Abstract
Crosstalk between cells in the blood vessel wall is vital to normal vascular function and is perturbed in diseases such as atherosclerosis and hypertension. Perivascular adipocytes reside at the adventitial border of blood vessels but until recently were virtually ignored in studies of vascular function. However, perivascular adipocytes have been demonstrated to be powerful endocrine cells capable of responding to metabolic cues and transducing signals to adjacent blood vessels. Accordingly, crosstalk between perivascular adipose tissue (PVAT) and blood vessels is now being intensely examined. Emerging evidence suggests that PVAT regulates vascular function through numerous mechanisms, but evidence to date suggests modulation of three key aspects that are the focus of this review: inflammation, vasoreactivity, and smooth muscle cell proliferation.
Characteristics of Perivascular Adipocytes
To begin to understand how PVAT potentially regulates vascular function, it is necessary to review the characteristics of this adipose depot. First, perivascular adipocytes are not separated from the blood vessel wall by a fascial layer or elastic lamina and actually encroach into the outer adventitial region [1,2]. The absence of an anatomic barrier suggests that mediators secreted by perivascular adipocytes can readily gain access into the blood vessel wall. Also, interspersed within the PVAT is the vasa vasorum, which proliferates during vascular inflammation and injury [3–5] and may transmit mediators released by PVAT to the inner vasculature. Second, perivascular adipocytes are morphologically and functionally distinct from adipocytes of other regional adipose depots, which likely have a direct bearing on vascular cell crosstalk [1,6]. Compared to adipocytes from other depots, perivascular adipocytes surrounding human coronary arteries are more heterogeneous in shape and smaller in size and exhibit a reduced state of adipogenic differentiation. They display a distinct profile of developmental and pattern-forming genes, as compared with subcutaneous and perirenal adipocytes isolated from the same subjects [1]. These observations are consistent with recent reports that adipocytes from various adipose depots are derived from discrete precursor cells [7,8].
Our analysis of gene expression profiles indicated that the human perivascular adipocytes surrounding coronary arteries are white, rather than brown, adipocytes. In contrast, Sacks et al. reported that epicardial adipose tissues harvested from the origin of human right coronary arteries express higher levels of brown adipocyte-related genes as compared with other regional adipose depots [9]. Also, Police et al. reported that perivascular adipocytes surrounding the thoracic aorta of mice exhibit morphological features of brown adipocytes, whereas adipocytes surrounding the abdominal aorta are predominately unilocular white adipocytes [10]. The heterogeneity of perivascular adipocytes is further demonstrated by a report showing that in rats, periaortic adipocytes are much smaller in size as compared with perimesenteric adipocytes [6]. Thus, it appears that perivascular adipocytes surrounding different blood vessels are biologically and functionally diverse, similar to observations of endothelial cells and SMC residing in different vascular beds. Whether this diversity reflects derivation of perivascular adipocytes from multiple precursor cell pools, or from a single precursor that adapts to different vascular environments, remains to be determined.
Role of PVAT in Inflammatory Crosstalk
Secretion of mediators that regulate inflammation is a characteristic feature of adipocytes. These mediators can be divided into two broad, interacting categories: adipokines and cytokines (summarized in Table). Within both categories, individual mediators can be classified as pro- or anti-inflammatory, although the distinction is often blurred. A primary function of pro-inflammatory cytokines is to recruit inflammatory cells, which are also capable of secreting cytokines and certain adipokines. Moreover, crosstalk between inflammatory cells and adipocytes plays a critical role in regulating adipose depot functions.
Table.
