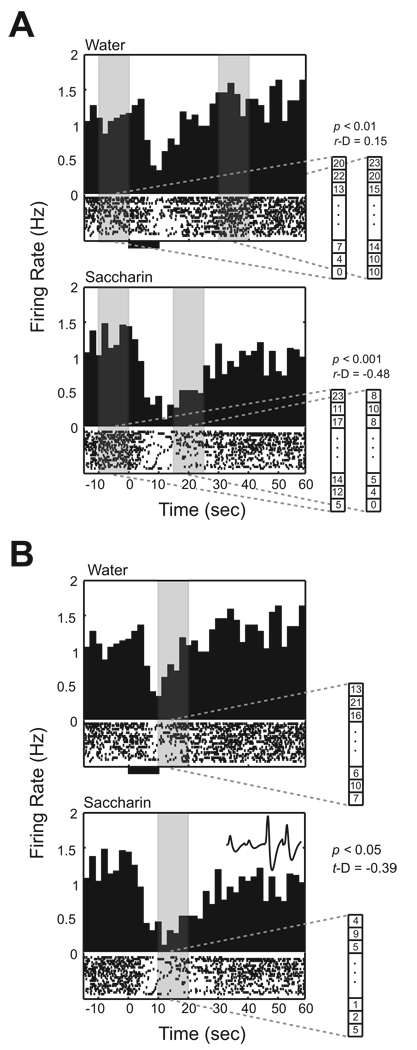
Figure 2. Taste responsiveness of BLA neuron.
The examples of unit activity illustrate how taste responsiveness is determined. Individual neuronal spikes obtained during randomly alternating trials between saccharin and water infusions are sorted according to trial type. The upper peristimulus time histogram (PSTH) represents data collected during water trials, and the lower PSTH shows unit firing during saccharin trials. At the bottom of each PSTH, a raster plot represents the spike activity for each trial. In the raster, the lowest line corresponds to the first trial and subsequent trials are stacked above. All trials are aligned with the initiation of water and saccharin infusions (T0). A thick line below the raster plot indicates the time period of the 10 sec infusion. An average waveform of the unit sampled by the tetrode is shown at the right-upper corner of the lowest PSTH. A. Baseline comparison. The baseline comparison was applied separately according to the trial type for a cell. The unit activity in this example was obtained from a conditioned rat. The sliding method detected an excitatory response (T30 to T40) for water and a suppressive response (T15 to T25) for taste trials. These time frames are indicated by the regions of gray shading. B. Taste comparison. The taste comparison was applied to the same unit in A. This unit demonstrates suppressive activity during saccharin trials compared to water trials. The largest suppression is detected 10 sec after the infusion started (p = 0.000592; D = −0.39) indicated by the shaded gray region.
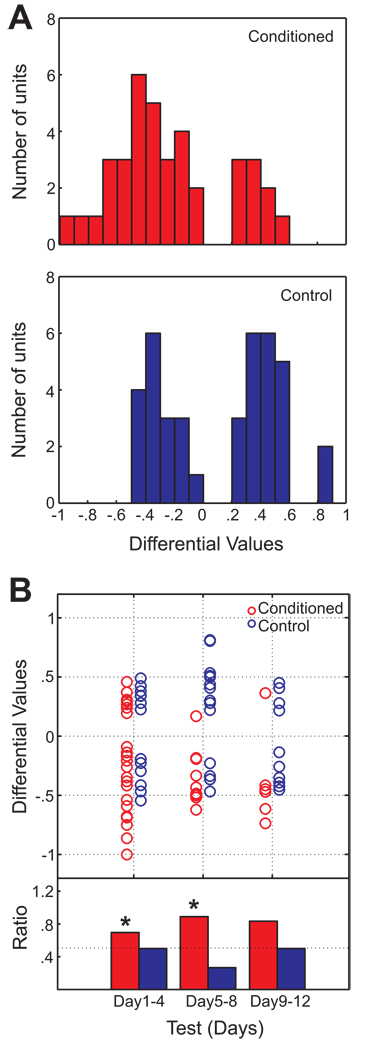
38 / 81 BLA cells from the conditioned rats, and 39 / 68 cells from controls, were classified as taste responsive cells. To determine whether taste responsive cells from conditioned rats differed from those of controls, t-D values were compared across conditions. Figure 3A displays the distribution of t-D values of BLA taste responsive cells obtained from conditioned and control animals. Nonparametric statistics indicate that the distribution of the conditioned group differed significantly from the control group (Mann-Whitney U test; p < 0.001). The t-D value distribution of the conditioned group was skewed toward negative t-D values, while that of the control group showed a bimodal distribution of t-D values. In other words, compared to the control group, more cells showed activity suppression to saccharin relative to water in the conditioned group. The group comparison was performed for taste responsive cells detected using a more conservative criterion, (p values < 0.02, rather than < 0.05). The distribution of t-D values of the conditioned group (n = 19) significantly differed from that of the control group (n = 21; Mann-Whitney U test; p < 0.05), indicating that suppressive responses were across taste responsive cells in the conditioned group. Table 2summarizes the mean firing rate of BLA units during