Abstract
Chemical shifts of complexes between paramagnetic lanthanide ions and macrocyclic chelates are sensitive to physiological variations (of temperature and/or pH). Here we demonstrate utility of a complex between thulium ion (Tm3+) and the macrocyclic chelate 1,4,7,10-tetramethyl 1,4,7,10-tetraazacyclodoecane-1,4,7,10-tetraacetate (or DOTMA4−) for absolute temperature mapping in rat brain. Feasibility of TmDOTMA− is compared with that of another Tm3+-containing biosensor which is based on the macrocyclic chelate 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(methylene phosphonate) (or DOTP8−). In general, the in vitro and in vivo results suggest that Biosensor Imaging of Redundant Deviation in Shifts (BIRDS) which originate from these agents (but exclude water) can provide temperature maps with good accuracy. While TmDOTP5− emanates three major distinct proton resonances which are differentially sensitive to temperature and pH, TmDOTMA− has a dominant pH-insensitive proton resonance from a −CH3 group to allow higher signal-to-noise ratio (SNR) temperature assessment. Temperature (and pH) sensitivities of these resonances are practically identical at low (4.0T) and high (11.7T) magnetic fields and at nominal repetition times only marginal SNR loss is expected at the lower field. Since these resonances have extremely short relaxation times, high-speed chemical shift imaging (CSI) is needed to detect them. Repeated in vivo CSI scans with BIRDS demonstrate excellent measurement stability. Overall, results with TmDOTP5− and TmDOTMA− suggest that BIRDS can be reliably applied, either at low or high magnetic fields, for functional studies in rodents.
Keywords: CEST, distribution, pH, paramagnetic, temperature, thulium
Introduction
Temperature and pH represent two systemic parameters which are tightly regulated under normal conditions and their deviations from normal values are usually indicators of abnormal situations. Temperature is a critical tool for prognosis of certain pathophysiological states (1–4). Hyperthermia, moreover, has been proposed for cancer treatment (5). pH changes are also common in pathology, e.g., tumors are characterized by decreased pH (6,7). Therefore temperature and pH measurements are extremely important for imaging stages of various pathophysiological states, especially in oncology.
Various in vivo methods can measure temperature and pH in animal brain. pH is measured by microelectrodes (8) and MR methods (9), while temperature is probed by thermocouple wires (10), infrared spectroscopy (11) and MR methods (12). Water-based MR methods for temperature depend on chemical shift changes observed by MRS (13) or by MRI using alterations in longitudinal relaxation time (14) or molecular diffusion coefficient (15). The most common MRS method for pH uses 31P chemical shifts of endogenous inorganic phosphate (Pi) and phosphocreatine (PCr) (16). Other MRS methods rely on small non-paramagnetic exogenous agents, but have relatively lower sensitivity (17,18). For higher spatial resolution pH a new MRI method, which generates contrast by chemical exchange, has been proposed (19). The chemical exchange saturation transfer (CEST) of bound water protons (20) or amide protons (21) measure pH indirectly by probing exchange between these proton pools and protons of bulk water, respectively. CEST contrasts of water and amide pools depend on exogenous (paramagnetic) and endogenous (diamagnetic) molecules, respectively. Some CEST agents can also be used as temperature sensors (22).
Since the water signal measured by MRI or MRS methods can also be simultaneously affected by factors other than temperature (and/or pH) in the brain, recent efforts have sought methods which exclude detection of water. A good majority of these approaches utilize exogenous agents composed of paramagnetic lanthanide ion complexes from derivatives of 1,4,7,10-tetraazacyclododecane or cyclen (23–28). The high temperature sensitivity of 1H and/or 31P chemical shifts of complexes containing lanthanide paramagnetic ions such as thulium (Tm3+) or ytterbium (Yb3+) and various macrocyclic chelates – such as cyclen-1,4,7,10-tetrakis(methylene phosphonate) (DOTP8−), cyclen-1,4,7,10-tetraacetate (DOTA4−), or 1,4,7,10-tetramethyl cyclen-1,4,7,10-tetraacetate (DOTMA4−) – provide attractive alternatives.
One of the best candidates for temperature is TmDOTP5− (26) because it emanates several proton chemical shifts that have higher temperature sensitivities than for other Tm3+ containing complexes, such as TmDOTA− (27) or TmDOTMA− (24). Moreover, the pH dependence of its proton chemical shifts represents an advantage when simultaneous measurement of both physiological parameters is desired. Recently, we demonstrated in rat brain that TmDOTP5− (a phosphonic acid derivative) infused into the blood stream can be used to obtain extracellular temperature (and pH) maps by high-speed 2D 1H chemical shift imaging (CSI) utilizing Biosensor Imaging of Redundant Deviation in Shifts (BIRDS) (29). The BIRDS method exploits the redundant temperature (and pH) information that is “stored” in the chemical shifts of multiple TmDOTP5− protons (excluding the water protons). Another potential for temperature sensing with BIRDS is TmDOTMA− (a carboxylic acid derivative) because of the presence of a high signal-to-noise ratio (SNR) proton resonance from its four −CH3 groups (24).
Since biodistribution of phosphonic and carboxylic acid derivatives in rodent body (30,31) show that the former deposit more favorably on bone and that lower charge on the latter allows improved access through the blood-brain barrier, we studied feasibility of TmDOTMA− for temperature mapping and compared these results with those from TmDOTP5−. We characterized TmDOTMA− for BIRDS, assessed its temperature sensitivities and SNR dependence at different magnetic fields, and obtained high resolution temperature maps, both in vitro and in vivo. In brief, the current results with TmDOTMA−, similar to results with TmDOTP5−, suggest that BIRDS can be reliably used for functional studies in rat brain.
Materials and Methods
MRS characterization of Tm3+ containing complexes
Temperature and pH dependencies for TmDOTP5− (a phosphonic acid derivative) and TmDOTMA− (a carboxylic acid derivative) were obtained from various samples with pH in the range of 6.9 to 7.7. The samples contained, as previously described (29,32), 4 mM of the agent, 3 mM of 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid (TSP), and 10% of D2O with different cation (e.g., K+, Na+, Ca2+) concentrations. We note that albumin (when ionized in water at pH 7.4, as found in the body) is negatively charged. Therefore it is unlikely that albumin or other negatively charged plasma proteins interacts with either of our agents.
