Abstract
Objective
In a randomized multi-center trial, we demonstrated that inhaled nitric oxide begun between 7 and 21 days and treated for 24 days significantly increased survival without bronchopulmonary dysplasia (BPD) in ventilated premature infants weighing < 1250 g. Since some preventative BPD treatments are associated with neurodevelopmental impairment, we designed a follow-up study to assess the safety of nitric oxide.
Study design
Our hypothesis was that inhaled nitric oxide will not increase neurodevelopmental impairment compared with placebo. We prospectively evaluated neurodevelopmental and growth outcomes at 24 months PMA in 477 (89%) of 535 surviving infants enrolled in the trial.
Results
In the treated group, 109 of 243 children (45%) had neurodevelopmental impairment (moderate or severe cerebral palsy, bilateral blindness, bilateral hearing loss or score of less than 70 on the Bayley Scales II), compared with 114 of 234 (49%) in the placebo group (Relative Risk 0.92; 95% confidence interval, 0.75 to 1.12; p = 0.39). No differences on any subcomponent of neurodevelopmental impairment or growth variables were found between inhaled nitric oxide or placebo.
Conclusions
Inhaled nitric oxide improved survival free of bronchopulmonary dysplasia with no adverse neurodevelopmental effects at 2 years of age.
Advances in neonatal-perinatal medicine have resulted in increased survival rates among premature infants. However, morbidities of prematurity, including bronchopulmonary dysplasia, a major contributor to neurodevelopmental impairment, have not decreased. In a single center controlled trial of inhaled nitric oxide in preterm infants Schreiber et al1 showed decreased intraventricular hemorrhage and found improved neurocognitive outcomes.2 Kinsella et al reported decreased abnormalities on head ultrasound examinations prior to discharge in another large trial.3 However, in the NICHD trial, critically ill infants less than 1000 g with hypoxic respiratory failure and treated with brief exposure to inhaled nitric oxide had increased mortality and no evidence of neuroprotection.4–5 The INNOVA trial found no differences in either pulmonary or neurologic outcomes.6
In the multicenter NO CLD Trial we demonstrated that inhaled nitric oxide significantly increased survival without bronchopulmonary dysplasia as determined at 36 weeks’ postmenstrual age in premature infants weighing less than 1250 g requiring ventilatory support between 7 and 21 days of life.7,8 Treated infants were more likely to be discharged or off respiratory support at 40 weeks postmenstrual age7, required fewer days of ventilatory support and hospitalization9, and were less likely to require treatment with pulmonary medications in the first year.10 In post hoc analyses infants entered between 7-14 days of age, compared with those enrolled later, had greater benefit (P=0.005), and non-white infants appeared to respond better than white infants (Interaction P=0.06).7 There were no short term safety concerns. Treatment was not associated with increased biomarkers for inflammation11 or oxidative stress,12 but was associated with a transient improvement in endogenous surfactant function.13 Because some treatments used to prevent bronchopulmonary dysplasia have been associated with unacceptable rates of neurodevelopmental impairment14, we assessed the neurodevelopmental outcome of NO CLD Trial infants through 2 years of age.
Methods
We hypothesized that treatment with inhaled nitric oxide in the NO CLD Trial would be safe as measured by survival without neurodevelopmental impairment at 2 years of age corrected for prematurity. We defined neurodevelopmental impairment as one or more of the following events: a score on the Mental or Psychomotor Developmental Index of the Bayley Scales of Infant Development II that was less than 70, disabling cerebral palsy (Palisano gross motor function score greater than or equal to 2), bilateral blindness, or bilateral hearing loss requiring amplification.
The initial study, conducted at 21 neonatal intensive care units, randomized ventilated infants with birth weight <1250 g to receive either nitric oxide or placebo beginning between 7 and 21 days of life. Infants initially received 20 parts per million (ppm) of study gas for 72±24 h, and subsequently were weaned to 10, 5, and 2 ppm at weekly intervals with a minimum treatment exposure of 24 days.7,8 Infants were stratified by birthweight (500–799 and 800–1250 g) and site in permuted blocks. If more than one infant from a multiple gestation was enrolled, all of the infants were assigned to the same treatment. Maternal race or ethnic group was self-reported. All of the infants in the trial initially received respiratory support. Severity of illness at entry to the trial was measured using a respiratory severity score (calculated from mean airway pressure multiplied by percentage of inspired oxygen) as well as an assessment of whether the infant had had a period of extubation prior to trial enrollment.
