Abstract
During the immune response to influenza infection, activated T cells are distributed to both lymphoid and extralymphoid tissues, including the infected airways where direct recognition of viral antigen-bearing cells takes place. The collagen binding α1β1 integrin VLA-1 is essential for the development of memory CD8+ T cells in the airways but while expressed by some CD4+ T cells, its significance has not been demonstrated. We investigated the role of VLA-1 on virus-specific CD4+ T cells during and after primary or secondary influenza infection of mice. The proportion of CD4+ cells expressing CD49a (α1 integrin) was low in all tissues sampled during primary infection, but increased in the airways after viral clearance. Furthermore, during the first 24hr of a secondary influenza challenge, the majority of IFN-γ secreting effector CD4+ T cells from the airways were in the CD49a+ population. Airway CD49a+ CD4+ cells also expressed reduced markers of apoptosis compared to CD49a− cells, and fewer memory or effector CD4+ cells could be recovered from airways of α1−/− mice, though lymphoid tissues appeared unaffected. These data suggest VLA-1 expression defines a population of tissue-memory CD4+ T cells that act as rapid effectors upon re-infection, and VLA-1 expression is integral to their accumulation in the airways.
Keywords: CD4+ T cells, influenza, adhesion molecules, α1 integrin, extracellular matrix
Introduction
Influenza virus poses a major health threat for humans worldwide. This is in large part due to the antigenic shift that occurs in the surface hemagglutinin and neuraminidase proteins, resulting in new virus variants that escape circulating neutralizing antibodies. The absence of immunity to variant influenza viruses greatly impacts human health, as highlighted by the emergence of several pandemic strains in the past century (1). Although the current flu vaccine is engineered to primarily elicit neutralizing antibody, a growing body of literature suggests that memory T cells, particularly in the mucosa, may be an effective means of limiting the morbidity and mortality resulting from infection with serologically distinct flu strains, also termed heterosubtypic immunity. T cell responses to conserved epitopes within internal viral proteins have been shown to be protective in mouse models of lethal heterosubtypic influenza infection (2, 3). More recent data suggests that memory T cells residing in extralymphoid tissues such as the lung seem especially capable of aiding in protection against serologically distinct flu variants in mice (4–7).
In cases of heterosubtypic flu infection, T cells are vital to viral clearance and host survival (8). CD4+ cells are important for the generation of neutralizing antibody and the utility of memory CD8+ cells that are effective in future heterosubtypic challenges (9–13). In addition, CD4+ cells aid in the effector response by recruiting immune cells to the site of infection and reducing viral burden through secretion of cytokines and potential cognate interactions with infected targets (14). Although there is obvious benefit to the host in retaining populations of memory CD4+ cells in both the lymphoid tissues and extralymphoid tissues such as the lung, little is known about the signals that select or retain memory cells to the extralymphoid compartment.
One T cell function that becomes increasingly important as lymphocytes move from the circulation and lymphoid tissues to extralymphoid sites is their interaction with extracellular matrix (ECM). Much of the ECM in the lung environment is available to infiltrating T cells, and T cell integrin-ECM interactions can promote T cell motility, survival and activation (15–17). Previous work has shown that the α1β1 integrin VLA-1 (detected by staining with antibody to the α1 integrin chain/CD49a) is expressed on primed CD8+ T cells in the lung and other extralymphoid tissues, and its interaction with type IV collagen may be important for CD8+ cell residence in the airways (18). In addition, VLA-1 contributes to the survival of memory CD8+ cells in the lung airways (16). When reduction in the number of flu-specific memory CD8+ cells in the airways is accelerated by antibody blockade or genetic deficiency of VLA-1, mice are more susceptible to mortality upon lethal heterosubtypic challenge (18). This is possibly due to a lack of effector memory cells localized to the lung airways at the time of challenge, where the flu infection occurs (8, 19, 20). These data suggest VLA-1 is an important mediator of memory T cell retention and survival in extralymphoid tissues, and may play a prominent role in the survival and extralymphoid tissue localization of T cells in inflammatory conditions.
Less is known concerning the role of VLA-1 on memory CD4+ T cells. A small proportion of CD4+ cells express CD49a during the acute viral response, while nearly half of airway CD4+ cells express CD49a one month after infection (21), suggesting either enrichment or development of the CD49a+ phenotype after viral infection. This population may be important in promoting inflammation, since blockade of CD49a resulted in less severe inflammation and resulting disease in several CD4+ T cell-dependent disease models in animals (22–24). However, these studies did not distinguish the effects of CD49a blockade on T cells versus other cell types such as monocytes, which express CD49a and can play a vital role in the inflammatory response (25, 26). CD49a+ CD4+ cells can be recovered from human PBL and airways (recovered by BAL) and express a highly activated phenotype in both of these sites (27, 28). Though CD49a+ CD4+ cells have been identified and characterized in mouse and human, it is largely unknown whether expression of VLA-1 is of any consequence to CD4+ T cells, specifically cells responding to a viral infection localized to extralymphoid tissue like the lung. Given the mixed phenotypes associated with CD4+ cells in the lung (29), an understanding of when and how populations of CD49a+ and CD49a− CD4+ cells contribute to the immune response could be valuable in targeting cells important for protection.
In this report we studied CD49a+ CD4+ T cells in both lymphoid and extralymphoid tissues following infection with influenza virus. CD49a is expressed on a subset of CD4+ T cells during infection that increase in their relative proportion following viral clearance. These cells can be distinguished from other CD4 memory T cells by both cell surface phenotype and function during secondary infection. Integrin α1−/− mice have a defect in the accumulation of this subset of primed CD4+ cells in the airway late after infection, suggesting a dependence on VLA-1 expression. Airway CD49a+ CD44hi CD62Llo CD4+ T cells also have low expression of apoptotic markers compared to their CD49a− counterparts. We have termed these CD44hi CD62Llow CD49a+ CD4 T cells as “tissue-memory” because of their distinct recirculation and effector capacity. These findings support the hypothesis that a subset of effector memory cells selectively accumulate in extralymphoid tissues, with unique features that allow them to survive and persist in extralymphoid tissues after infection. This knowledge has important implications to understanding secondary influenza immunity and the design of optimized vaccination strategies targeting influenza viruses whose emergence is unpredictable.
Materials and Methods
Mice
C57Bl/6 (Bl/6) and congenic CD45.1+ B6.SJL mice were purchased from the National Cancer Institute. Integrin α1−/− mice (gift from Biogen, Inc.) (30), TCR Cα −/− mice on the C57Bl/6 background (gift from Dr. Deb Fowell) (31) and TCR transgenic OT-II mice on the B6.PL (CD90.1) background (gift from Dr. Linda Bradley) (32) were bred and maintained at the University of Rochester Animal Housing Facility (Rochester, NY) in specific pathogen-free conditions.