The 1H spectra of TmDOTP5− and TmDOTMA− (Figs. 1A and 1B, respectively) were acquired at temperatures between 26 and 40 °C. The spectra, obtained either at 4.0T or 11.7T, were line-broadened (50 Hz) and then baseline (first order) and phase (zero order) corrected. The proton chemical shifts were measured by fitting each resonance to a Lorentzian function. Longitudinal (T1) and transverse (T2) relaxation times of the different proton resonances were measured at 35 °C and pH of 7.4 using conventional inversion recovery and spin echo methods, respectively. The T1 and T2 relaxation times (Supplementary Fig 1) were obtained by fitting the time dependencies of the intensities to a single exponential function.
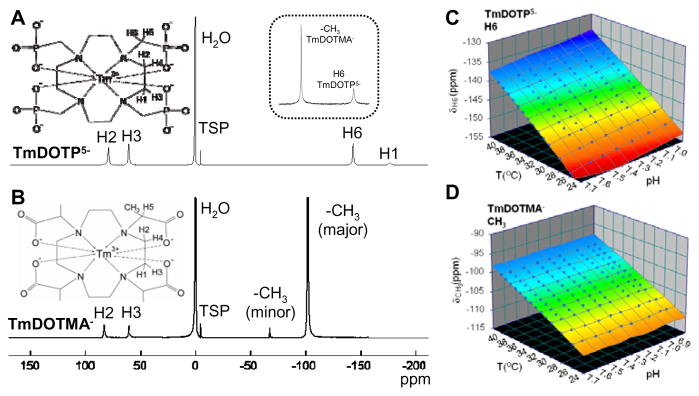
Figure 1. 1H spectra and temperature calibration of Tm3+ containing complexes.
Structures and 1H spectra of (A) TmDOTP5− and (B) TmDOTMA− (at 11.7T, 35 °C, pH 7.4). The inset (in A) compares sensitivity of BIRDS with TmDOTMA− vs. TmDOTP5− (spectrum of a sample containing equivalent concentration of each compound). The −CH3 protons of TmDOTMA− have ~5× higher SNR than the H6 proton of TmDOTP5−. Temperature and pH dependencies of (C) H6 proton of TmDOTP5− (for H2 and H3 protons see ref. (29)) and (D) −CH3 protons of TmDOTMA−. Temperature and/or pH dependencies of proton resonances of Tm3+ containing complexes (eq. [1]) are derived from a multi-parametric database (at 11.7T) consisting of chemical shift (δ), temperature (T) and pH and can be suitably portrayed in 3D surface plots. Calibration for TmDOTMA− is simplified by the fact that the resonance of the −CH3 protons is unaffected by pH (eq. [4]; Supplementary Tab. 1). In contrast, calibration of TmDOTP5− has to simultaneously account for relationships between δ vs. T as well as δ vs. pH (29).
Temperature and pH dependencies for multiple proton resonances of TmDOTP5− and a major peak representing the −CH3 protons of TmDOTMA− are based on a second-order model derived from multi-parametric data consisting of chemical shift (δ) , temperature (T) and pH,
| [1] |
where A–F are coefficients that can be determined analytically (29). All experiments for the multi-parametric database were conducted on a vertical bore 11.7T Bruker system which has a variable temperature controller in the radio frequency (RF) probe for independent temperature assessment. The pH of each sample in the database was accurately measured by a Corning 430 pH meter with a Beckman glass electrode (5×178 mm, Calomel).
This model can be conveniently depicted by a 3D surface plot where each of the parameters (δ, T, pH) represents an axis (Figs. 1C and 1D, respectively). While exact values of the coefficients A–F in eq. [1] are not critical, the inter-dependence of one parameter on the other(s) is crucial. For example, as we show in eq. [4], TmDOTMA− is all but pH-insensitive and therefore coefficients C, E, and F disappear to allow a second-order calibration with regard to δ vs. T only. On the other hand, as we showed previously (29), each proton resonance of TmDOTP5− is differentially sensitive to temperature and pH, and therefore calibration requires a two-step process that accounts for relationships between δ vs. T as well as δ vs. pH.
Effect of spectral SNR on temperature determination
Consequence of SNR on chemical shift determination was assessed by adding uniformly distributed random noise to the free induction decay (FID) (29). Briefly, uniform distributed random noise of various amplitudes was added to the FID and then Fourier transformed with no extra line-broadening. After first order baseline and zero order phase corrections to the spectrum the peak of interest was fitted to a Lorentzian lineshape. The SNR was calculated from the “signal” and “noise” values
| [2] |
where the “signal” was given by the height of the resonance and the “noise” was estimated from the standard deviation of the signal in a spectral region away from any resonances of interest. For each designated noise amplitude the chemical shifts of interest were calculated 100 times. Then the temperature values were calculated by BIRDS using TmDOTP5− (29) and TmDOTMA− (see eq. [4]). Finally, the standard deviation in temperature was estimated for each SNR value.
Effect of magnetic field strength on BIRDS
A 4 mM sample in 10% D2O and at pH of 7.4 was used to measure the field dependence of BIRDS at various repetition times (TR). The 4.0T and 11.7T data were acquired on two Bruker horizontal bore spectrometers using two 1H surface coil RF probes of 1.0 and 1.4 cm diameter, respectively. The number of averages was chosen such that the total experiment duration at each TR value was the same. The SNR values were calculated as in eq. [2].
In vitro 1H CSI
The TmDOTP5− phantom used for in vitro experiments consisted of two parallel glass tubes containing 4 mM TmDOTP5− and 10% D2O with 1 mM Ca2+ at two different pH values, 7.0 and 7.4, respectively (see ref. (29) for justification of Ca2+). For the TmDOTMA− phantom we used similar parallel glass tubes containing 4 mM TmDOTMA− and 10% D2O, with the two tubes at pH of 7.0 and 7.4, respectively (no Ca2+ effects were observed).
2D 1H CSI data were acquired on a 11.7T Bruker horizontal bore spectrometer using a 1H surface coil RF probe (1.4 cm), 16×16 encoding steps, field of view of 2.56×2.56 cm, and 100 averages. A 200 μs gaussian excitation pulse was used for excitation of a 6 mm slice. The phase encode gradient duration was 100 μs. The TR was 22 ms for TmDOTP5− because of two excitations on either side of water (see Fig. 1A and ref. (29) for details) and 11 ms for the TmDOTMA−, resulting in a total acquisition times of 9 and 4.5 min, respectively. The temperature was controlled by a water-heating blanket wrapped around the phantom. Two different CSI datasets were obtained at two different water bath temperatures, 45 and 35 °C.