Children were evaluated at one and two years of age corrected for prematurity with standardized outcome assessments including ascertainment of pulmonary health status and medication use using a structured interview, growth measurements, neurological examination14, gross motor function using the method of Palisano15, and developmental outcomes (Bayley Scales of Infant Development II)16. Disabling cerebral palsy was defined by a Palisano score greater than or equal to 2. Patients were screened for visual impairment by means of a parental report, and direct observation. Deafness was defined as impairment requiring amplification. The Bayley Scales of Infant Development II were administered by certified examiners trained to reliability using standardized cases. A score of 49 was assigned in cases where the child was too impaired to complete the testing. Infants were assessed at 2 years (22–26 months) of age.
At study inception procedures were designed to ensure the highest possible ascertainment of outcomes in all study participants. Families who were unable to complete visits at 2 years of age were offered additional in-person visits until 3 years of age. Failing this, families were invited to participate in alternative evaluations which included some or all of the following: completion of a structured telephone questionnaire on infant health resource use and functional outcomes, abstraction of records from inpatient or outpatient ambulatory visits and inpatient admissions, abstraction of records from subspecialty evaluations in neurology, developmental clinics, or early intervention evaluations. Information from these alternative evaluations were independently reviewed by a three-member adjudication committee (MCW, AMH, RAB), masked to treatment assignment. Each adjudicator assigned the outcome as normal; impaired; or unable to classify using predetermined criteria. Infants who could not be classified were counted as lost to follow-up. An example of the use of the adjudication process is an infant seen at one year of age by the study assessment team, but who was not seen at two years in clinic, but detailed notes were reviewed from neurology assessment.
Statistical Analyses
Clinical and demographic characteristics of the treatment and placebo groups were compared using Chi-squared, t, and Wilcoxon rank sum tests as appropriate. Since there was no difference in survival between treatment groups, we assessed incidence of neurodevelopmental impairment in survivors. Linear and log-link Bernoulli models were used to assess the effects of treatment on continuous and binary neurodevelopmental outcomes. Fifty eight infants were excluded from the analyses: 39 who were lost to follow-up, and 19 in whom the outcome could not be classified by adjudication. Analyses of outcome were performed in two ways: (1) limited to the 411 of 497 (83%) survivors with complete outcome assessments at 2 years of age (physical exam, neurologic exam, Bayley MDI, Bayley PDI, Palisano Gross Motor Scales); and (2) complete cohort analyses that included 477 infants (411 complete outcome exams PLUS 66 infants in whom outcomes were determined by adjudication). We also used Bernoulli models to assess the differences in incidence of neurodevelopmental impairment according to clinical characteristics including age at trial entry, birth weight, sex, race, maternal education, neurological injury on head ultrasound (Grade III/IV hemorrhage, periventricular leukomalacia or hydrocephalus requiring a shunt), and extubation prior to study entry. Finally, we assessed modification of the effect of treatment on neurodevelopmental impairment by eight baseline factors. We also estimated treatment effects stratified by three post-randomization factors: exposure to more than 3 days of postnatal dexamethasone, and chronic lung disease at 36 and 40 weeks. These subgroup analyses, only one of which was preplanned, were conducted to assess evidence for adverse effects of treatment within high-risk subgroups. Wald tests of the equality of the treatment effects in each subgroup and its complement were used to evaluate effect modification. No adjustments for multiple comparisons were made. To account for correlation between outcomes for twins or triplets, all regressions were performed using generalized estimating equations with compound symmetric working correlation and robust standard errors. A two-sided P value of less than 0.05 was considered to indicate statistical significance.
The study was approved by the institutional review boards of each of the participating institutions. Written informed consent was obtained from the children's parents. An independent data safety and monitoring board reviewed the outcome results twice during the outcome period. Registered at ClinicalTrials.gov (NCT00000548).
Results
Of the 582 infants (294 iNO and 288 placebo) enrolled in the NO CLD Trial, 539 survived to hospital discharge, and 4 died later, leaving 535 survivors at two years of age (270 of 294 in the group given inhaled nitric oxide and 265 of 288 in the placebo group; Figure). Fifty-eight infants (27 in nitric group and 31 in placebo; 7.3% of survivors) were lost to follow-up. Complete assessment data were available for 411 of the 535 surviving infants (77%) and an additional 66 (12%) had adjudicated outcome data. Thus, 477 of 535 surviving infants (89%, range by center 82–100%) were evaluated.
Figure 1.