Mediators released from PVAT, along with their functions and primary cellular sources
| Mediator | Function | Primary Source | References |
|---|---|---|---|
| Adiponectin | ↓ TNF- α, IFNγ, IL-6, NF-κB, phagocytosis, endothelial adhesion molecules ↑ IL-10, IL-1RA |
Adipocytes | [11] |
| Adrenomedullin | ↓ inflammation | Adipocytes | [21] |
| IL-6 | ↓ adiponectin secretion ↓ lipoprotein lipase activity ↑ lipolysis, suppressor of cytokine signaling type-3 (SOCS-3) |
Macrophages, inflammatory cells, fibroblasts, endothelial cells, adipocytes |
[13] |
| IL-8 | ↑ Chemotaxis of neutrophils, monocytes, T-lymphocytes, ↑ reactive oxygen species (ROS) |
Adipocytes, inflammatory cells |
[1,46–49] |
| Leptin | ↑ TNF-α, IL-6, IL-12, ROS ↑ macrophage and monocyte activation, ↑ smooth muscle cell proliferation and migration |
Adipocytes | [11,41] |
| MCP-1 | ↑ Chemotaxis and transendothelial migration of monocytes |
Adipocytes, inflammatory cells |
[1,45,46] |
| PAI-1 | ↓ plasminogen activation | Platelets, vascular endothelium, adipocytes |
[13] |
| Resistin | ↑ Endothelial adhesion molecules, Pentraxin-3, TNF- α, IL-6, IL-12, IL-1β , NF-κB |
Macrophages, adipocytes |
[11,12] |
| Visfatin | ↑ IL-6, IL-8, TNF-α ↓ apoptosis of neutrophils ↑ smooth muscle cell proliferation and migration |
Adipocytes, macrophages |
[11,41] |
Leptin was one of the first adipokines to be discovered and has been shown to function primarily as a pro-inflammatory mediator by increasing production of the pro-inflammatory cytokines tumor-necrosis factor-α (TNF-α), IL-6 and IL-12, enhancing macrophage phagocytosis, and inducing the activation, proliferation, and migration of monocytes [11]. Adiponectin is the prototypical anti-inflammatory adipokine secreted by healthy adipose tissue. It functions to suppress the synthesis of TNF-α and interferon-γ (IFNγ), and to induce the production of anti-inflammatory cytokines such as IL-10 and IL-1 receptor antagonist (IL-1RA) [11]. Resistin, another pro-inflammatory adipokine, has effects antagonistic to that of adiponectin [12]. It is secreted by both adipocytes and macrophages and is thought to promote insulin resistance and atherosclerosis. Plasminogen activator inhibitor-1 (PAI-1), a fibrinolysis inhibitor, is up-regulated primarily in the visceral adipose tissue during obesity and insulin resistance and might contribute to increased atherothrombosis under these conditions [13]. Similarly, obesity is associated with increased release of MCP-1, RANTES, and other chemokines/cytokines from adipose tissues that promote inflammatory cell infiltration and enhance inflammation. The balance between leptin, adiponectin, resistin, and other adipokines, as well as the pro- and anti-inflammatory cytokines, governs the overall extent of inflammation within adipose depots [11]. Inflammation, in turn, triggers insulin resistance, particularly in visceral adipose tissues, which is a key component of obesity-related metabolic disease. On the other hand, in lean states, adipose tissue inflammation is kept at a low level, and insulin sensitivity is maintained.
A number of studies have examined the extent of inflammation in PVAT obtained from humans. Inflammatory cell infiltration was reported to be markedly increased in PVAT surrounding atherosclerotic human aorta as compared with non-diseased aorta [14]. Moreover, inflammatory gene expression was shown to be upregulated, and expression of adiponectin downregulated, in PVAT surrounding diseased human coronary arteries [15–17]. In animal studies, balloon injury of porcine coronary arteries led to inflammation extending into the adventitia and PVAT [18]. Also, in aged mice fed a high fat diet, adventitial T cell accumulation far exceeded that observed in the intimal layer of the abdominal aorta [19]. These observational studies indicate that PVAT is highly inflamed in the setting of vascular injury and atherosclerosis. However, the extent to which perivascular adipocytes are active versus passive participants in this process remains to be established.