In vivo studies
In vivo CSI acquisition parameters were the same as for the in vitro experiments (see above). Typical in vivo concentrations for the two agents in a CSI voxel was estimated by comparison with CSI experiments on phantoms of known concentrations (29). The chosen voxel for comparison of in vitro and in vivo CSI datasets was equidistant from the surface coil. The two CSI datasets (i.e., in vivo and in vitro) were acquired under identical conditions and areas under corresponding resonances were used for quantification. For TmDOTP5− the H2 and H3 protons were used (29), whereas for TmDOTMA− the −CH3 protons was used. We removed blood from the sagittal sinus, as previously described (29), to measure concentration of each agent in the blood. We assumed that the total MR signal in a CSI voxel (Ct) represents the sum of signals from the extracellular space (Cex) and the blood (Cbl). Therefore Cex was inferred from measured values of Ct and Cbl according to
| [3] |
where fex = 0.87 and fbl = 0.13 represent fractions of the extracellular and blood spaces, respectively (29).
All animal experimental procedures on rats were approved by the Institutional Animal Care and Use Committee (IACUC). Sixteen Sprague-Dawley rats (200–300 g) were tracheotomized and artificially ventilated (70% N2O, 30% O2). Isoflurane (1.5–2 %) was used for induction and surgery. An intraperitoneal line was inserted for administration of α-chloralose (46±4 mg/kg/hr) and an intravenous line was used for administration of D-tubocurarine chloride (1 mg/kg/hr), TmDOTP5− (1.0 mmol/kg) and TmDOTMA− (0.5 mmol/kg). Eight animals each were used for TmDOTP5− and TmDOTMA− experiments. The infusion rate was adjusted to keep the animal within the autoregulatory range of cerebral perfusion. An arterial line was used for monitoring physiology (blood pH, pO2, pCO2) throughout the experiment. The anesthetized rats were prepared with renal ligation as previously described (29,32). A water-heating blanket was used to control and maintain the body temperature.
Results
1H spectra and relaxation times of Tm3+ containing complexes
Structures and 1H spectra (at 11.7T, 35 °C, pH 7.4) of TmDOTP5− and TmDOTMA− are shown in Figs. 1A and 1B, respectively. The four observable protons of TmDOTP5− (H1, H2, H3, and H6) are detectable within ±200 ppm of the water resonance. The TmDOTP5− chemical shifts are sensitive to both temperature and pH (29,32). Since the H1 proton has a much smaller SNR than the other three protons, the H2, H3, and H6 protons were used to calculate temperature and pH (29). Under normal conditions (26–40 °C; pH 6.9 to 7.7) TmDOTMA− has two isomers (24), with the minor conformation comprising about 3 %. Although four proton resonances are detectable (i.e., H2, H3, as well as −CH3 protons of minor and major conformations), all results are based on the −CH3 protons for the major conformation because of significantly higher SNR. Comparing sensitivity of BIRDS for TmDOTMA− vs. TmDOTP5− using a sample of equivalent concentrations of each compound (Fig. 1A inset) shows that the −CH3 protons of TmDOTMA− have ~5× higher SNR than the H6 proton of TmDOTP5−.
Linewidths for H1, H2, H3, and H6 protons of TmDOTP5− were approximately 1100, 620, 550, and 470 Hz, respectively, corresponding to T2 values of less than 1 ms (Supplementary Figs. 1A–C). The −CH3 protons of TmDOTMA− produce a single peak and its linewidth was about 100 Hz, corresponding to T1 and T2 values of less than 5 ms (Supplementary Fig. 1D). The enhanced relaxation rates are due to proximity of the nuclear spin to unpaired electrons (33). Therefore extremely short TR is needed for TmDOTMA− and TmDOTP5− imaging to alleviate relaxation during data acquisition.
Temperature calibration of Tm3+ containing complexes
Temperature and/or pH dependencies of detectable protons of Tm3+ containing complexes can be represented by eq. [1] derived from a multi-parametric database (at 11.7T) consisting of chemical shift (δ) , temperature (T) and pH. This can be conveniently depicted in 3D surface plots, as shown in Figs. 1C and 1D respectively for TmDOTP5− (H6 proton only) and TmDOTMA− (the −CH3 protons). 1H spectra with different cations (and concentration) showed very weak dependence (data not shown), except for TmDOTP5− where a stable adduct with Ca2+ is known to form and was therefore accounted within the calibration (29).
Because each proton resonance of TmDOTP5− is independently sensitive to temperature and pH, the second-order calibration has to simultaneously account for relationships between δ vs. T as well as δ vs. pH (29). Calibration for TmDOTMA− is simpler because the major peak representing the −CH3 protons is practically unaffected by pH. Therefore the calculated temperature (Tc) depends only on the chemical shift of the −CH3 protons (δCH3) according to
| [4] |
where δ0 = −103.0 ppm and the coefficients a0 (34.45±0.01), a1 (1.460±0.003), and a2 (0.0152±0.0009) were calculated from linear least-squares fit of temperature as a function of chemical shift δCH3.
Uncertainties in temperature (εT) and/or pH (εpH) are proportional to uncertainties in the chemical shift (εδ). When εδ is considered the same for all TmDOTP5− protons (34), at 35 °C and pH of 7.4, the errors in temperature and pH for TmDOTP5− are given by εT = 2.63×εδ and εpH = 0.48×εδ, respectively (29). With TmDOTP5−, excellent correlations between the calculated temperature and pH (using equations similar to eq. [4]; see ref. (29)) were observed when compared to measured temperature (by a thermocouple) and pH (by a pH probe). If εδ is considered to be the same for TmDOTMA− as that for TmDOTP5−, then at 35 °C and pH of 7.4, the error in temperature is given by εT = 1.47×εδ. With TmDOTMA−, a very good correlation (R=0.99998) was obtained by linear least-square fit
| [5] |
between calculated (Tc; using eq. [4]) and measured (Tm; by a thermocouple) temperatures.