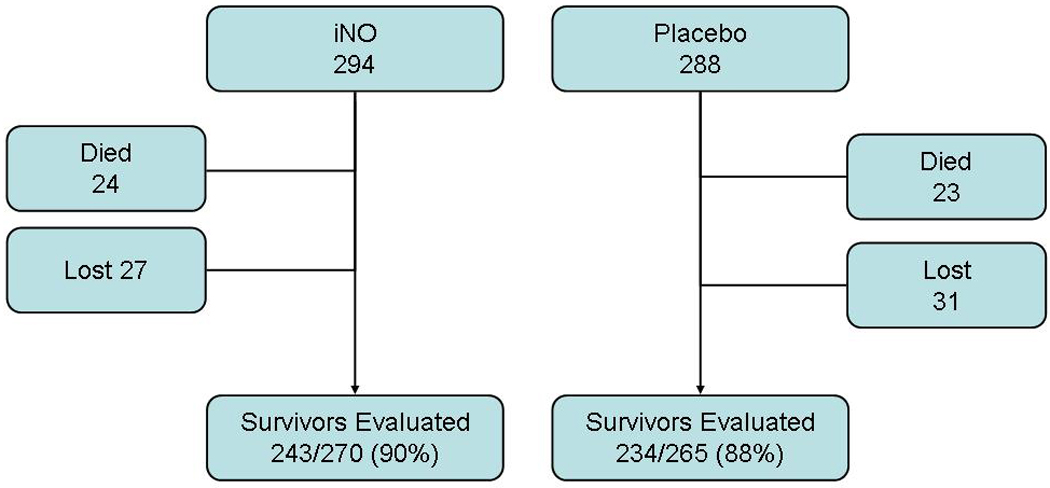
Flow Chart of Study Population
Compared with infants who were lost, infants followed had similar birth weights (764±158 grams versus 776±151; p=0.59), gestational ages (25.7±1.4 weeks versus 25.7±1.4; p=0.73) and percentage of males (54.7% versus 51.7%; p=0.67) but were more likely to be white (57.7% white versus 37.9%; p<0.01)
Characteristics of the Follow-up Cohort
In the follow-up cohort, 270 of the 535 patients who were alive at two years of age (50.5%) had received inhaled nitric oxide. Table I gives the baseline characteristics of the 477 infants evaluated. There were no significant differences between groups in gestational age, birth weight, sex, or the co-morbidities of prematurity, but treated infants were more likely to be white (Table I).7 The median age at entry was 16 days and the median respiratory severity score was 3.5 for both groups. There was no significant difference in the mean corrected age at follow-up: 25.2 months in the group given nitric oxide and 25.0 months in the placebo group. Maternal education beyond high school did not differ between groups (Table I).
Table 1.
Baseline Characteristics of the Infants and Mothers in the Follow-Up Cohort
| Characteristics | Inhaled | ||
|---|---|---|---|
| Nitric Oxide | Placebo | P value | |
| (N=243) | (N=234) | ||
| Infant Characteristics | |||
| Birth weight – g1 | 765 ± 163 | 764± 153 | 0.85 |
| 500–799g – no. (%) | 165 (68%) | 157 (67%) | |
| 800–1250g – no. (%) | 78 (32%) | 77 (33%) | |
| Gestational age – wk | 25.8 ± 1.4 | 25.7± 1.5 | 0.52 |
| Male sex – no. (%) | 126 (52%) | 135 (58%) | 0.20 |
| Antenatal corticosteroids – no. (%) | 203 (84%) | 189(81%) | 0.23 |
| Age at entry | |||
| Age 7–14 days at entry – no. (%) | 89 (37%) | 87 (37%) | |
| 0.90 | |||
| Days – median (IQR) | 17(13–19) | 16 (13–20) | |
| Respiratory severity score at entry2 | |||
| 0.40 | |||
| <3.5 | 136 (56%) | 122 (52%) | |
| ≥3.5 | 107 (44%) | 112(48%) | |
| Severe intraventricular hemorrhage prior to entry3(%) | 28(11.5%) | 36(15.4%) | 0.22 |
| Maternal Characteristics | |||
| Maternal Age mean (yr) | 28±7 | 28±6 | 0.50 |
| Maternal Education n, (%) | 0.32 | ||
| Less than high school | 37(15%) | 25(11%) | |
| High school or more | 167 (69%) | 167(71%) | |
| Unknown | 39(16%) | 42(18%) | |
| Mother’s race or ethnic group 4–no. (%) | 0.04 | ||
| White | 151(62%) | 124 (53%) | |
| Non-white | 92 (38%) | 110(47%) |
Plus-minus values are means ± SD.
The respiratory severity score was calculated as the fraction of inspired oxygen × mean airway pressure (in centimeters of water). The median value for infants in this trial was 3.5, which represents less severe disease.
Severe was defined as grade 3 or 4 intraventricular hemorrhage using the criteria of Papile.