To address this latter question, Chatterjee et al. examined inflammatory responses in perivascular adipocytes cultured from PVAT of subjects without underlying atherosclerotic disease [1]. Expression and secretion of adiponectin by perivascular adipocytes was markedly reduced, while secretion of pro-inflammatory IL-6 and IL-8 was increased, as compared with subcutaneous and perirenal adipocytes cultured from the same subjects. Moreover, secretion of MCP-1, a key pathogenic cytokine in atherosclerosis [20], was increased by up to 40-fold in perivascular as compared with perirenal and subcutaneous adipocytes. In fact, perivascular adipocytes released substantially more MCP-1 as compared with omental adipocytes. On the other hand, Silaghi et al. reported that adrenomedullin, a potent anti-inflammatory factor, is synthesized by PVAT, suggesting a protective role on the adjacent vasculature [21]. Thus, while PVAT releases large quantities of pro-inflammatory cytokines, it also releases anti-inflammatory cytokines that could counterbalance inflammation, depending on the physiological context [22].
Obesity is a common denominator in dysfunction of adipose tissues. In obese humans, the thickness of epicardial fat was positively correlated with insulin resistance, suggesting local expansion of this depot in the setting of obesity-related disease [23]. Moreover, several recent studies have demonstrated a positive correlation between the volume of epicardial adipose tissues and the presence and/or extent of underlying coronary disease [24–27]. Recently, obesity in mice was demonstrated to lead to increased macrophage infiltration and cytokine expression in PVAT surrounding the abdominal aorta, and to enhanced angiotensin II-induced abdominal aortic aneurysm formation [11]. PVAT from obese animals was also shown to activate CD8(+) T cells and to promote the recruitment and activation of macrophages [28]. Hosogai et al. demonstrated that the hypoperfusion and hypoxia characteristic of white adipose tissues in obesity underlies the dysregulated production of adipokines and cytokines in PVAT of obese mice [29]. These studies suggest that obesity-related states may directly impact inflammation in PVAT. In support of this notion, feeding mice a high-fat diet for just two weeks augmented adipocyte dysfunction and increased expression of MIP1α, a pro-inflammatory cytokine, in PVAT [1]. Infiltration of macrophages into PVAT was not detected at this early time point, suggesting that the perivascular adipocytes responded to high-fat feeding and linked the metabolic signals to inflammation of the blood vessel wall. Collectively, these observations suggest that in obesity, PVAT predominately exerts a pro-inflammatory influence that contributes to vascular disease.
Exactly how PVAT communicates with the surrounding vasculature has not been well-established. Mediators released from PVAT could diffuse across the arterial wall to interact with cells in each layer of the vessel in a paracrine manner [2]. In this regard, Nishumara and colleagues examined leukocyte adhesion in lean and obese adipose tissue in mice by intravital microscopy [30]. They observed increased leukocyte-endothelial cell-platelet interactions in the vasculature of obese visceral adipose tissue compared to adipose tissue of lean mice. These endothelial-leukocyte interactions are the initial steps in the leukocyte adhesion cascade, a hallmark of inflammation. Whether such interactions are restricted to the microvasculature and/or extend to conduit vessels remains to be determined. Conversely, adipokines might be released from perivascular adiopocytes directly into the vasa vasorum and transported downstream into the arterial wall, thereby modulating vascular function [31].
Paracrine Effects of PVAT on Vasoreactivity
The initial studies demonstrating that PVAT is biologically active were centered on modulation of vasoreactivity. Under normal physiological conditions, healthy perivascular adipocytes produce an as yet unidentified factor termed adipocyte-derived relaxing factor (ADRF), which activates SMC K+ channels and inhibits vasoconstriction [32]. Recently PVAT was reported to inhibit contraction of aortic rings prepared from the rat thoracic aorta [33]. Interestingly, the supernatant from donor aortic rings with intact PVAT induced relaxation in vessels with intact endothelium. When the PVAT was stripped from the donor rings, or the endothelium was removed from the recipient aortic rings, this effect was lost. Finally, the supernatant from incubated donor aortic rings with stripped PVAT but intact endothelium did not induce vasorelaxation. These results indicate that the origin of the transferable relaxing factor (i.e. ADRF) is the PVAT, but its activity is dependent upon an intact endothelium. The authors further demonstrated that this effect depended on endothelial nitric oxide release and subsequent calcium-dependent K+ channel activation in SMC. Lastly, the authors described an endothelial-independent mechanism whereby PVAT-derived reactive oxygen species (ROS) can directly inhibit SMC contraction by activating soluble guanylyl cylcase. Thus, endothelial cells and perivascular adipocytes can determine vascular tone both separately and in concert with each other.