the time intervals in which the responsiveness was detected according to two different comparisons. Taken together, we conclude that the alteration of taste responsiveness of BLA cells after CTA can primarily be characterized as suppression which is specific to the conditioned taste. Figure 3B plots individual t-D values according to test sessions to reveal that the negatively skewed t-D value distribution persisted across days of testing. Data were segmented according to days in order to test whether or not cells with positive and negative t-D values were equally distributed among taste responsive cells (one-tailed binomial test). There was a tendency for a greater number of units to have a negative t-D value for Days 1–4 and Days 5–8 (ps < 0.05) in the paired group which was not the case in the control group.
Figure 3. The distribution of t-D values for BLA cells from conditioned and control groups.
A. (Top) The distribution of t-D values for taste responsive cells in conditioned animals (n = 38). Cells with a positive t-D value showed preferential firing for saccharin, while a negative value indicates suppressive firing toward saccharin relative to water. (Bottom) A distribution of t-D values for the control group (n = 39). Note that the distribution of t-D values from the conditioned group is skewed towards negative values, compared to the control group (p < 0.001). B. Individual t-D values according to test days. Individual t-D values from the two groups are plotted side by side. Data were combined and grouped into bins of three experimental sessions. The ratio of cells with a negative t-D value relative to the total number of cells is shown in the bar graphs. Asterisks indicate p values of one-tailed binomial test that were less than 0.05. The tendency toward suppression in the conditioned taste subjects persisted throughout the test sessions.
Table 2.
Spike activity of responsive BLA cells
| Saccharin | Water | |||
|---|---|---|---|---|
| Baseline | Infusion | Baseline | Infusion | |
| Baseline comparison | ||||
| Conditioned | 0.34 (0.03 – 4.21) | 0.19 (0.00 – 3.03) | 0.40 (0.01 – 3.95) | 0.19 (0.02 – 3.18) |
| Control | 0.25 (0.02 – 9.10) | 0.20 (0.00 – 6.89) | 0.28 (0.03 – 9.69) | 0.13 (0.01 – 7.65) |
| Taste comparison | ||||
| Conditioned | 0.19 (0.00 – 3.71) | 0.32 (0.03 – 4.45) | ||
| Control | 0.23 (0.07 – 5.22) | 0.24 (0.02 – 7.18) | ||
* Numbers represent medians of mean firing rate (Hz) of responsive BLA neurons during 10 sec when the most responsiveness was detected by different comparison methods. Numbers within the parentheses indicate the range of mean firing rate.
Although the taste comparison revealed that more BLA cells were suppressed by saccharin infusion compared to water following conditioning, this analysis did not directly characterize BLA responsiveness to infusions. Therefore, we further analyzed the responsiveness of a cell to the infusion by comparing activity between baseline and post-infusions.