Race or ethnic group was self-reported
Neurodevelopmental and Growth Outcomes
Patients treated with nitric oxide had similar risk of neurodevelopmental impairment (NDI) as those in the placebo group (Table II). Mean mental developmental indices and psychomotor indices were similar, as were the percentage of infants with indices exceeding 85 [MDI: 95 (39%) infants treated with nitric oxide and 83 (35%) infants receiving placebo; PDI: 75 (31%) infants with nitric oxide and 73 (31%) infants with placebo].
TABLE 2.
Neurodevelopmental and Growth Outcomes at 22–26 Months Postmenstrual Age*
| Nitric | Placebo | P value, or Relative Risk and 95% | |
|---|---|---|---|
| Oxide | confidence Intervals | ||
| Neurodevelopmental | 109 (45%) | 114(49%) | 0.92(0.75,1.12) |
| Impairment1 | |||
| Died (n, %) | 24 (8%) | 23 | 1.02(0.59,1.77) |
| (8%) | |||
| Mental Developmental Index2 | (n=210) | (n=214) | 0.35 |
| 81 ±20 | 79 ±22 | ||
| Psychomotor Developmental | (n=207) | (n=212) | 0.87 |
| Index2 | 76 ±20 | 77±21 | |
| Unable to crawl or walk3 | 15 (6%) | 12(5%) | 1.23(0.59 2.55) |
| Bilateral deafness4 | 8 (3%) | 3 (1%) | 2.56 (0.68,9.52) |
| Bilateral blindness | 9 (4%) | 9 (4%) | 0.97 (0.40,2.40) |
| Head Circumference (cm) | (n=217) | (n=205) | 0.22 |
| 47.6 ±2.1 | 47.8 ± 1.9 | ||
| Weight (kg) | (n=222) | (n = 204) | 0.65 |
| 11.4 ± 1.7 | 11.5 ± 1.7 | ||
| Length (cm) | (n=216) | (n=204) | 0.99 |
| 85.2 ±5.2 | 85.3 ±6.0 |
Neurodevelopmental impairment is defined as one or more of the following at 2 years of age: MDI < 70, PDI <70, unable to crawl or walk as defined by a Palisano gross motor level 2 or more, bilateral blindness, or bilateral deafness requiring amplification.
Bayley Scales of Infant Development II. Values are mean ± 1 standard deviation. 82 children with severe neurodevelopmental impairment could not be tested and were assigned a score of 49.
Palisano examination score of 2 or more.
Bilateral deafness requiring amplification
Outcomes were similar for all subcomponents of the neurodevelopmental assessment (Table II). To ensure that our conclusions were not sensitive to the inclusion of subjects who underwent the adjudication process, a second analysis was performed that included only the subset with complete in person 2-year follow-up evaluations. This analysis yielded similar results: inhaled nitric oxide 48% with NDI versus placebo 51% with NDI (relative risk 0.93; 95% confidence interval 0.76, 1.14). Growth variables were also examined at 2 years of age. There were no significant differences between treated and placebo infants for weight, length or head circumference (Table II).
In the main clinical trial, we observed an interaction between treatment and age at study entry (p<0.005) and race (p=0.06). Infants treated with nitric oxide beginning between 7 and 14 days of age (early), but not those treated between 15 and 21 days (late), had increased survival free of bronchopulmonary dysplasia (early treated 49.1 % versus placebo 27.0%; late treated 40.7% versus placebo 42.8%). We compared neurodevelopmental outcome for all infants based on early versus later trial entry and found no interaction with treatment (P = 0.84). Similarly, there was no significant treatment interaction in NDI for white versus non-white infants (P = 0.28), male versus female (P = 0.81), birth weight 500–799 versus 800–1250 (P = 0.28), RSS at entry <3.5 versus ≥3.5 (P = 0.28), prior extubation versus no prior extubation (P = 0.28), neurologic injury (P = 0.81), or maternal education <high school versus ≥high school (P = 0.54). Stratifying by two post-randomization factors, there was no treatment interaction in NDI for postnatal dexamethasone >3 days versus ≤3 days (P = 0.0.19) or bronchopulmonary dysplasia at 36 wk versus no bronchopulmonary dysplasia (P = 0.48).
Prediction of impairment by clinical characteristics
In an analysis of the entire study cohort of infants, we examined the influence of clinical characteristics on neurodevelopmental outcome (Table III). Using single-predictor models, the incidence of neurodevelopmental impairment was significantly increased in males versus females, in infants with more severe lung disease (Respiratory Severity Score >3.5 and no period of prior extubation), postnatal treatment with dexamethasone for more than 3 days, bronchopulmonary dysplasia at 36 or 40 weeks postmenstrual age, and infants with evidence of neurological injury on head ultrasound (severe intraventricular hemorrhage, periventricular leukomalacia or hydrocephalus requiring a shunt). In a multivariate model for neurodevelopmental impairment, neurologic injury and bronchopulmonary dysplasia at 40 weeks were independently associated with neurodevelopmental impairment (both p<0.0005, data not shown). As noted above, none of the clinical characteristics examined was found to modify the effect of treatment with nitric oxide on neurodevelopmental impairment.