Viral Infection and Cell Transfer
Stocks of influenza A/WSN-OVAII (H1N1) (33) and A/HKx31/OVAII (H3N2) (gift from R. Webby) (34) viruses in allantoic fluid were thawed from −80°C. Virus was diluted in cold DPBS so that a 30μl volume would contain 500–1000 PFU (for WSN) or ~2.8×104 PFU (for x31) of virus. Recipient mice were first sedated with avertin (2,2,2-tribromoethanol) i.p., then given a 30μl intranasal inoculation of virus. In experiments involving OT-II cells, 5×105–1×106 OT-II cells from spleen enriched via bead depletion of class II+ and CD8+ cells were transferred intravenously 24hr prior to viral infection. In all cases, the frequency of OT-IIs in the cell preparation was determined by flow cytometry prior to transfer to take into account day-to-day variations in the enrichment procedure.
Organ Harvest
Mice were sacrificed via lethal dose of Avertin and exsanguinated via brachial artery. Bronchoalveolar lavage (BAL) samples were collected by three intratracheal lung washes (with C-mem) using a Teflon cannula attached to a 1ml syringe. Lung tissue, mediastinal lymph node (MLN) and spleen were removed separately. BAL samples were subjected to 45min. plastic adherence prior to use. Lung tissue was ground through a tea strainer and lymphocytes isolated via centrifugation with a Histopaque underlay (Sigma). MLN and spleen were homogenized and filtered through nylon mesh. All organ cells were maintained in C-mem prior to use.
Flow Cytometry and Apoptosis
1–2×106 cells from each organ were placed in individual wells of a 96-well round-bottom plate for staining. Fc receptors were blocked with anti-CD16/32 (clone 2.4G2, from BD) for 15min. prior to antibody staining. Cells were washed and surface stained with antibody panels containing combinations of CD4-APC/Cy5.5 (eBioscience), CD90.1-Biotin/Pacific Orange 2° (eBioscience, Invitrogen 2°), CD44-Pacific Blue (Biolegend), CD62L-APC/Cy7 (Biolegend), CD11a-PE/Cy7 (BD), CD49a-Alexa 488 (BD), CD49b-FITC (BD), and CD49d-PE (BD); cells were incubated for 30min. on ice in the dark. For apoptotic marker analysis: Stained cells were washed in Annexin buffer (10× buffer of 0.1M Hepes, 1.4M NaCl and 25mM CaCl2 diluted to 1× in dH2O) and resuspended in 100μl Annexin buffer with 5μl per well Annexin V-APC (BD). After 5min. incubation of cells at room temperature in the dark, 5μl per well 7AAD (BD) was added for an additional 10min. incubation time. Cells were washed and resuspended in Annexin buffer for FACS. Samples were run on an LSRII (BD) cytometer, and analyzed with FlowJo (Treestar) software.
Intracellular Staining
Spleen cells from naive B6.SJL (CD45.1+) mice were used as APCs and pulsed with either OVA323–339, control peptide or nothing for 90min. at 37°C. To study the response to whole virus, APCs were infected with influenza (MOI=1) in 1ml serum-free media for 60min. Infected cells were then washed and resuspended in C-mem. 1×106 APCs were added to 1×106 responders (prepared as described above) for a total volume of 100μl. Golgi Plug (BD) was then diluted 1μl/ml in C-mem and 100μl added to each well. Cells were incubated for 5–6hr at 37°C. Samples were then surface stained as described above. Samples were washed and resuspended in 100μl/well Cytofix/Cytoperm (BD) for 15min. After one Perm/Wash (BD), IFN-γ-PE antibody was added in Perm/Wash, and cells incubated for 30min. on ice in the dark. Samples were resuspended in PBS/BSA for FACS. For staining of 5’ Bromodeoxyuridine (BrdU)+ cells: After surface staining and permeabilization, the APC anti-BrdU kit (BD) was used to detect BrdU+ DNA in cells. In short, fixed and permeabilized cells were incubated with Cytoperm Plus buffer to permeabilize nuclei, and then treated a second time with Cytofix/Cytoperm for re-fixation of cells. Cells were then treated with DNase to expose BrdU, and subsequently stained with APC anti-BrdU for detection via cytometry. Gating of BrdU+ cells was determined by parallel staining of cells that did not receive BrdU in the experiment as a negative staining control.
Statistical Analysis
Groups of data were compared using two-tailed student’s T test or Wilcoxon signed rank test; resulting p values lower than 0.05 were considered significant.
Results
CD49a is expressed on a population of effector CD4+ cells following infection
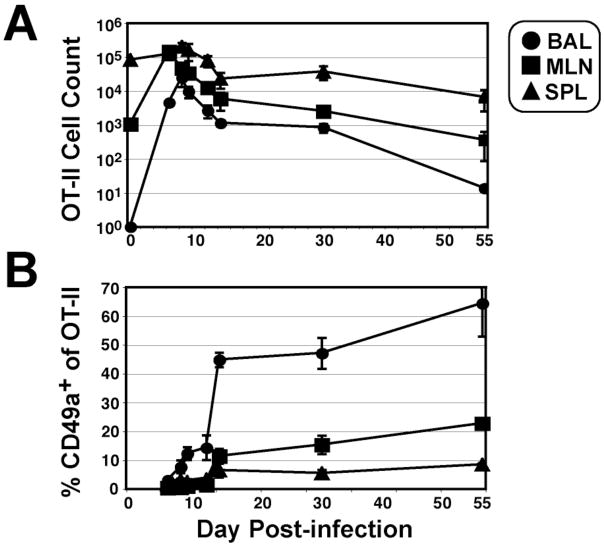
In order to follow a population of virus-primed T cells, as well as the CD4+ population as a whole, we studied the influenza response from both endogenous CD4+ T cells and adoptively transferred OT-II cells following infection with A/WSN-OVAII (33). Very few CD49a-expressing OT-II cells were detectable in the early stages of infection (Fig. 1), which is consistent with the time course observed for endogenous CD4+ cells during X-31 infection (21). However, the proportion of CD49a+ CD4+ cells increased gradually through the peak immune infiltrate and more substantially after viral clearance, most strikingly on those recovered from BAL where >50% of the CD4 T cells were positive, with a smaller proportion (<10%) of primed cells expressing CD49a in the lymphoid tissues (Fig. 1). The enrichment of CD49a+ CD4+ cells in the airways after viral clearance suggested to us this population of cells may be uniquely regulated. Therefore, we compared the phenotypes of CD49a+ and CD49a− CD4+ cells to determine their relative contribution to the effector and memory populations present after infection.
Figure 1. Kinetics of OT-II response and CD49a expression following recombinant A/WSN-OVAII infection.
5×105 OT-II cells on the CD90.1 B6.PL congenic background were transferred to C57Bl/6 mice prior to infection with influenza. A) To obtain OT-II cell counts, bronchoalveolar lavage (BAL; circles), mediastinal lymph node (MLN; squares) and spleen (SPL; triangles) samples were processed at several time points post-infection and CD4+ Thy1.1+ cells detected by flow cytometry. B) The frequency of CD49a+ OT-II cells was determined by staining samples with α-CD49a antibody (Ha31/8). Data are shown as +/− SEM of n≥4 animals per time point.