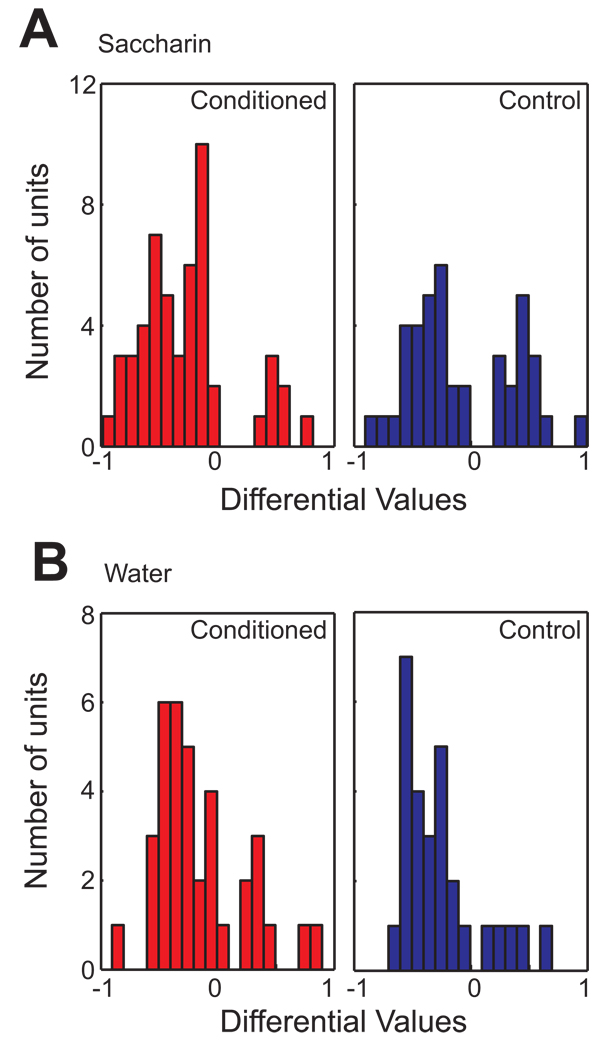
To determine whether a cell changed activity after the infusion, a baseline comparison was separately performed for saccharin and water trials (Figure 2A). The responsiveness of a cell was initially determined by the statistical difference in spike activity between baseline and post infusion. 51/81 BLA cells obtained from the conditioned rats, and 41/68 cells from controls, were classified as saccharin responsive cells. To determine if conditioning affected the responsiveness to saccharin differently, r-D values were compared between conditioned and control groups. Figure 4A displays the distribution of r-D values of BLA saccharin responsive cells obtained from conditioned and control animals. Nonparametric statistics indicate that the distribution of the conditioned group significantly differed from the control group (Mann-Whitney U test; p < 0.05). The r-D value distribution of the conditioned group was skewed toward negative r-D values, while that of the control group showed a bimodal distribution of r-D values. In other words, compared to the control group, more cells showed a suppressed response to saccharin infusion relative to the baseline in the conditioned group. In contrast, no group difference was found in the r-D value distribution of water responsive cells between conditioned and control groups (Figure 4B).
Figure 4. The distribution of r-D values for BLA cells from the conditioned and control groups.
A. (Left) A distribution of r-D values for saccharin responsive cells in conditioned animals (n = 51). Cells with a positive r-D value show preferential firing during saccharin infusion, while a negative value indicates suppressive firing toward saccharin infusion relative to baseline. (Right) A distribution of r-D values for the control group (n = 41). Note that the distribution of r-D values from the conditioned group is significantly skewed towards negative values, compared to the control group (p < 0.05). B. (Left) A distribution of r-D values for water responsive cells in conditioned animals (n = 36). Cells with a positive r-D value show preferential firing for water infusion, while a negative value indicates suppressive firing toward water infusion relative to baseline. (Right) A distribution of r-D values for the control group (n = 28). Note that there is no significant difference in the distribution of r-D values of water responsive cells between the conditioned and the control groups (p > 0.5).