Table 3.
Effect of Nitric Oxide on Neurodevelopmental Impairment at 2 years Overall and Stratified by Baseline and Post-Randomization Factors
| Characteristic | RR (95% CI) | P Value for Interaction1 |
|---|---|---|
| Overall n=477 | 0.92(0.75, 1.12) | 0.40 |
| Stratified by Baseline Factors | ||
| Entry 7–14 d, n=176 | 0.89(0.62, 1.28) | 0.84 |
| Entry 15–21 d, n=301 | 0.93 (0.74, 1.17) | |
| Female n=216 | 0.96(0.69, 1.34) | 0.81 |
| Male n=261 | 0.91 (0.72, 1.16) | |
| White n=275 | 1.02(0.78, 1.32) | 0.28 |
| Non-white n=202 | 0.81(0.60, 1.10) | |
| Birthweight 500–799 n=322 | 0.85(0.67, 1.08) | 0.28 |
| Birthweight 800–1250 n=155 | 1.07(0.76, 1.50) | |
| Respiratory Severity Score2< 3.5 n=258 | 0.93 (0.69, 1.26) | 0.98 |
| Respiratory Severity Score ≥ 3.5 n=219 | 0.93 (0.72, 1.19) | |
| Neurologic injury3 yes n=81 | 0.90(0.64, 1.25) | 0.81 |
| Neurologic injury no n=396 | 0.94(0.75, 1.18) | |
| Maternal Education <High School n=62 | 0.77(0.46, 1.30) | 0.54 |
| Maternal Education ≥High School n=334 | 0.92(0.72, 1.17) | |
| Prior extubation n= 128 | 0.75 (0.48, 1.19) | 0.28 |
| No prior extubation n=349 | 0.99(0.80, 1.23) | |
| Stratified by Post-Randomization Factors | ||
| Postnatal dexamethasone>3 days4 n=57 | 0.70(0.46, 1.06) | 0.19 |
| Postnatal dexamethasone ≤ 3 days n=420 | 0.96(0.77, 1.20) | |
| Bronchopulmonary Dysplasia5 at 36 wk n=270 | 0.90(0.72, 1.11) | 0.48 |
| No Bronchopulmonary Dysplasia at 36 wk n=207 | 1.05 (0.71, 1.56) | |
| BPDat40w n=188 | 0.93 (0.75, 1.16) | 0.61 |
| No BPD at 40 wk n=289 | 1.03 (0.74, 1.43) |
P-value for modification of treatment effect
Respiratory Severity Score (mean airway pressure multiplied by fraction of inspired oxygen) at trial entry. Score > 3.5 was predictive of 80% likelihood of oxygen treatment at 36 weeks of age.
Neurologic injury: occurrence of intraventricular hemorrhage, periventricular leukomalacia on head ultrasound or hydrocephalus requiring a shunt.
Infant was treated with a course of postnatal dexamethasone for more than 3 days after enrollment.
Infant was discharged or off all respiratory support at 36 or 40 weeks postmenstrual age.
Discussion
We assessed neurodevelopment and growth at 2 years of age for premature infants who participated in the NO CLD Trial for prevention of bronchopulmonary dysplasia. Previously, we reported benefit of inhaled nitric oxide treatment for survival without bronchopulmonary dysplasia at 36 weeks, clinical status at 40 weeks (discharged or off respiratory support) and requirement for respiratory medications during the first year.7,8,10 With regard to the initial safety of therapy, no adverse effects on the co-morbidities of prematurity or biomarkers of inflammation and oxidative stress were found.11–13 Because many premature infants have impaired neurological development, longer term follow-up is important to evaluate the safety of this new therapy. Bronchopulmonary dysplasia is known to be associated with poor neurodevelopmental outcome18–21 and, in this trial as expected, infants who had bronchopulmonary dysplasia were significantly more likely to have neurodevelopmental impairment (56 versus 35%; p <0.001). However, despite a significant reduction in the incidence of bronchopulmonary dysplasia in treated infants, we did not find improved neurodevelopment at 2 years. Infants treated with inhaled nitric oxide had a similar rate of impairment for each component of the developmental assessment compared with those receiving placebo. These data suggest that nitric oxide has no independent adverse impact on neurological development and is a safe treatment to reduce bronchopulmonary dysplasia.