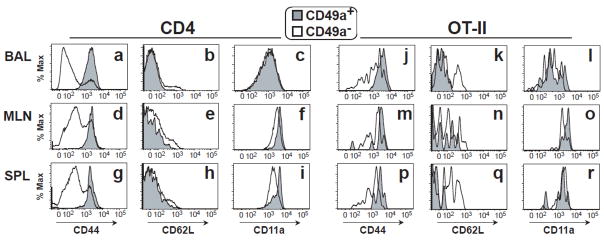
CD4+ T cells from primed mice were segregated by CD49a expression and studied for phenotypic differences one month post-infection. As expected after infection, CD49a+ cells were almost exclusively CD44hi in all organs studied (Fig. 2a, d, g). CD62L expression was lower among CD49a+ CD44hi CD4+ cells in all organs, particularly for BAL cells (p = 0.018; Table 1). CD11a was also higher among CD49a+ CD44hi CD4 cells from MLN and spleen (Table 1). Results were similar within the flu-primed OT-II cells after infection, including higher CD44 expression (Fig. 2j-r). This is consistent with other reports that describe VLA-1 expression on CD4+ cells with an effector phenotype in mouse and human (27, 28, 35), and our data shows this phenotype is generated during an acute viral infection. It is important to note that while not all cells with an effector-memory phenotype expressed CD49a, the CD49a+ population was uniformly of effector phenotype in the organs studied.
Figure 2. CD49a+ CD4+ cells have a highly activated phenotype.
One month post-infection, total CD4+ cells (a–i) or CD4+ Thy1.1+ OT-II cells (j–r) were analyzed by flow cytometry for expression of CD44 within the CD49a+ (gray histograms) and CD49a− (white histograms) subsets. Gated CD44hi CD4+ or CD44hi Thy1.1+ CD4+ OT-II cells were then analyzed for CD62L and CD11a expression within the CD49a+ and CD49a− subsets. BAL (a–c, j–l), MLN (d–f, m–o) and SPL (g–i, p–r) samples were collected from the same animals. Histogram plots shown are representative results from multiple experiments.
Table 1.
Comparison of CD62L and CD11a expression on CD49a+ and CD49a-cells*
| Organ | BAL | MLN | SPL | |||
|---|---|---|---|---|---|---|
| Gate | CD49a+ | CD49a− | CD49a+ | CD49a− | CD49a+ | CD49a− |
| CD62L MFI | 20±18 | 71±25 | 36±20 | 74±14 | 25±7 | 65±4 |
| p value | 0.018 | 0.012 | 0.043 | |||
| CD11a MFI | 947±172 | 1098±249 | 4577±1195 | 3855±1032 | 4109±682 | 3092±812 |
| p value | 0.173 | 0.038 | 0.004 | |||
All groups gated on CD44hi CD4+ cells prior to segregation by CD49a expression; MFI = mean fluorescence intensity.
Paired data comparing CD49a+ and CD49a- populations were subjected to Wilcoxon signed- rank test.
Influenza-primed CD49a+ CD4+ cells rapidly secrete IFN-γ during a recall response
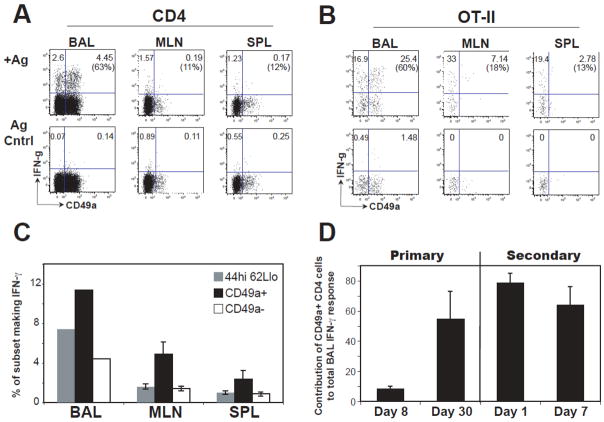
Effector-memory T cells rapidly secret cytokine in response to antigen. We questioned whether CD49a+ CD4 T cells were functionally different than the effector-memory population as a whole. The effector function of CD49a+ and CD49a−CD4+ T cells was first studied by intracellular IFN-γ staining of mouse BAL, MLN and spleen cells taken at several time points following primary infection. In order to compare endogenous CD4+ and transgenic OT-II cell responses, cells were re-stimulated with whole virus or OVA323–339 peptide, respectively. During the peak (d8) of the acute primary response, CD49a+ OT-II cells composed less than 10% of the total OT-II cells (identified as Thy1.1+ CD4+ cells) secreting IFN-γ (Fig. 3D). However, the majority (70% +/− 2%) of CD49a+ OT-II cells made IFN-γ, and there was more cytokine production per cell in the CD49a+ subset from the BAL (MFI of CD49a+ IFN-γ+ OT-IIs: 17185+/−1768; CD49a− IFN-γ+ OT-IIs: 9917+/−1348; p<0.02). In contrast, at one month post-infection, CD49a+ cells accounted for about 55–60% of the total IFN-γ secreting endogenous CD4+ or OT-II populations in the BAL upon ex vivo re-stimulation, while in contrast making a minor contribution to the response from lymphoid tissues (Fig. 3A, B).
Figure 3. Contribution of CD49a+ CD4+ cells to the IFN-γ recall response after influenza infection.
A–B) One month after influenza infection, BAL, MLN and SPL samples were collected and cells re-stimulated ex vivo by splenic APCs pulsed with live influenza virus at an MOI of 1 (for endogenous CD4+ response) or OVA323–339 peptide (for OT-II response) in separate experiments. Representative dot plots of CD49a and IFN-γ staining for CD44hi CD4+ cells (A) or OT-II cells (B) are shown. Numbers report the frequency of cells in the two upper quadrants; the number in parentheses is the percentage of the total IFN-γ response found in the upper right quadrant. Upper plots show results with APCs pulsed with specific antigen, lower plots show results with APCs pulsed with control peptide. C) IFN-γ potential of CD4+ cell subsets was determined by calculating the frequency of CD44hi CD62Llo cells that made IFN-γ during the 5hr. re-stimulation ex vivo. The same analysis was also performed on CD49a+ and CD49a− populations within the CD44hi CD62Llo gate. Data are n=2 (pooled samples from independent experiments) for BAL, n=8 for MLN, n=10 for spleen. D) Contribution of CD49a+ CD4+ cells to the IFN-γ response detected in the BAL. Intracellular cytokine staining was performed on BAL samples from days 8 and 30 post-infection by re-stimulation of primed OT-II cells with OVA323–339-pulsed APCs. At day 30, a cohort of mice was given a heterosubtypic challenge with A/x31-OVAII. Intracellular staining was performed days 1 and 7 post-secondary infection. Data are shown as the proportion of the total OT-II IFN-γ response in the BAL contributed by CD49a+ OT-II cells. Data are +/− SEM of an n ≥3 for each time point.
CD49a+ cells were further analyzed for effector potential by comparison with the classic CD44hi CD62Llo effector-memory phenotype. We first gated CD4+ cells for CD44hi CD62Llo; then the CD44hi CD62Llo cells were fractionated by CD49a expression and measured the proportion of cells that made IFN-γ in response to whole virus ex vivo. Among the CD44hi CD62Llo cells, we found the CD49a+ subset was relatively enriched for IFN-γ potential in all organs studied (Fig. 3C). Although the number of these highly antigen-reactive cells was greatest in the airways, their presence in the lymphoid organs suggests that the lung environment is not strictly required to maintain this phenotype. Furthermore, these observations suggest that this is a recirculating population with a program of differentiation that results in preferential though not exclusive localization to the lung.