Alteration of short interspike intervals (ISI) after CTA conditioning
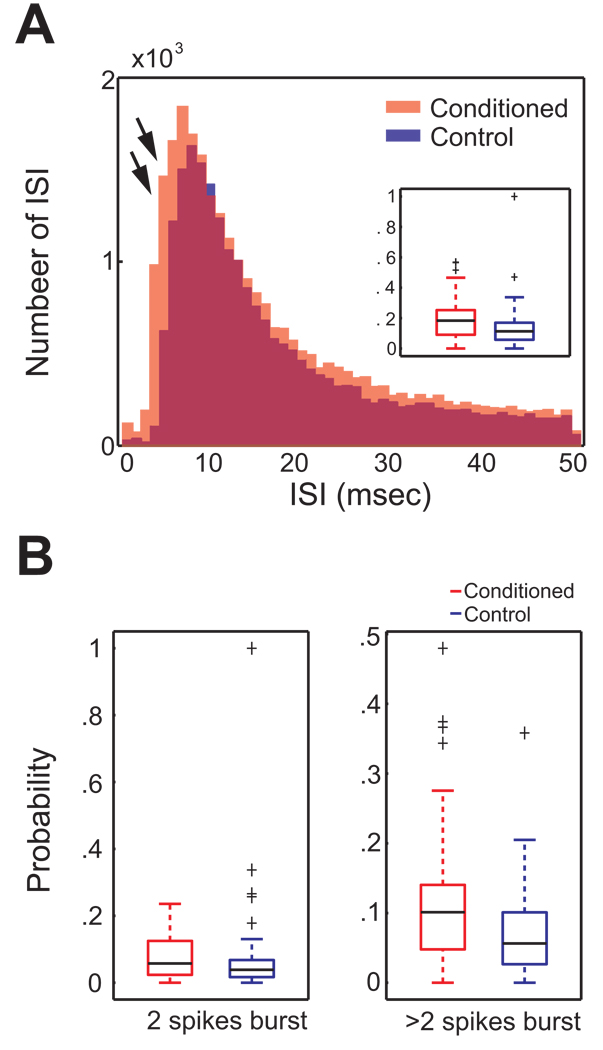
We investigated whether there were alterations in the temporal dynamics of spiking in BLA neurons as a consequence of CTA conditioning. From the conditioned group (mean = 0.36; SE = 0.042), units having similar mean firing rates to those of controls were selected for comparison of temporal effects (n = 66 pairs; mean = 0.32; SE = 0.036). The two superimposed frequency histograms of all ISIs obtained from both groups in Figure 5A reveal that the conditioned animals demonstrate more ISI occurrences ranging from 3 to 7 msec compared to the control group. The probability of occurrence of 3~7 msec burst ISIs of the conditioned group (median = 0.18) was significantly higher than that of the controls (median = 0.11; inset, Figure 5A; Mann-Whitney U test; p < 0.01). The probability of occurrence of 3~7 msec burst ISIs for a unit was calculated by dividing the number of 3~7 msec ISIs by the number of 50 msec ISIs.
Figure 5. Conditioning effect on short interspike intervals of BLA cells.
A. The interspike interval (ISI) frequency histogram (range = 0 ~ 50 msec) was generated for the total number of ISIs for both groups (conditioned group = red; control = blue). Superimposed ISI frequency histogram reveals a higher occurrence of 3~7 msec ISIs in the conditioned group than the control (double arrows). Inset. The boxplot shows that the probability of occurrence of 3~7 msec burst ISI was higher in the conditioned animals. Group medians of the probability and extreme values (cross symbols) are indicated with black. B. Comparisons of the probability of occurrence of 2 spike bursts and > 2 spike bursts across the conditioned and control groups. (Left) A boxplot depicting the probability of occurrence of 3~7 msec ISI bursts containing only 2 spikes. No statistically significant, but a marginal group difference is detected (p = 0.07). (Right) A boxplot representing the probability of occurrence of bursts with more than 2 spikes. The probability of occurrence of 3~7 msec ISIs containing more than 2 spikes was significantly higher in the conditioned group than the control.
Burst activity of BLA units was investigated in further detail to specify the nature of the temporal alteration in ISI after CTA learning. The probability of occurrence of 3~7 msec burst ISIs was compared according to the burst size. As seen Figure 5B, a group difference was found when the burst consisted of more than 2 spikes (Mann-Whitney U test, p < 0.005). The probability of occurrence of these 3~7 msec burst ISIs in the conditioned group (median = 0.10) was significantly higher than that of the controls (median = 0.05).