Although ADRF has primarily been detected in conduit vessels, similar PVAT-dependent vasorelaxation responses have been observed in rat mesenteric microvessels [34]. The mechanism of action of ADRF in the rat mesenteric microvasculature was determined to be activation of delayed rectifier K+ channels. Thus, the influence of ADRF extends to microvessels that control arterial resistance. Moreover, the quantity of mesenteric adipose tissue, and its counter-regulatory influence on vasoconstriction, was reported to be diminished in spontaneously-hypertensive rats, suggesting that PVAT may play a role in regulating blood pressure [35].
While most studies performed in rodents indicate that PVAT produces vasorelaxing factors, others have reported the production of vasoconstricting factors. For example, Gao et al. reported that electrical field stimulation of rat mesenteric artery rings produced greater contractile responses in the presence of PVAT [36]. The PVAT-dependent contraction was determined to be mediated by ROS and was blocked by inhibitors of NADPH oxidase. Also, studies of PVAT in dogs indicate that a contractile factor is produced that inhibits nitric oxide bioactivity [37]. The mechanism of contraction was determined to be protein kinase C-dependent phosphorylation of endothelial nitric oxide synthase [38]. As is the case for ADRF, the identity of these contractile factors, known as adipocyte-derived constricting factors (ADCF), has yet to be established.
A recent study in humans confirmed that in healthy individuals, PVAT increases nitric oxide bioavailability and inhibits vascular contraction [39]. This dilator effect was lost in obese patients with metabolic syndrome, and was accompanied by an increase in inflammation and adipocyte size. The obese phenotype could be reproduced by adding the inflammatory cytokines TNF-α or IL-6 to the PVAT around healthy blood vessels, which in turn could be blocked by free radical scavengers or cytokine antagonists. Finally, low oxygen, or hypoxia stimulated an inflammatory phenotype in PVAT and a loss of the anti-contractile properties. Hypoxia is a prominent feature of adipose tissue in obese individuals and is thought to cause adipocyte dysfunction and tissue inflammation [29]. The effects of hypoxia could be mitigated by free radical scavengers and cytokine inhibition, further supporting a prominent role for PVAT-derived ROS and inflammatory cytokines in adversely modulating vascular tone. Thus, factors produced by PVAT can elicit relaxation or constriction of the adjacent vasculature, dependent upon species, vascular bed, and/or pathophysiologic state.
Effects of PVAT on SMC Proliferation
In addition to modulating vascular tone, conditioned media from perivascular adipocytes can stimulate SMC proliferation in culture, supporting a paracrine role for perivascular adipocytes in modulating vascular SMC growth [40]. PVAT secretes adipokines such as visfatin and leptin that are potent stimulators of SMC proliferation and migration [41]. Further animal studies are required to establish the extent to which PVAT in vivo modulates SMC phenotype. However, a recent study in humans found that the carotid extra-media thickness (EMT) – which includes the arterial adventitia but not the intima or media – was increased in diabetes and dyslipidemia [42]. The EMT is comprised of a significant amount of adipose tissue, both within and surrounding the adventitia, suggesting that PVAT is expanding in response to metabolic stimuli in obese individuals [43]. EMT correlated with intima-media thickness (IMT), suggesting that PVAT may contribute to SMC migration and proliferation in human atherosclerotic blood vessels. Thus, PVAT is an endocrine tissue that potentially integrates metabolic signals with SMC proliferation in vascular disease.