In the single-center study by Schreiber, infants treated with nitric oxide had significantly improved cognitive outcomes and reduced neurodevelopmental impairment at 2 years of age.2 These infants were enrolled at approximately 13 hours of age, treated initially with 10 ppm and weaned to 5 ppm, iNO for a maximum of 7 days. Kinsella et al treated intubated infants with 5 ppm in their trial but results for neurodevelopmental outcome are not yet reported.3 In the NICHD Network Trial, severely ill newborn premature infants received a relatively brief exposure to nitric oxide and treatment affected neither the incidence of BPD nor 2-year neurodevelopmental outcome.4,5 In our study, infants were enrolled at a median age of 16 days and treated for 24 days resulting in a higher cumulative dose and time of exposure to nitric oxide. The failure to observe significantly improved neurodevelopmental outcome in treated infants of the NO CLD Trial, in spite of a reduction in bronchopulmonary dysplasia, may reflect, in part, the later initiation of therapy. In the Schreiber1 and Kinsella3 trials, which reported less intraventricular hemorrhage in treated infants, nitric oxide therapy began prior to the time that most hemorrhages occur. By contrast, entry for the NO CLD Trial was between 7 and 21 days which may be too late to impact intraventricular hemorrhage.
The lack of observed benefit of iNO on neurodevelopmental impairment in this trial may also reflect limited impact of iNO to reduce episodes of transient respiratory deteriorations or inflammation that may injure the brain. During iNO administration there was no significant effect on the severity of lung disease as indicated by respiratory severity scores or time of extubation, consistent with the proposed mechanism for iNO to promote repair and growth of lung parenchyma over time rather than acutely affecting pulmonary vascular tone. Thus, our results may represent an “uncoupling” of BPD from NDI for iNO-treated infants. It is also possible that there was inadequate power to detect a small but significant effect of iNO on NDI; the 95% confidence intervals include the possibility of an important improvement (25%) or a small worsening (11%) of NDI. Many factors contribute to later neurodevelopmental impairment in the premature infant including lower gestational age and birth weight, male sex, perinatal asphyxia, intraventricular hemorrhage, periventricular leukomalacia, postnatal dexamethasone treatment, and bronchopulmonary dysplasia.18 Our analysis of these characteristics among surviving infants (Table III) confirms these associations and in addition shows that relatively early indicators of severe lung disease (e.g., continued ventilation at trial entry and high Respiratory Severity Score at entry) are strongly associated with poor neurodevelopmental outcome. Treatment with dexamethasone was also associated with neurodevelopmental impairment. However, no evidence for modification of the effects of treatment was found for any clinical characteristics examined. Thus, our subgroup analyses gave no persuasive evidence that iNO therapy as used in the NO CLD Trial is associated with adverse events within any subgroup.
About 12% of the surviving infants were either lost to follow-up or could not be classified. This group differed significantly from the followed group with more non-white and early trial entry infants. Because these characteristics favor a better response to nitric oxide for prevention of BPD, our follow-up data may be somewhat biased against a positive effect of treatment and may slightly over-estimate the incidence of neurodevelopmental impairment in this population.
In the main trial outputation, a cluster randomization analysis method was used to adjust for the known non-independent effect of the enrollment of multiples with highly correlated outcomes in biologically determined diseases such as BPD. In this followup analysis a different clustering methodology of generalized estimating equations was used as this is methodologically less burdensome yet yields similar outcomes. Our group has published comparisons of these two methods.22
We conclude that exposure to 24 days of inhaled nitric oxide treatment initiated at 20 ppm between 7 and 21 days in ventilated preterm infants is both safe and effective with improved survival free of bronchopulmonary dysplasia and no adverse effects on growth or neurodevelopmental status at 2 years of age.
Acknowledgments
We are indebted to the families and babies who participated in the trial; to the nurses, respiratory therapists, residents, fellows and attendings who made the trial possible; to National Heart Lung and Blood Institute for funding; and to INO Therapeutics (now Ikaria) for providing study equipment and gas.
Supported by grants (U01-HL62514, P50-HL56401, P30- HD26979, MRDDRC-P30, and HD26979) from the National Institutes of Health and grants (M01-RR00240, M01-RR00084, M01-RR00425, M01-RR001271, M01-RR00064, and M01-RR00080) from the General Clinical Research Centers Program. Inhaled nitric oxide and the delivery system for the initial trial as well as support for completion and analysis of follow-up data were provided by INO Therapeutics (now IKARIA). The company was not involved in study design, safety monitoring, data analysis, data interpretation, or manuscript preparation. A.M.H. was supported by a Career Development award (NICHD K23 HD056299). R.A.B. received unrestricted grant support for additional biostatistical analyses of the trial from iNO Therapeutics (now IKARIA). M.C.W. received support from iNO Therapeutics to fund the research nurse coordinator for follow-up of this trial.