Temperature accuracy as a function of spectral SNR
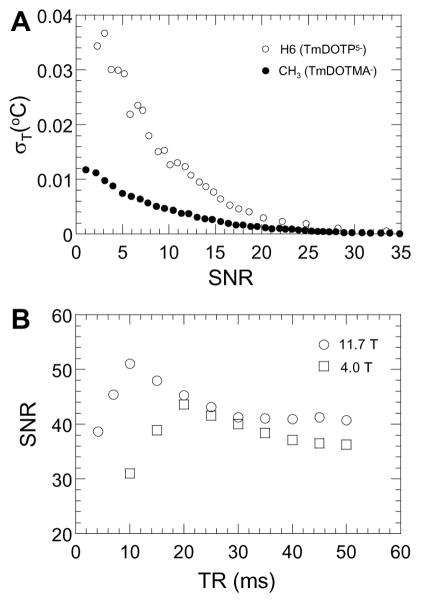
Error in temperature determination depends on how precisely we can measure chemical shifts, which in turn is related to SNR of each resonance. There is a strong dependence between the standard deviation in temperature (and pH) estimation and the SNR of the TmDOTP5− protons (see ○ for H6 proton in Fig. 2A; for H2 and H3 protons see ref. (29)). Similar simulations were conducted with the major peak representing the −CH3 protons of TmDOTMA− (see ● in Fig. 2A). Comparison of spectral SNR for TmDOTP5− and TmDOTMA− shows that the standard deviation for the latter is smaller than the standard deviation for the former (Fig. 2A). The range of in vivo SNR values for TmDOTP5− and TmDOTMA− can be quite wide (5 to 35). However typical SNR values in the cerebral cortex of the rat for TmDOTP5− and TmDOTMA− in 1.6×1.6×6 mm3 CSI voxels is about 15 and 25 (e.g., see Fig. 4A), respectively, for similar data acquisition times. At these respective SNR values, the standard deviations of temperature for TmDOTP5− and TmDOTMA− were 0.008 and 0.002 °C, respectively (Fig. 2A).
Figure 2. SNR effects on BIRDS.
(A) Effect of spectral SNR on temperature determination. Temperature standard deviations (σT) calculated at various SNR values were used to estimate temperature accuracy. Open and closed symbols, respectively, represent the H6 proton of TmDOTP5− and −CH3 protons of TmDOTMA−. See ref. (29) for pH standard deviations (σpH) calculated at various SNR values. (B) Sensitivity of BIRDS at different magnetic fields assessed by SNR of the proton resonance from the agent in a capillary tube sample. Squares and circles, respectively, represent data from −CH3 protons of TmDOTMA− at 4.0T and 11.7T. Similar trends were observed with H6 protons of TmDOTP5− (data not shown). Total data acquisition time at each TR was the same. See Tab. 1 (and Supplementary Tab. 1) for temperature (and pH) sensitivities of BIRDS with TmDOTP5− and TmDOTMA−.
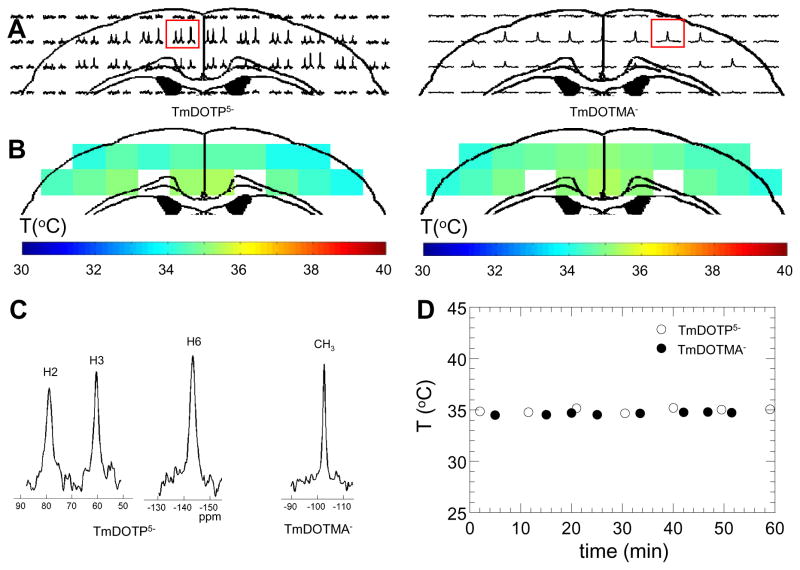
Figure 4. Temperature (and pH) mapping in vivo with BIRDS.
(A) 2D 1H CSI datasets after infusion of TmDOTP5− (left) and TmDOTMA− (right) in the rat. Concentrations of TmDOTP5− and TmDOTMA− in the CSI voxel were about 4 and 3 mM, respectively, at infusion doses of 1.0 and 0.5 mmol/kg (Tab. 2). (B) Temperature distributions in cerebral cortex of the rat measured by TmDOTP5− (left) and TmDOTMA− (right). (C) Examples of 1H spectra from CSI voxels (boxed in A), one from the TmDOTP5− infused brain (left) and the other from the TmDOTMA− infused brain (right). (D) Temporal stability of temperature (±0.21 and ±0.13 °C, respectively, for TmDOTP5− and TmDOTMA−) in the CSI voxels (boxed in A), where open and filled symbols represent data from TmDOTP5− and TmDOTMA−, respectively. See Supplementary Fig. 2 for pH mapping in vivo.
Sensitivity of BIRDS at different magnetic fields
We examined the magnetic field dependence on sensitivity of BIRDS with regard to SNR of the resonance from the agent (Fig. 2B) and temperature sensitivity of the resonance (Tab. 1). Experiments were conducted at 11.7T and 4.0T with the same samples and parameters.
Table 1.
Temperature sensitivities of TmDOTP5− and TmDOTMA− at 4.0T and 11.7T.
| Agent | Resonance | Temperature sensitivity (ppm/°C) | |
|---|---|---|---|
| 4.0T | 11.7T | ||
| TmDOTP5− | H2 | −0.6±0.1 | −0.6±0.1 |
| H3 | −0.5±0.1 | −0.5±0.1 | |
| H6 | 1.1±0.1 | 1.0±0.1 | |
| TmDOTMA− | −CH3 (major) | 0.7±0.1 | 0.7±0.1 |
The SNR comparison (Fig. 2B) shows that each magnetic field has an optimal TR (e.g., ~10 and ~20 ms, respectively, at 11.7T and 4.0T), which is expected due to field-dependent relaxation time variations (33). However at TR of about 20 ms (i.e., optimal TR at 4.0T) there is negligible SNR difference between data at the different magnetic fields. Since the total data acquisition time at each TR was the same, the SNR comparison shows that TR of 20 ms can allow approximately similar level SNR in μL sized CSI voxels at low and high magnetic fields.
Temperature sensitivities for multiple protons of TmDOTP5− and −CH3 protons of TmDOTMA− at 4.0T and 11.7T (Tab. 1) are nearly identical. The minor variations in sensitivities could be due to slight SNR differences at the two magnetic fields and/or slightly different ambient conditions inside the two magnet bores. The H6 proton of TmDOTP5− and −CH3 protons of TmDOTMA− have comparable temperature sensitivities (i.e., ~1 and 0.7 ppm/°C, respectively). However the −CH3 protons of TmDOTMA− offer much higher SNR compared to the H6 proton of TmDOTP5− (Fig. 1A inset). Similar constancy of pH sensitivity was observed with multiple protons of TmDOTP5− at 4.0T and 11.7T (Supplementary Tab. 1). These results demonstrate that while each proton of TmDOTP5− is differentially sensitive to temperature and pH, TmDOTMA− has a dominant pH-insensitive proton to allow higher SNR measurements.