Contribution of CD49a+ CD4+ cells during secondary infection
Since CD49a+ CD4+ cells were enriched in the BAL after infection and capable of rapid effector cytokine secretion ex vivo, we postulated that they play a central role during secondary infection of the lung. To test this, OT-II cells were transferred to mice that were subsequently primed by infection with H1N1 A/WSN-OVAII.One month after infection, mice were given a secondary heterosubtypic challenge with H3N2 A/X31/OVAII (34).CD49a+ OT-II cells in the BAL accounted for approximately 80% of the cytokine positive CD4+ cells 24 hours after secondary challenge (Fig. 3D). This is in contrast to the primary response, where activated CD49a+ T cells are not even detectable in the BAL until day 5 (Fig. 1, (18) and data not shown). The relative contribution of primary day 8, day 30, and day 1 secondary CD49a+ cells show an increasingly dominant role in the IFN-γ response detected by ex vivo re-stimulation (Fig. 3D). These data show that CD49a+ CD4+ cells are reactivated in the first day of secondary challenge in the BAL, and there is a dominant early contribution of CD49a+ cells to the secondary IFN-γ response.
Defect in the accumulation of primed CD4+ T cells to the BAL in α1−/− mice
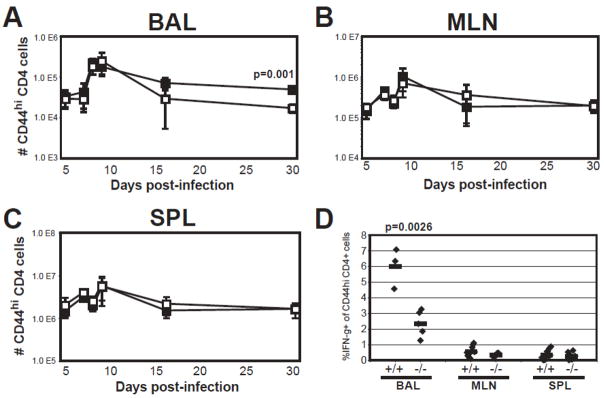
VLA-1 is the major T cell binding partner for collagen IV (30, 36–39), which is abundant in basement membrane. Previous data showed a strong correlation between CD49a expression on BAL CD4+ T cells and lung localization to collagen IV (21). Taken together, these data suggest a dependence on VLA-1 in CD4+ T cell localization in the BAL. To test this, we infected integrin α1−/− mice with influenza and compared CD4+ cell recoveries from various organs to wild-type mice. We found comparable cell recoveries from BAL, draining MLN, and spleen during the acute response (Fig. 4A-C). However, one month after infection, fewer CD4+ T cells were recovered from the BAL of α1−/− mice, suggesting a defect in retention of cells at that site (Fig. 4A), which was not reflected in the lymphoid tissues (Fig. 4B-C). Upon re-stimulation ex vivo, a lower proportion of BAL CD4+ T cells from α1−/− mice were capable of secreting IFN-γ (Fig. 4D), suggesting the loss of CD44hi CD4+ T cells from the BAL of α1−/− mice primarily impacted the tissue-memory population in that organ.
Figure 4. Reduced recovery of effector CD4+ cells from BAL of integrin α1−/− mice.
Wild-type (closed boxes) or α1−/− (open boxes) mice were infected with influenza and monitored over several time points for cell recovery from BAL (A), MLN (B), and SPL (C). Data represented as number of CD44hi CD4+ cells recovered per organ; +/− SEM of n≥ 3 per time point. D) At day 30 post-infection, intracellular cytokine staining was done on Wild-type (+/+) or α1−/− (−/−) cells to determine the frequency of CD4+ cells secreting IFN-γ in a 5hr. ex vivo re-stimulation. Data represented as percent IFN-γ+ cells in the CD44hi CD4+ gate; each point represents a pool of 4–7 mice (for BAL) or individual mice (MLN and SPL). P–values were determined by student’s T-test.
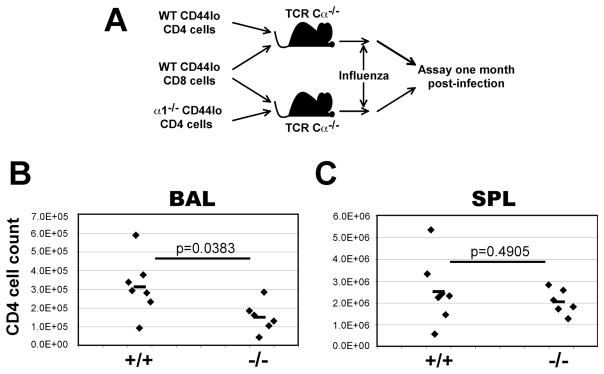
VLA-1 is expressed on a variety of hematopoietic and non-hematopoietic cells (25). Therefore, it was unknown whether the defect in CD4+ T cell recovery in α1−/− mice was a direct effect of α1 deficiency on CD4+ cells, or indirect through another cell type. Adoptive transfer experiments were performed to determine the importance of VLA-1 on CD4+ T cells. CD3+ CD4+ CD44lo T cells were FACS sorted from naive wild-type or α1−/− mice. To limit competition from endogenous T cells, these donor cells were adoptively transferred into separate cohorts of TCR Cα−/− host mice, along with equal numbers of sorted wild-type CD3+ CD8+ CD44lo cells (Fig. 5A). The recipients contain no endogenous TCRα-expressing T cells (31) but do have some TCRβ+ cells (40). Donor cells were from congenic B6.PL animals, so endogenous and adoptively transferred cells were distinguished post-transfer by CD90. Mice were challenged with influenza and sampled one month later. At this time, TCR Cα−/− mice that received α1−/− CD4+ T cells had fewer CD4+ T cells in the BAL, and similar cell recovery from the spleen (Fig. 5B-C), suggesting VLA-1 expression on the CD4+ T cell itself is important for accumulation to the BAL one month post-infection.
Figure 5. CD4+ cell-intrinsic defect in BAL accumulation in the absence of α1 integrin.
A) Experimental setup: CD3+ CD44lo CD8+ T cells were FACS sorted from B6.PL (CD90.1) mice, and CD3+ CD44lo CD4+ cells were sorted from α1+/+ or α1−/− mice on a B6.PL background. Equal numbers (2–3×106 per population) of cells were intravenously transferred to TCR Cα−/− (CD90.2) recipients. 48hr post-transfer, recipients were infected with influenza. B-C) CD4+ cell recovery from TCR Cα−/− recipient mice one month post-infection. BAL (B) or SPL (C) cell recoveries are shown as the number of CD4+ CD90.1+ cells per organ; each point represents an individual mouse. Values from T test are above the corresponding data.