Although these alterations in temporal processing could reflect differential responses during the two types of trials (water and saccharin) this did not appear to be the case. When we examined whether the alterations in short ISIs or bursts were related to trial types, we found no differences as a function of whether cells were taste responsive. Furthermore, no differences were detected as a function of the type of trials (saccharin CS versus water). Thus alterations in short ISIs and bursts were induced across all types of BLA units as a result of the behavioral conditioning per se, and were not specific to taste responsive or CS responsive units.
Histological verification of recording site
Figure 6 (upper) illustrates a reconstruction of electrode tracks in the BLA obtained from seven subjects. Unit data collected from outside the BLA (dashed line) were not included in the data analysis. Histological verification of the electrolytic lesions revealed that the tips of electrodes were positioned in the BLA. As examples, photographs of three coronal sections obtained from different subjects were also included in Figure 6 (bottom).
Figure 6. Histological verification of recording.
(Top) A reconstruction of the recording tracks in the left BLA from seven animals. The left illustration shows the anterior portion of the BLA, while the right depiction represents the posterior BLA. Dashed tracks represent tetrodes that were implanted outside the BLA. Data from dashed sections are not included in the analysis. (Bottom) Photomicrographs of cresyl violet stained sections from three different rats, depicting the electrolytic marking lesions within the BLA.
Discussion
The present study developed a modified CTA behavioral paradigm suitable for characterizing the electrophysiological properties of taste-responsive unit activity during and after taste aversion learning. Robust and persistent taste aversions developed after a single conditioning trial as demonstrated by aversive responses (forelimb flails) to saccharin infusions in the conditioned animals, responses which were not observed in controls. For taste responsive units in the basolateral nucleus of amygdala (BLA), CTA training resulted in suppressed neural activity during subsequent exposure to the CS (saccharin infusion). The suppression of BLA neurons to the CS was mainly due to alteration in CS responsiveness, and was not secondary to changes in responsiveness to water. In addition, the overall excitability of BLA units regardless of taste responsiveness was slightly increased following conditioning. These conditioning effects were quite resistant to extinction.
The amygdala complex is comprised of several subnuclei, each of which has different afferent/efferent patterns of connectivity, cell composition, and functional roles (McDonald, 1998; Pitkänen, 2000; Sah et al., 2003). The BLA in the rat receives gustatory and visceral information via cortical and subcortical pathways including heavy projections from the primary gustatory and the primary visceral cortices of the insular cortex. In addition, BLA forms a complex circuitry by interconnection within the region itself (Pitkänen et al., 1997). Anatomically, 85% of the cells in BLA are pyramidal-like projecting neurons and 15% inhibitory interneurons (McDonald, 1982). BLA neurons in rats have been characterized in in vitro and in vivo studies (Washburn & Moises, 1992; Pratt & Mizumori, 1998; Schoenbaum et al., 1998). Based on the histological data (electrode tracts shown in Figure 2), the majority of our unit data was obtained from the BLA rather than the lateral nucleus of the amygdala (LA). Even if the tips of our electrodes traveled through the LA region, it was likely that LA units were not sampled due to their lack of spontaneous activity (Sah et al., 2003). Our data are consistent with prior electrophysiological characterization in this region including low spontaneous firing (mean firing rate of < 1Hz) and burst firing. We therefore conclude that the majority of the recordings represented here came from pyramidal-like projecting cells in the BLA.