Temperature (and pH) measurements in phantoms
All in vitro studies (Fig. 3), mimicking in vivo situations, were conducted on a phantom consisting of two parallel glass tubes with different pH. The left and right tubes had pH of 7.4 and 7.0, respectively. A water-heating blanket was used to raise or cool both tubes equally. Identical phantoms were made with TmDOTP5− (Fig. 3, left) and TmDOTMA− (Fig. 3, right).
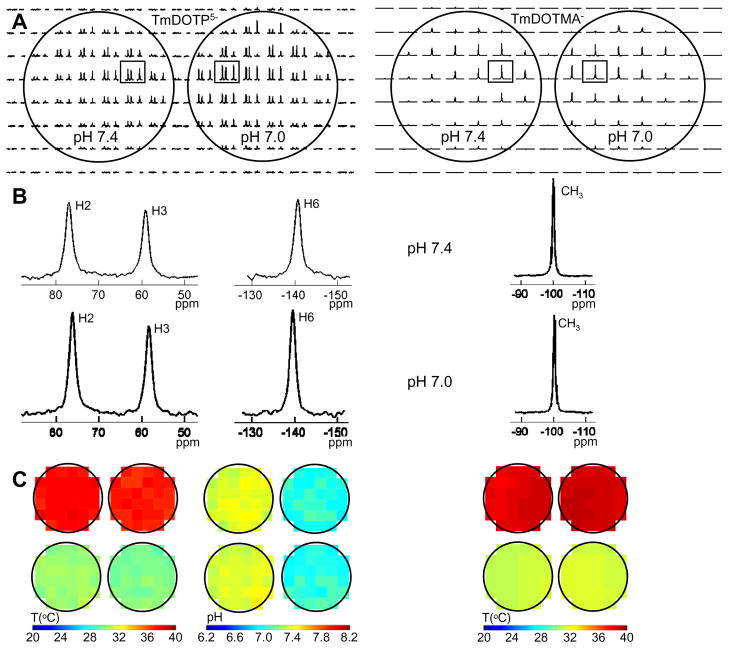
Figure 3. Temperature (and pH) mapping in vitro with BIRDS.
The phantom – containing either TmDOTP5− (left) or TmDOTMA− (right) – consisted of two parallel glass tubes with different pH (left 7.4, right 7.0) and using a water-heating blanket the temperature was changed in both tubes identically. (A) 2D 1H CSI datasets with TmDOTP5− (left) and TmDOTMA− (right) phantoms. (B) Examples of 1H spectra from CSI voxels (boxed in A), a pair from the TmDOTP5− phantom (left) and another pair from the TmDOTMA− phantom (right), at pH of 7.4 (top) and 7.0 (bottom). (C) Temperature distributions in TmDOTP5− (left) and TmDOTMA− (right) phantoms at two different water-bath temperatures, 45 °C (top) and 35 °C (bottom). Note that the TmDOTP5− phantom shows similar temperature distributions as with the TmDOTMA− phantom, but in addition it allows accurate distribution of pH.
Well resolved 1H spectra were obtained from 2D CSI datasets of TmDOTP5− (left) and TmDOTMA− (right) phantoms (Fig. 3A). The chemical shifts of the three protons of TmDOTP5− (left) were slightly different in the two different pH tubes, whereas the chemical shift of the −CH3 protons of TmDOTMA− were identical in the two different pH tubes (Fig. 3B). Calculated temperature distributions in TmDOTP5− (left) and TmDOTMA− (right) phantoms at two different water-bath temperatures, 45 °C (top) and 35 °C (bottom), showed clear sensitivity of TmDOTP5− for temperature and pH, whereas TmDOTMA− was responsive to temperature only (Fig. 3C).
The TmDOTP5− CSI data (Fig. 3, left) allowed calculation of both temperature and pH values for each voxel. When the water-bath was at 45 °C, the average phantom temperatures were 37.7±0.3 °C and 37.2±0.3 °C for the tubes with pH of 7.4 and 7.0, respectively, whereas the corresponding average pH values were 7.39±0.04 and 7.02±0.06 in left and right tubes, respectively (Fig. 3C, left and top). When the water-bath was at 35 °C, the average phantom temperatures were 30.6±0.3 °C and 30.0±0.3 °C for the tubes with pH of 7.4 and 7.0, respectively, whereas the corresponding average pH values were 7.39±0.04 and 7.03±0.06 in left and right tubes, respectively (Fig. 3C, left and bottom).
Likewise, the TmDOTMA− CSI data (Fig. 3, right) allowed calculation of only temperature for each voxel. When the water-bath was at 45 °C, the average phantom temperatures were 38.6±0.2 °C and 38.9±0.2 °C for the tubes with pH of 7.4 and 7.0, respectively (Fig. 3C, right and top). When the water-bath was at 35 °C, the average phantom temperatures were 31.4±0.1 °C and 31.7±0.2 °C for the tubes with pH of 7.4 and 7.0, respectively (Fig. 3C, right and bottom).
Temperature (and pH) measurements in rat brain
All in vivo studies (Fig. 4) were conducted in renaly ligated rats (29,32) with otherwise normal physiology for the duration of the experiment (data not shown). Well resolved 1H spectra of the cerebral cortex were obtained from 2D CSI datasets after infusion of TmDOTP5− (left) and TmDOTMA− (right) in the rat (Fig. 4A).
Calculated temperature maps with TmDOTP5− (left) and TmDOTMA− (right) in the rat brain (Fig. 4B) showed similar distributions, with ~0.6 °C difference between deep (34.9±0.3 and 35.0±0.2 °C by TmDOTP5− and TmDOTMA−, respectively) and superficial (34.3±0.2 and 34.4±0.2 °C by TmDOTP5− and TmDOTMA−, respectively) regions. Average temperatures with TmDOTP5− and TmDOTMA− over all voxels were 34.6±0.4 and 34.8±0.4 °C, respectively. Similar pH distributions were observed in vivo with TmDOTP5− (Supplementary Fig. 2A).