To test the local influence of adipose tissue in regulating SMC proliferation, a novel experimental model of transplanting adipose tissue to the blood vessel wall was developed [44]. In this study, the investigators transplanted subcutaneous fat harvested from donor mice fed a normal or high-fat, high-sucrose diet to the femoral artery immediately following endovascular injury. Fat transplanted from mice fed a normal diet inhibited neointimal formation after injury, whereas fat transplanted from high fat-, high sucrose-fed mice had no effect [44]. Transplanting visceral (epididymal) fat failed to attenuate neointimal formation. These observations offer direct evidence that adipose tissue associated with the blood vessel wall can regulate SMC proliferation in vivo. It is important to point out, however, that the modulatory influences were observed with subcutaneous adipose tissues. Since perivascular adipose tissue is phenotypically distinct from subcutaneous adipose tissue [1], these observations cannot be directly extrapolated to the normal in vivo blood vessel. Additional experiments using this novel transplantation model should provide insight into how PVAT regulates the SMC response to injury.
Conclusions
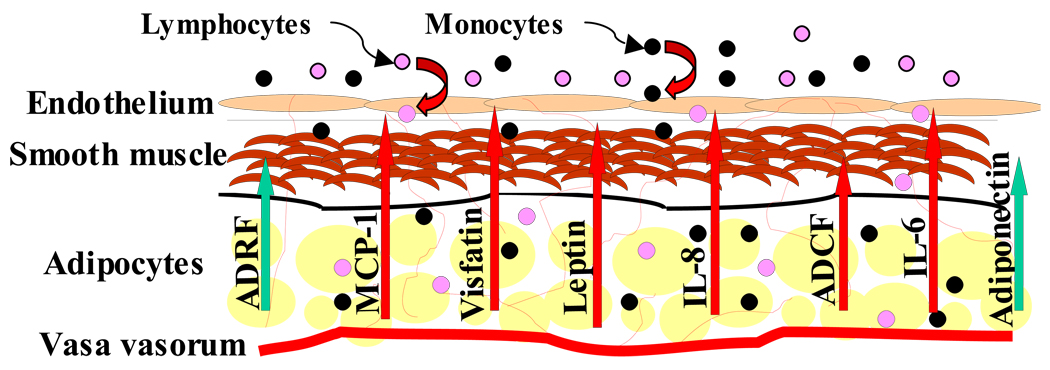
Crosstalk between PVAT and cells of the blood vessel wall likely occurs on multiple levels. While some functions of PVAT may contribute to vascular homeostasis, it appears that PVAT primarily plays a pathological role in vascular disease (Figure). Substantial published data support a role for PVAT in modulating vascular inflammation, vasoreactivity and SMC proliferation. A number of issues related to the biology of PVAT remain unresolved. For example, the crosstalk between PVAT and blood vessels may also occur in the reverse direction; i.e., vascular dysfunction could potentially modulate perivascular adipocyte phenotype. Also, inflammatory cells or perhaps adipose-derived stem cells might traffic from PVAT into the vessel wall. Such trafficking could trigger inflammation, angiogenesis, neointimal formation, or perhaps vascular repair mechanisms. Testing these hypotheses will require systematic studies of adipose tissue transplantation to the blood vessel wall in the setting of models of vascular injury and atherosclerosis. Finally, since perivascular adipose tissue likely contributes to vascular disease, it might prove to be a useful target for treatment of atherosclerosis or post-angioplasty restenosis. For example, targeting mediators such as MCP-1, visfatin and or leptin in perivascular adipose tissue might decrease lesional inflammation and SMC proliferation, respectively. Recently developed adventitial delivery catheters potentially could facilitate this form of local perivascular therapy. Also, novel molecular imaging techniques designed to characterize atherosclerotic plaque might be employed to image the perivascular adipose tissue, thereby providing a tool to assess cardiovascular risk based on the molecular characteristics of this unique adipose depot.
Figure.