Comparable expansion between wild-type and α1−/− CD4 T cells following infection
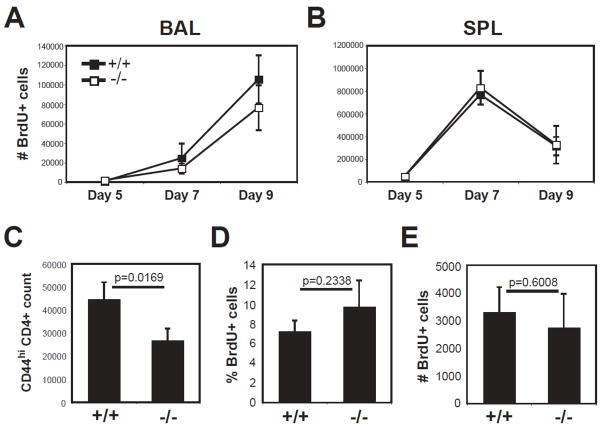
One possibility for reduced cell recovery in CD49a deficient mice is a defect in the ability of the T cells to proliferate. To test this, we fed infected mice BrdU starting at day 2 post-infection, and identified dividing cells by BrdU incorporation during the acute primary response. We found no significant differences between knockout and wild-type in the number or phenotype of BrdU+ CD4+ cells in the BAL and spleen through acute primary infection (Fig. 6A, B and data not shown). Since the defect in α1−/− BAL cell recovery was observed after infection (Fig. 4), we also fed mice BrdU days 20–25 post- infection to examine the accumulation of BrdU+ CD4+ cells to the airways after viral clearance. As seen before (Fig. 4A), fewer CD4+ cells were recovered from α1−/− BAL (Fig. 6C). However, the proportions of BrdU+ CD4+ T cells from α1−/− mice were similar if not greater (Fig. 6D), resulting in the same number of BrdU+CD4+ cells being recovered in both groups (Fig. 6E). This suggests the reduced number of CD4+ T cells in α1−/− BAL is not due to a defect in expansion, and must be explained by other mechanisms.
Figure 6. Turnover of primed CD4+ cells is comparable between wild-type and α1−/− mice.
A–B) Wild-type (black squares) and α1−/− (white squares) mice were infected with influenza. Two days post-infection, mice were administered BrdU by intraperitoneal injection of 1mg per animal, followed by continuous feeding of 0.8mg/ml BrdU in drinking water. At day 5, 7, and 9 post-infection, mice were sacrificed and BAL (A) and SPL (B) analyzed by flow cytometry for BrdU+ CD44hi CD4+ cells. Data are shown as +/− SEM of n≥3 per group. C–E) Mice that recovered from influenza infection were administered BrdU as above from day 20–25 post-infection. At day 25, BAL samples from wild-type (+/+) and α1−/− (−/−) mice were analyzed for recovery of total CD44hi CD4+ cells (C), and the frequency (D) and number (E) of BrdU+ CD44hi CD4+ cells. Data are shown as +/− SEM of n=5 per group. Values from T test are above the corresponding data.
CD49d+ CD4+ cells are reduced in α1−/− mice
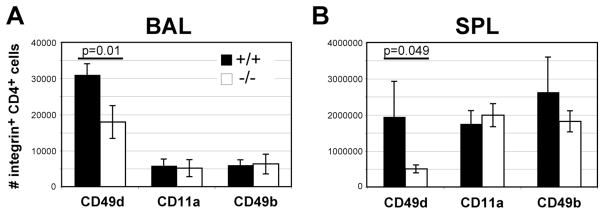
The integrins LFA-1 and VLA-4 are integral in the extravasation of primed lymphocytes out of circulation. Although acute cell recoveries suggest α1−/− CD4+ cells are not defective in their ability to traffic to the lung (Fig. 4A), we wondered whether differences existed after viral clearance. Primed CD44hi cells from wild-type and α1−/− mice were stained for the integrins CD11a, CD49b and CD49d. While the frequency of CD49d+ cells was the same, CD11a and CD49b integrin-positive CD4+ T cells were actually higher in proportion from the BAL of α1−/− mice (not shown). However, the reduced cell recovery in the deficient mice (Fig. 6C) resulted in numbers of CD11a+ or CD49b+ cells comparable to wild-type mice (Fig. 7A). In contrast, the frequency and CD49d+ CD4+ cells was lower in α1−/− spleen, and the numbers of CD49d+ CD4+ cells was lower in both the BAL and spleen (Fig. 7 and data not shown). We also found the mean fluorescence intensity of CD49d reduced among CD4+ spleen cells in α1−/− mice, which was not found with CD11a or CD49b (data not shown). These data show a CD49d-expressing population of CD4+ cells is reduced in both the BAL and spleen of α1−/− mice.
Figure 7. α1−/− mice have reduced numbers of CD49d+ CD4+ cells after infection.
Wild-type (black bars) and α1−/− mice (white bars) were infected with influenza, and 25 days later analyzed by flow cytometry for expression of integrins on CD4+ cells. BAL (A) and SPL (B) data are represented as the number of CD4+ cells co-staining positive for the indicated integrin antibody; +/− SEM for n=5 per group. Significant values from T test are shown above corresponding data.
Differences in apoptotic marker expression between CD49a+ and CD49a− CD4+ cells
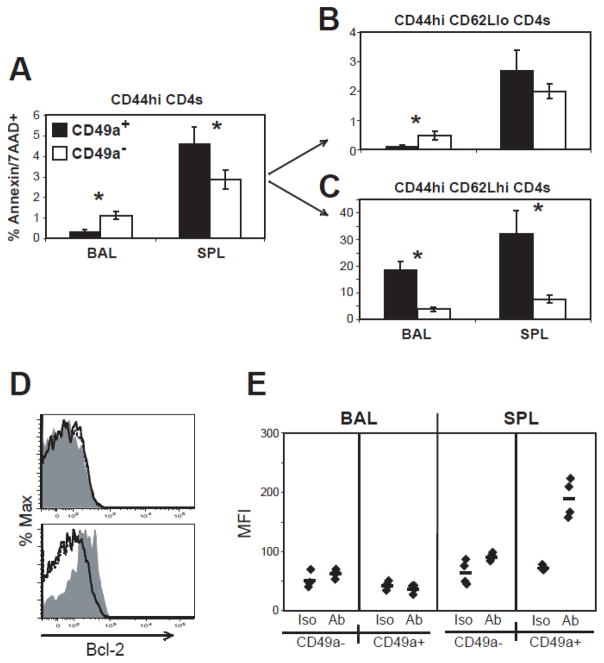
Another possible explanation for the reduced recovery after BAL in α1−/− mice was the inability of a specific cell population to survive and/or persist in the lung. To address this, we stained primed CD4+ cells with apoptotic markers both during and after infection. Figure 8 shows that a lower proportion of CD49a+ CD4+ cells in the BAL express markers of apoptosis compared to CD49a− cells after infection. Surprisingly, this was not observed in the spleen, where a higher proportion of CD49a+ CD4+ cells express apoptotic markers (Fig. 8A). This phenotype was also consistent in the OT-II cell population (data not shown). These populations were further gated on CD62Lhi or CD62Llo cells. The results show that CD44hi CD62Llo CD49a+ CD4+ cells are lowest for apoptotic markers in the BAL (Fig. 8B-C).
Figure 8. Reduced frequency of CD49a+ effector cells express apoptotic markers in the BAL.