1H spectra from CSI voxels, one from a TmDOTP5− infused brain (left) and the other from a TmDOTMA− infused brain (right), showed high SNR (Fig. 4C). For TmDOTP5−, the SNR was in the range of 5 to 30, while for TmDOTMA− the SNR was in the range of 5 to 35. Estimated concentrations of TmDOTP5− and TmDOTMA− in the CSI voxel were about 4 and 3 mM, respectively, with current infusion doses (Tab. 2). By including the measured concentrations of TmDOTP5− and TmDOTMA− in blood, the extracellular concentrations were calculated to be 3.8 and 2.8 mM, respectively, which represented the majority of the 1H signal observed in the CSI voxel. Static field shimming differences between in vitro and in vivo conditions did not affect the concentration determination process as these peaks are intrinsically broad due to their extremely short relaxation times. Assessment of temporal stability of temperature mapping with TmDOTP5− (open symbol) and TmDOTMA− (filled symbol) in rat brain was measured over an hour long scanning session. Most cortical voxels showed very small fluctuations (±0.1 °C) around the respective average values (Fig. 4D). Similar level pH fluctuations were observed in vivo with TmDOTP5− (Supplementary Fig. 2B).
Table 2.
In vivo TmDOTP5− and TmDOTMA− concentrations with respective doses of 1.0 and 0.5 mmol/kg, respectively.
| Compartment | [TmDOTP5−] (mM) | [TmDOTMA−] (mM) |
|---|---|---|
| Blood | 4.8±0.4 | 3.4±0.2 |
| Extracellular space | 3.8±0.2 | 2.8±0.2 |
| Total | 4.0±0.2 | 2.9±0.2 |
Discussion
In the present study, we compared temperature mapping capabilities in rat brain by BIRDS using two Tm3+ containing complexes, TmDOTP5− and TmDOTMA−. Both agents are derivatives of cyclen (Figs. 1A and 1B). Except for the obvious difference in functional groups (i.e., phosphonic and carboxylic acids, respectively), the two structures only differ by replacement of the H6 proton (in TmDOTP5−) with a −CH3 group (in TmDOTMA−). However this small structural difference in the cyclen part of the complex introduces divergent properties which are detectable by BIRDS.
TmDOTP5− has three dominant proton resonances, whereas TmDOTMA− has a major signal from a −CH3 group. Because each proton of TmDOTP5− is uniquely affected by temperature and pH, both can be mapped by BIRDS. The H2 and H3 protons are attached to carbons within the cyclen itself, whereas the H6 proton is attached to a carbon one bond away from the cyclen. Therefore the H2/H3 protons and H6 proton have slightly different temperature (and pH) sensitivities (Tab. 1; Supplementary Tab. 1). Since the major TmDOTMA− signal is pH-insensitive, temperature is mapped by BIRDS with good accuracy (Figs. 1C and 1D). The −CH3 group of TmDOTMA− is in a similar position as the H6 proton of TmDOTP5−, whereas the other weaker proton signals are attached to carbons within the cyclen itself.
Temperature sensitivity of the TmDOTMA− −CH3 protons is slightly weaker than that of the TmDOTP5− H6 proton (i.e., 0.7 ppm/°C vs. 1 ppm/°C; Tab. 1). However the TmDOTMA− signal has about ×5 higher SNR than that of the TmDOTP5− signal, due to more observable protons and/or much longer T2 (i.e., TmDOTMA− > TmDOTP5−). Moreover, because the T2 for the observable protons of TmDOTP5− are extremely short compared to those of TmDOTMA− (Supplementary Fig. 1), it is possible that more SNR loss occurs during data acquisition with TmDOTP5− than for TmDOTMA−. Therefore the CSI scans are conducted at high-speed.
Precision of BIRDS with TmDOTMA− and TmDOTP5− were compared by adding simulated noise to FIDs because error in chemical shift measurement is the main source of error for temperature (or pH in the case of TmDOTP5−) determination (Fig. 2A). Simulated results showed that at any given SNR the standard deviation in temperature determination is smaller for the −CH3 protons of TmDOTMA− than for any of the three TmDOTP5− protons. Within the SNR range observed in CSI voxels in vivo (i.e., 15 to 20), the standard deviation in temperature determination with TmDOTMA− is about ×2–4 lower (than with TmDOTP5−). These theoretical predictions were confirmed by in vitro (Fig. 3) and in vivo (Fig. 4) results.
The standard deviations in the calculated average temperature in phantoms (measured over the entire sample) were about 0.30 and 0.15 °C, respectively, with TmDOTP5− and TmDOTMA−. Similarly, the standard deviations in calculated average temperature of the cerebral cortex (measured over an hour) were 0.21 and 0.15 °C, respectively, with TmDOTP5− and TmDOTMA−. While these results suggest that TmDOTMA− may be more ideal for temperature mapping, it should be noted that the accuracy for TmDOTP5− are within or better than expectations of other MR methods (see ref. (29) for details). Furthermore TmDOTP5− also allows reliable pH measurements in vivo (Supplementary Fig. 2).
Temperature sensitivities (Tab. 2) and SNR (Fig. 2B) of BIRDS were nearly independent of the magnetic field strength. Similar results were obtained for pH sensitivity (Supplementary Tab. 1). These findings encourage the use of BIRDS even at a lower magnetic field with nominal SNR loss from a higher magnetic field, and furthermore calibration of an agent can be used rather reliably at different magnetic fields. Therefore experimental results from BIRDS with agents such as TmDOTMA− and TmDOTP5− could easily be translated across laboratories. Comparisons of different MR methods that measure temperature and pH (29) reveal that BIRDS (using TmDOTP5−) is quite accurate in absolute measurements, albeit these are made at slightly lower spatial resolution. We note that other MRI methods that utilize multiple tracers with ratiometric approach have much superior spatial resolution (35). However BIRDS is an easier method to apply for in vivo studies (i.e., single-shot, single agent).
Toxicity, calculated as half lethal dose (LD50), of some lanthanide ion complexes such as YbDOTMA− (23) or GdDOTA− (36) have been measured (e.g., 10.5 and 11.4 mmol/kg, respectively). Since these LD50 values are more than an order of magnitude higher than infusion doses used in our experiments (i.e., 1.0 and 0.5 mmol/kg for TmDOTP5− and TmDOTMA−, respectively), it is unlikely that conditions for renaly ligated rats ever reach fatal levels. However biodistribution studies with lanthanide ion complexes (30,31) show that phosphonic acids deposit more favorably on bone, whereas carboxylic acids (because of their lower charge compared to phosphonic acids) have better access through the blood-brain barrier. Previously, we showed that TmDOTP5− is observed in cerebral spinal fluid at mM level (32), and because of its high negative charge, proposed fenestrated vessels of circumventricular organs, not necessarily the blood-brain barrier, as the delivery route for entry into extracellular space (29). The dose for TmDOTP5− was twice that for TmDOTMA−, but we detected about 4 and 3 mM of each agent, respectively, in the extracellular space (Tab. 2). This suggests, as hypothesized, that a lower charge on TmDOTMA− (compared to TmDOTP5−) may allow partial access through the blood-brain barrier. However the delivery route of ventricular zones may still be operative with TmDOTMA− (and perhaps other agents that are uncharged). Moreover due to minimized excretion of any agent with renal ligation it is possible that the concentration gradient is increased for entry into the extracellular milieu, via the blood-brain barrier and/or the fenestrated vessels of circumventricular organs.