Proposed model of crosstalk between perivascular adipose tissue and blood vessels. Mediators that primarily promote vascular health and homeostasis are indicated by green arrows, whereas those that induce vascular disease are indicated by straight red arrows. Chemotactic cytokines released by PVAT recruit monocytes and lymphocytes to the blood vessel wall (curved red arrows). Abbreviations: ADRF, adipocyte-derived relaxing factor; ADCF, adipocyte-derived constricting factor.
Acknowledgements
Dr. Weintraub is funded by HL076684 and HL62984 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. This paper highlighted the unique characteristics of human perivascular adipocytes and demonstrated the influence of high fat feeding on PVAT in mice.
- 2.Iacobellis G, Sharma AM. Epicardial adipose tissue as new cardio-metabolic risk marker and potential therapeutic target in the metabolic syndrome. Curr Pharm Des. 2007;13:2180–2184. doi: 10.2174/138161207781039670. [DOI] [PubMed] [Google Scholar]
- 3.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Jr, Schwartz RS, Lerman A. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest. 1998;101:1551–1556. doi: 10.1172/JCI1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon HM, Sangiorgi G, Ritman EL, Lerman A, McKenna C, Virmani R, Edwards WD, Holmes DR, Schwartz RS. Adventitial vasa vasorum in balloon-injured coronary arteries: visualization and quantitation by a microscopic three-dimensional computed tomography technique. J Am Coll Cardiol. 1998;32:2072–2079. doi: 10.1016/s0735-1097(98)00482-3. [DOI] [PubMed] [Google Scholar]
- 5.Gössl M, Herrmann J, Tang H, Versari D, Galili O, Mannheim D, Rajkumar SV, Lerman LO, Lerman A. Prevention of vasa vasorum neovascularization attenuates early neointima formation in experimental hypercholesterolemia. Basic Res Cardiol. 2009;104:695–706. doi: 10.1007/s00395-009-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gálvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, Ruiz-Gayo M, Huber M, Wehland M, Kreutz R, Fernandez-Alfonso MS. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol. 2008;197:55–64. doi: 10.1677/JOE-07-0284. [DOI] [PubMed] [Google Scholar]
- 7.Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 9.Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, Karas J, Optican R, Bahouth SW, Garrett E, Wolf RY, Carter RA, Robbins T, Wolford D, Samaha J. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94:3611–3615. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- 10. Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. The authors of this study demonstrated that increased macrophage accumulation in periaortic adipose tissue of angiotensin II-infused obese mice is associated with enhanced abdominal aortic aneurysm formation.
- 11.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 12.Jung HS, Park KH, Cho YM, Chung SS, Cho HJ, Cho SY, Kim SJ, Kim SY, Lee HK, Park KS. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc Res. 2006;69:76–85. doi: 10.1016/j.cardiores.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Bray GA, Clearfield MB, Fintel DJ, Nelinson DS. Overweight and obesity: the pathogenesis of cardiometabolic risk. Clin Cornerstone. 2009;9:30–40. doi: 10.1016/s1098-3597(09)80003-3. [DOI] [PubMed] [Google Scholar]
- 14.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 15.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 16.Baker AR, da Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1–7. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, Gallo P, Rosaria C, di Gioia T. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 19.Moos MP, John N, Gräbner R, Nossmann S, Günther B, Vollandt R, Funk CD, Kaiser B, Habenicht AJ. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 20.Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714–721. [PubMed] [Google Scholar]
- 21.Silaghi A, Achard V, Paulmyer-Lacroix O, Scridon T, Tassistro V, Duncea I, Clément K, Dutour A, Grino M. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. Am J Physiol Endocrinol Metab. 2007;293:E1443–E1450. doi: 10.1152/ajpendo.00273.2007. [DOI] [PubMed] [Google Scholar]
- 22.Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res. 2008;40:442–445. doi: 10.1055/s-2008-1062724. [DOI] [PubMed] [Google Scholar]
- 23.Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005;90:6300–6302. doi: 10.1210/jc.2005-1087. [DOI] [PubMed] [Google Scholar]
- 24.Djaberi R, Schuijf JD, van Werkhoven JM, Nucifora G, Jukema JW, Bax JJ. Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol. 2008;102:1602–1607. doi: 10.1016/j.amjcard.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Gorter PM, de Vos AM, van der Graaf Y, Stella PR, Doevendans PA, Meijs MF, Prokop M, Visseren FL. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol. 2008;102:380–285. doi: 10.1016/j.amjcard.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008;94:e7. doi: 10.1136/hrt.2007.118471. [DOI] [PubMed] [Google Scholar]
- 27.Wang CP, Hsu HL, Hung WC, Yu TH, Chen YH, Chiu CA, Lu LF, Chung FM, Shin SJ, Lee YJ. Increased epicardial adipose tissue volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol (Oxf) 2009;70:876–882. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 29.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yudkin JS, Eringa E, Stehouwer CD. "Vasocrine" signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- 32.Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol. 2004;286:H1107–H1113. doi: 10.1152/ajpheart.00656.2003. [DOI] [PubMed] [Google Scholar]
- 33. Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. This paper demonstrates the vasorelaxant effects of PVAT through an endothelium independent and endothelium dependent process.