APPENDIX
In addition to the authors, the following members of the NO CLD Study Group participated in this study:
Study Centers
Alta Bates Summit Medical Center, Berkeley, Calif., and Children’s Hospital and Research Center Oakland, Oakland, CA - D. Durand, J Asselin, L. Pacello, R. Ratcliff, V. Daly, A. Espinoza
Brigham and Women’s Hospital and Children’s Hospital, Boston, MA - E. Eichenwald, K. Puopolo, A. Hansen, T. Cimini, D. Beadles, C. Pantano
Cedars-Sinai Medical Center, Los Angeles, CA - A Puri, W. Bunuan, A. Verne, J. Raber, S. Sehgal
Children’s Hospital of Philadelphia and the Hospital of the University of Pennsylvania, Philadelphia, PA - J. Merrill, L. Corcoran, J. Fricko, S. Zirin, A. Hedgman, K. Kelly, B. Hubble, K. Mooney, L. Brown, R. Scarborough, J. Bernbaum, H. Hurt
Children’s Hospital and Regional Medical Center and University of Washington Medical Center, Seattle, WA - D. Mayock, C. Gleason, S. Jacques, H. Meo, F. Bennett
Children’s Mercy Hospital, Kansas City, MO. - I. Ekekezie, C. Castor, P. Johnson, K. Meinert, D. Taylor, H. Kilbride
Columbus Children’s Hospital, Columbus, OH - S. Welty, S. Farley, T. Preston, C. Timan
Kosair Children’s Hospital, Louisville, KY - D. Stewart, S. Daugherty, J. Foos, K. Sheeley, S. Polston, S. Wilkerson
North Shore–Long Island Jewish Health System and Schneider Children’s Hospital, New Hyde Park, NY - S. Courtney, A. Steele, D. Potak, B. Wilkens, S. Pollard, A. Adesman
Primary Children’s Medical Center and the University of Utah Hospital and Clinics, Salt Lake City, UT - D. Null, R. Milley, S. Baker, L. Cole, K. Hillier, L. Hiatt, A. Bodnar
Rainbow Babies & Children’s Hospital, Case Western Reserve University, Cleveland, OH - M.Tracy, J. Di Fiore, M. Hack, D. Wilson-Costello
Wolfson Children’s Hospital and Shands Jacksonville Hospital, Jacksonville, FL - M.Hudak, S. Osbeck, A. Kellum, L. Hogans, D. Childers
University of California at San Francisco Medical Center, San Francisco, CA - R. Phibbs, S., Sehring, J. Imamura-Ching, N. Newton, C. Kelly, R. Piecuch
Westchester Medical Center, Valhalla, NY - S. Golembek, L. Parton, N. Dweck, J. Weissleder, J. Kase
Data Coordinating Center - X. Luan, T. Alvarado-Taylor, J. Valliyil, M. Davis, K. Gibbs
Data and Safety Monitoring Committee — Vanderbilt University School of Medicine, Nashville, TN - T. Hazinski (chair, deceased)
New York Academy of Medicine, New York, NY - A. Fleischman; Children’s Hospital of Buffalo, Buffalo, NY - F. Morin; Women and Infants Hospital, Brown University School of Medicine, Providence, RI - B. Vohr; Emmes, Rockville, MD - S. Carter; National Heart, Lung, and Blood Institute, Bethesda, MD - M. Berberich, N. Geller, C. Hunt, G. Zheng
Clinical Steering Committee — R. Ballard, W. Truog, R. Martin, P. Ballard, J. Merrill, M. Walsh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no other conflicts of interest.
Registered at ClinicalTrials.gov (NCT00000548).