Current and prior (29,32) in vivo studies with BIRDS have been limited to primarily the rat’s cerebral cortex because of RF surface coils. Since surface coils yield high efficiency RF receptive fields near the probe, short duration excitation pulses can be used to detect those regions near the coil far more easily than deeper regions. To alleviate concerns about competitive relaxation during data acquisition and also push future investigations beyond superficial regions of the brain, RF technology development is needed to increase RF efficiency of birdcage-like coils. Furthermore the current high-speed CSI pulse sequence has been limited to cubical k-space. Since majority of the signal is concentrated in the center of k-space, replacing cubical with spherical encoding will have very small effect on the overall SNR because higher spatial frequencies in k-space have negligible contributions to the total SNR. Therefore future studies with improved RF coils and k-space trajectory could improve SNR for CSI datasets which encompass the whole brain.
Since BIRDS can provide much higher temperature sensitivity (i.e., 1.0 or 0.7 ppm/°C with TmDOTP5− and TmDOTMA−, respectively, compared to 0.01 ppm/°C with water; see ref. (29) for a detailed discussion), use of this technique in clinical imaging is highly desirable. However human studies are currently limited by concerns about agent efficacy and poor performance of human head resonators. Radio-labeled version of a methylated DOTP8− has been used for clinical imaging because phosphonates deposit on bone (37). We believe that similar structural modification to DOTP8− for enhanced BIRDS sensitivity may allow imaging of bone by high-speed CSI.
In summary, we showed in vitro and in vivo results which suggest that BIRDS with TmDOTP5− and TmDOTMA− can be reliably applied, either at low or high magnetic fields, for functional brain studies in rats (38–40). Since many CEST agents have cyclen-based structures (20,22), designing lanthanide complexes that combine BIRDS as well as CEST effects – similar to the idea of using two CEST agents in the same study for improved biosensing (41) – would be highly attractive for cancer studies which could be aided by quantitative determination of temperature (and/or pH).
Supplementary Material
Acknowledgments
Thanks to Drs. Basavaraju G. Sanganahalli and Peter Herman for consultations. We thank Peter Brown and Bei Wang for engineering and surgical help. Supported by National Institutes of Health grants (R01 MH-067528, P30 NS-52519).
References
- 1.Ginsberg MD, Busto R. Combating hyperthermia in acute stroke: a significant clinical concern. Stroke. 1998;29(2):529–534. doi: 10.1161/01.str.29.2.529. [DOI] [PubMed] [Google Scholar]
- 2.Thompson HJ, Pinto-Martin J, Bullock MR. Neurogenic fever after traumatic brain injury: an epidemiological study. J Neurol Neurosurg Psychiatry. 2003;74(5):614–619. doi: 10.1136/jnnp.74.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson HJ, Tkacs NC, Saatman KE, Raghupathi R, McIntosh TK. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol Dis. 2003;12(3):163–173. doi: 10.1016/s0969-9961(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 4.Yager JY, Armstrong EA, Jaharus C, Saucier DM, Wirrell EC. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011(1):48–57. doi: 10.1016/j.brainres.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 5.van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13(8):1173–1184. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- 6.Raghunand N, Altbach MI, van Sluis R, et al. Plasmalemmal pH-gradients in drug-sensitive and drug-resistant MCF-7 human breast carcinoma xenografts measured by 31P magnetic resonance spectroscopy. Biochem Pharmacol. 1999;57(3):309–312. doi: 10.1016/s0006-2952(98)00306-2. [DOI] [PubMed] [Google Scholar]
- 7.Stubbs M, Bhujwalla ZM, Tozer GM, et al. An assessment of 31P MRS as a method of measuring pH in rat tumours. NMR Biomed. 1992;5(6):351–359. doi: 10.1002/nbm.1940050606. [DOI] [PubMed] [Google Scholar]
- 8.de Curtis M, Manfridi A, Biella G. Activity-dependent pH shifts and periodic recurrence of spontaneous interictal spikes in a model of focal epileptogenesis. J Neurosci. 1998;18(18):7543–7551. doi: 10.1523/JNEUROSCI.18-18-07543.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillies RJ, Raghunand N, Garcia-Martin ML, Gatenby RA. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag. 2004;23(5):57–64. doi: 10.1109/memb.2004.1360409. [DOI] [PubMed] [Google Scholar]
- 10.Young CC, Sladen RN. Temperature monitoring. Int Anesthesiol Clin. 1996;34(3):149–174. doi: 10.1097/00004311-199603430-00009. [DOI] [PubMed] [Google Scholar]
- 11.Brugge JF, Poon PW, So AT, Wu BM, Chan FH, Lam FK. Thermal images of somatic sensory cortex obtained through the skull of rat and gerbil. Exp Brain Res. 1995;106(1):7–18. doi: 10.1007/BF00241352. [DOI] [PubMed] [Google Scholar]
- 12.Denis de Senneville B, Quesson B, Moonen CT. Magnetic resonance temperature imaging. Int J Hyperthermia. 2005;21(6):515–531. doi: 10.1080/02656730500133785. [DOI] [PubMed] [Google Scholar]
- 13.De Poorter J. Noninvasive MRI thermometry with the proton resonance frequency method: study of susceptibility effects. Magn Reson Med. 1995;34(3):359–367. doi: 10.1002/mrm.1910340313. [DOI] [PubMed] [Google Scholar]
- 14.Parker DL, Smith V, Sheldon P, Crooks LE, Fussell L. Temperature distribution measurements in two-dimensional NMR imaging. Med Phys. 1983;10(3):321–325. doi: 10.1118/1.595307. [DOI] [PubMed] [Google Scholar]