A–C) At day 26 post-infection, BAL and SPL cells were analyzed by flow cytometry for Annexin V and 7AAD. Cells were gated by CD44hi CD4+ and then segregated by CD49a expression (A). Fig. A shows the proportion of CD49a+ (black bars) or CD49a− (white bars) cells that were Annexin V+ 7AAD+. B–C) CD44hi CD4+ cells on day 26 were further segregated by CD62L expression and analyzed for apoptotic marker expression on CD49a+ (black bars) and CD49a− (white bars) cells. Data are +/− SEM of n 4 per group; *=p<0.05 comparing CD49a+ and CD49a− groups. D–E) Two weeks post-infection, BAL and spleen cells were stained for Bcl-2 expression. D) Representative histograms for Bcl-2 staining on CD62Llo (dashed line), CD49a+ (gray filled), and CD49a− (solid line) subsets gated on CD44hi CD4+ cells from BAL (top) or spleen (bottom). E) Bcl-2 MFI was determined for CD49a+ and CD49a− subsets in isotype (Iso) or Bcl-2 antibody (Ab) staining conditions. Each point represents one mouse, with the average displayed as a horizontal line.
A reduced proportion of apoptotic CD49a+ cells in the airways may be related to expression of cytokine receptors or anti-apoptotic proteins by these cells. In order to further describe the survival phenotype of CD49a+ cells, we stained BAL, MLN and spleen cells two weeks post-infection with IL-2Rα (CD25), IL-7Rα (CD127), and Bcl-2. CD127 was comparably expressed on all populations studied, and there was a trend toward increased CD25 expression on CD49a+ CD4 cells (data not shown). Interestingly, while Bcl-2 levels were the same in BAL CD49a+, CD49a−, and CD44hi CD62Llo populations after infection, CD49a+ CD4 cells in the spleen expressed high levels of Bcl-2 (Fig. 8D, E). The difference in apoptotic phenotype and Bcl-2 expression between airway and spleen suggests that, although CD49a+ cells in both locations are of tissue-memory phenotype, they may be regulated by different mechanisms. Taken together, these results show that highly activated, CD49a+ CD4+ cells in the BAL are comparatively low in the expression of markers of apoptosis, which may be one explanation for the enrichment of these cells in the lung after infection. However, reduced apoptotic marker expression does not appear to correlate with differences in expression of Bcl-2, CD25 or CD127, and must be explained by other mechanisms.
Discussion
In this report we have demonstrated an important role for the α1β1 integrin VLA-1 in the accumulation of tissue memory CD4+ cells in the airways following influenza infection. Although CD49a+ CD4+ cells share some attributes of the effector-memory T cell subset (41, 42), they also have distinct features. CD49a+ CD4+ T cells are highly enriched for effector potential, and in the airway are the dominant rapid effectors within the first 24 hours of secondary infection. In the absence of VLA-1, there is a deficit in tissue-memory CD4+ T cells and reduced cytokine effectors in the airways upon re-stimulation. The presence of a low proportion of CD49a+ CD4+ T cells in the lymphoid organs suggests this population is re-circulating through the body, though selectively accumulates in the airways. Taken together, this report shows that a population of effector CD4+ T cells accumulates in the airway in a α1-dependent fashion, and mediates the rapid secondary effector response in this location. As noted previously (18), we have no evidence showing that VLA-1 is important for recruitment or trafficking into the lung or airways during priming, rather that its role becomes apparent after the virus is cleared. Similar to flu-specific tissue memory CD8+ T cells, there is a population of tissue memory CD4+ T cells in the airways that depend on VLA-1 expression to become established.
While the precise mechanism of VLA-1-mediated CD4+ T cell accumulation in the airways and other peripheral tissues remains unclear, our hypothesis is that VLA-1 expression increases the half-life of the cells in extralymphoid sites, and this is most evident in the airways and other mucosal sites (43). This is supported by the selective enrichment of CD49a+ cells in the airways after the contraction phase of the immune response (Fig. 1), as well as the specific loss of effector cells from the airways in α1−/− mice after viral clearance (Fig. 4). The simplest explanation is that adhesion to ECM slows migration or loss from the airway environment, and there is evidence of a close association of T cells with areas of basement membrane in the lung (21). However, it is also possible that matrix interactions act to increase the survival of T cells in these environments. This hypothesis is supported by reduced expression of apoptotic markers on CD49a-expressing T cell populations after infection (Fig. 8). In many extralymphoid cells, matrix interactions are essential for preventing apoptosis (16, 44).
We found that α1−/− mice have reduced numbers of CD49d+ CD4+ cells in both the airways and spleen (Fig. 7). There was not a general defect in β1 or other integrins inα1−/− mice, since the expression of CD49b and CD11a was the same compared to wild-type mice (Fig 7). While differences in CD49d may be a factor in reduced α1−/− CD4+ cell recovery by BAL, it does not by itself explain the defect. First, recruitment of primed α1−/− CD4+ cells to the BAL is normal during acute infection (Fig. 4). Second, the same number of BrdU+ CD4+ cells was recovered from BAL of wild-type and α1−/− after viral clearance (Fig. 6). Both pieces of evidence suggest entry of new cells into the airways is not defective, consistent with recent data that BAL T cells are continually replaced after viral infection by newly recruited cells (45). If VLA-4 expression was responsible for reduced extravasation and thus recovery of primed BAL CD4+ cells after viral clearance, we would expect to see this reflected in the recovery of BrdU+ cells. Instead we favor the view that VLA-1 functions after the cells have entered the airways, and the reduced CD49d population and expression reflects the fact that high levels of CD49a and CD49d are co-expressed by the same cells. Each integrin then functions at distinct steps in the process of getting to and staying in the airways.
One important point is that only about half of the primed CD4+ cells in the BAL express CD49a after influenza infection, while nearly all of the BAL CD8+ cells express CD49a (18, 21). This seems to be a general feature of BAL CD4+ cells, since the proportions of CD49a+ CD4 T cells is similar after infection with vaccinia virus, systemic versus respiratory infection, priming with peptide plus lipopolysaccharide, or sensitization and challenge with OVA aerosol (as a model for airway hyper reactivity), even though BAL cell recoveries varied between treatments (data not shown and 21). This may be the result of homeostatic differences between primed CD4+ and CD8+ cells, since the turnover of primed CD4+ and CD8+ cells and their rate of lung accumulation have been shown to be different (45, 46). Alternatively, VLA-1 expression on both CD4+ and CD8+ cells may be a consequence of strong versus weak TCR stimulation at priming, such that a subset of T cells expressing the ‘fittest’ TCRs are programmed for VLA-1 expression. A recent report by the Bank lab showed that the TCR repertoires of VLA-1+ CD4 cells in human inflamed synovium were largely distinct from VLA-1-synovial cells or those in peripheral blood (47). This suggests the enrichment of VLA-1+ cells to extralymphoid tissues may represent a distinct T cell sub-population within the memory pool. However, it is still unknown whether any potential unique specificities found in the CD49a+ fraction have high affinity T cell receptors compared to other specificities. Further work must be done to address this interesting hypothesis.