It has been reported that unit activity of gustatory cortex, amygdala, and ventromedial hypothalamus significantly decreased after one or two licks of a conditioned taste in rats (Burešová et al., 1979). Thus, our results are in agreement with the finding of overall reductions in amygdala activity to the conditioned taste, where a greater proportion of cells showed suppressed activity to saccharin in the conditioned group. However, the findings we report here are inconsistent with the results reported in Yasoshima et al. (1995) and Grossman et al. (2008). The former reported that conditioning increases excitatory responsiveness to the CS while the latter observed that the gain and loss of BLA responsiveness are equally distributed and concluded no overall detectable change in the population response. This contrasts with our findings which suggest that conditioning increases inhibitory responsiveness to the CS. Several factors could contribute to the different outcomes observed. First of all, even though the rats utilized for behavioral testing demonstrated the ability to reliably acquire CTA through the modified paradigm during both extended (~ 1 hr) and frequent (60 trials) presentation of CS, the overall behavioral state induced by the conditioned taste in our study appears to differ from the two studies. Namely, in those studies subjects clearly demonstrated gaping responses to the brief infusion of the conditioned taste, which were not observed in our study. Additionally, saccharin infusions were delivered with alternating water infusions during testing in our paradigm. More importantly, the two previous studies examined the CTA effect on encoding of the conditioned taste in BLA by focusing on the spike discharges during infusion or the timing of early spikes related to the onset of infusion. Compared to those, our study did not restrict the analysis to the infusion time and used a broader window (10 sec) to determine taste responsiveness. Our data demonstrated that differential activity often appeared later, even after the infusion has ended. The low firing rate (median around 0.3 Hz) of BLA cells required a broader window to include a sufficient number of spikes in the temporal windows. Moreover, in response to slower infusions (~ 80 µl/10 sec), rats often initiated mouth related movement with a ~1 sec delay. The response latency varied across animals and even across trials. Due to the variation in response timing, temporal precision was difficult to investigate with our data. As proposed by Grossman et al., if the early spikes related to the infusion onsets are associated with taste related sensory information of CTA, the differential activities which occurs at a later time point could reflect a cognitive or affective evaluation function by BLA. Alternatively, the overall reduction of spikes in response to the conditioned taste might be a part of an adaptive mechanism that creates a situation in which the effects of a subtle change in the temporal pattern of firing is accentuated to support the persistence of a learned aversion.
As mentioned in the previous paragraph, conditioned animals in the unit recording study did not show clear signs of aversive taste reactivity when their behavior was monitored during the recording sessions. This contrasted with observations in the behavioral experiment where significant aversive responses to the CS were detected following CTA conditioning. Two features of the unit recording paradigm may have contributed to this discrepancy. The infusion parameters utilized for neural recording consisted of multiple 10 sec pulses of CS employing a slow infusion rate. The delivery of solution may have been too brief to elicit detectable aversive behavioral reactions as compared to the 10 min of continuous CS infusion in the behavioral study. Previous studies performed in our lab have shown that animals often start exhibiting rejection behavior over seconds or even minutes after the infusion has started (Schafe et al., 1998; Navarro et al., 2000). Recent observations in our laboratory suggest that the effectiveness of a CS in eliciting negative taste reactivity varies with infusion parameters. Conditioned animals showed clear gaping responses during a 10 min continuous infusion but not during a series of 10 sec infusions (unpublished data). In addition, alternating the delivery of the tastant with water may further influence the pattern of taste reactions. It is also possible that, unit recording subjects were constrained by the presence of the recording device on their head and were therefore less able to express aversive behaviors. In view of the fact that conditioning procedures were identical in the behavioral and recording studies, we believe that the absence of aversive responses during unit recording is not due to a failure to acquire significant aversions but rather to conditions during testing which affect behavioral expression. Interestingly, the lack of aversive behavioral responses in the recording subjects supports the idea that the observed differential activity in BLA cells in this study is related to CS responsiveness and not to the emission of behavioral reactivity.
Our main claim is based on the observation of persistent aversive reactivity in behavior and differential BLA activity due to the suppression to the conditioned taste. Given the fact that rats commonly show a fast extinction of CTA with forced consumption of the conditioned taste (Cantora et al., 2006), the current result indicating lack of extinction is somewhat puzzling. During the pilot study, we examined whether or not the infusion parameters of CS delivery affect behavioral expression of CTA. When a 10 min infusion was used on the conditioning day, the paired group showed a fast extinction to the conditioned taste as previously reported. Therefore, it seems that the behavioral paradigm used in the current study (short infusion durations) yields conditioned responses that persist over multiple test days. This supports that the suppressed responses of BLA neurons to the conditioned taste obtained during multiple test days are related to the aversiveness of the stimulus.