- 15.Le Bihan D, Delannoy J, Levin RL. Temperature mapping with MR imaging of molecular diffusion: application to hyperthermia. Radiology. 1989;171(3):853–857. doi: 10.1148/radiology.171.3.2717764. [DOI] [PubMed] [Google Scholar]
- 16.Petroff OA, Prichard JW, Behar KL, Alger JR, Shulman RG. In vivo phosphorus nuclear magnetic resonance spectroscopy in status epilepticus. Ann Neurol. 1984;16(2):169–177. doi: 10.1002/ana.410160203. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Martin ML, Herigault G, Remy C, et al. Mapping extracellular pH in rat brain gliomas in vivo by 1H magnetic resonance spectroscopic imaging: comparison with maps of metabolites. Cancer Res. 2001;61(17):6524–6531. [PubMed] [Google Scholar]
- 18.Gillies RJ, Liu Z, Bhujwalla Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am J Physiol. 1994;267(1 Pt 1):C195–203. doi: 10.1152/ajpcell.1994.267.1.C195. [DOI] [PubMed] [Google Scholar]
- 19.Ward KM, Balaban RS. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST) Magn Reson Med. 2000;44(5):799–802. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Merritt M, Woessner DE, Lenkinski RE, Sherry AD. PARACEST agents: modulating MRI contrast via water proton exchange. Acc Chem Res. 2003;36(10):783–790. doi: 10.1021/ar020228m. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Malloy CR, Sherry AD. MRI thermometry based on PARACEST agents. J Am Chem Soc. 2005;127(50):17572–17573. doi: 10.1021/ja053799t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aime S, Botta M, Fasano M, et al. A new ytterbium chelate as contrast agent in chemical shift imaging and temperature sensitive probe for MR spectroscopy. Magn Reson Med. 1996;35(5):648–651. doi: 10.1002/mrm.1910350504. [DOI] [PubMed] [Google Scholar]
- 24.Hekmatyar SK, Hopewell P, Pakin SK, Babsky A, Bansal N. Noninvasive MR thermometry using paramagnetic lanthanide complexes of 1,4,7,10-tetraazacyclodoecane-alpha,alpha’,alpha”,alpha”’-tetramethyl-1, 4,7,10-tetraacetic acid (DOTMA4-) Magn Reson Med. 2005;53(2):294–303. doi: 10.1002/mrm.20345. [DOI] [PubMed] [Google Scholar]
- 25.Hekmatyar SK, Poptani H, Babsky A, Leeper DB, Bansal N. Non-invasive magnetic resonance thermometry using thulium-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate (TmDOTA(−)) Int J Hyperthermia. 2002;18(3):165–179. doi: 10.1080/02656730110098598. [DOI] [PubMed] [Google Scholar]
- 26.Zuo CS, Bowers JL, Metz KR, Nosaka T, Sherry AD, Clouse ME. TmDOTP5-: a substance for NMR temperature measurements in vivo. Magn Reson Med. 1996;36(6):955–959. doi: 10.1002/mrm.1910360619. [DOI] [PubMed] [Google Scholar]
- 27.Zuo CS, Mahmood A, Sherry AD. TmDOTA-: a sensitive probe for MR thermometry in vivo. J Magn Reson. 2001;151(1):101–106. doi: 10.1006/jmre.2001.2356. [DOI] [PubMed] [Google Scholar]
- 28.Zuo CS, Metz KR, Sun Y, Sherry AD. NMR temperature measurements using a paramagnetic lanthanide complex. J Magn Reson. 1998;133(1):53–60. doi: 10.1006/jmre.1998.1429. [DOI] [PubMed] [Google Scholar]
- 29.Coman D, Trubel HK, Rycyna RE, Hyder F. Brain temperature and pH measured by (1)H chemical shift imaging of a thulium agent. NMR Biomed. 2009;22(2):229–239. doi: 10.1002/nbm.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geraldes CF, Sherry AD, Lazar I, et al. Relaxometry, animal biodistribution, and magnetic resonance imaging studies of some new gadolinium (III) macrocyclic phosphinate and phosphonate monoester complexes. Magn Reson Med. 1993;30(6):696–703. doi: 10.1002/mrm.1910300607. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard DS, Houlne MP, Kiefer GE, McMillan K, Bornhopz DJ. Endoscopic fluorescence imaging of tissue selective lanthanide chelates. Bioimaging. 1998;6:63–70. [Google Scholar]
- 32.Trubel HK, Maciejewski PK, Farber JH, Hyder F. Brain temperature measured by 1H-NMR in conjunction with a lanthanide complex. J Appl Physiol. 2003;94(4):1641–1649. doi: 10.1152/japplphysiol.00841.2002. [DOI] [PubMed] [Google Scholar]
- 33.Abragam A. Principles of Nuclear Magnetism. Oxford, UK: Oxford University Press; 1961. [Google Scholar]
- 34.Bevington PR. Data Reduction and Error Analysis for The Physical Sciences. New York, NY: McGraw-Hill Book Company; 1969. p. 336. [Google Scholar]
- 35.Garcia-Martin ML, Martinez GV, Raghunand N, Sherry AD, Zhang S, Gillies RJ. High resolution pH(e) imaging of rat glioma using pH-dependent relaxivity. Magn Reson Med. 2006;55(2):309–315. doi: 10.1002/mrm.20773. [DOI] [PubMed] [Google Scholar]
- 36.Bousquet JC, Saini S, Stark DD, et al. Gd-DOTA: characterization of a new paramagnetic complex. Radiology. 1988;166(3):693–698. doi: 10.1148/radiology.166.3.3340763. [DOI] [PubMed] [Google Scholar]
- 37.Breitz HB, Wendt RE, 3rd, Stabin MS, et al. 166Ho-DOTMP radiation-absorbed dose estimation for skeletal targeted radiotherapy. J Nucl Med. 2006;47(3):534–542. [PubMed] [Google Scholar]
- 38.Trubel H, Herman P, Kampmann C, et al. A novel approach for selective brain cooling: implications for hypercapnia and seizure activity. Intensive Care Med. 2004;30(9):1829–1833. doi: 10.1007/s00134-004-2350-1. [DOI] [PubMed] [Google Scholar]
- 39.Trubel HK, Sacolick LI, Hyder F. Regional temperature changes in the brain during somatosensory stimulation. J Cereb Blood Flow Metab. 2006;26(1):68–78. doi: 10.1038/sj.jcbfm.9600164. [DOI] [PubMed] [Google Scholar]
- 40.Maandag NJ, Coman D, Sanganahalli BG, et al. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci U S A. 2007;104(51):20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali MM, Liu G, Shah T, Flask CA, Pagel MD. Using two chemical exchange saturation transfer magnetic resonance imaging contrast agents for molecular imaging studies. Acc Chem Res. 2009;42(7):915–924. doi: 10.1021/ar8002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.