REFERENCES
- 1.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349:2099–2107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 2.Mestan KK, Marks JD, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. N Engl J Med. 2005;353:23–32. doi: 10.1056/NEJMoa043514. [DOI] [PubMed] [Google Scholar]
- 3.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 4.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med. 2005;353:13–22. doi: 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 5.Hintz SR, Van Meurs KP, Perritt R, Poole WK, Das A, Stevenson DK, et al. Neurodevelopmental outcomes of premature infants with severe respiratory failure enrolled in a randomized controlled trial of inhaled nitric oxide. J Pediatr. 2007;151:16–22. doi: 10.1016/j.jpeds.2007.03.017. 22.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huddy CL, Bennett CC, Hardy P, et al. The INNOVO multicentre randomized controlled trial: neonatal ventilation with inhaled nitric oxide versus ventilatory support without nitric oxide for severe respiratory failure in preterm infants: follow up at 4–5 years. Arch Dis Child Fetal Neonatal Ed. 2008;93:F430–F435. doi: 10.1136/adc.2007.129353. [DOI] [PubMed] [Google Scholar]
- 7.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 8.Ballard RA. Inhaled nitric oxide in preterm infants – correction. N Engl J Med. 2007;357:1444–1445. doi: 10.1056/NEJMc076350. [DOI] [PubMed] [Google Scholar]
- 9.Zupancic J, Hibbs A, Palermo L, Martin R, Truog W, Cnaan A, et al. Economic Evaluation of Inhaled Nitric Oxide in Ventilated Preterm Infants; Pediatrics. 2009 doi: 10.1542/peds.2008-3214. in press. [DOI] [PubMed] [Google Scholar]
- 10.Hibbs AM, Walsh MC, Martin RJ, Truog WE, Lorch SA, Alessandrini E, et al. One-year respiratory outcomes of preterm infants enrolled in the Nitric Oxide [to prevent] Chronic Lung Disease Trial. J Pediatr. 2008;153:525–529. doi: 10.1016/j.jpeds.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truog WE, Ballard PL, Norbert M, Golombek S, Savani RC, Merrill JD, et al. Inflammatory markers and mediators in tracheal fluid of premature infants treated with inhaled nitric oxide. Pediatrics. 2007;119:670–678. doi: 10.1542/peds.2006-2683. [DOI] [PubMed] [Google Scholar]
- 12.Ballard PL, Truog WE, Merrill JD, Gow A, Posencheg M, Golombek SG, et al. Plasma biomarkers of oxidative stress: relationship to lung disease and inhaled nitric oxide therapy in premature infants. Pediatrics. 2008;121:555–561. doi: 10.1542/peds.2007-2479. [DOI] [PubMed] [Google Scholar]
- 13.Ballard PL, Merrill JD, Truog WE, Godinez RI, Godinez MH, McDevitt TM, et al. Surfactant function and composition in premature infants treated with inhaled nitric oxide. Pediatrics. 2007;120:346–353. doi: 10.1542/peds.2007-0095. [DOI] [PubMed] [Google Scholar]
- 14.Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr. 2001;1:1. doi: 10.1186/1471-2431-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobaly K, Schluchter M, Minich N, Friedman H, Taylor HG, Wilson-Costello D, Hack M. Outcomes of extremely low birth weight [<1 kg] and extremely low gestational age [<28 weeks] infants with bronchopulmonary dysplasia: effects of practice changes in 2000 to 2003. Pediatrics. 2008;121:73–81. doi: 10.1542/peds.2007-1444. [DOI] [PubMed] [Google Scholar]
- 16.Bayley N. Bayley Scales of Infant Development. 2nd. San Antonio, TX: Psychological Corp; 1993. [Google Scholar]
- 17.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF. Trial of Indomethacin Prophylaxis in Preterms (TIPP) Investigators. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289:1124–1129. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- 18.Majnemer A, Riley P, Shevell M, Birnbaum R, Greenstone H, Coates AL. Severe bronchopulmonary dysplasia increases risk for later neurological and motor sequelae in preterm survivors. Dev Med Child Neurol. 2000;42:53–60. doi: 10.1017/s001216220000013x. [DOI] [PubMed] [Google Scholar]
- 19.Palta M, Sadek-Badawi M, Evans M, Weinstein MR, McGuinnes G. Functional assessment of a multicenter very low-birth-weight cohort at age 5 years: Newborn Lung Project. Arch Pediatr Adolesc Med. 2000;154:23–30. [PubMed] [Google Scholar]
- 20.Hughes CA, O’Gorman LA, Shyr Y, Schork MA, Bozynski ME, McCormick MC. Cognitive performance at school age of very low birth weight infants with bronchopulmonary dysplasia. J Dev Behav Pediatr. 1999;20:1–8. doi: 10.1097/00004703-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Skidmore MD, Rivers A, Hack M. Increased risk of cerebral palsy among very low-birthweight infants with chronic lung disease. Dev Med Child Neurol. 1990;32:325–332. doi: 10.1111/j.1469-8749.1990.tb16944.x. [DOI] [PubMed] [Google Scholar]
- 22.Hibbs AM, Black D, Palermo L, Cnaan A, Truog W, Martin RJ, et al. The Importance of accounting for Multiple Births in Neonatal and Perinatal Studies: A Case Study and Systematic Review. J Pediatrics. 2009 doi: 10.1016/j.jpeds.2009.08.049. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