Burst activity, which is typically defined by the presence of consecutive spikes comprised of short interspike intervals, has been identified in various brain regions, including the amygdala. It has been speculated that the information carried by bursts could be more reliable than single spikes (Lisman, 1997). Burst activity of neurons could play a role in network stability by maintaining synaptic strength (Buzsáki et al., 2002). Also, experience-induced temporal shifts within a short time scale (< 10 msec) have been interpreted as a temporal code for spatial information (Mehta et al., 2002). However, functional implications remain unresolved since there is little in vivo work in amygdala in association with learning. Our data demonstrated that overall firing rates of BLA units were not affected by CTA acquisition. However, the temporal dynamics of spiking of BLA units was altered by conditioning. First, 3~7 msec ISIs occurred more frequently across BLA cells in the conditioned animals. Next, the temporal alteration occurred in the bursts consisted of more than two spikes. Although the occurrence of two spike bursts was not statistically different following learning in the conditioned group, they possessed a higher probability of occurrence during a 3~7 msec (p = 0.07) interval. It seemed that those two spike bursts may also contribute to the temporal alteration of those bursts that have more than two spikes. Therefore, it is reasonable to speculate that the overall excitability was slightly increased after learning based on a higher incidence of short ISIs (3~7 msec). Additionally, the occurrence of bursts has been shown to be related to the state of the animal (i.e., higher burst occurrence during non-theta sleep; Steriade et al., 2001) rather than a specific event or stimulus (i.e., more information is not carried via bursts compared to single spikes; Harris et al., 2001). Our data also demonstrated that the temporal alteration of BLA neuronal spiking was a general change following CTA learning, rather than stimulus or cell type specific. Burst occurrence differences were not observed for either taste responsive units or CS trials. Therefore, it is possible that the different level of arousal due to the learned aversion might be reflected in the altered temporal dynamics (i.e. elevated arousal could increase cellular excitability). In addition, the change in excitability could be utilized within a neural network to maintain homeostasis as proposed by Buzsáki and colleagues (Buzsáki et al., 2002). With our current dataset, the interpretation regarding the functional role of burst activity is limited. Future work is needed to address the functional implication of the altered burst activity and whether the increased excitability could contribute to the suppressive representation of the conditioned taste.
It has been suggested that the amygdala is one of the central loci of plasticity which underlies CTA learning. However, the investigation of ongoing neural processes during CTA acquisition is in its early phase. The Bernstein laboratory has shown a robust c-Fos expression in the amygdala (Koh & Bernstein, 2005) and convergent neuronal activation in the BLA utilizing catFISH after a CS-US pairing (Barot et al., 2008). These findings suggest that the amygdala is a site of neuronal plasticity during CTA acquisition. However, the conditioned taste itself does not appear to produce robust c-Fos activation in the amygdala (CTA expression, Navarro et al., 2000). The lack of c-Fos expression by the conditioned taste could be explained by the present findings, namely that the learned aversion is encoded as a reduction in overall firing to the CS rather than an increase in firing to the CS. This level of alteration in neuronal firing would not be detected utilizing c-Fos methodology. Our current study highlights the ongoing process of how the conditioned taste is represented by BLA units of behaving rats. Future studies will address BLA activity during the different phases of CTA learning to provide further insight into how BLA is engaged in the process of CTA learning, and the neural mechanisms of acquiring the memory.
Acknowledgements
We thank SiWei Luo for important feedback on earlier drafts of this manuscript. This work was funded by grants NS37040 and MH58755. M.J.K. was also supported by Royal Research Fund at the University of Washington.