An important feature of the CD49a+ population in the BAL is the ability to respond to secondary infection. While at most approximately half of CD4+ cells express CD49a, they are dominant contributors to the rapid effector cytokine response in a 5hr re-stimulation ex vivo (Fig. 3). Furthermore, the relative contribution of the CD49a+ subset to the cytokine responders 24 hours after secondary infection is even greater than at one month post-infection. Rapid recruitment of new cells from lung parenchyma or circulation, induction of CD49a expression on resident cells, or rapid loss of CD49a− cells could all potentially account for this phenotype in the secondary. Additionally, if CD49a+ cells are localized to regions of the lung where infection is prevalent, they may receive increased stimulation in vivo from the second infection compared to their CD49a− counterparts. Our current experiments cannot distinguish among these possibilities. In either event, the relative abundance of CD49a+ cells and their activity in the BAL during acute secondary infection suggests a central role for these cells in the rapid recall response in the lung. A detailed analysis of the secondary response in the airways will give additional insight into the nature of the rapid recall response.
In general, the high proportion of extralymphoid T cells that have an effector memory phenotype has led to the hypothesis that unique populations of primed T cells are poised for rapid response to secondary antigenic encounter in the tissue (6). While this hypothesis is an attractive model, there is little published evidence demonstrating such a role for extralymphoid cells. Data in this report support the ‘sentinel’ hypothesis and suggest that specific memory cell populations are uniquely able to accumulate in extralymphoid tissues for rapid response in case of future infectious challenge. While CD4+ cells are not strictly required for protection from influenza (8, 48, 49), they may directly engage infected targets in certain conditions (50, 51). Alternatively, they may play a dominant role in orchestrating the early innate response in the tissue through local secretion of chemokines that modulate the inflammatory environment. To this end, primed CD4+ cells have been shown to alter the lung innate inflammatory response to respiratory challenge with Sendai virus (52). Further study into the functions of extralymphoid CD4+ cells will be helpful in assessing their utility in protective tissue responses.
The identification of a population of tissue memory T cells that become rapid effectors upon secondary encounter with virus is an important correlate of protection from influenza disease. It may be useful to monitor the CD49a+ populations after infection or vaccination, or devise strategies to increase the prevalence of this memory population. The potential therapeutic impact of targeting VLA-1 on T cells could also prove valuable in models of allergy and autoimmunity, where effector CD4+ cells in extralymphoid tissues can be crucial inducers of inflammation and damage that cause debilitating disease over time. There is a body of literature suggesting a role for VLA-1 in several autoimmune disease models (22–24, 35). However, these studies used global blockade of CD49a in vivo or mice deficient in α1 integrin, so it is not known whether the reduced inflammation observed was a direct effect on inflammatory CD4+ cells or some other cell type. An elegant study of the interaction of VLA-1 and Semaphorin 7A showed an integral role for VLA-1 expression on monocytes for inflammatory responses in models of contact hypersensitivity and experimental autoimmune encephalomyelitis (26). It is clear that several inflammatory cell types may be affected by blockade of CD49a. This report confirms a role for VLA-1 on a subset of effector CD4+ cells. Further investigation into the mechanisms of how CD49a blockade (or deficiency) results in decreased inflammation in tissue sites will be valuable in the potential discovery of effective therapies for debilitating immune disease.
Acknowledgments
We would like to thank Nathan Laniewski for his skillful assistance with flow cytometric sorting of T cell populations.
Footnotes
This work was supported by the National Institutes of Health grants R01-AG021970 and N01-AI50020 and HHSN266200700008C. T. Chapman is supported by NIH/NRSA training grant #2T32AI007169
References
- 1.Hsieh YC, Wu TZ, Liu DP, Shao PL, Chang LY, Lu CY, Lee CY, Huang FY, Huang LM. Influenza pandemics: past, present and future. J Formos Med Assoc. 2006;105:1–6. doi: 10.1016/S0929-6646(09)60102-9. [DOI] [PubMed] [Google Scholar]
- 2.Bennink J, Effros RB, Doherty PC. Influenzal pneumonia: early appearance of cross-reactive T cells in lungs of mice primed with heterologous type A viruses. Immunology. 1978;35:503–509. [PMC free article] [PubMed] [Google Scholar]
- 3.Effros RB, Doherty PC, Gerhard W, Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977;145:557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol. 2005;175:4677–4685. doi: 10.4049/jimmunol.175.7.4677. [DOI] [PubMed] [Google Scholar]
- 5.Ely KH, Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells in the lung airways retain the potential to mediate recall responses. J Immunol. 2003;171:3338–3342. doi: 10.4049/jimmunol.171.7.3338. [DOI] [PubMed] [Google Scholar]
- 6.Hikono H, Kohlmeier JE, Ely KH, Scott I, Roberts AD, Blackman MA, Woodland DL. T-cell memory and recall responses to respiratory virus infections. Immunol Rev. 2006;211:119–132. doi: 10.1111/j.0105-2896.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 7.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 9.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riberdy JM, Christensen JP, Branum K, Doherty PC. Diminished primary and secondary influenza virus-specific CD8(+) T-cell responses in CD4-depleted Ig(−/−) mice. J Virol. 2000;74:9762–9765. doi: 10.1128/jvi.74.20.9762-9765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocha B, Tanchot C. Towards a cellular definition of CD8+ T-cell memory: the role of CD4+ T-cell help in CD8+ T-cell responses. Curr Opin Immunol. 2004;16:259–263. doi: 10.1016/j.coi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Welsh RM, Selin LK, Szomolanyi-Tsuda E. Immunological memory to viral infections. Annu Rev Immunol. 2004;22:711–743. doi: 10.1146/annurev.immunol.22.012703.104527. [DOI] [PubMed] [Google Scholar]
- 14.Swain SL, Agrewala JN, Brown DM, Jelley-Gibbs DM, Golech S, Huston G, Jones SC, Kamperschroer C, Lee WH, McKinstry KK, Roman E, Strutt T, Weng NP. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dustin ML, de Fougerolles AR. Reprogramming T cells: the role of extracellular matrix in coordination of T cell activation and migration. Curr Opin Immunol. 2001;13:286–290. doi: 10.1016/s0952-7915(00)00217-x. [DOI] [PubMed] [Google Scholar]
- 16.Richter MV, Topham DJ. The alpha1beta1 integrin and TNF receptor II protect airway CD8+ effector T cells from apoptosis during influenza infection. J Immunol. 2007;179:5054–5063. doi: 10.4049/jimmunol.179.8.5054. [DOI] [PubMed] [Google Scholar]