- 34.Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, Gollasch M. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- 35.Gálvez B, de Castro J, Herold D, Dubrovska G, Arribas S, González MC, Aranguez I, Luft FC, Ramos MP, Gollasch M, Fernández Alfonso MS. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertens ive rats. Arterioscler Thromb Vasc Biol. 2006;26:1297–1302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- 36.Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, Lee RM. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Payne GA, Borbouse L, Bratz IN, Roell WC, Bohlen HG, Dick GM, Tune JD. Endogenous adipose-derived factors diminish coronary endothelial function via inhibition of nitric oxide synthase. Microcirculation. 2008;15:417–426. doi: 10.1080/10739680701858447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC-beta-dependent phosphorylation of nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H460–H465. doi: 10.1152/ajpheart.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. The authors here demonstrated the loss of the physiological function of PVAT with the development of obesity leading to adipocyte hypertrophy, hypoxia, inflammation and oxidative stress.
- 40.Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–H1813. doi: 10.1152/ajpheart.01259.2004. [DOI] [PubMed] [Google Scholar]
- 41.Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res. 2009;81:370–380. doi: 10.1093/cvr/cvn288. [DOI] [PubMed] [Google Scholar]
- 42. Skilton MR, Serusclat A, Sethu AH, Brun S, Bernard S, Balkau B, Moulin P, Bonnet F. Noninvasive measurement of carotid extra-media thickness: associations with cardiovascular risk factors and intima-media thickness. JACC Cardiovasc Imaging. 2009;2:176–182. doi: 10.1016/j.jcmg.2008.09.013. This paper shows the association between carotid extra-media thickness and cardiovascular risk factors.
- 43.Falk E, Thim T, Kristensen IB. Atherosclerotic plaque, adventitia, perivascular fat, and carotid imaging. JACC Cardiovasc Imaging. 2009;2:183–186. doi: 10.1016/j.jcmg.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 44. Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–911. doi: 10.1161/CIRCRESAHA.109.199653. This study showed that healthy subcutaneous adipose tissue attenuated neointimal formation in a wire injury femoral artery mouse model when compared to adipose tissue from high fat diet fed mice.
- 45.Melgarejo E, Medina MA, Sánchez-Jiménez F, Urdiales JL. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol. 2009;41:998–1001. doi: 10.1016/j.biocel.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Reape TJ, Groot PHE. Chemokines and atherosclerosis. Atherosclerosis. 1999;147:213–225. doi: 10.1016/s0021-9150(99)00346-9. [DOI] [PubMed] [Google Scholar]
- 47.Daniels RH, Finnen MJ, Hill ME, Lackie JM. Recombinant human monocyte IL-8 primes NADPH-oxidase and phospholipase A2 activation in human neutrophils. Immunology. 1992;75:157–163. [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703–11706. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- 49.Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]