Abbreviations
- BLA
basolateral amygdala
- CTA
conditioned taste aversion
- CS
conditioned stimulus
- FLI
Fos-like immunoreactivity
- I/O
intraoral
- ISI
interspike interval
- LA
lateral nucleus of amygdala
- PSTH
peristimulus time histogram
- US
unconditioned stimulus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Mishkin M. The amygdala: sensory gateway to the emotions. In: Plutchik R, Kellerman H, editors. Emotion: Theory, Research and Experience, Vol. 3: Biological foundations of emotion. Orlando: Academic Press, Inc; 1986. pp. 281–299. [Google Scholar]
- Barot SK, Kyono Y, Clark EW, Bernstein IL. Visualizing stimulus convergence in amygdala neurons during associative learning. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20959–20963. doi: 10.1073/pnas.0808996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nature reviews. Neuroscience. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- Brožek G, Siegfried B, Klimenko VM, Bureš J. Lick triggered intracranial stimulation interferes with retrieval of conditioned taste aversion. Physiology & behavior. 1979;23:625–631. doi: 10.1016/0031-9384(79)90150-1. [DOI] [PubMed] [Google Scholar]
- Burešová O, Aleksanyan ZA, Bureš J. Electrophysiological analysis of retrieval of conditioned taste aversion in rats. Unit activity changes in critical brain regions. Physiologia Bohemoslovaca. 1979;28:525–536. [PubMed] [Google Scholar]
- Buzsáki G, Csicsvari J, Dragoi G, Harris K, Henze D, Hirase H. Homeostatic maintenance of neuronal excitability by burst discharges in vivo. Cerebral cortex. 2002;12:893–899. doi: 10.1093/cercor/12.9.893. [DOI] [PubMed] [Google Scholar]
- Cantora R, López M, Aguado L, Rana S, Parker LA. Extinction of a saccharin-lithium association: assessment by consumption and taste reactivity. Learning & Behavior. 2006;34:37–43. doi: 10.3758/bf03192869. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. Journal of neuroscience methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science. 1974;185:824–831. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- Gill KM, Mizumori SJ. Context-dependent modulation by D(1) receptors: differential effects in hippocampus and striatum. Behavioral neuroscience. 2006;120:377–392. doi: 10.1037/0735-7044.120.2.377. [DOI] [PubMed] [Google Scholar]
- Grossman SE, Fontanini A, Wieskopf JS, Katz DB. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. The Journal of neuroscience. 2008;28:2864–2873. doi: 10.1523/JNEUROSCI.4063-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Hirase H, Leinekugel X, Henze DA, Buzsáki G. Temporal interaction between single spikes and complex spike bursts in hippocampal pyramidal cells. Neuron. 2001;32:141–149. doi: 10.1016/s0896-6273(01)00447-0. [DOI] [PubMed] [Google Scholar]
- Koh MT, Bernstein IL. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behavioral neuroscience. 2005;119:388–398. doi: 10.1037/0735-7044.119.2.388. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends in neurosciences. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. The Journal of comparative neurology. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Mehta MR, Lee AK, Wilson MA. Role of experience and oscillations in transforming a rate code into a temporal code. Nature. 2002;417:741–746. doi: 10.1038/nature00807. [DOI] [PubMed] [Google Scholar]
- Navarro M, Spray KJ, Cubero I, Thiele TE, Bernstein IL. cFos induction durng conditioned taste aversion expression varies with aversion strength. Brain research. 2000;887:450–453. doi: 10.1016/s0006-8993(00)03032-8. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala: a functional analysis. New York: Oxford University Press; 2000. pp. 31–115. [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in neurosciences. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Mizumori SJ. Characteristics of basolateral amygdala neuronal firing on a spatial memory task involving differential reward. Behavioral neuroscience. 1998;112:554–570. doi: 10.1037//0735-7044.112.3.554. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Rozin P, Kalat JW. Specific hungers and poison avoidance as adaptive specializations of learning. Psychological review. 1971;78:459–486. doi: 10.1037/h0031878. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiological reviews. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Thiele TE, Bernstein IL. Conditioning method dramatically alters the role of amygdala in taste aversion learning. Learning & memory. 1998;5:481–492. [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature neuroscience. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. Journal of Neurophysiology. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. The Journal of neuroscience. 1992;12:4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Sensory inputs from the oral region to the cerebral cortex in behaving rats: an analysis of unit responses in cortical somatosensory and taste areas during ingestive behavior. Journal of neurophysiology. 1988;60:1303–1321. doi: 10.1152/jn.1988.60.4.1303. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Shimura T, Yamamoto T. Single unit responses of the amygdala after conditioned taste aversion in conscious rats. Neuroreport. 1995;6:2424–2428. doi: 10.1097/00001756-199511270-00034. [DOI] [PubMed] [Google Scholar]