- 17.Thatte J, Dabak V, Williams MB, Braciale TJ, Ley K. LFA-1 is required for retention of effector CD8 T cells in mouse lungs. Blood. 2003;101:4916–4922. doi: 10.1182/blood-2002-10-3159. [DOI] [PubMed] [Google Scholar]
- 18.Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 19.Eichelberger MC, Wang ML, Allan W, Webster RG, Doherty PC. Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J Gen Virol. 1991;72(Pt 7):1695–1698. doi: 10.1099/0022-1317-72-7-1695. [DOI] [PubMed] [Google Scholar]
- 20.Walker JA, Sakaguchi T, Matsuda Y, Yoshida T, Kawaoka Y. Location and character of the cellular enzyme that cleaves the hemagglutinin of a virulent avian influenza virus. Virology. 1992;190:278–287. doi: 10.1016/0042-6822(92)91214-f. [DOI] [PubMed] [Google Scholar]
- 21.Richter M, Ray SJ, Chapman TJ, Austin SJ, Rebhahn J, Mosmann TR, Gardner H, Kotelianski V, deFougerolles AR, Topham DJ. Collagen distribution and expression of collagen-binding alpha1beta1 (VLA-1) and alpha2beta1 (VLA-2) integrins on CD4 and CD8 T cells during influenza infection. J Immunol. 2007;178:4506–4516. doi: 10.4049/jimmunol.178.7.4506. [DOI] [PubMed] [Google Scholar]
- 22.Abraham WM, Ahmed A, Serebriakov I, Carmillo AN, Ferrant J, de Fougerolles AR, Garber EA, Gotwals PJ, Koteliansky VE, Taylor F, Lobb RR. A monoclonal antibody to alpha1beta1 blocks antigen-induced airway responses in sheep. Am J Respir Crit Care Med. 2004;169:97–104. doi: 10.1164/rccm.200304-543OC. [DOI] [PubMed] [Google Scholar]
- 23.de Fougerolles AR, Sprague AG, Nickerson-Nutter CL, Chi-Rosso G, Rennert PD, Gardner H, Gotwals PJ, Lobb RR, Koteliansky VE. Regulation of inflammation by collagen-binding integrins alpha1beta1 and alpha2beta1 in models of hypersensitivity and arthritis. J Clin Invest. 2000;105:721–729. doi: 10.1172/JCI7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorucci S, Mencarelli A, Palazzetti B, Sprague AG, Distrutti E, Morelli A, Novobrantseva TI, Cirino G, Koteliansky VE, de Fougerolles AR. Importance of innate immunity and collagen binding integrin alpha1beta1 in TNBS-induced colitis. Immunity. 2002;17:769–780. doi: 10.1016/s1074-7613(02)00476-4. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Horin S, Bank I. The role of very late antigen-1 in immune-mediated inflammation. Clin Immunol. 2004;113:119–129. doi: 10.1016/j.clim.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, Kitao T, Takagi J, Rennert PD, Kolodkin AL, Kumanogoh A, Kikutani H. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 27.Braun RK, Foerster M, Grahmann PR, Haefner D, Workalemahu G, Kroegel C. Phenotypic and molecular characterization of CD103+ CD4+ T cells in bronchoalveolar lavage from patients with interstitial lung diseases. Cytometry B Clin Cytom. 2003;54:19–27. doi: 10.1002/cyto.b.10021. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein I, Ben-Horin S, Li J, Bank I, Jiang H, Chess L. Expression of the alpha1beta1 integrin, VLA-1, marks a distinct subset of human CD4+ memory T cells. J Clin Invest. 2003;112:1444–1454. doi: 10.1172/JCI19607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 31.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 32.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 33.Chapman TJ, Castrucci MR, Padrick RC, Bradley LM, Topham DJ. Antigen-specific and non-specific CD4+ T cell recruitment and proliferation during influenza infection. Virology. 2005;340:296–306. doi: 10.1016/j.virol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 34.Thomas PG, Brown SA, Yue W, So J, Webby RJ, Doherty PC. An unexpected antibody response to an engineered influenza virus modifies CD8+ T cell responses. Proc Natl Acad Sci U S A. 2006;103:2764–2769. doi: 10.1073/pnas.0511185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein I, Ben-Horin S, Koltakov A, Chermoshnuk H, Polevoy V, Berkun Y, Amariglio N, Bank I. alpha1beta1 Integrin+ and regulatory Foxp3+ T cells constitute two functionally distinct human CD4+ T cell subsets oppositely modulated by TNFalpha blockade. J Immunol. 2007;178:201–210. doi: 10.4049/jimmunol.178.1.201. [DOI] [PubMed] [Google Scholar]
- 36.Bank I, Book M, Ware R. Functional role of VLA-1 (CD49A) in adhesion, cation-dependent spreading, and activation of cultured human T lymphocytes. Cell Immunol. 1994;156:424–437. doi: 10.1006/cimm.1994.1187. [DOI] [PubMed] [Google Scholar]
- 37.Bank I, Koltakov A, Goldstein I, Chess L. Lymphocytes expressing alpha1beta1 integrin (very late antigen-1) in peripheral blood of patients with arthritis are a subset of CD45RO(+) T-cells primed for rapid adhesion to collagen IV. Clin Immunol. 2002;105:247–258. doi: 10.1006/clim.2002.5286. [DOI] [PubMed] [Google Scholar]
- 38.Bank I, Rapman E, Shapiro R, Schiby G, Goldberg I, Barzilai A, Trau H, Gur H. The epidermotropic mycosis fungoides associated alpha1beta1 integrin (VLA-1, CD49a/CD29) is primarily a collagen IV receptor on malignant T cells. J Cutan Pathol. 1999;26:65–71. doi: 10.1111/j.1600-0560.1999.tb01804.x. [DOI] [PubMed] [Google Scholar]
- 39.Roberts AI, Brolin RE, Ebert EC. Integrin alpha1beta1 (VLA-1) mediates adhesion of activated intraepithelial lymphocytes to collagen. Immunology. 1999;97:679–685. doi: 10.1046/j.1365-2567.1999.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eichelberger M, McMickle A, Blackman M, Mombaerts P, Tonegawa S, Doherty PC. Functional analysis of the TCR alpha- beta+ cells that accumulate in the pneumonic lung of influenza virus-infected TCR-alpha−/− mice. J Immunol. 1995;154:1569–1576. [PubMed] [Google Scholar]
- 41.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 42.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 43.Meharra EJ, Schon M, Hassett D, Parker C, Havran W, Gardner H. Reduced gut intraepithelial lymphocytes in VLA1 null mice. Cell Immunol. 2000;201:1–5. doi: 10.1006/cimm.2000.1630. [DOI] [PubMed] [Google Scholar]
- 44.Wang XZ, Stepp SE, Brehm MA, Chen HD, Selin LK, Welsh RM. Virus-specific CD8 T cells in peripheral tissues are more resistant to apoptosis than those in lymphoid organs. Immunity. 2003;18:631–642. doi: 10.1016/s1074-7613(03)00116-x. [DOI] [PubMed] [Google Scholar]
- 45.Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- 46.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein I, Simon AJ, Ben Horin S, Matzri S, Koltakov A, Langevitz P, Rechavi G, Amariglio N, Bank I. Synovial VLA-1+ T cells display an oligoclonal and partly distinct repertoire in rheumatoid and psoriatic arthritis. Clin Immunol. 2008;128:75–84. doi: 10.1016/j.clim.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- 49.Topham DJ, Doherty PC. Clearance of an influenza A virus by CD4+ T cells is inefficient in the absence of B cells. J Virol. 1998;72:882–885. doi: 10.1128/jvi.72.1.882-885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 51.Jellison ER, Kim SK, Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174:614–618. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 52.Zhong W, Marshall D, Coleclough C, Woodland DL. CD4+ T cell priming accelerates the clearance of Sendai virus in mice, but has a negative effect on CD8+ T cell memory. J Immunol. 2000;164:3274–3282. doi: 10.4049/jimmunol.164.6.3274. [DOI] [PubMed] [Google Scholar]